The Influence of Seasonal Freeze–Thaw in Northeast China on Greenhouse Gas Emissions and Microbial Community Structure in Peat Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Frozen Soil Samples
2.2. Soil Freeze–Thaw Experiment
2.3. Determination of Soil’s Soluble Organic Carbon Content
2.4. Determination of Greenhouse Gas Emissions from Soil
2.5. Microbial Sampling and Detection Methods
2.6. Analytical Statistical Methods
3. Results and Discussion
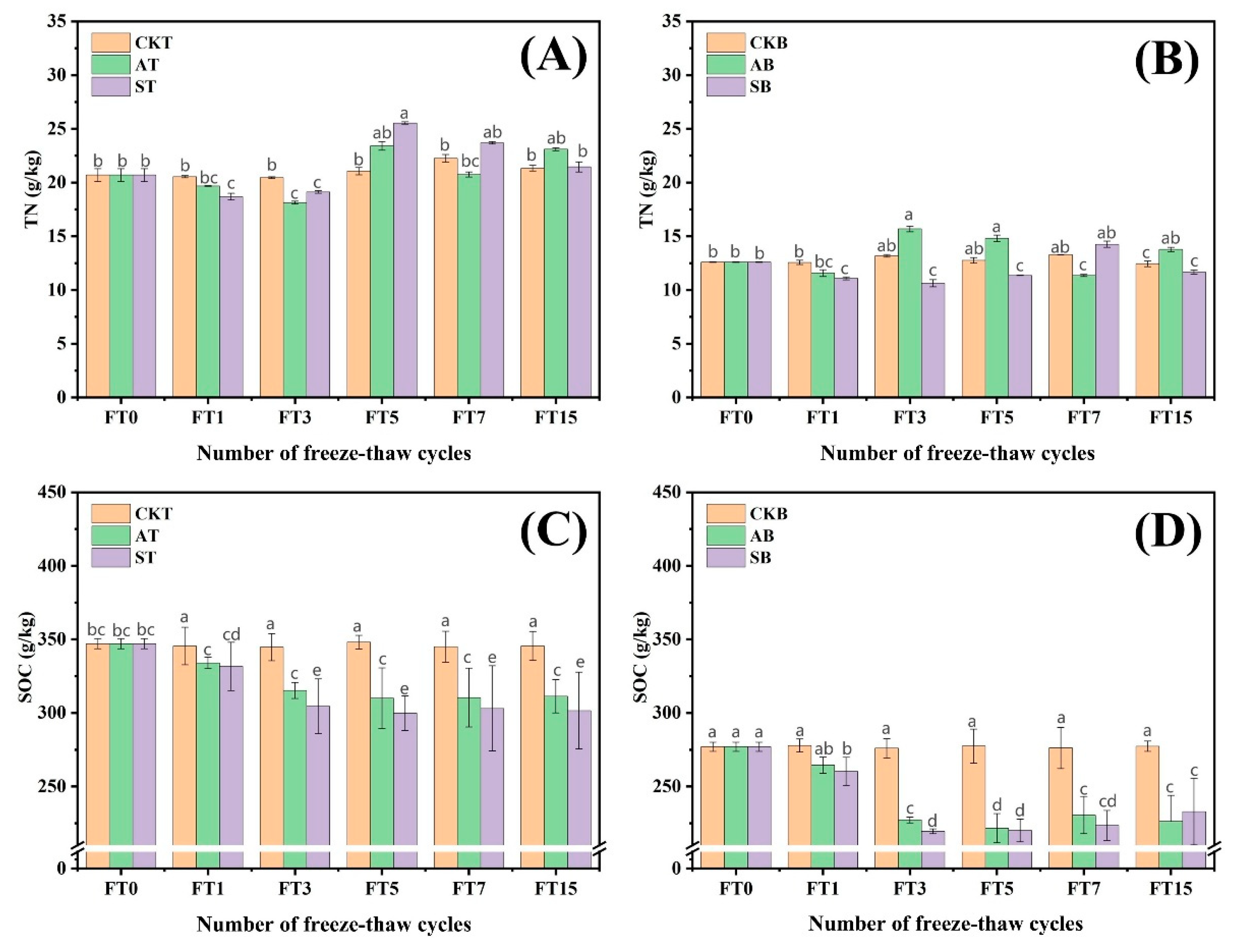
3.1. Seasonal Freeze–Thaw Changes the Carbon and Nitrogen Content in Peat Soil
3.1.1. Total Nitrogen, Ammonium Nitrogen, and Nitrate Nitrogen
3.1.2. Total Organic Carbon Content
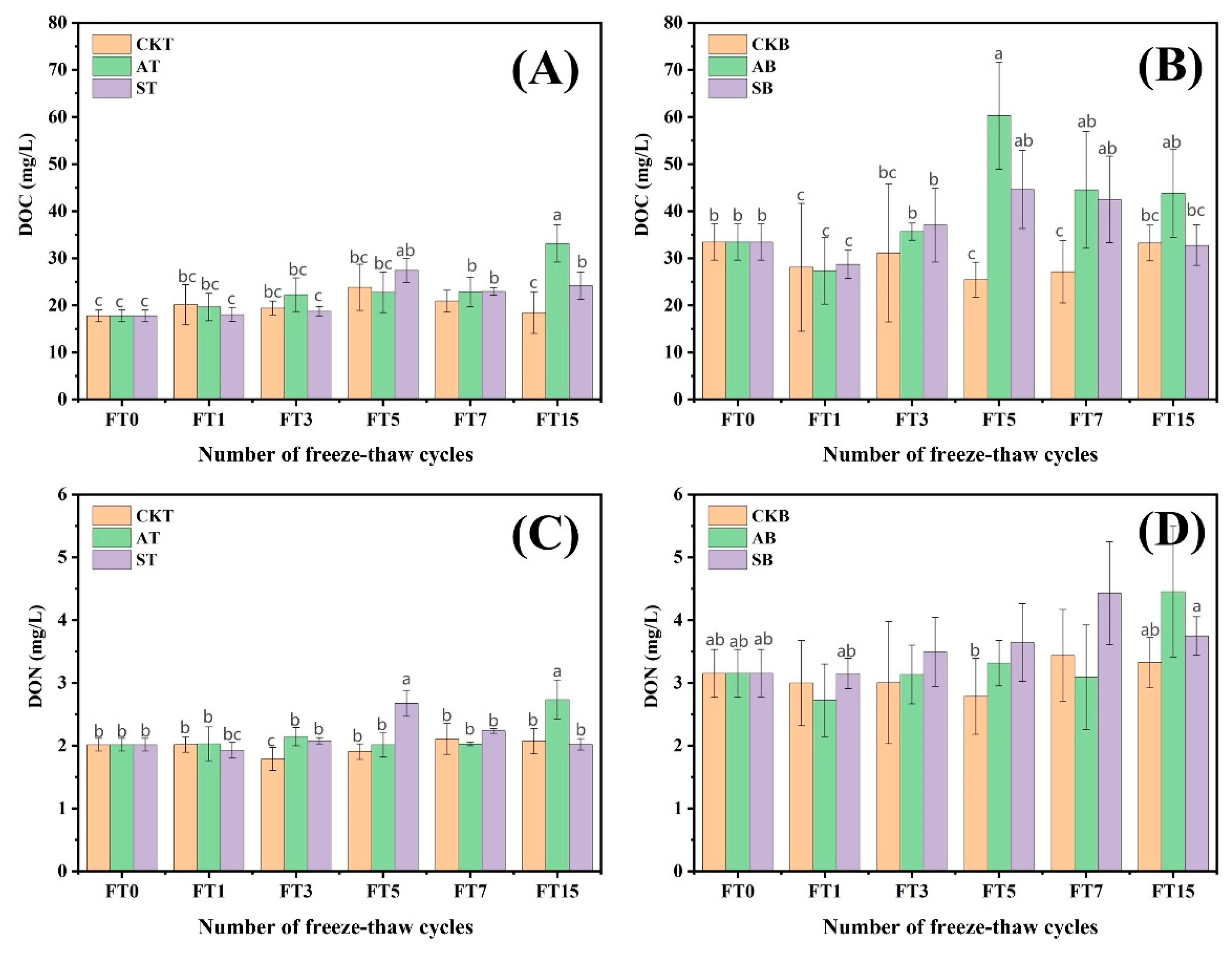
3.1.3. Soluble Organic Carbon and Soluble Organic Nitrogen
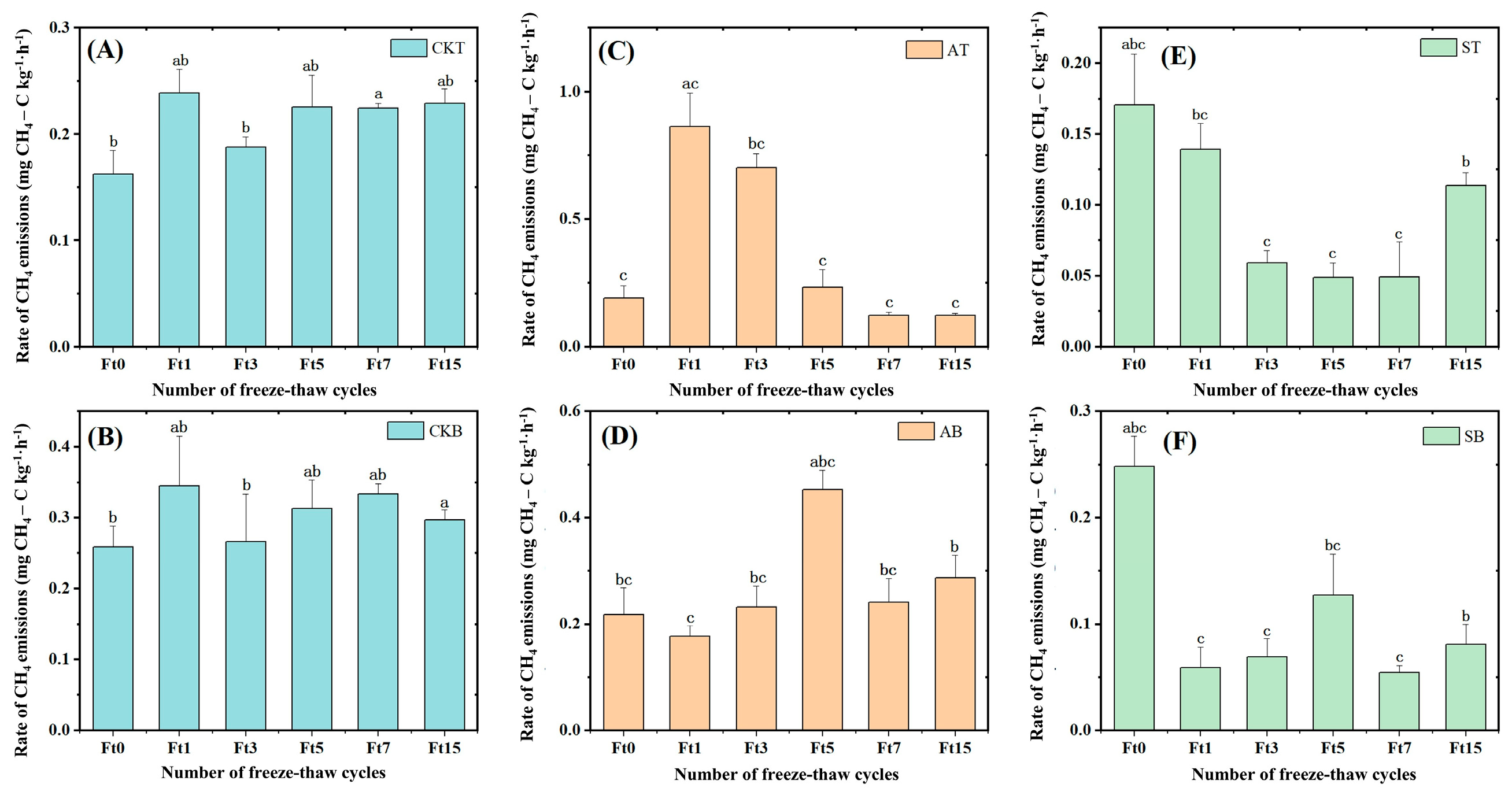
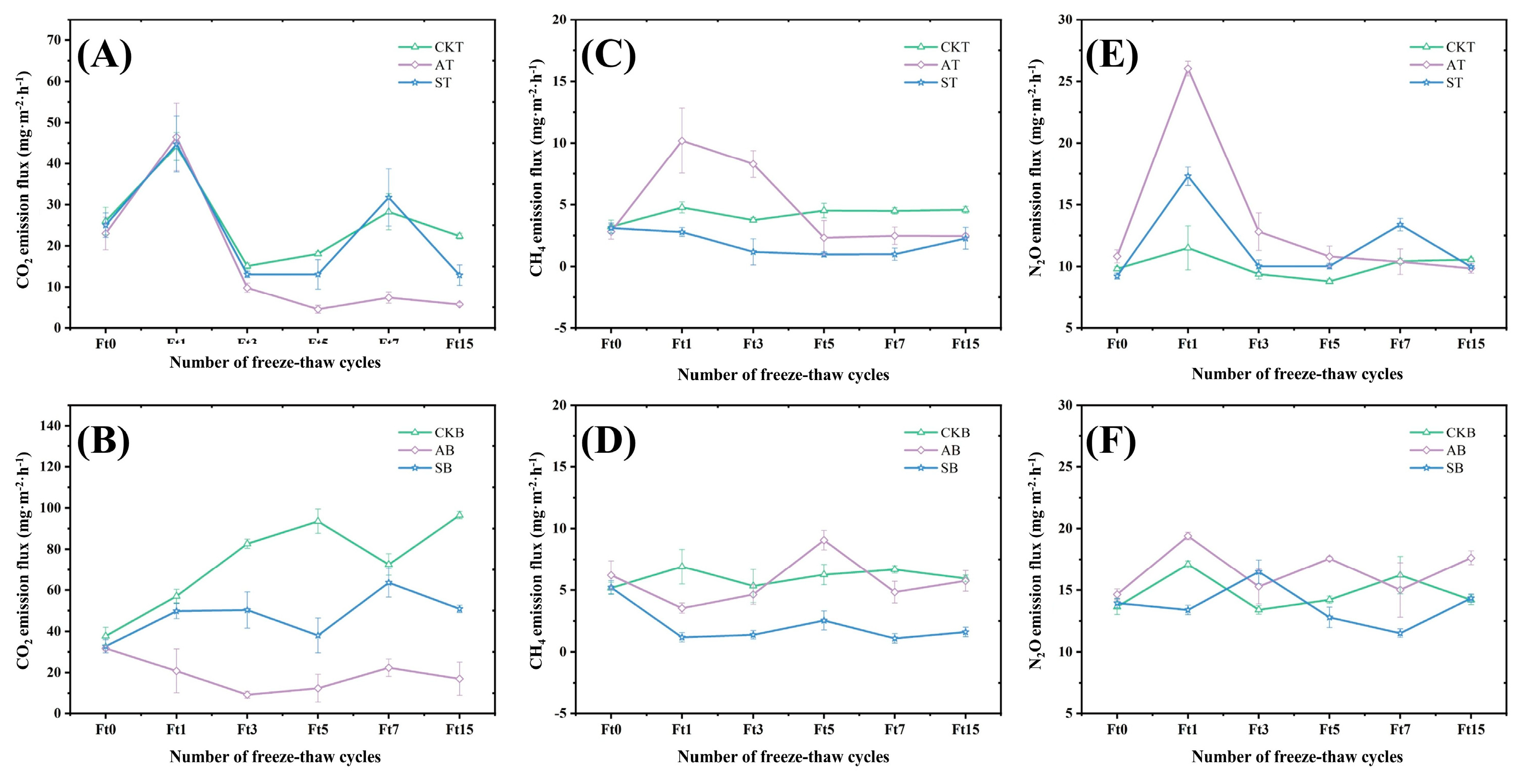
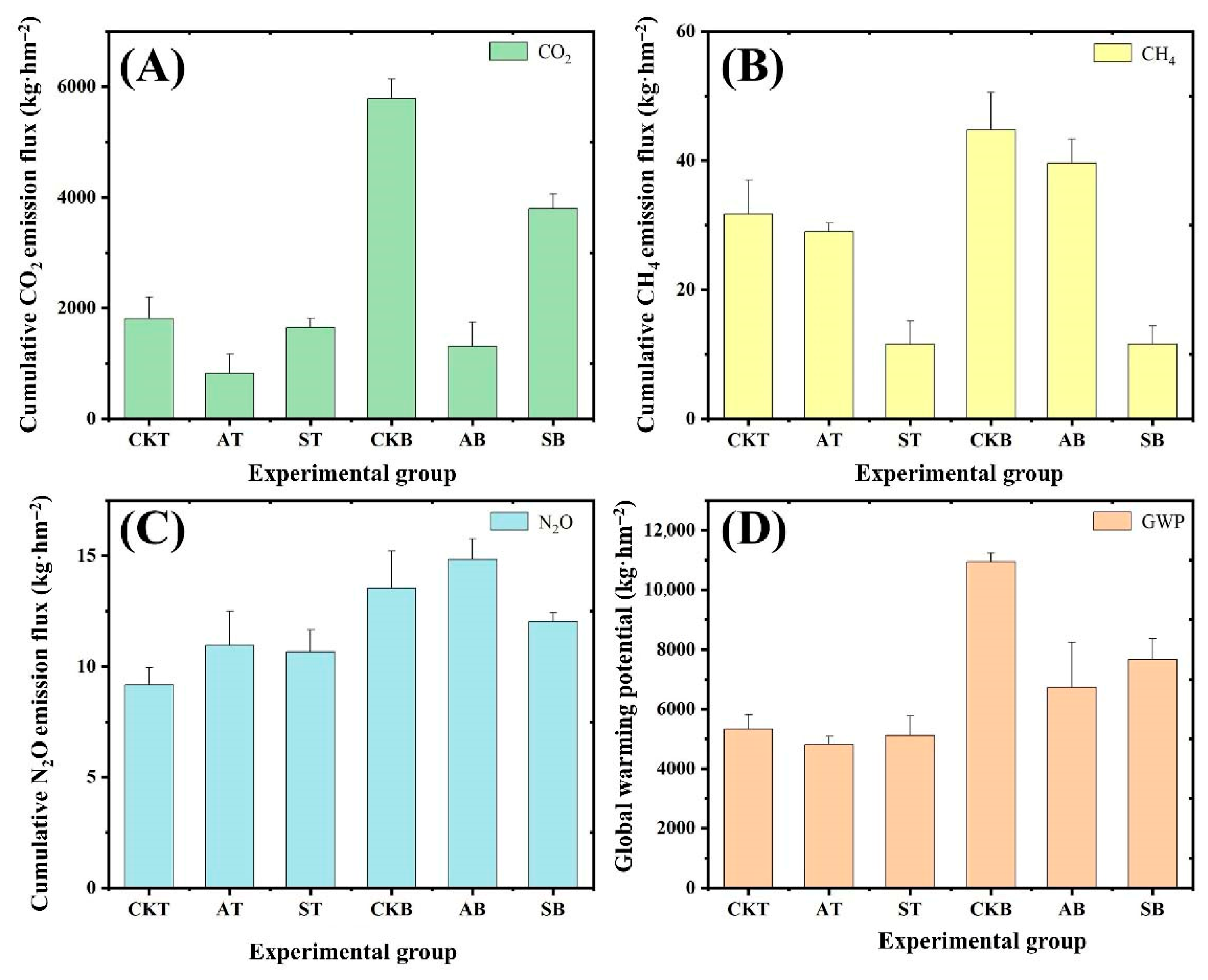
3.2. The Freeze–Thaw Cycle Suppresses Greenhouse Gas Emissions from Peat Soil
3.3. The Freeze–Thaw Cycle Reduces the Global Warming Potential of Greenhouse Gases in Peat Soil in Seasonally Frozen Ground Regions
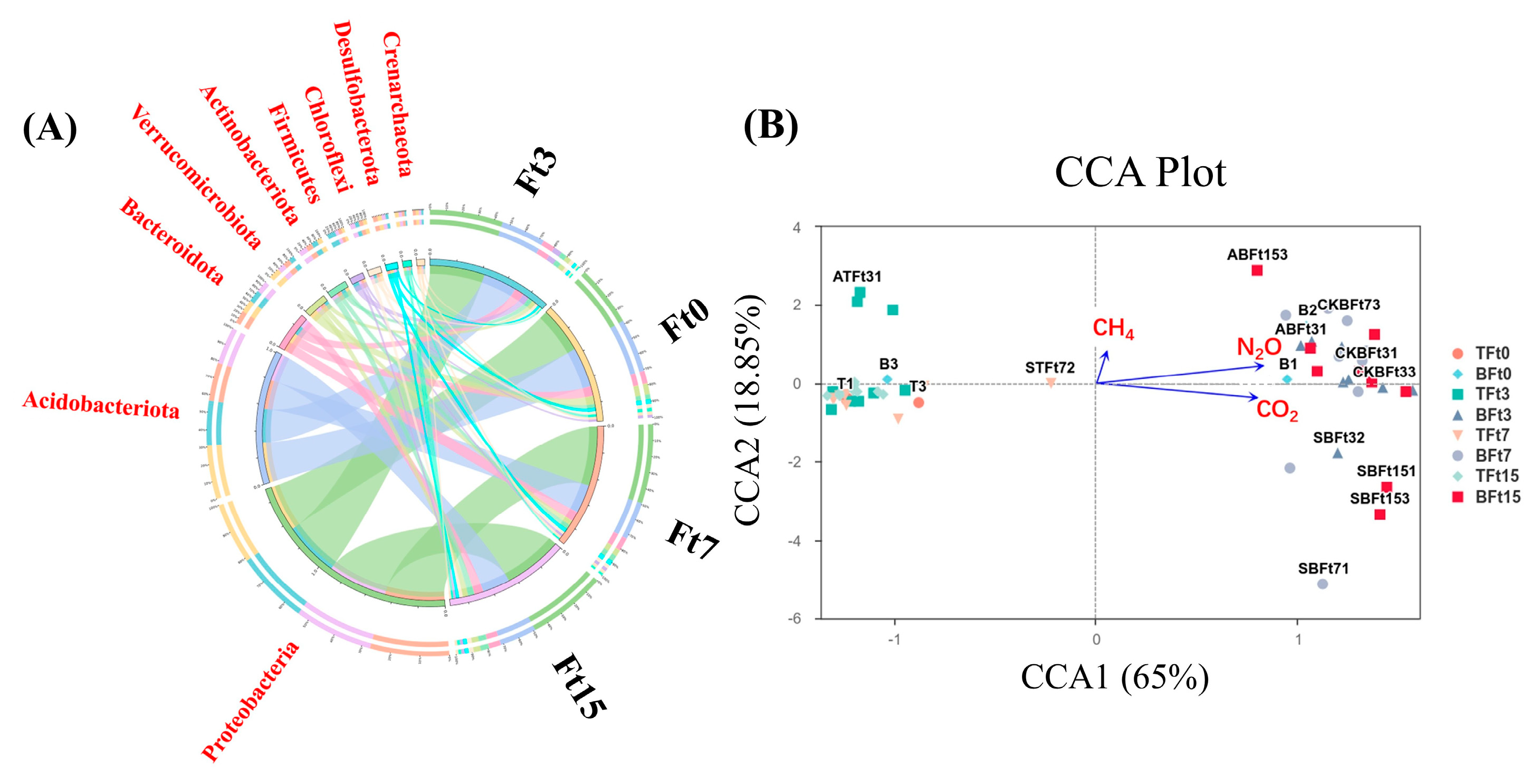
3.4. The Freeze–Thaw Cycle Has Changed the Microbial Community Structure of Peat Soil
3.5. The Relationship Between Soil Microbial Community Structure and Greenhouse Gas Emissions
3.6. Prediction and Analysis of Functional Pathways of Soil Microbial Communities Under Seasonal Freezing and Thawing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Grogan, P.; Michelsen, A.; Ambus, P.; Jonasson, S. Freeze-thaw regime effects on carbon and nitrogen dynamics in sub-arctic heath tundra mesocosms. Soil Biol. Biochem. 2004, 36, 641–654. [Google Scholar] [CrossRef]
- Yu, X.F.; Zou, Y.C.; Jiang, M.; Lu, X.G.; Wang, G.P. Response of soil constituents to freeze-thaw cycles in wetland soil solution. Soil Biol. Biochem. 2011, 43, 1308–1320. [Google Scholar] [CrossRef]
- Maljanen, M.; Hytönen, J.; Martikainen, P.J. Cold-season nitrous oxide dynamics in a drained boreal peatland differ depending on land-use practice. Can. J. For. Res. 2010, 40, 565–572. [Google Scholar] [CrossRef]
- Risk, N.; Snider, D.; Wagner-Riddle, C. Mechanisms leading to enhanced soil nitrous oxide fluxes induced by freeze-thaw cycles. Can. J. Soil Sci. 2013, 93, 401–414. [Google Scholar] [CrossRef]
- Cong, J.X.; Gao, C.Y.; Zhao, H.Y.; Han, D.X.; Meng, F.; Wang, G.P. Chemical stability of carbon pool in peatlands dominated by different plant types in Jilin province (China) and its potential influencing factors. Front. Ecol. Evol. 2023, 11, 12. [Google Scholar] [CrossRef]
- Gorham, E.; Lehman, C.; Dyke, A.; Clymo, D.; Janssens, J. Long-term carbon sequestration in North American peatlands. Quat. Sci. Rev. 2012, 58, 77–82. [Google Scholar] [CrossRef]
- Cong, J.X.; Gao, C.Y.; Han, D.X.; Li, Y.H.; Wang, G.P. Stability of the permafrost peatlands carbon pool under climate change and wildfires during the last 150 years in the northern Great Khingan Mountains, China. Sci. Total Environ. 2020, 712, 136476. [Google Scholar] [CrossRef]
- Groenevelt, P.H.; Grant, C.D. Heave and Heaving Pressure in Freezing Soils: A Unifying Theory. Vadose Zone J. 2013, 12, 11. [Google Scholar] [CrossRef]
- Xia, H.; Peng, Y.; Yan, W.; Ning, W. Effect of Snow Depth and Snow Duration on Soil N Dynamics and Microbial Activity in the Alpine Areas of the Eastern Tibetan Plateau. Russ. J. Ecol. 2014, 45, 263–268. [Google Scholar] [CrossRef]
- Feng, X.; Xia, X.; Chen, S.T.; Lin, Q.M.; Zhang, X.H.; Cheng, K.; Liu, X.Y.; Bian, R.J.; Zheng, J.F.; Li, L.Q.; et al. Amendment of crop residue in different forms shifted micro-pore system structure and potential functionality of macroaggregates while changed their mass proportion and carbon storage of paddy topsoil. Geoderma 2022, 409, 13. [Google Scholar] [CrossRef]
- Zhou, W.J.; Wang, Q.Z.; Fang, J.H.; Wang, K.J.; Zhao, X.Q. Study of the Mechanical and Microscopic Properties of Modified Silty Clay under Freeze-Thaw Cycles. Geofluids 2022, 2022, 9613176. [Google Scholar] [CrossRef]
- Matzner, E.; Borken, W. Do freeze-thaw events enhance C and N losses from soils of different ecosystems? A review. Eur. J. Soil Sci. 2008, 59, 274–284. [Google Scholar] [CrossRef]
- Wu, X.; Brüggemann, N.; Gasche, R.; Shen, Z.Y.; Wolf, B.; Butterbach-Bahl, K. Environmental controls over soil-atmosphere exchange of N2O, NO, and CO2 in a temperate Norway spruce forest. Glob. Biogeochem. Cycle 2010, 24, 16. [Google Scholar] [CrossRef]
- Brooks, P.D.; Schmidt, S.K.; Williams, M.W. Winter production of CO2 and N2O from Alpine tundra: Environmental controls and relationship to inter-system C and N fluxes. Oecologia 1997, 110, 403–413. [Google Scholar] [CrossRef]
- Elberling, B.; Brandt, K.K. Uncoupling of microbial CO2 production and release in frozen soil and its implications for field studies of arctic C cycling. Soil Biol. Biochem. 2003, 35, 263–272. [Google Scholar] [CrossRef]
- Wu, X.; Brüggemann, N.; Butterbach-Bahl, K.; Fu, B.J.; Liu, G.H. Snow cover and soil moisture controls of freeze-thaw-related soil gas fluxes from a typical semi-arid grassland soil: A laboratory experiment. Biol. Fertil. Soils 2014, 50, 295–306. [Google Scholar] [CrossRef]
- Panikov, N.S.; Dedysh, S.N. Cold season CH4 and CO2 emission from boreal peat bogs (West Siberia): Winter fluxes and thaw activation dynamics. Glob. Biogeochem. Cycle 2000, 14, 1071–1080. [Google Scholar] [CrossRef]
- Herrmann, A.; Witter, E. Sources of C and N contributing to the flush in mineralization upon freeze-thaw cycles in soils. Soil Biol. Biochem. 2002, 34, 1495–1505. [Google Scholar] [CrossRef]
- Groffman, P.M.; Driscoll, C.T.; Fahey, T.J.; Hardy, J.P.; Fitzhugh, R.D.; Tierney, G.L. Effects of mild winter freezing on soil nitrogen and carbon dynamics in a northern hardwood forest. Biogeochemistry 2001, 56, 191–213. [Google Scholar] [CrossRef]
- Hanson, R.S.; Hanson, T.E. Methanotrophic bacteria. Microbiol. Rev. 1996, 60, 439–471. [Google Scholar] [CrossRef]
- Thauer, R.K. Biochemistry of methanogenesis: A tribute to Marjory!Stephenson. Microbiology 1998, 144, 2377–2406. [Google Scholar] [CrossRef]
- Müller, C.; Martin, M.; Stevens, R.J.; Laughlin, R.J.; Kammann, C.; Ottow, J.C.G.; Jäger, H.J. Processes leading to N2O emissions in grassland soil during freezing and thawing. Soil Biol. Biochem. 2002, 34, 1325–1331. [Google Scholar] [CrossRef]
- Costello, E.K.; Schmidt, S.K. Microbial diversity in alpine tundra wet meadow soil: Novel Chloroflexi from a cold, water-saturated environment. Environ. Microbiol. 2006, 8, 1471–1486. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.Q.; Wang, M.; Zhou, J.H.; Huang, Y.J.; Zhang, J.; Wang, S.Z. Soil bacteria, archaea, and enzymatic activity of natural and rewetted peatlands display varying patterns in response to water levels. Catena 2023, 228, 10. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Yang, W.Q.; Wu, F.Z.; Xu, Z.F.; Tan, B.; Zhang, L.; He, X.H.; Guo, L. Canopy gaps accelerate soil organic carbon retention by soil microbial biomass in the organic horizon in a subalpine fir forest. Appl. Soil Ecol. 2018, 125, 169–176. [Google Scholar] [CrossRef]
- Chang, X.L.; Jin, H.J.; Zhang, Y.L.; He, R.X.; Luo, D.L.; Wang, Y.P.; Lü, L.Z.; Zhang, Q.L. Thermal impacts of boreal forest vegetation on active layer and permafrost soils in northern Da Xing’anling (Hinggan) Mountains, Northeast China. Arct. Antarct. Alp. Res. 2015, 47, 267–279. [Google Scholar] [CrossRef]
- Wang, X.; Bai, X.Y.; Ma, L.; He, C.G.; Jiang, H.B.; Sheng, L.X.; Luo, W.B. Snow depths’ impact on soil microbial activities and carbon dioxide fluxes from a temperate wetland in Northeast China. Sci. Rep. 2020, 10, 11962. [Google Scholar] [CrossRef]
- Lang, M.; Cai, Z.C.; Chang, S.X. Effects of land use type and incubation temperature on greenhouse gas emissions from Chinese and Canadian soils. J. Soils Sediments 2011, 11, 15–24. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Stange, F.; Papen, H.; Li, C.S. Regional inventory of nitric oxide and nitrous oxide emissions for forest soils of southeast Germany using the biogeochemical model PnET-N-DNDC. J. Geophys. Res.-Atmos. 2001, 106, 34155–34166. [Google Scholar] [CrossRef]
- Vinther, F.P.; Eiland, F.; Lind, A.M.; Elsgaard, L. Microbial biomass and numbers of denitrifiers related to macropore channels in agricultural and forest soils. Soil Biol. Biochem. 1999, 31, 603–611. [Google Scholar] [CrossRef]
- Kværno, S.H.; Oygarden, L. The influence of freeze-thaw cycles and soil moisture on aggregate stability of three soils in Norway. Catena 2006, 67, 175–182. [Google Scholar] [CrossRef]
- Lovett, G.M.; Mitchell, M.J. Sugar maple and nitrogen cycling in the forests of eastern North America. Front. Ecol. Environ. 2004, 2, 81–88. [Google Scholar] [CrossRef]
- Christopher, S.F.; Shibata, H.; Ozawa, M.; Nakagawa, Y.; Mitchell, M.J. The effect of soil freezing on N cycling: Comparison of two headwater subcatchments with different vegetation and snowpack conditions in the northern Hokkaido Island of Japan. Biogeochemistry 2008, 88, 15–30. [Google Scholar] [CrossRef]
- Deluca, T.H.; Keeney, D.R.; McCarty, G.W. Effect of freeze-thaw events on mineralization of soil nitrogen. Biol. Fertil. Soils 1992, 14, 116–120. [Google Scholar] [CrossRef]
- Zhang, X.; Bai, W.; Gilliam, F.S.; Wang, Q.; Han, X.; Li, L. Effects of in situ freezing on soil net nitrogen mineralization and net nitrification in fertilized grassland of northern China. Grass Forage Sci. 2011, 66, 391–401. [Google Scholar] [CrossRef]
- Song, Y.Z.; Song, T.J.; An, Y.; Shan, L.P.; Su, X.S.; Yu, S.D. Soil ecoenzyme activities coupled with soil properties and plant biomass strongly influence the variation in soil organic carbon components in semi-arid degraded wetlands. Sci. Total Environ. 2024, 922, 11. [Google Scholar] [CrossRef] [PubMed]
- Mikan, C.J.; Schimel, J.P.; Doyle, A.P. Temperature controls of microbial respiration in arctic tundra soils above and below freezing. Soil Biol. Biochem. 2002, 34, 1785–1795. [Google Scholar] [CrossRef]
- Allaire, S.E.; Roulier, S.; Cessna, A.J. Quantifying preferential flow in soils: A review of different techniques. J. Hydrol. 2009, 378, 179–204. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Lal, R. Mechanisms of carbon sequestration in soil aggregates. Crit. Rev. Plant Sci. 2004, 23, 481–504. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, Q.; Li, J.; Jian, S.G.; Ren, H. Mangrove succession enriches the sediment microbial community in South China. Sci. Rep. 2016, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Holtan-Hartwig, L.; Dörsch, P.; Bakken, L.R. Low temperature control of soil denitrifying communities: Kinetics of N2O production and reduction. Soil Biol. Biochem. 2002, 34, 1797–1806. [Google Scholar] [CrossRef]
- Goldberg, S.D.; Borken, W.; Gebauer, G. N2O emission in a Norway spruce forest due to soil frost: Concentration and isotope profiles shed a new light on an old story. Biogeochemistry 2010, 97, 21–30. [Google Scholar] [CrossRef]
- Kariyapperuma, K.A.; Furon, A.; Wagner-Riddle, C. Non-growing season nitrous oxide fluxes from an agricultural soil as affected by application of liquid and composted swine manure. Can. J. Soil Sci. 2012, 92, 315–327. [Google Scholar] [CrossRef]
- Hobbie, S.E.; Nadelhoffer, K.J.; Högberg, P. A synthesis: The role of nutrients as constraints on carbon balances in boreal and arctic regions. Plant Soil 2002, 242, 163–170. [Google Scholar] [CrossRef]
- Conant, R.T.; Steinweg, J.M.; Haddix, M.L.; Paul, E.A.; Plante, A.F.; Six, J. Experimental warming shows that decomposition temperature sensitivity increases with soil organic matter recalcitrance. Ecology 2008, 89, 2384–2391. [Google Scholar] [CrossRef]
- Robroek, B.J.M.; Heijboer, A.; Jassey, V.E.J.; Hefting, M.M.; Rouwenhorst, T.G.; Buttler, A.; Bragazza, L. Snow cover manipulation effects on microbial community structure and soil chemistry in a mountain bog. Plant Soil 2013, 369, 151–164. [Google Scholar] [CrossRef]
- Tokida, T.; Mizoguchi, M.; Miyazaki, T.; Kagemoto, A.; Nagata, O.; Hatano, R. Episodic release of methane bubbles from peatland during spring thaw. Chemosphere 2007, 70, 165–171. [Google Scholar] [CrossRef]
- Dutaur, L.; Verchot, L.V. A global inventory of the soil CH4 sink. Glob. Biogeochem. Cycle 2007, 21, 7949–7950. [Google Scholar] [CrossRef]
- Campbell, J.L.; Socci, A.M.; Templer, P.H. Increased nitrogen leaching following soil freezing is due to decreased root uptake in a northern hardwood forest. Glob. Change Biol. 2014, 20, 2663–2673. [Google Scholar] [CrossRef]
- Smith, J.; Wagner-Riddle, C.; Dunfield, K. Season and management related changes in the diversity of nitrifying and denitrifying bacteria over winter and spring. Appl. Soil Ecol. 2010, 44, 138–146. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, Y.; Yang, T.; Yan, J.; Yu, X. The Influence of Seasonal Freeze–Thaw in Northeast China on Greenhouse Gas Emissions and Microbial Community Structure in Peat Soil. Water 2025, 17, 2395. https://doi.org/10.3390/w17162395
Gong Y, Yang T, Yan J, Yu X. The Influence of Seasonal Freeze–Thaw in Northeast China on Greenhouse Gas Emissions and Microbial Community Structure in Peat Soil. Water. 2025; 17(16):2395. https://doi.org/10.3390/w17162395
Chicago/Turabian StyleGong, Yanru, Tao Yang, Jiawen Yan, and Xiaofei Yu. 2025. "The Influence of Seasonal Freeze–Thaw in Northeast China on Greenhouse Gas Emissions and Microbial Community Structure in Peat Soil" Water 17, no. 16: 2395. https://doi.org/10.3390/w17162395
APA StyleGong, Y., Yang, T., Yan, J., & Yu, X. (2025). The Influence of Seasonal Freeze–Thaw in Northeast China on Greenhouse Gas Emissions and Microbial Community Structure in Peat Soil. Water, 17(16), 2395. https://doi.org/10.3390/w17162395





