Diatom Biosilica: A Useful Natural Material for Biomedical Engineering
Abstract
1. Introduction
2. Biological Properties of DB and Its Biofabrication
2.1. DB Biosynthesis
2.2. Ecological Functions of DB
2.3. Environmental Stimuli for DB
2.4. Purification
3. Application of DB in DDS
3.1. Sustained Drug Release
3.2. Targeted Delivery
3.3. Biodegradability
4. Use of DB in Biosensor
4.1. Electrochemical Signal Detection
4.2. Optical Signal Detection
4.3. Fluorescent Signal Detection
5. Theragnosis
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rozan, H.E.; Wu, G.; Zhou, Z.; Li, Q.; Sharaf, M.; Chen, X. The complex hydrogel based on diatom biosilica and hydroxybutyl chitosan for wound healing. Colloids Surf. B Biointerfaces 2022, 216, 112523. [Google Scholar] [CrossRef]
- Dalgic, A.D.; Atila, D.; Tezcaner, A.; Gürses, S.; Keskin, D. Diatom silica frustules-doped fibers for controlled release of melatonin for bone regeneration. Eur. Polym. J. 2023, 186, 111858. [Google Scholar] [CrossRef]
- Dolganyuk, V.; Belova, D.; Babich, O.; Prosekov, A.; Ivanova, S.; Katserov, D.; Patyukov, N.; Sukhikh, S. Microalgae: A Promising Source of Valuable Bioproducts. Biomolecules 2020, 10, 1153. [Google Scholar] [CrossRef] [PubMed]
- Calijuri, M.L.; Silva, T.A.; Magalhães, I.B.; de Paula Pereira, A.S.A.; Marangon, B.B.; de Assis, L.R.; Lorentz, J.F. Bioproducts from Microalgae Biomass: Technology, Sustainability, Challenges and Opportunities. Chemosphere 2022, 305, 135508. [Google Scholar] [CrossRef] [PubMed]
- Siddiki, S.Y.A.; Mofijur, M.; Kumar, P.S.; Ahmed, S.F.; Inayat, A.; Kusumo, F.; Badruddin, I.A.; Khan, T.M.Y.; Nghiem, L.D.; Ong, H.C. Microalgae Biomass as a Sustainable Source for Biofuel, Biochemical and Biobased Value-Added Products: An Integrated Biorefinery Concept. Fuel 2022, 307, 121782. [Google Scholar] [CrossRef]
- Gautam, P.; Upadhyay, S.N.; Dubey, S.K. Bio-Methanol as a Renewable Fuel from Waste Biomass: Current Trends and Future Perspective. Fuel 2020, 273, 117783. [Google Scholar] [CrossRef]
- Hussein, H.A.; Nazir, M.S.; Azra, N.; Qamar, Z.; Seeni, A.; Tengku Din, T.A.D.A.-A.; Abdullah, M.A. Novel Drug and Gene Delivery System and Imaging Agent Based on Marine Diatom Biosilica Nanoparticles. Mar. Drugs 2022, 20, 480. [Google Scholar] [CrossRef]
- Kröger, N.; Poulsen, N. Diatoms—From Cell Wall Biogenesis to Nanotechnology. Annu. Rev. Genet. 2008, 42, 83–107. [Google Scholar] [CrossRef]
- Maher, S.; Kumeria, T.; Aw, M.S.; Losic, D. Diatom Silica for Biomedical Applications: Recent Progress and Advances. Adv. Heal. Mater. 2018, 7, 1800552. [Google Scholar] [CrossRef]
- Bak, M.; Molnár, F.; Rákosa, R.; Németh, Z.; Németh, R. Dimensional Stabilization of Wood by Microporous Silica Aerogel Using In-Situ Polymerization. Wood Sci. Technol. 2022, 56, 1353–1375. [Google Scholar] [CrossRef]
- Terracciano, M.; De Stefano, L.; Rea, I. Diatoms Green Nanotechnology for Biosilica-Based Drug Delivery Systems. Pharmaceutics 2018, 10, 242. [Google Scholar] [CrossRef]
- Sardo, A.; Orefice, I.; Balzano, S.; Barra, L.; Romano, G. Mini-Review: Potential of Diatom-Derived Silica for Biomedical Applications. Appl. Sci. 2021, 11, 4533. [Google Scholar] [CrossRef]
- Delalat, B.; Sheppard, V.C.; Rasi Ghaemi, S.; Rao, S.; Prestidge, C.A.; McPhee, G.; Rogers, M.-L.; Donoghue, J.F.; Pillay, V.; Johns, T.G. Targeted Drug Delivery Using Genetically Engineered Diatom Biosilica. Nat. Commun. 2015, 6, 8791. [Google Scholar] [CrossRef]
- Uthappa, U.T.; Brahmkhatri, V.; Sriram, G.; Jung, H.-Y.; Yu, J.; Kurkuri, N.; Aminabhavi, T.M.; Altalhi, T.; Neelgund, G.M.; Kurkuri, M.D. Nature Engineered Diatom Biosilica as Drug Delivery Systems. J. Control. Release 2018, 281, 70–83. [Google Scholar] [CrossRef]
- Liu, Q.; Cai, W.; Zhen, T.; Ji, N.; Dai, L.; Xiong, L.; Sun, Q. Preparation of Debranched Starch Nanoparticles by Ionic Gelation for Encapsulation of Epigallocatechin Gallate. Int. J. Biol. Macromol. 2020, 161, 481–491. [Google Scholar] [CrossRef]
- Zhu, H.; Zhu, E.; Xie, Y.; Liu, D.; Hu, Y.; Shi, Z.; Xiong, C.; Yang, Q. Hydrangea-like Nanocellulose Microspheres with High Dye Adsorption and Drug Encapsulation Prepared by Emulsion Method. Carbohydr. Polym. 2022, 296, 119947. [Google Scholar] [CrossRef]
- Moretta, R.; De Stefano, L.; Terracciano, M.; Rea, I. Porous Silicon Optical Devices: Recent Advances in Biosensing Applications. Sensors 2021, 21, 1336. [Google Scholar] [CrossRef]
- Liao, X.; Liu, J.; Yang, M.; Ma, H.; Yuan, B.; Huang, C.-H. Evaluation of Disinfection By-Product Formation Potential (DBPFP) during Chlorination of Two Algae Species—Blue-Green Microcystis Aeruginosa and Diatom Cyclotella Meneghiniana. Sci. Total Environ. 2015, 532, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Agustí, S. Fast Adaptation of Tropical Diatoms to Increased Warming with Trade-Offs. Sci. Rep. 2018, 8, 17771. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.-M.; Zhang, S.-F.; Xie, Z.-X.; Li, D.-X.; Lin, L.; Wang, M.-H.; Wang, D.-Z. Metabolic Adaptation of a Globally Important Diatom Following 700 Generations of Selection under a Warmer Temperature. Environ. Sci. Technol. 2022, 56, 5247–5255. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Koester, J.A.; Liefer, J.D.; Irwin, A.J.; Finkel, Z.V. Molecular Mechanisms of Temperature Acclimation and Adaptation in Marine Diatoms. ISME J. 2019, 13, 2415–2425. [Google Scholar] [CrossRef]
- Delasoie, J.; Zobi, F. Natural Diatom Biosilica as Microshuttles in Drug Delivery Systems. Pharmaceutics 2019, 11, 537. [Google Scholar] [CrossRef]
- Mayzel, B.; Aram, L.; Varsano, N.; Wolf, S.G.; Gal, A. Structural Evidence for Extracellular Silica Formation by Diatoms. Nat. Commun. 2021, 12, 4639. [Google Scholar] [CrossRef]
- Tesson, B.; Hildebrand, M. Dynamics of Silica Cell Wall Morphogenesis in the Diatom Cyclotella Cryptica: Substructure Formation and the Role of Microfilaments. J. Struct. Biol. 2010, 169, 62–74. [Google Scholar] [CrossRef]
- Tesson, B.; Lerch, S.J.L.; Hildebrand, M. Characterization of a New Protein Family Associated with the Silica Deposition Vesicle Membrane Enables Genetic Manipulation of Diatom Silica. Sci. Rep. 2017, 7, 13457. [Google Scholar] [CrossRef]
- Lechner, C.C.; Becker, C.F.W. Silaffins in Silica Biomineralization and Biomimetic Silica Precipitation. Mar. Drugs 2015, 13, 5297–5333. [Google Scholar] [CrossRef] [PubMed]
- Trofimov, A.A.; Pawlicki, A.A.; Borodinov, N.; Mandal, S.; Mathews, T.J.; Hildebrand, M.; Ziatdinov, M.A.; Hausladen, K.A.; Urbanowicz, P.K.; Steed, C.A. Deep Data Analytics for Genetic Engineering of Diatoms Linking Genotype to Phenotype via Machine Learning. NPJ Comput. Mater. 2019, 5, 67. [Google Scholar] [CrossRef]
- Görlich, S.; Pawolski, D.; Zlotnikov, I.; Kröger, N. Control of Biosilica Morphology and Mechanical Performance by the Conserved Diatom Gene Silicanin-1. Commun. Biol. 2019, 2, 245. [Google Scholar] [CrossRef]
- Nemoto, M.; Iwaki, S.; Moriya, H.; Monden, Y.; Tamura, T.; Inagaki, K.; Mayama, S.; Obuse, K. Comparative Gene Analysis Focused on Silica Cell Wall Formation: Identification of Diatom-Specific SET Domain Protein Methyltransferases. Mar. Biotechnol. 2020, 22, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Hamm, C.E.; Merkel, R.; Springer, O.; Jurkojc, P.; Maier, C.; Prechtel, K.; Smetacek, V. Architecture and Material Properties of Diatom Shells Provide Effective Mechanical Protection. Nature 2003, 421, 841–843. [Google Scholar] [CrossRef]
- De Tommasi, E.; Congestri, R.; Dardano, P.; De Luca, A.C.; Managò, S.; Rea, I.; De Stefano, M. UV-Shielding and Wavelength Conversion by Centric Diatom Nanopatterned Frustules. Sci. Rep. 2018, 8, 16285. [Google Scholar] [CrossRef]
- Musenich, L.; Origo, D.; Gallina, F.; Buehler, M.J.; Libonati, F. Revealing Diatom-Inspired Materials Multifunctionality. Adv. Funct. Mater. 2025, 35, 2407148. [Google Scholar] [CrossRef]
- Pawolski, D.; Heintze, C.; Mey, I.; Steinem, C.; Kröger, N. Reconstituting the Formation of Hierarchically Porous Silica Patterns Using Diatom Biomolecules. J. Struct. Biol. 2018, 204, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Kléparski, L.; Beaugrand, G.; Edwards, M.; Schmitt, F.G.; Kirby, R.R.; Breton, E.; Gevaert, F.; Maniez, E. Morphological Traits, Niche-Environment Interaction and Temporal Changes in Diatoms. Prog. Oceanogr. 2022, 201, 102747. [Google Scholar] [CrossRef]
- Petrucciani, A.; Moretti, P.; Ortore, M.G.; Norici, A. Integrative Effects of Morphology, Silicification, and Light on Diatom Vertical Movements. Front. Plant Sci. 2023, 14, 1143998. [Google Scholar] [CrossRef]
- Tréguer, P.; Bowler, C.; Moriceau, B.; Dutkiewicz, S.; Gehlen, M.; Aumont, O.; Bittner, L.; Dugdale, R.; Finkel, Z.; Iudicone, D. Influence of Diatom Diversity on the Ocean Biological Carbon Pump. Nat. Geosci. 2018, 11, 27–37. [Google Scholar] [CrossRef]
- Ryderheim, F.; Grønning, J.; Kiørboe, T. Thicker Shells Reduce Copepod Grazing on Diatoms. Limnol. Oceanogr. Lett. 2022, 7, 435–442. [Google Scholar] [CrossRef]
- Rajabi-Abhari, A.; Kim, J.N.; Lee, J.; Tabassian, R.; Mahato, M.; Youn, H.J.; Oh, I.K. Diatom bio-silica and cellu-lose nanofibril for bio-triboelectric nanogenerators and self-powered breath monitoring masks. ACS Appl. Mater. Interfaces 2020, 13, 219–232. [Google Scholar] [CrossRef]
- Bondoc-Naumovitz, K.G.; Crosato, E.; Wan, K.Y. Functional Morphology of Gliding Motility in Benthic Diatoms. Proc. Natl. Acad. Sci. USA 2025, 122, e2426910122. [Google Scholar] [CrossRef]
- Hansen, A.N.; Visser, A.W. The Seasonal Succession of Optimal Diatom Traits. Limnol. Oceanogr. 2019, 64, 1442–1457. [Google Scholar] [CrossRef]
- Fan, J.; Li, F.; Hu, S.; Gao, K.; Xu, J. Larger Diatoms Are More Sensitive to Temperature Changes and Prone to Succumb to Warming Stress. Limnol. Oceanogr. 2023, 68, 2512–2528. [Google Scholar] [CrossRef]
- Shimakawa, G.; Matsuda, Y. Extra O2 evolution reveals an O2-independent alternative electron sink in pho-tosynthesis of marine diatoms. Photosynth. Res. 2024, 159, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Smerilli, A.; Orefice, I.; Corato, F.; Gavalás Olea, A.; Ruban, A.V.; Brunet, C. Photoprotective and Antioxidant Responses to Light Spectrum and Intensity Variations in the Coastal Diatom Skeletonema Marinoi. Environ. Microbiol. 2017, 19, 611–627. [Google Scholar] [CrossRef]
- Palanisamy, K.M.; Rahim, M.H.A.; Govindan, N.; Ramaraj, R.; Kuppusamy, P.; Maniam, G.P. Effect of Blue Light Intensity and Photoperiods on the Growth of Diatom Thalassiosira Pseudonana. Bioresour Technol. Rep. 2022, 19, 101152. [Google Scholar] [CrossRef]
- Liu, M.; Hua, W.; Yu, C.; Zhang, S.; Li, W.; Li, C.; Peng, J.; Liu, R.; Liu, H.; Qu, J. Toxicity Mechanism of Microplastics on the Growth Traits and Metabolic Pathways of Vallisneria Natans under Different Light Environments. Ecotoxicol. Environ. Saf. 2025, 291, 117772. [Google Scholar] [CrossRef]
- Muñoz-López, C.L.; Rivera-Rondón, C.A. Diatom Response to Environmental Gradients in the High Mountain Lakes of the Colombia’s Eastern Range. Aquat. Sci. 2022, 84, 15. [Google Scholar] [CrossRef]
- Fu, H.; Wang, P.; Wu, X.; Zhou, X.; Ji, G.; Shen, Y.; Gao, Y.; Li, Q.Q.; Liang, J. Distinct Genome-Wide Alternative Polyadenyl-ation during the Response to Silicon Availability in the Marine Diatom Thalassiosira pseudonana. Plant J. 2019, 99, 67–80. [Google Scholar] [CrossRef]
- Naseema Rasheed, R.; Pourbakhtiar, A.; Mehdizadeh Allaf, M.; Baharlooeian, M.; Rafiei, N.; Alishah Aratboni, H.; Moro-nes-Ramirez, J.R.; Winck, F.V. Microalgal Co-Cultivation -Recent Methods, Trends in Omic-Studies, Applications, and Fu-ture Challenges. Front. Bioeng. Biotechnol. 2023, 11, 1193424. [Google Scholar] [CrossRef]
- Schneider, R.J.; Roe, K.L.; Hansel, C.M.; Voelker, B.M. Species-Level Variability in Extracellular Production Rates of Reac-tive Oxygen Species by Diatoms. Front. Chem. 2016, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Zepernick, B.N.; Gann, E.R.; Martin, R.M.; Pound, H.L.; Krausfeldt, L.E.; Chaffin, J.D.; Wilhelm, S.W. Elevated pH Conditions Associated with Microcystis spp. Blooms Decrease Viability of the Cultured Diatom Fragilaria crotonensis and Natural Diatoms in Lake Erie. Front. Microbiol. 2021, 12, 598736. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Bhattacharjya, R.; Marella, T.K.; Saxena, A.; Mishra, B.; Savio, S.; Tiwari, A. Production of Lipids and Proteins from Marine Diatoms under Changing pH and Silica. Bioresour. Technol. 2022, 362, 127766. [Google Scholar] [CrossRef]
- Hervé, V.; Derr, J.; Douady, S.; Quinet, M.; Moisan, L.; Lopez, P.J. Multiparametric Analyses Reveal the pH-Dependence of Silicon Biomineralization in Diatoms. PLoS ONE 2012, 7, e46722. [Google Scholar] [CrossRef]
- Petrou, K.; Baker, K.G.; Nielsen, D.A.; Hancock, A.M.; Schulz, K.G.; Davidson, A.T. Acidification Diminishes Diatom Silica Production in the Southern Ocean. Nat. Clim. Change 2019, 9, 781–786. [Google Scholar] [CrossRef]
- Roubeix, V.; Lancelot, C. Effect of Salinity on Growth, Cell Size and Silicification of an Euryhaline Freshwater Diatom: Cyclotella meneghiniana Kütz. Transit. Waters Bull. 2008, 1, 1–38. [Google Scholar]
- Hu, J.; Zheng, Y.; Yang, S.; Yang, L.; You, Q.; Wang, Q. Transcriptomic Analysis Reveals the Mechanism Underlying Salinity-Induced Morphological Changes in Skeletonema subsalsum. Front. Microbiol. 2024, 15, 1476738. [Google Scholar] [CrossRef]
- Johnston, M.R.; Gascooke, J.R.; Ellis, A.V.; Leterme, S.C. Diatoms Response to Salinity Changes: Investigations Using Single Pulse and Cross Polarisation Magic Angle Spinning 29Si NMR Spectra. Analyst 2018, 143, 4930–4935. [Google Scholar] [CrossRef]
- Javaheri, N.; Dries, R.; Burson, A.; Stal, L.J.; Sloot, P.M.A.; Kaandorp, J.A. Temperature Affects the Silicate Morphology in a Diatom. Sci. Rep. 2015, 5, 11652. [Google Scholar] [CrossRef]
- Yi, Z.; Su, Y.; Cherek, P.; Nelson, D.R.; Lin, J.; Rolfsson, O.; Fu, W. Combined Artificial High-Silicate Medium and LED Illumination Promote Carotenoid Accumulation in the Marine Diatom Phaeodactylum tricornutum. Microb. Cell. Fact. 2019, 18, 209. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Fabris, M.; Baart, G.; Kim, M.K.; Goossens, A.; Vyverman, W.; Falkowski, P.G.; Lun, D.S. Flux Balance Analysis of Primary Metabolism in the Diatom Phaeodactylum tricornutum. Plant J. 2016, 85, 161–176. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Cai, J.; Pan, J.; Chen, M.; Li, A.; Jiang, Y. Biosilica Structures Obtained from Nitzschia, Ditylum, Skeletonema, and Coscinodiscus Diatom by a Filtration-Aided Acid Cleaning Method. Appl. Microbiol. Biotechnol. 2012, 95, 1165–1178. [Google Scholar] [CrossRef]
- Kröger, N.; Bergsdorf, C.; Sumper, M. A New Calcium Binding Glycoprotein Family Constitutes a Major Diatom Cell Wall Component. EMBO J. 1994, 13, 4676–4683. [Google Scholar] [CrossRef] [PubMed]
- Gholami, P.; Khataee, A.; Bhatnagar, A. Environmentally Superior Cleaning of Diatom Frustules Using Sono-Fenton Process: Facile Fabrication of Nanoporous Silica with Homogeneous Morphology and Controlled Size. Ultrason. Sonochem. 2020, 64, 105044. [Google Scholar] [CrossRef]
- Abate, R.; Song, S.; Patil, V.; Chen, C.; Liang, J.; Sun, L.; Li, X.; Huang, B.; Gao, Y. Enhancing the Production of a Marine Diatom (Skeletonema costatum) with Low-Frequency Ultrasonic Irradiation. J. Appl. Phycol. 2020, 32, 3711–3722. [Google Scholar] [CrossRef]
- Annunziata, R.; Balestra, C.; Marotta, P.; Ruggiero, A.; Manfellotto, F.; Benvenuto, G.; Biffali, E.; Ferrante, M.I. An Optimised Method for Intact Nuclei Isolation from Diatoms. Sci. Rep. 2021, 11, 1681. [Google Scholar] [CrossRef]
- Desai, A.R.; Maulvi, F.A.; Desai, D.M.; Shukla, M.R.; Ranch, K.M.; Vyas, B.A.; Shah, S.A.; Sandeman, S.; Shah, D.O. Multiple Drug Delivery from the Drug-Implants-Laden Silicone Contact Lens: Addressing the Issue of Burst Drug Release. Mater. Sci. Eng. C. 2020, 112, 110885. [Google Scholar] [CrossRef]
- Seo, Y.; Woo, Y.; Oh, B.; Yoo, D.; Kwon, H.K.; Park, C.; Cho, H.Y.; Kim, H.S.; Lee, T. Microfluidic Fabrication of Oleosin-Coated Liposomes as Anticancer Drug Carriers with Enhanced Sustained Drug Release. Materials 2024, 17, 5550. [Google Scholar] [CrossRef]
- Lim, H.; Seo, Y.; Kwon, D.; Kang, S.; Yu, J.; Park, H.; Lee, S.D.; Lee, T. Recent Progress in Diatom Biosilica: A Natural Nanoporous Silica Material as Sustained Release Carrier. Pharmaceutics 2023, 15, 2434. [Google Scholar] [CrossRef]
- Dou, J.; Zhao, F.; Fan, W.; Chen, Z.; Guo, X. Preparation of Non-Spherical Vaterite CaCO3 Particles by Flash Nano Precipitation Technique for Targeted and Extended Drug Delivery. J. Drug Deliv. Sci. Technol. 2020, 57, 101768. [Google Scholar] [CrossRef]
- Wojtczak, I.; Brzozowska, W.; Bekissanova, Z.; Trykowski, G.; Rybczyński, P.; Ośmiałowski, B.; Sprynskyy, M. Diatom Biosilica Modified with Ce-Tb Mixed Oxide Twinning Nanoparticles and with Polyphases Quasi-Crystalline Tb Oxide Nanoparticles. Colloids. Surf. A Physicochem. Eng. Asp. 2025, 704, 135463. [Google Scholar] [CrossRef]
- Saxena, A.; Dutta, A.; Kapoor, N.; Kumar, A.; Tiwari, A. Envisaging Marine Diatom Thalassiosira weissflogii as a “SMART” Drug Delivery System for Insoluble Drugs. J. Drug Deliv. Sci. Technol. 2022, 68, 102983. [Google Scholar] [CrossRef]
- Kang, S.; Woo, Y.; Seo, Y.; Yoo, D.; Kwon, D.; Park, H.; Lee, S.D.; Yoo, H.Y.; Lee, T. A Descriptive Review on the Potential Use of Diatom Biosilica as a Powerful Functional Biomaterial: A Natural Drug Delivery System. Pharmaceutics 2024, 16, 1171. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, J.; Lin, D.; Yang, J.; Jiao, N.; Wang, Y.; Liu, L. A diatom-based biohybrid microrobot with a high drug-loading capacity and pH-sensitive drug release for target therapy. Acta Biomater. 2022, 154, 443–453. [Google Scholar] [CrossRef]
- Siepmann, J.; Faisant, N.; Akiki, J.; Richard, J.; Benoit, J.P. Effect of the Size of Biodegradable Microparticles on Drug Release: Experiment and Theory. J. Control. Release 2004, 96, 123–134. [Google Scholar] [CrossRef]
- Le, T.D.H. Hydrophobic and Hydrophilic Drug Loading Capacity of Micro Diatom Frustule from Diatomite. J. Tech. Educ. Sci. 2021, 16, 35–40. [Google Scholar] [CrossRef]
- Ormerod, C. Opening a Window on Diffusion Polar Mixed Boundary Simulations for Diatom Controlled Release. Ph.D. Thesis, University of Wollongong, Wollongong, Australia, 2021. [Google Scholar]
- Seo, Y.; Lim, H.; Park, H.; Yu, J.; An, J.; Yoo, H.Y.; Lee, T. Recent Progress of Lipid Nanoparticles-Based Lipophilic Drug Delivery: Focus on Surface Modifications. Pharmaceutics 2023, 15, 772. [Google Scholar] [CrossRef]
- Ruan, S.; Zhou, Y.; Jiang, X.; Gao, H. Rethinking CRITID Procedure of Brain Targeting Drug Delivery: Circulation, Blood Brain Barrier Recognition, Intracellular Transport, Diseased Cell Targeting, Internalization, and Drug Release. Adv. Sci. 2021, 8, 2004025. [Google Scholar] [CrossRef]
- Ulbrich, K.; Holá, K.; Šubr, V.; Bakandritsos, A.; Tuček, J.; Zbořil, R. Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef]
- Cicco, S.R.; Vona, D.; De Giglio, E.; Cometa, S.; Mattioli-Belmonte, M.; Palumbo, F.; Ragni, R.; Farinola, G.M. Chemically Modified Diatoms Biosilica for Bone Cell Growth with Combined Drug-Delivery and Antioxidant Properties. Chempluschem 2015, 80, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Xu, J.; Xie, S.; Zhang, W.; Gong, D.; Peng, G.; Du, Z.; Rong, J.; Li, X.; Liang, C.; et al. Biotemplated Diatom Microrobots for Dual-Drug Delivery in Targeted Neuroblastoma Therapy. ACS Appl. Mater. Interfaces 2025, 19, 24807–24819. [Google Scholar] [CrossRef] [PubMed]
- Story, B.D.; Park, S.; Roszak, K.; Shim, J.; Motta, M.; Ferneding, M.; Rudeen, K.M.; Blandino, A.; Ardon, M.; Le, S.; et al. Safety and Biocompatibility of a Novel Biodegradable Aflibercept-Drug Delivery System in Rhesus Macaques. Drug Deliv. 2025, 32, 2460671. [Google Scholar] [CrossRef]
- Sprynskyy, M.; Szczyglewska, P.; Wojtczak, I.; Nowak, I.; Witkowski, A.; Buszewski, B.; Feliczak-Guzik, A.; Martins, M. Diatom Biosilica Doped with Palladium(II) Chloride Nanoparticles as New Efficient Photocatalysts for Methyl Orange Degradation. Int. J. Mol. Sci. Artic. 2021, 22, 6734. [Google Scholar] [CrossRef]
- Miao, Y.; Feng, Y.; Bai, J.; Liu, Z.; Zhao, X. Optimized Mesoporous Silica Nanoparticle-Based Drug Delivery System with Removable Manganese Oxide Gatekeeper for Controlled Delivery of Doxorubicin. J. Colloid Interface Sci. 2021, 592, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Slowing, I.I.; Vivero-Escoto, J.L.; Wu, C.W.; Lin, V.S.Y. Mesoporous Silica Nanoparticles as Controlled Release Drug Delivery and Gene Transfection Carriers. Adv. Drug Deliv. Rev. 2008, 60, 1278–1288. [Google Scholar] [CrossRef]
- Kempen, P.J.; Greasley, S.; Parker, K.A.; Campbell, J.L.; Chang, H.Y.; Jones, J.R.; Sinclair, R.; Gambhir, S.S.; Jokerst, J.V. Theranostic Mesoporous Silica Nanoparticles Biodegrade after Pro-Survival Drug Delivery and Ultrasound/Magnetic Resonance Imaging of Stem Cells. Theranostics 2015, 5, 631. [Google Scholar] [CrossRef]
- Ashour, M.M.; Mabrouk, M.; Soliman, I.E.; Beherei, H.H.; Tohamy, K.M. Mesoporous Silica Nanoparticles Prepared by Different Methods for Biomedical Applications: Comparative Study. IET Nanobiotechnol. 2021, 15, 291–300. [Google Scholar] [CrossRef]
- Putri, R.M.; Almunadya, N.S.; Amri, A.F.; Afnan, N.T.; Nurachman, Z.; Devianto, H.; Saputera, W.H. Structural Characterization of Polycrystalline Titania Nanoparticles on C. Striata Biosilica for Photocatalytic POME Degradation. ACS Omega 2022, 7, 44047–44056. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ma, K.; Kao, T.; Spoth, K.A.; Sai, H.; Zhang, D.; Kourkoutis, L.F.; Elser, V.; Wiesner, U. Formation Pathways of Mesoporous Silica Nanoparticles with Dodecagonal Tiling. Nat. Commun. 2017, 8, 252. [Google Scholar] [CrossRef] [PubMed]
- Spitzmüller, L.; Nitschke, F.; Rudolph, B.; Berson, J.; Schimmel, T.; Kohl, T. Dissolution Control and Stability Improvement of Silica Nanoparticles in Aqueous Media. J. Nanoparticle Res. 2023, 25, 40. [Google Scholar] [CrossRef]
- Sprynskyy, M.; Pomastowski, P.; Hornowska, M.; Król, A.; Rafińska, K.; Buszewski, B. Naturally Organic Functionalized 3D Biosilica from Diatom Microalgae. Mater. Des. 2017, 132, 22–29. [Google Scholar] [CrossRef]
- Maher, S.; Alsawat, M.; Kumeria, T.; Fathalla, D.; Fetih, G.; Santos, A.; Habib, F.; Losic, D. Luminescent Silicon Diatom Replicas: Self-Reporting and Degradable Drug Carriers with Biologically Derived Shape for Sustained Delivery of Therapeutics. Adv. Funct. Mater. 2015, 25, 5107–5116. [Google Scholar] [CrossRef]
- Heidegger, S.; Gößl, D.; Schmidt, A.; Niedermayer, S.; Argyo, C.; Endres, S.; Bourquin, C. Immune response to functionalized mesoporous silica nanoparticles for targeted drug delivery. Nanoscale 2016, 8, 938–948. [Google Scholar] [CrossRef]
- Zhang, H.; Shahbazi, M.A.; Mäkilä, E.M.; da Silva, T.H.; Reis, R.L.; Salonen, J.J.; Santos, H.A. Diatom silica microparticles for sustained release and permeation enhancement following oral delivery of prednisone and mesalamine. Biomaterials 2013, 34, 9210–9219. [Google Scholar] [CrossRef]
- Moodley, T.; Singh, M. Sterically Stabilised Polymeric Mesoporous Silica Nanoparticles Improve Doxorubicin Efficiency: Tailored Cancer Therapy. Molecules 2020, 25, 742. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi Shayan, R.; Jalaei, D.; Dobakhti, F. Modified diatom-based ocular suspension for sustained diclofenac sodium delivery: A novel drug carrier approach. BMC Pharmacol. Toxicol. 2025, 26, 77. [Google Scholar]
- Kolimi, P.; Narala, S.; Youssef, A.A.A.; Nyavanandi, D.; Dudhipala, N. A systemic review on development of mesoporous nanoparticles as a vehicle for transdermal drug delivery. Nanotheranostics 2023, 7, 70. [Google Scholar] [CrossRef]
- An, J.; Park, H.; Ju, M.; Woo, Y.; Seo, Y.; Min, J.; Lee, T. An Updated Review on the Development of a Nanomaterial-Based Field-Effect Transistor-Type Biosensors to Detect Exosomes for Cancer Diagnosis. Talanta 2024, 279, 126604. [Google Scholar] [CrossRef]
- Li, M.; Liu, D.; Wang, S.; Guo, H.; Losic, D.; Deng, L.; Wu, S.; Yuan, P. Efficient Removal of Cd2+ by Diatom Frustules Self-Modified in Situ with Intercellular Organic Components. Environ. Pollut. 2023, 319, 121005. [Google Scholar] [CrossRef]
- Kumari, E.; Görlich, S.; Poulsen, N.; Kröger, N. Genetically Programmed Regioselective Immobilization of Enzymes in Biosilica Microparticles. Adv. Funct. Mater. 2020, 30, 2000442. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, C.W.; Wang, D.; Wei, N. A Whole-Cell Biosensor for Point-of-Care Detection of Waterborne Bacterial Pathogens. ACS Synth. Biol. 2021, 10, 333–344. [Google Scholar] [CrossRef]
- Messaabi, A.; Merindol, N.; Bohnenblust, L.; Fantino, E.; Meddeb-Mouelhi, F.; Desgagné-Penix, I. In Vivo Thrombin Activity in the Diatom Phaeodactylum tricornutum: Biotechnological Insights. Appl. Microbiol. Biotechnol. 2024, 108, 481. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Peng, Y.; Sheng, M.; Wang, Q.; Huang, J.; Yang, X. Sensitive and Amplification-Free Electrochemiluminescence Biosensor for HPV-16 Detection Based on CRISPR/Cas12a and DNA Tetrahedron Nanostructures. ACS Sens. 2023, 8, 2852–2858. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Huang, Q.; Yan, Y.; Yao, J.; Zhong, F.; Xie, S.; Zhang, M.; Zhang, H.; Jin, M.; Shui, L. A Multi-Unit Integrated Electrochemical Biosensor Array for Synergistic Signal Enhancing Carbohydrate Antigen 125 Detection. Sens. Actuators B Chem. 2023, 393, 134224. [Google Scholar] [CrossRef]
- Cvjetinovic, J.; Merdalimova, A.A.; Kirsanova, M.A.; Somov, P.A.; Nozdriukhin, D.V.; Salimon, A.I.; Korsunsky, A.M.; Gorin, D.A. A SERS Platform Based on Diatomite Modified by Gold Nanoparticles Using a Combination of Layer-by-Layer Assembly and a Freezing-Induced Loading Method. Phys. Chem. Chem. Phys. 2022, 24, 8901–8912. [Google Scholar] [CrossRef]
- Saridag, A.M.; Karagoz, I.D.; Wachsmann-Hogiu, S.; Kahraman, M. Diatomite-Based, Flexible SERS Immunosensor Platform for Rapid, Specific, and Sensitive Detection of Circulating Cancer-Specific Protein Biomarkers in Serum Using Raman Probes. ACS Appl. Bio Mater. 2024, 7, 1878–1887. [Google Scholar] [CrossRef]
- Tabassum, R.; Sarkar, P.P.; Jalal, A.H.; Ashraf, A.; Islam, N. Laser-induced electrochemical biosensor modified with graphene-based ink for label-free detection of alpha-fetoprotein and 17β-estradiol. Polymers 2024, 16, 2069. [Google Scholar] [CrossRef]
- Li, P.; Li, T.; Feng, X.; Liu, D.; Zhong, Q.; Fang, X.; Wang, L. A micro-carbon nanotube transistor for ultra-sensitive, label-free, and rapid detection of Staphylococcal enterotoxin C in food. J. Hazard. Mater. 2023, 449, 131033. [Google Scholar] [CrossRef]
- Wang, H.; Cai, J.; Wang, T.; Yan, R.; Shen, M.; Zhang, J.; Wang, J. Functionalized gold nanoparticle enhanced nanorod hyperbolic metamaterial biosensor for highly sensitive detection of carcinoembryonic antigen. Biosens. Bioelectron. 2024, 257, 116295. [Google Scholar] [CrossRef]
- Beck, F.; Horn, C.; Baeumner, A.J. Dry-reagent microfluidic biosensor for simple detection of NT-proBNP via Ag nanoparticles. Anal. Chim. Acta 2022, 1191, 339375. [Google Scholar] [CrossRef]
- Wang, W.; Ma, Z.; Shao, Q.; Wang, J.; Wu, L.; Huang, X.; He, L. Multi-MXene assisted large-scale manufacturing of electrochemical biosensors based on enzyme-nanoflower enhanced electrodes for the detection of H2O2 secreted from live cancer cells. Nanoscale 2024, 16, 12586–12598. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kwon, N.; Park, G.; Jang, M.; Kwon, Y.; Yoon, Y.; An, J.; Min, J.; Lee, T. Fast-Response Electrochemical Biosensor Based on a Truncated Aptamer and MXene Heterolayer for West Nile Virus Detection in Human Serum. Bioelectrochemistry 2023, 154, 108540. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Lee, M.; Kaushik, N.K.; Yoo, H.Y.; Park, C.; Lee, M.H.; Lee, T. Electrochemical Cell-SELEX Monitoring and Its Application to Electrochemical Aptasensor for Colorectal Cancer Detection. Chem. Eng. J. 2025, 506, 159935. [Google Scholar] [CrossRef]
- Kim, G.; Li, Y.G.; Seo, Y.; Baek, C.; Choi, J.H.; Park, H.; An, J.; Lee, M.; Noh, S.; Min, J.; et al. Fabrication of Graphene Oxide-Based Pretreatment Filter and Electrochemical-CRISPR Biosensor for the Field-Ready Cyanobacteria Monitoring System. Biosens. Bioelectron. 2023, 237, 115474. [Google Scholar] [CrossRef]
- dos Reis, G.S.; Dotto, G.L.; Vieillard, J.; Oliveira, M.L.S.; Lütke, S.F.; Silva, L.F.O.; Lima, É.C.; Salau, N.P.G.; Lassi, U. Uptake the Rare Earth Elements Nd, Ce, and La by a Commercial Diatomite: Kinetics, Equilibrium, Thermodynamic and Adsorption Mechanism. J. Mol. Liq. 2023, 389, 122862. [Google Scholar] [CrossRef]
- Zakariah, E.I.; Ariffin, E.Y.; Nokarajoo, D.; Mohamed Akbar, M.A.; Lee, Y.H.; Hasbullah, S.A. Highly Sensitive of an Electrochemical DNA Biosensor Detection towards Toxic Dinoflagellates Alexandrium minutum (A. minutum). Microchem. J. 2024, 199, 109997. [Google Scholar] [CrossRef]
- Silva, M.; Briceño, S.; Vizuete, K.; Debut, A.; González, G. Structural and Electrochemical Characterization of Diatomite with Lignin, Glucose, Activated Carbon and Magnetic Nanoparticles. Carbon Trends 2025, 19, 100492. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, W.; Gong, D.; Liang, C.; Su, Y.; Peng, G.; Deng, X.; Xu, W.; Cai, J. Biotemplated Janus Magnetic Microrobots Based on Diatomite for Highly Efficient Detection of Salmonella. ACS Appl. Mater. Interfaces 2024, 16, 49030–49040. [Google Scholar] [CrossRef]
- Yoon, J.; Kwon, N.; Lee, Y.; Kim, S.; Lee, T.; Choi, J.W. Nanotechnology-Based Wearable Electrochemical Biosensor for Disease Diagnosis. ACS Sens. 2025, 10, 1675–1689. [Google Scholar] [CrossRef] [PubMed]
- Ghobara, M.; Oschatz, C.; Fratzl, P.; Reissig, L. Numerical Analysis of the Light Modulation by the Frustule of Gomphonema parvulum: The Role of Integrated Optical Components. Nanomaterials 2023, 13, 113. [Google Scholar] [CrossRef]
- De Tommasi, E.; Rea, I.; Ferrara, M.A.; De Stefano, L.; De Stefano, M.; Al-Handal, A.Y.; Stamenković, M.; Wulff, A. Multiple-Pathways Light Modulation in Pleurosigma Strigosum Bi-Raphid Diatom. Sci. Rep. 2024, 14, 6476. [Google Scholar] [CrossRef]
- León-Valencia, A.; Briceño, S.; Reinoso, C.; Vizuete, K.; Debut, A.; Caetano, M.; González, G. Photochemical Reduction of Silver Nanoparticles on Diatoms. Mar. Drugs 2023, 21, 185. [Google Scholar] [CrossRef]
- Ide, Y.; Matsukawa, Y. Fundamental Research on Functionalization of Diatomite Using DNA-Coated Single-Walled Carbon Nanotubes. J. Photochem. Photobiol. A Chem. 2024, 452, 115550. [Google Scholar] [CrossRef]
- Chen, T.; Wu, F.; Li, Y.; Rozan, H.E.; Chen, X.; Feng, C. Gold nanoparticle-functionalized diatom biosilica as label-free biosensor for biomolecule detection. Front. Bioeng. Biotechnol. 2022, 10, 894636. [Google Scholar] [CrossRef] [PubMed]
- Kamińska, A.; Sprynskyy, M.; Winkler, K.; Szymborski, T. Ultrasensitive SERS immunoassay based on diatom biosilica for detection of interleukins in blood plasma. Anal. Bioanal. Chem. 2017, 409, 6337–6347. [Google Scholar] [CrossRef] [PubMed]
- Shapturenka, P.; Isaac Zakaria, N.; Birkholz, F.; Gordon, M.J. Extending the Diatom’s Color Palette: Non-Iridescent, Disorder-Mediated Coloration in Marine Diatom-Inspired Nanomembranes. Opt. Express 2023, 31, 21658–21671. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Cao, C.; Ying, Y.L.; Li, S.; Wang, M.B.; Huang, J.; Long, Y.T. Rationally Designed Sensing Selectivity and Sensitivity of an Aerolysin Nanopore via Site-Directed Mutagenesis. ACS Sens. 2018, 3, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Zobi, F. Diatom Biosilica in Targeted Drug Delivery and Biosensing Applications: Recent Studies. Micro 2022, 2, 342–360. [Google Scholar] [CrossRef]
- Park, S.; Cho, E.; Chueng, S.T.D.; Yoon, J.S.; Lee, T.; Lee, J.H. Aptameric Fluorescent Biosensors for Liver Cancer Diagnosis. Biosensors 2023, 13, 617. [Google Scholar] [CrossRef]
- Broccoli, A.; Carnevale, L.; Mayorga González, R.; Dorresteijn, J.M.; Weckhuysen, B.M.; Olthuis, W.; Odijk, M.; Meirer, F. Accessibility Study of Porous Materials at the Single-Particle Level as Evaluated within a Microfluidic Chip with Fluorescence Microscopy. Chem. Catal. 2023, 3, 100791. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, J.; Jiang, Y.; Jiang, X.; Zhang, D. Preparation of Biosilica Structures from Frustules of Diatoms and Their Applications: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2013, 97, 453–460. [Google Scholar] [CrossRef]
- Gilic, M.; Ghobara, M.; Reissig, L. Tuning SERS Signal via Substrate Structuring: Valves of Different Diatom Species with Ultrathin Gold Coating. Nanomaterials 2023, 13, 1594. [Google Scholar] [CrossRef]
- Mu, Y.; Zhang, M.; Sun, H.; Zhang, Q.; Chen, X.; Feng, C. Composite Diatom Fluorescent Sensor Substrate Enriched with CdSe/ZnS Quantum Dots on the Surface by Biofabrication. Colloids Surf. B Biointerfaces 2025, 246, 114396. [Google Scholar] [CrossRef]
- Wang, Z.; Gong, D.; Cai, J. Diatom Frustule Array for Flow-through Enhancement of Fluorescent Signal in a Microfluidic Chip. Micromachines 2021, 12, 1017. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, M.; Liu, J.; Hui, G.; Chen, X.; Feng, C. The art of exploring diatom biosilica biomaterials: From biofabrication perspective. Adv. Sci. 2024, 11, 2304695. [Google Scholar] [CrossRef]
- Esfandyari, J.; Shojaedin-Givi, B.; Hashemzadeh, H.; Mozafari-Nia, M.; Vaezi, Z.; Naderi-Manesh, H. Capture and Detection of Rare Cancer Cells in Blood by Intrinsic Fluorescence of a Novel Functionalized Diatom. Photodiagnosis Photodyn. Ther. 2020, 30, 101753. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.K.; Seibert, M. Prospects for Commercial Production of Diatoms. Biotechnol. Biofuels 2017, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, L.; Zhao, X.; Hou, S.; Guo, B.; Ma, P.X. Self-Healing Supramolecular Bioelastomers with Shape Memory Property as a Multifunctional Platform for Biomedical Applications via Modular Assembly. Biomaterials 2016, 104, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Nakielski, P.; Pawłowska, S.; Rinoldi, C.; Ziai, Y.; De Sio, L.; Urbanek, O.; Zembrzycki, K.; Pruchniewski, M.; Lanzi, M.; Salatelli, E.; et al. Multifunctional Platform Based on Electrospun Nanofibers and Plasmonic Hydrogel: A Smart Nanostructured Pillow for Near-Infrared Light-Driven Biomedical Applications. ACS Appl. Mater. Interfaces 2020, 12, 54328–54342. [Google Scholar] [CrossRef]
- Park, G.; Kang, S.; Kwon, Y.; An, J.; Park, H.; Lee, M.H.; Lee, T. Electrical Capacitance-Based Cancer Cell Viability Monitoring Device for Accelerated Drug Development. Sens. Actuators B Chem. 2024, 409, 135566. [Google Scholar] [CrossRef]
- Lee, D.E.; Koo, H.; Sun, I.C.; Ryu, J.H.; Kim, K.; Kwon, I.C. Multifunctional Nanoparticles for Multimodal Imaging and Theragnosis. Chem. Soc. Rev. 2012, 41, 2656–2672. [Google Scholar] [CrossRef]
- Aboagye, E.O.; Barwick, T.D.; Haberkorn, U. Radiotheranostics in Oncology: Making Precision Medicine Possible. CA Cancer J. Clin. 2023, 73, 255–274. [Google Scholar] [CrossRef]
- Medeiros Borsagli, F.G.L.; de Souza, A.J.M.; Paiva, A.E. Ecofriendly Multifunctional Thiolated Carboxymethyl Chitosan-Based 3D Scaffolds with Luminescent Properties for Skin Repair and Theragnostic of Tissue Regeneration. Int. J. Biol. Macromol. 2020, 165, 3051–3064. [Google Scholar] [CrossRef]
- Conklin, B.; Conley, B.M.; Hou, Y.; Chen, M.; Lee, K.B. Advanced Theragnostics for the Central Nervous System (CNS) and Neurological Disorders Using Functional Inorganic Nanomaterials. Adv. Drug Deliv. Rev. 2023, 192, 1114636. [Google Scholar] [CrossRef] [PubMed]
- Yi, K.; Rong, Y.; Huang, L.; Tang, X.; Zhang, Q.; Wang, W.; Wu, J.; Wang, F. Aptamer-Exosomes for Tumor Theranostics. ACS Sens. 2021, 6, 1418–1429. [Google Scholar] [CrossRef]
- Sze, J.Y.Y.; Ivanov, A.P.; Cass, A.E.G.; Edel, J.B. Single Molecule Multiplexed Nanopore Protein Screening in Human Serum Using Aptamer Modified DNA Carriers. Nat. Commun. 2017, 8, 1552. [Google Scholar] [CrossRef]
- Alshaer, W.; Hillaireau, H.; Vergnaud, J.; Ismail, S.; Fattal, E. Functionalizing Liposomes with Anti-CD44 Aptamer for Selective Targeting of Cancer Cells. Bioconjug. Chem. 2015, 26, 1307–1313. [Google Scholar] [CrossRef]
- Kim, D.M.; Kim, M.; Park, H.B.; Kim, K.S.; Kim, D.E. Anti-MUC1/CD44 Dual-Aptamer-Conjugated Liposomes for Cotargeting Breast Cancer Cells and Cancer Stem Cells. ACS Appl. Bio. Mater. 2019, 2, 4622–4633. [Google Scholar] [CrossRef] [PubMed]
- Hess, K.L.; Medintz, I.L.; Jewell, C.M. Designing Inorganic Nanomaterials for Vaccines and Immunotherapies. Nano Today 2019, 27, 73–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhong, X.; Li, J.; Liu, Z.; Cheng, L. Inorganic Nanomaterials with Rapid Clearance for Biomedical Applications. Chem. Soc. Rev. 2021, 50, 8669–8742. [Google Scholar] [CrossRef]
- Frickenstein, A.N.; Hagood, J.M.; Britten, C.N.; Abbott, B.S.; McNally, M.W.; Vopat, C.A.; Patterson, E.G.; Maccuaig, W.M.; Jain, A.; Walters, K.B.; et al. Mesoporous Silica Nanoparticles: Properties and Strategies for Enhancing Clinical Effect. Pharmaceutics 2021, 13, 570. [Google Scholar] [CrossRef]
- Živojević, K.; Mladenović, M.; Djisalov, M.; Mundzic, M.; Ruiz-Hernandez, E.; Gadjanski, I.; Knežević, N. Advanced Mesoporous Silica Nanocarriers in Cancer Theranostics and Gene Editing Applications. J. Control. Release 2021, 337, 193–211. [Google Scholar] [CrossRef]
- Zhou, S.; Zhong, Q.; Wang, Y.; Hu, P.; Zhong, W.; Huang, C.B.; Yu, Z.Q.; Ding, C.D.; Liu, H.; Fu, J. Chemically Engineered Mesoporous Silica Nanoparticles-Based Intelligent Delivery Systems for Theranostic Applications in Multiple Cancerous/Non-Cancerous Diseases. Coord. Chem. Rev. 2022, 452, 214309. [Google Scholar] [CrossRef]
- Xu, B.; Li, S.; Shi, R.; Liu, H. Multifunctional Mesoporous Silica Nanoparticles for Biomedical Applications. Signal Transduct. Target. Ther. 2023, 8, 435. [Google Scholar] [CrossRef]
- Wells, C.M.; Harris, M.; Choi, L.; Murali, V.P.; Guerra, F.D.; Jennings, J.A. Stimuli-Responsive Drug Release from Smart Polymers. J. Funct. Biomater. 2019, 10, 34. [Google Scholar] [CrossRef]
- Kashin, A.D.; Sedelnikova, M.B.; Chebodaeva, V.V.; Uvarkin, P.V.; Luginin, N.A.; Dvilis, E.S.; Bakina, O.V. Diatomite-based ceramic biocoating for magnesium implants. Ceram. Int. 2022, 48, 28059–28071. [Google Scholar] [CrossRef]
- Han, Y.; Du, L.; Wu, J.; Zhang, H.; Yang, G.; Zheng, Y.; Wu, C. Diatomaceous cross-species constructs for tendon-to-bone regeneration. Mater. Today 2025, 83, 64–84. [Google Scholar] [CrossRef]
- Brzozowska, W.; Sprynskyy, M.; Wojtczak, I.; Dąbek, P.; Markuszewski, M.J.; Witkowski, A.; Buszewski, B. Metabolically Doping of 3D Diatomaceous Biosilica with Titanium. Materials 2022, 15, 5210. [Google Scholar] [CrossRef]
- Tramontano, C.; Chianese, G.; Terracciano, M.; de Stefano, L.; Rea, I. Nanostructured Biosilica of Diatoms: From Water World to Biomedical Applications. Appl. Sci. 2020, 10, 6811. [Google Scholar] [CrossRef]
- Mu, Y.; Fu, Y.; Li, J.; Shao, K.; Pang, J.; Su, C.; Cai, Y.; Sun, X.; Cong, X.; Chen, X.; et al. Thrombin Immobilized Polydopamine-Diatom Biosilica for Effective Hemorrhage Control. Biomater. Sci. 2021, 9, 4952–4967. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.A.; Abdullah, M.A. Anticancer Compounds Derived from Marine Diatoms. Mar. Drugs 2020, 18, 356. [Google Scholar] [CrossRef]






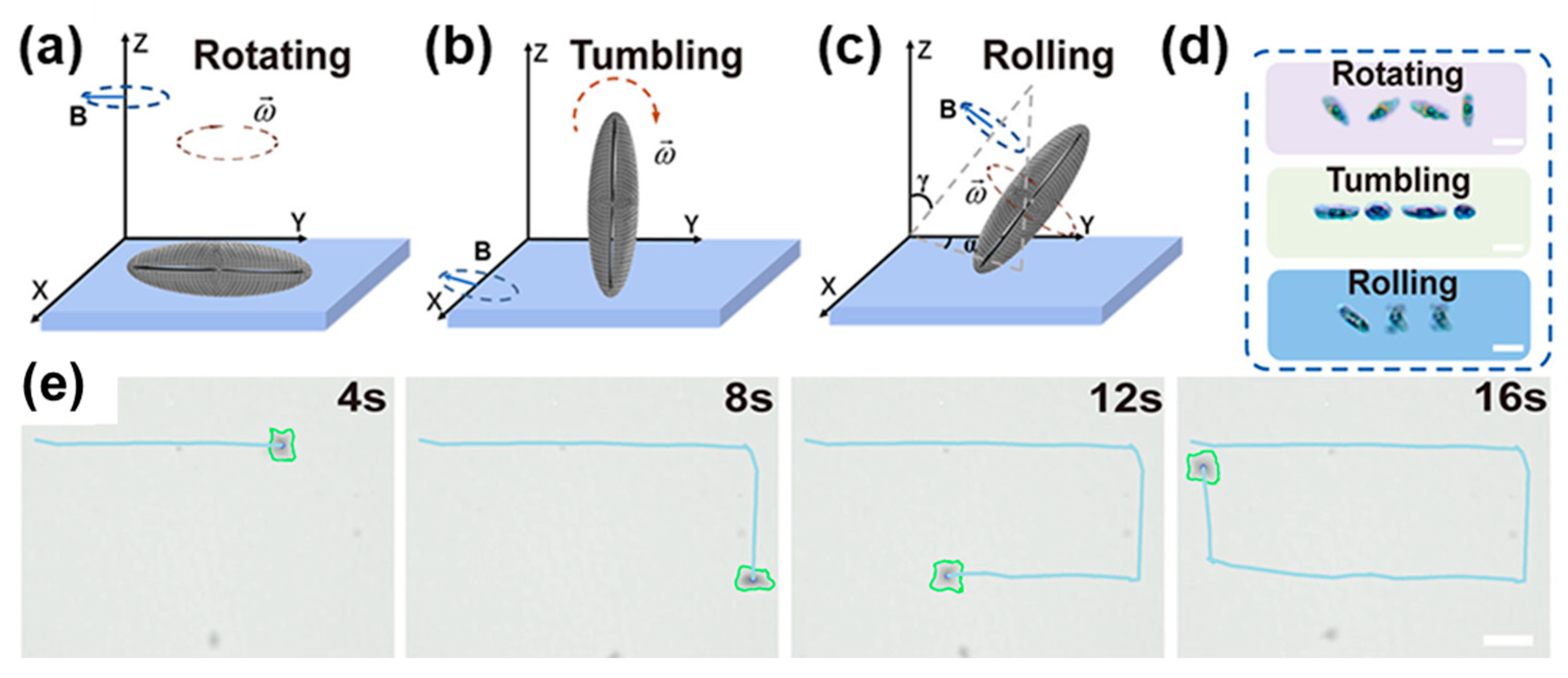







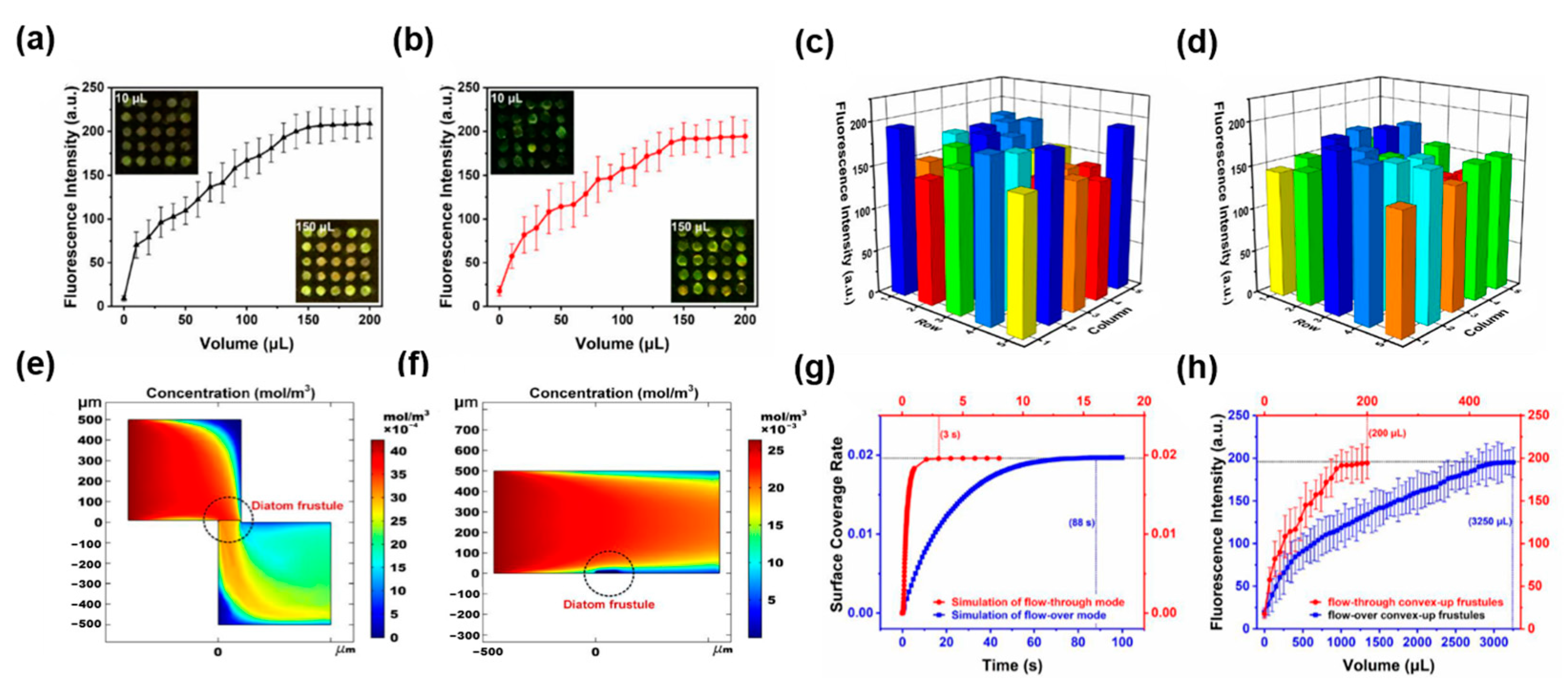
| Condition | Species | Range | Effect | Reference |
|---|---|---|---|---|
| pH | Fragilaria crotonensis | 7.7 | Enhanced growth rate and silica accumulation. | [50] |
| pH | Thalassiosira sp., Skeletonema sp., and Chaetoceros sp. | 8.5 | Enhanced growth rate. | [51] |
| pH | T. weissflogii | 7.8 | Enhanced silicate accumulation. | [52] |
| pH | Fragilariopsis cylindrus | 8.1 | Enhanced growth rate | [53] |
| Salinity | Cyclotella meneghiniana | 18 PSU | Enhanced growth rate and silica accumulation | [54] |
| Salinity | Skeletonema subsalsum | 0–12 PSU | As salinity increases, the length of diatoms decreases, and their diameter and pore size increase. | [55] |
| Salinity | T. pseudonana, Chaetoceros muelleri | 36 PSU | Enhanced silicate accumulation | [56] |
| Temperature and salinity | T. pseudonana | 23 °C and Si limited | Enhanced silicate accumulation. | [57] |
| 14–23 °C and Si replete | ||||
| Light and salinity | P. tricornutum | 50:50 Red:Blue LED and 3.0 mM Na2SiO3 | Enhanced growth rate | [58] |
| Condition | NH4F Reaction Time | Sonication Power (W: Watt) | Pulse Condition |
|---|---|---|---|
| A | 3–5 min | 20 W | 1 pulse every 30 s for 3 min |
| B | 3–5 min | 40 W | 1 pulse every 30 s for 3 min |
| C | 10 min | 20 W | 1 pulse every 30 s for 3 min |
| D | 10 min | 40 W | 1 pulse every 30 s for 2 min |
| E | 10 min | 40 W | 1 pulse every 15 s for 3–11 pulse |
| Category | Material | Experimental Model | Property | Reference |
|---|---|---|---|---|
| Biocompatibility | MSN | In vitro | No significant apoptotic response in the splenic cell up to 100 g/mL. | [92] |
| DB | In vitro | No cytotoxicity in colon cells up to 100 g/mL. | [93] | |
| Sustained release | MSN | In vitro | After 12 h, approximately 40% of the drug was released. | [94] |
| DB | In vitro | After 8 h, 68.5% of the drug was released from DB. | [95] | |
| Biodegradability | MSN | In vitro | Degraded after 24 days in PBS. | [85] |
| DB | In vitro | Degraded after 30 days in PBS. | [91] | |
| Synthetic accessibility and cost | MSN | – | Surfactant-based and acid-base synthesis system offers a high-cost process. | [96] |
| DB | – | Sustainable and eco-friendly biosynthesis-based culturing system offers a low-cost process. | [12] |
| Scaffold Material Properties | Biosensor Performances | Reference | ||||
|---|---|---|---|---|---|---|
| Material Type | Biocompatibility | Cost Efficiency | Detection Limit | Detection Range | Detection Method | |
| DB | Biodegradable, low immunotoxicity; suitable for POC/in vivo use | Natural material-based, overwhelming cost efficiency | Under 0.1 ng/mL | Under 0.1 ng/mL | SERS | [105] |
| Graphene | High controllability in surface modification; applicable in neuro/cardiovascular biosensing | Cost effective | 1.15 ng/mL | 4–400 ng/mL | Differential pulse voltammetry (DPV) | [106] |
| Carbon nanotubes | Poor biocompatibility due to fibrous structure and ROS inducibility | High cost relative to performance | 8.15 × 10−6 ng/mL | Under 8.15 × 10−6 ng/mL | Field-effect transistor | [107] |
| AuNP | Low immunogenicity; biologically validated | Expensive; limited for large-scale use | 0.25 ng/mL | 1–500 ng/mL | LSPR | [108] |
| AgNP | Oxidative, cytotoxic; restricted in biomedical use | Inexpensive; requires oxidation stabilization | 0.57 ng/mL | 0.6–1 ng/mL | DPV | [109] |
| MXene | High-performing; long-term biocompatibility uncertain | Excellent requires optimization for cost-effectiveness | 0.29 µM | 0.5 µM–3 mM | Cyclic voltammetry (CV) | [110] |
| Target Antigen | Raman Marker Peak (cm−1) | Calibration Curve Equation (y = ax + b) | Slope (Signal Amplification) | R2 (Linearity) | LOD (ng/mL) |
|---|---|---|---|---|---|
| HER2 | 1579 | y = 18.346x + 602.7 | 18.35 | 0.948 | Under 0.1 |
| CA15-3 | 1333 | y = 82.76x + 1754.1 | 82.76 | 0.9703 | Under 0.1 |
| MUC4 | 1066 | y = 72.625x + 4578.6 | 72.63 | 0.9913 | Under 0.1 |
| PSA | 1333 | y = 175.96x + 3066.8 | 175.96 | 0.9995 | Under 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, D.; Lee, M.; Seo, Y.; Yoon, J.; Jang, E.; Lee, G.; Kwon, D.; Lee, S.D.; Min, J.; Lee, T. Diatom Biosilica: A Useful Natural Material for Biomedical Engineering. Water 2025, 17, 2373. https://doi.org/10.3390/w17162373
Yoo D, Lee M, Seo Y, Yoon J, Jang E, Lee G, Kwon D, Lee SD, Min J, Lee T. Diatom Biosilica: A Useful Natural Material for Biomedical Engineering. Water. 2025; 17(16):2373. https://doi.org/10.3390/w17162373
Chicago/Turabian StyleYoo, Daehyeon, Minyoung Lee, Yoseph Seo, Jinwook Yoon, Eunseok Jang, Gaeun Lee, Daeryul Kwon, Sang Deuk Lee, Junhong Min, and Taek Lee. 2025. "Diatom Biosilica: A Useful Natural Material for Biomedical Engineering" Water 17, no. 16: 2373. https://doi.org/10.3390/w17162373
APA StyleYoo, D., Lee, M., Seo, Y., Yoon, J., Jang, E., Lee, G., Kwon, D., Lee, S. D., Min, J., & Lee, T. (2025). Diatom Biosilica: A Useful Natural Material for Biomedical Engineering. Water, 17(16), 2373. https://doi.org/10.3390/w17162373








