Abstract
Gehu Lake in the lower reaches of the Taihu Lake Basin has experienced water quality degradation due to increasing human activities, pollutant discharge, and non-point source pollution, which requires ecosystem restoration. Currently, the community structure of aquatic organisms and their influencing environmental factors remain poorly understood. Thus, in this study, we conducted comprehensive fieldwork in June 2024 and analyzed the community structures of plankton (i.e., phytoplankton and zooplankton) and macroinvertebrates, and their influencing environmental factors in Gehu Lake and the inflowing river. The trophic level index (TLI) and biodiversity indices (Shannon–Wiener, Pielou, and Margalef) were utilized to assess water quality status. Pearson correlation analysis and redundancy analysis (RDA) were applied to identify key factors influencing plankton and macroinvertebrate community structures. The dominant phytoplankton species included Merismopedia tranquilla, Microcystis aeruginosa, Aphanizomenon flos-aquae, Aphanocapsa elachista, and Aulacoseira granulata. The dominant zooplankton species were mainly Brachionus diversicornis, Brachionus calyciflorus, and Asplanchna priodonta. The dominant macroinvertebrate species were Microchironomus tabarui and Chironomus flaviplumus. The findings suggest that Gehu Lake exhibited moderate pollution levels, while the diversity indices were significantly correlated with environmental factors. The Shannon–Wiener index of zooplankton displayed a markedly negative correlation with Chl-a (p < 0.05). The results from redundancy analysis showed that TP, TN, SD, CODMn, and Chl-a were key environmental factors shaping the aquatic community structure in the lake.
1. Introduction
Plankton and macroinvertebrates are key functional groups in freshwater ecosystems, reflecting water quality and ecosystem health through their community structure dynamics [1,2]. Plankton, including phytoplankton and zooplankton, as primary producers and primary consumers, are essential components of aquatic ecosystems [3]. The phytoplankton community drives carbon, nitrogen, and phosphorus cycling via photosynthesis [4,5]. Zooplankton regulate algal abundance and maintain food web structure. Macroinvertebrates accelerate the decomposition of organic matter and the mineralization of carbon, nitrogen, and phosphorus by feeding on humus and organic detritus, thereby driving nutrient cycling in ecosystems. They also serve as vital connectors between primary producers and higher-trophic-level consumers [6,7]. Under the impact of global climate change and increasing human activities, shallow lakes are facing eutrophication, frequent cyanobacterial blooms, and declining biodiversity [8,9]. Therefore, it is important to investigate the aquatic community structures and their influencing environmental factors in shallow lakes, and to make corresponding treatment measures.
Existing studies demonstrate that spatial shifts in the distribution of dominant phytoplankton species and structural changes in zooplankton and macroinvertebrate communities are typical features of shallow lake ecosystems in response to environmental stresses [10,11]. This study focuses on Gehu Lake, a representative shallow eutrophic lake in the Taihu Basin, which serves as an ideal model system for systematically analyzing the structural distribution of aquatic biota and its driving mechanisms. We focused on sampling in June because June marks the start of the peak eutrophication period in the Taihu Lake Basin, when temperatures rise and algal blooms begin, making it crucial for understanding the initial stages of eutrophication-related aquatic changes. As for large aquatic plants, due to increased pollutant loads entering the lake and the expansion of aquaculture, emergent plants are scattered only in the northern part of the lake, while submerged plants are almost non-existent [12,13,14,15], making collection difficult. Additionally, previous studies have mainly focused on the relationship between single species and environmental factors [16,17]. Systematic research on the structure of biological communities and the environmental factors that influence them is still insufficient.
Thus, this study seeks to (1) identify spatial distribution characteristics of water quality parameters and aquatic community structures; (2) evaluate water trophic status using the trophic level index (TLI) and biodiversity indices; and (3) investigate the environmental factors that influence biological community structures. These results help to emphasize the main environmental factors and thus develop targeted measures for the ecological health of lake environments [18].
2. Materials and Methods
2.1. Site Description
Gehu Lake (31°29′–31°42′ N, 119°44′–119°53′ E) is the second-largest freshwater lake in the southern part of Jiangsu Province and is one of the main lakes in the Taihu Lake Basin [19]. Gehu Lake connects Changdang Lake and Taihu Lake. It receives water from the South Jiangsu Canal and Yixing City’s three waterways. The Wuyi Canal and Mengjin River also traverse its banks. With a surface area of approximately 164 km2, the lake extends 25 km from north to south and has an average width of 6.6 km from east to west. The annual average precipitation is 1066 mm [20]. Gehu Lake is located in a subtropical monsoon climate zone, which has four distinct seasons. Summers are hot and rainy, while winters are mild and dry. It is a typical shallow lake that has a shallow water depth (1.2 m), shallow sediment deposits, and a dish-shaped basin morphology [21]. It serves as a source for critical economic functions, including aquaculture, flood regulation, irrigation, water transportation, and tourism [22]. However, rapid urbanization, increasing nutrient inputs, hydrological modifications, and land-use changes have significantly degraded its water quality in recent years. According to the China Surface Water Environmental Quality Standard, the water quality of Gehu Lake has remained at Grade IV to Grade V level in recent years, exhibiting pronounced eutrophication and recurrent algal blooms [23,24].
2.2. Sample Collection
2.2.1. Water Sampling
We conducted comprehensive fieldwork in June 2024 (the wet season), collecting plankton and macroinvertebrates samples and measuring environmental factors. Eight sampling sites (GH1-GH8) were set up based on the lake’s hydrological conditions and environmental characteristics, and two sampling sites were set up on the rivers flowing into the lake (GH9-GH10). The specific location is shown in Figure 1. Water temperature (WT, °C), dissolved oxygen (DO, mg/L), pH, and electrical conductivity (EC, in µS/cm) were measured on site using a portable multi-parameter water quality analyzer (YSI ProQuatro, Columbus, OH, USA). Samples were collected at a depth of 0.5 m below the water surface and transported to the laboratory for further physicochemical analysis, including total phosphorus (TP), total nitrogen (TN), nitrate nitrogen (NO3-N), ammonia nitrogen (NH3-N), permanganate index (CODMn), suspended solids (SS), and Chlorophyll-a (Chl-a). TP was determined via the ammonium molybdate spectrophotometric method. TN was analyzed using alkaline potassium persulfate digestion followed by ultraviolet spectrophotometry. NO3-N was measured by ultraviolet spectrophotometry, while NH3-N was quantified using the Nessler reagent spectrophotometric method. CODMn was assessed through potassium permanganate oxidation and sodium oxalate titration. SS was determined gravimetrically after drying at 105 °C, and Chl-a was extracted with hot ethanol and analyzed via spectrophotometry [25,26]. All samples were measured at the National Engineering Laboratory for Lake Pollution Control and Ecological Restoration, Chinese Research Academy of Environmental Sciences.
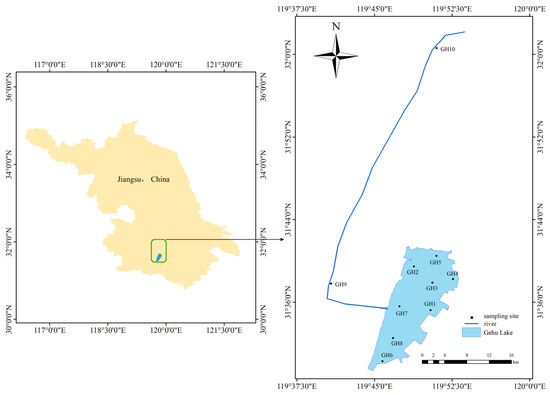
Figure 1.
Geographic location of sampling sites.
2.2.2. Sample Collection and Identification
Phytoplankton samples were collected using a 25# plankton net at 0.5 m depth for 3–5 min. The filtrate was transferred into 50 mL sample bottles and preserved with 4% Lugol’s reagent. We collected 1 L samples of water using a plexiglass water sampler, fixed on-site with 15 mL Lugol’s reagent, and transported to the laboratory for quantitative analysis. After 48 h of sedimentation, the samples were concentrated to 30 mL for phytoplankton identification [27,28]. Zooplankton samples were collected with a 13# plankton net in the surface layer (0–50 cm) and preserved in 4% formaldehyde reagent. About 10 L of water samples were collected using a plexiglass water sampler, filtered through a plankton net, and fixed with 4% formaldehyde reagent for quantitative analysis. The samples were transported to the laboratory for sedimentation, concentration, and identification of zooplankton [29,30]. Macroinvertebrate samples were collected by pouring sediment samples through a macroinvertebrate aquatic sieve with a mesh size of 450 µm. After the mud was washed away, the remaining material was transferred into a 500 mL plastic bottle and preserved in a 10% formalin reagent for subsequent identification and counting [31].
2.3. Data Processing and Analysis
The trophic level index (TLI) serves as a comprehensive metric for assessing water body eutrophication [32,33]. The TLI is calculated using total phosphorus (TP), total nitrogen (TN), chlorophyll a (Chl-a), chemical oxygen demand (CODMn), and Secchi depth (SD). The formulae are as follows:
among them, is a comprehensive measure of trophic state, where Wj denotes the weighting coefficient for the j-th parameter, and TLI(j) represents the trophic level index of j. These weightings are typically assigned according to each parameter’s relative contribution to eutrophication [34,35]. ranges from 0 to 100, with specific ranges corresponding to distinct trophic states. Trophic levels correspond to oligotrophic ( < 30), mesotrophic (30 ≤ ≤ 50), lightly eutrophic (50 < ≤ 60), moderately eutrophic (60 < T ≤ 70), and hypertrophic (T > 70).
Three indices are used to measure species diversity in biological communities. The Shannon–Wiener index (H) integrates species richness, reflecting the overall diversity of the community by considering both the number of species and their proportional distribution. The Pielou evenness index (J) focuses on the uniformity of individual distribution among species, indicating whether the community is dominated by a few species or evenly distributed. The Margalef richness index (D) primarily reflects species richness, i.e., the number of species present in the community. These indices are calculated using Equations (7)–(9).
In the formulae, i equals i = 1 to S, with S being the number of groups, where ni is the abundance of the i-th group, and N is the total abundance [36,37,38].
The dominant species was determined by the McNaughton dominance index (Y), calculated using Equation (10) [27,39]. Y ≥ 0.02 is screened as a dominant species [40].
where ni is the number of individuals of species i at each sampling location, N is the total number of individuals of all species, ni/N represents the relative proportion of each species, and fi is the occurrence frequency of species i among all sampling locations [41]. The assessment criteria for water quality using diversity indices are outlined in Table 1 [36,37,38].

Table 1.
Water quality assessment standards based on diversity indices.
2.4. Statistical Analysis
Prior to Pearson correlation analysis, the Shapiro–Wilk test was used to verify the normality of variables, and Levene’s test was applied to check homogeneity of variance. Variables with non-normal distributions were log10-transformed to meet parametric assumptions. Pearson correlation coefficients were calculated between environmental variables and diversity indices. A significant correlation was defined as having a p-value of less than 0.05. The detrended correspondence analysis (DCA), including canonical correspondence analysis (CCA) or redundancy analysis (RDA), was applied to analyze the relationship between aquatic biological communities and environmental factors. The gradient length derived from the DCA results determined the appropriate analytical method. For example, when the first axis value exceeded 4.0, canonical correspondence analysis (CCA) was selected; when the first axis value was between 3.0 and 4.0, either RDA or CCA was applicable; when the first axis value was below 3.0, redundancy analysis (RDA) was preferred for the following analysis. Environmental variables with variance inflation factors (VIF) of higher than 10 were eliminated from the following analysis [42]. The analyses were performed using SPSS 27 and CANOCO 5.0 [43], while the graphs were plotted using Origin 2024 and ArcGIS 10.8.
3. Results and Analysis
3.1. Characteristics of Environmental Factors
The statistics of the environmental variables in Gehu Lake and the inflowing river are shown in Table 2. Overall, the lake had lower average values of WT, TN, NH3-N, NO3-N, SD, and EC than those in the inflowing river. Conversely, average values of pH, DO, TP, Chl-a, CODMn, SS, and TLI were higher in the lake than in the inflowing river. The TLI was between 50 and 60 at all sampling sites. Figure 2 illustrates the spatial distribution of the main environmental factors that were chosen in the RDA. TP levels were significantly higher in the northwest of the lake than in the other regions. SD was higher in the southern part of the lake than in the northern part. TN and Chl-a contents were higher in the western part of the lake than in other areas. The western part of the lake had higher CODMn levels than in the eastern part.

Table 2.
Statistics of environmental variables in Gehu Lake and the inflowing river.
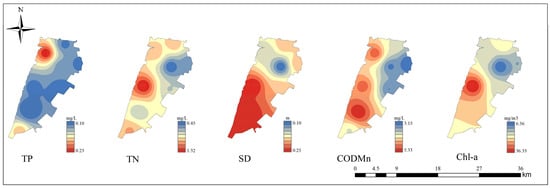
Figure 2.
Spatial distribution of main environmental factors (TP, TN, SD, CODMn, and Chl-a) in Gehu Lake.
3.2. The Aquatic Biological Community Structure of the Lake
3.2.1. Composition of Species and Dominant Species
A total of 94 phytoplankton species belonging to seven phyla were identified. Chlorophyta had 45 species, accounting for 47.87% of all species. Bacillariophyta and Cyanobacteria had 21 species and 19 species, accounting for 22.34% and 20.21% of all species, respectively. Cryptophyta had 3 species, accounting for 3.19%. Pyrrophyta, Euglenophyta, and Chrysophyta had 2 species each, accounting for 2.13% separately.
Zooplankton had three groups and 36 species in total. Rotifera had 16 species, accounting for 44.4% of all species. Copepoda had 11 species, accounting for 30.6% of all species. Cladocera had 9 species, accounting for 25%.
Macroinvertebrates had 17 species in total, belonging to three phyla and six classes. Insecta had the highest number of species, with 8 species accounting for 47.06%. Oligochaeta had 3 species, accounting for 17.65% of all species. Both Polychaeta and Crustacea had 2 species each, representing 11.76% of all species, respectively. Bivalvia and Hirudinea had 1 species each, representing 5.88% of all species separately.
There were 5 dominant phytoplankton species belonging to Cyanobacteria and Bacillariophyta. Four of these species belonged to Cyanobacteria, including Merismopedia tranquilla, Microcystis aeruginosa, Aphanizomenon flos-aquae and Aphanocapsa elachista. One of the species belonged to Bacillariophyta, i.e., Aulacoseira granulata. There were four dominant zooplankton species, including Brachionus diversicornis, Brachionus calyciflorus, Asplanchna priodonta, and Moina micrura. There were five dominant macroinvertebrate species, including Microchironomus tabarui, Chironomus flaviplumus, Tanypus chinensis, Grandidierella japonica, and Limnodrilus hoffmeisteri. The dominant species and their degree of dominance are shown in Table 3.

Table 3.
Dominant species and dominance.
3.2.2. Spatial Variation in Abundance and Biomass
The average abundance of phytoplankton at all sampling points was 2.89 × 107 cells/L, with an average biomass of 2.45 mg/L. Cyanobacteria had the highest abundance, while Chlorophyta and Bacillariophyta had less abundance (Figure 3). Bacillariophyta had the greatest biomass, followed by Cyanobacteria and Chlorophyta. The highest phytoplankton abundance was observed at GH10 (8.68 × 107 cells/L), followed by GH1 (5.86 × 107 cells/L) and GH6 (4.78 × 107 cells/L). Phytoplankton biomass was relatively higher at GH6, GH2, GH8, and GH7 and lower at GH9, GH3, and GH10. The average abundance of the zooplankton community was 990.50 ind./L, with an average biomass of 4.80 mg/L.
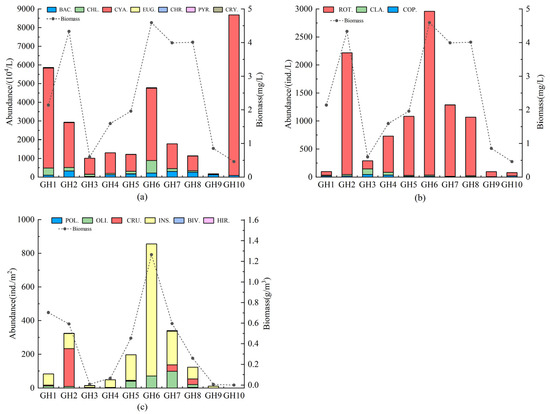
Figure 3.
Spatial variation in the abundance and biomass of phytoplankton (a), zooplankton (b), and macroinvertebrates (c).
The zooplankton community was dominated by rotifers, followed by Cladocera and Copepoda. The highest zooplankton abundance was recorded at GH6 (2956.7 ind./L), followed by GH2 (2215.1 ind./L), whereas GH1, GH9, and GH10 showed extremely low zooplankton abundance. Biomass was relatively high at GH6 and GH2, reaching 11.30 mg/L and 11.16 mg/L, respectively, while GH9 and GH10 had the lowest biomass.
For macroinvertebrates, the mean abundance and biomass were 1996.30 ind./m2 and 3.95 g/m2, respectively. Insecta had the highest abundance and biomass, followed by Crustacea. Polychaeta, Bivalvia, and Hirudinea displayed lower abundance, while Polychaeta and Hirudinea had minimal biomass. Spatially, GH6 showed the highest macroinvertebrate abundance and biomass, with GH2 ranking second in abundance and GH1 seconds in biomass. In contrast, GH3, GH9, and GH10 showed low abundance and biomass.
3.2.3. Evaluation of Biodiversity Indices
The spatial distribution of biodiversity indices in Gehu Lake were presented in Table 4. For phytoplankton, the Shannon–Wiener index ranged from 1.38 to 2.44, indicating β-mesotrophic pollution. The Pielou index ranged from 0.36 to 0.67, corresponding to light pollution status. The Margalef index ranged from 1.04 to 3.28, indicating β-mesotrophic pollution. GH9 and GH10 exhibited significantly lower diversity indices than average. For zooplankton, the Shannon–Wiener index ranged from 0.78 to 1.90, reflecting α-mesotrophic pollution. The Pielou index ranged from 0.26 to 0.61, indicating moderate pollution. The Margalef index ranged from 2.44 to 4.23, indicative of light or no pollution. GH9 and GH10 showed slightly higher indices than the average value of lake water. For macroinvertebrates, the Shannon–Wiener index ranged from 0.87 to 1.75, corresponding to α-mesotrophic pollution. The Pielou index ranged from 0.54 to 0.84, indicating moderate pollution. The Margalef index ranged from 0.55 to 0.98, which reflects heavy pollution. GH9 and GH10 exhibited significantly lower indices than the average value of lake water.

Table 4.
Diversity indices of aquatic organisms.
3.3. Pearson Correlation Analysis of Biomes with Environmental Factors
As shown in Figure 4, phytoplankton’s H index exhibited negative correlations with WT, NH3-N, and SD (p < 0.05). The abundance of Bacillariophyta displayed positive correlations with pH, Chl-a, and CODMn (p < 0.05). For zooplankton, H, J, and D were all negatively correlated with DO (p < 0.05); H and J were negatively correlated with CODMn (p < 0.05). J and H exhibited negative correlations with Chl-a, and D was negatively correlated with SD (p < 0.05). The abundance of Copepods showed negative correlations with pH, WT, TN, SD, and Chl-a, while the abundance of Cladoceran had significantly negative correlations with TP and SD. For macroinvertebrates, H and D exhibited positive correlations with CODMn. D was also negatively correlated with WT. There were positive correlations between Polychaeta and Chl-a, Crustacea and TP, and between Bivalvia and WT (p < 0.05). Additionally, Polychaeta demonstrated a significantly positive correlation with Chl-a (p < 0.01). Insecta showed significantly positive correlations with Chlorophyta and Rotifera. There were significantly positive correlations between Polychaeta and Chrysophyta, and between Hirudinea and Cladocera (p < 0.001).
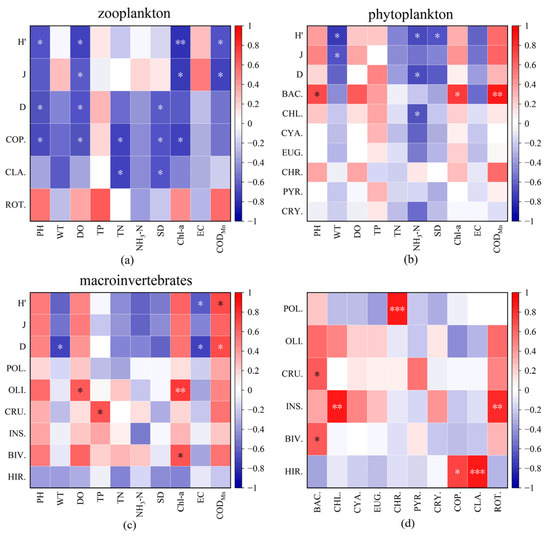
Figure 4.
Correlations between diversity indices, abundance of zooplankton (a), phytoplankton (b), macroinvertebrates (c), and environmental factors; correlations between abundances of phytoplankton, zooplankton, and macroinvertebrates (d) (Note: CHL: Chlorophyta, CYA: Cyanobacteria, BAC: Bacillariophyta, CRY: Cryptophyta, EUG: Euglenophyta, PYR: Pyrrophyta, CHR: Chrysophyta. ROT: Rotifera, CLA: Cladocera, COP: Copepoda. POL: Polychaeta, OLI: Oligochaeta, CRU: Crustacea, INS: Insecta, BIV: Bivalvia, HIR: Hirudinea. * p < 0.05; ** p < 0.01; *** p < 0.001).
3.4. Relationships Between Aquatic Organisms and Environmental Factors
The axis lengths of DCA1 were below 3.0, suggesting that RDA was applicable for the following analyses. Figure 5a shows the RDA ordination of phytoplankton abundance and environmental factors. The first and second principal component axes explained 54.27% and 29.64% of the variance. CODMn and Chl-a exhibited significantly positive correlations with the abundance of Chrysophyta. TP exhibited strong positive correlations with Cyanobacteria and Cryptophyta, but a strong negative correlation with Bacillariophyta. SD and TN had positive correlations with Bacillariophyta, but negative correlations with all other phytoplankton groups. The RDA ordination of zooplankton abundance and environmental factors is shown in Figure 5b. The first and second RDA axes explained 86.69% and 13.03% of the variance. The five chosen environmental factors exhibited positive correlations with Rotifera, but significantly negative correlations with Copepoda and Cladocera. Figure 5c illustrates the RDA ordination of macroinvertebrate abundance and environmental factors. The first and second RDA axes explained 39.27% and 25.95% of the variance. SD displayed significantly negative correlations with all macroinvertebrate groups, while CODMn and Chl-a showed positive correlations with all groups except Polychaeta. The correlation between aquatic biome community structure and environmental factors is shown in Table 5.
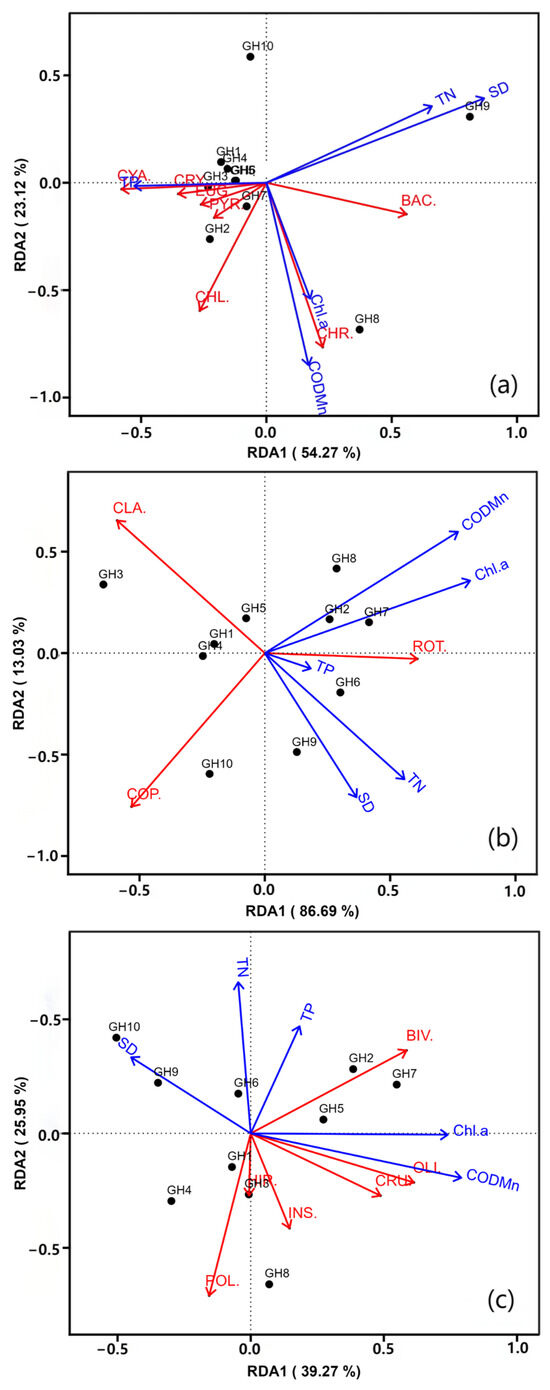
Figure 5.
Redundant analysis of (a) phytoplankton, (b) zooplankton, (c) macroinvertebrates, and main environmental factors (Note: The abbreviations refer to Figure 4).

Table 5.
Monte Carlo test results for community structures of phytoplankton, zooplankton, and macroinvertebrates with environmental factors.
4. Discussion
4.1. Analysis of Water Quality in Gehu Lake
Lakes often have complex water flow patterns. Areas near inflows are likely to have different nutrient and contaminant loads. If a river is carrying agricultural runoff with high nutrients, the lake area closest to the inflow will have elevated concentrations of these nutrients. As the water moves further into the lake, processes like dilution and mixing occur. The shape and depth can also influence the distribution of indicators such as CODMn and Chl-a. Shallow areas may experience more sediment–water interactions. The resuspension of sediments during windy conditions can release nutrients, increasing their concentrations. As a typical shallow eutrophic lake in the Taihu Lake Basin, Gehu Lake exhibits significant spatial heterogeneity and a moderate water pollution state. The average TLI for the entire lake was 58.20, indicating mild eutrophication. The TLI of the inflowing rivers (GH9 and GH10) were slightly lower than that of the lake, but still within the eutrophic range. Its eutrophication aligns with global patterns in agriculturally impacted shallow lakes [44]. Chl-a concentrations were about 19.8 mg/m3, which was above the eutrophication threshold of 10 mg/m3. This demonstrated that the overgrowth of phytoplankton, including cyanobacteria, became a key indicator of deteriorating water quality [45]. The average SS content was higher in the lake than in the inflowing river, which is due to the resuspension-prone nature of sediments in shallow lakes [46]. This may further increase water turbidity, affect light conditions, and inhibit the growth of submerged plants. In terms of spatial distribution, TP was higher along the northern coast than in the south. This may be because the northern area is close to an urban industrial zone and receives agricultural non-point source pollution input. The SD was high in the south. This suggests that human activity has a minimal impact on this area. The distribution of TN may be due to the weaker hydrodynamic conditions and longer retention times in the middle of the lake than in the lakeshore area. CODMn and Chl-a reflected higher pollution loads in the western lake inlet area. This could be due to pollutants entering the lake from the inflowing river.
In recent years, there has been a clear improvement in water quality through the “fencing and net reduction” [47] project and the “fishing to control algae” ecological aquaculture project. Although the submerged vegetation restoration project implemented in 2010 has partially improved water transparency in the northern area of the lake, the high nutrient loads of the entire lake and very scarce submerged plants in the southern area has caused poor lake water quality [13,14,15]. Additionally, the ecological dredging project has reduced the release of pollutants from the lake sediment, resulting in a decrease in nutrient loads [48].
4.2. Characterizing the Spatial Distribution of Aquatic Abundance and Diversity
The abundance and diversity of the aquatic community show significant spatial differences. The phytoplankton groups were dominated by Cyanobacteria (e.g., Microcystis aeruginosa), reflecting their competitive advantage in phosphorus-rich eutrophic waters, a pattern consistent with cyanobacterial proliferation globally [49,50,51]. Spatially, the highest phytoplankton abundance was at GH10, possibly due to high nutrient loads (TN and TP) from the Xinmeng River. The SD was high in the north, which may provide suitable light conditions for Bacillariophyta [21]. Rotifers (e.g., Brachionus diversicornis) dominated the zooplankton community, reflecting the shift towards pollution-tolerant species in eutrophic waters [52]. The zooplankton diversity indices showed medium pollution of the lake water. The zooplankton diversity indices of the inflowing river were slightly higher than that of the lake, which may be related to the higher DO and lower Chl-a concentration brought by river scouring. Insecta dominated among macroinvertebrates, indicating typical characteristics of organic pollution in the bottom environment [53]. GH6 had the highest abundance of macroinvertebrates. This may be related to the high organic matter content and weak hydrodynamics of the sediments. Meanwhile, GH3 and GH9 had an extremely low abundance of macroinvertebrates, which may be related to the strong disturbance of the bottom sediments [54]. The Margalef index was only 0.76, indicating heavy pollution of the lake water. The spatial distribution of biodiversity indices further reveals the spatial distribution of pollution magnitude. The diversity indices of macroinvertebrates were lowest in the southern part of the lake, consistent with the disappearance of submerged plants and frequent algal blooms.
Restoring submerged plants can increase phytoplankton diversity indices. This suggests that submerged plants can locally increase biodiversity by raising SD and hindering cyanobacteria growth [55]. The dominance of pollution-tolerant species was a typical feature of shallow eutrophic lakes [56,57]. It is necessary to improve habitat heterogeneity and promote diversity of species (e.g., Cladocera, Mollusca) through targeted management measures such as pollution source control, substrate remediation, and revegetation.
4.3. Factors Influencing Biome Structure
The Monte Carlo test showed that CODMn was a consistently significant factor across all three biological groups, highlighting its critical role in regulating community structure, due to its association with organic matter content, which directly affects food availability and habitat quality. SD significantly influenced phytoplankton because they are more sensitive to light availability. TN and Chl-a approached significance for zooplankton. They may indirectly affect zooplankton through trophic interaction but effects were weaker than CODMn. RDA analysis explained 83.91%, 99.72%, and 65.22% of the variation in phytoplankton, zooplankton, and macroinvertebrate abundance, respectively. The spatial distribution of TN and TP directly affected phytoplankton community composition [58]. Among zooplankton, Rotifers showed a significant positive correlation with Chl-a, reflecting the adaptation of pollution-tolerant species to high algal concentrations in eutrophic waters [59]. Cyanobacterial abundance was high in areas with low SD, whereas high SD significantly promoted the growth of Bacillariophyta [60]. For macroinvertebrates, SD was negatively correlated with the abundance of all taxa, possibly due to low transparency inhibiting the growth of submerged vegetation and reducing habitat complexity. CODMn was significantly positively correlated with zooplankton abundance and pollution-tolerant species of macroinvertebrates (e.g., Limnodrilus hofmeisteri), suggesting that high organic matter contents support the reproduction of species that tolerate low oxygen levels and have high nutritional requirements [61]. These conclusions are consistent with those of Whatley et al. [53], who found that Insecta dominate macroinvertebrate communities in organically polluted freshwater systems due to their tolerance of low-oxygen environments. Additionally, CODMn was significantly positively correlated with Chrysophyta, probably due to Chrysophyta’s high adaptability to organic matter-rich habitats. This corroborates the impact of exogenous pollutant inputs on community structure. The key factors driving the structure of the biological community are attributed to exogenous nutrient inputs, endogenous sediment release, insufficient hydrodynamic conditions, and degradation of submerged vegetation. In future, it will be necessary to strengthen pollution control, decrease lake sediments, and restore submerged vegetation across the whole lake in order to promote the health of water ecological environment [62,63].
5. Conclusions
This study systematically analyzes the structural characteristics of the plankton and macroinvertebrate communities in Gehu Lake and their influencing environmental factors. The results indicate that the lake was in a mild eutrophic state, with exogenous inputs from the inflowing river and the resuspension of bottom sediments. The latter led to high concentrations of suspended solids and low transparency of the lake water. These factors exacerbate the steady-state cycle of turbid water. The aquatic biological communities showed spatial variations. The phytoplankton community was dominated by Cyanobacteria and Bacillariophyta. The zooplankton and macroinvertebrate communities were dominated by pollution-tolerant Rotifers and Insecta, respectively. Shannon–Wiener and Margalef diversity indices revealed that the entire lake was moderately polluted, whereas Chl-a and CODMn inhibited zooplankton diversity. RDA analysis, combined with Monte Carlo tests identified CODMn was the most consistent primary factor influencing all aquatic communities. Additionally, SD determined light utilization efficiency which significantly shaped phytoplankton and macroinvertebrates. TN and Chl-a had effects on zooplankton. TP, though not individually significant, can contribute to community dynamics through synergistic effects with other nutrients. The three-in-one strategy of “pollution control, sediment desilting, and vegetation restoration” is recommended, such as reducing exogenous nutrient loads, suppressing endogenous releases, and expanding submerged vegetation coverage to restore sensitive species (e.g., Copepoda and Mollusca), could facilitate the transition of Gehu Lake into a clear-water state.
Author Contributions
Z.Y.: Formal analysis, data curation, writing—original draft preparation, writing—review and editing. Q.Z.: Conceptualization, methodology, investigation, supervision, writing—review and editing. C.L.: Resources, supervision, writing—review and editing. C.Y.: Resources, validation, Y.W.: Investigation, data curation. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Open Research Fund of Key Laboratory for Lake Pollution Control of the Ministry of Ecology and Environment, Chinese Research Academy of Environmental Sciences (2024HPYKFYB07), the Second Phase of the Joint Research Project on Ecological Environment Protection and Restoration of the Yangtze River (2022-Y1-02-0502-02), and the Fundamental Research Funds for the Central Public-interest Scientific Institution (2024YSKY-01).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Reynolds, C.S. The Ecology of Phytoplankton; Cambridge University Press: New York, NY, USA, 2006. [Google Scholar]
- Suthers, I.; Rissik, D.; Richardson, A. (Eds.) Plankton: A Guide to Their Ecology and Monitoring for Water Quality; CSIRO Publishing: Collingwood, Australia, 2019. [Google Scholar]
- Li, Z.; Bai, M.; Yao, L.; Ma, J.; He, F.; Bian, G.; Li, W. Phytoplankton and Zooplankton Community Dynamics in an Alpine Reservoir: Environmental Drivers and Ecological Implications in Daqing Reservoir, China. Water 2025, 17, 1202. [Google Scholar] [CrossRef]
- Falkowski, P.G. The role of phytoplankton photosynthesis in global biogeochemical cycles. Photosynth. Res. 1994, 39, 235–258. [Google Scholar] [CrossRef]
- Arrigo, K.R. Marine microorganisms and global nutrient cycles. Nature 2005, 437, 349–355. [Google Scholar] [CrossRef]
- Dias, E.; Morais, P.; Antunes, C.; Hoffman, J.C. The benthic food web connects the estuarine habitat mosaic to adjacent ecosystems. Food Webs 2023, 35, e00282. [Google Scholar] [CrossRef]
- Kang, C.K.; Park, H.J.; Choy, E.J.; Choi, K.S.; Hwang, K.; Kim, J.B. Linking intertidal and subtidal food webs: Consumer-mediated transport of intertidal benthic microalgal carbon. PLoS ONE 2015, 10, e0139802. [Google Scholar] [CrossRef]
- Brasil, J.; Attayde, J.L.; Vasconcelos, F.R.; Dantas, D.D.F.; Huszar, V.L. Drought-induced water-level reduction favors cyanobacteria blooms in tropical shallow lakes. Hydrobiologia 2016, 770, 145–164. [Google Scholar] [CrossRef]
- Li, D.; Wu, N.; Tang, S.; Su, G.; Li, X.; Zhang, Y.; Wang, G.; Zhang, J.; Liu, H.; Hecker, M.; et al. Factors associated with blooms of cyanobacteria in a large shallow lake, China. Environ. Sci. Eur. 2018, 30, 27. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Li, M.; Yu, R.; Sun, H.; Zhang, L.; Sun, L.; Lv, C.; Xu, J. Response of planktonic diversity and stability to environmental drivers in a shallow eutrophic lake. Ecol. Indic. 2022, 144, 109560. [Google Scholar] [CrossRef]
- Borics, G.; Görgényi, J.; Grigorszky, I.; László-Nagy, Z.; Tóthmérész, B.; Krzsznai, e.; Várbíró, G. The role of phytoplankton diversity metrics in shallow lake and river quality assessment. Ecol. Indic. 2022, 45, 28–36. [Google Scholar] [CrossRef]
- Xu, H.L.; Pan, J.Z.; Xu, L.G.; Lu, X.J.; Zhao, M.; Yang, H.S.; Wu, X.D. Ecological system health evaluation of lacustrine wetland in Taihu Basin. J. Lake Sci. 2019, 31, 1279–1288. [Google Scholar] [CrossRef]
- Tao, H.; Pan, J.Z.; Shen, Y.L.; Li, W.Z.; Huang, F. Overview and Degradation Reasons of Submerged Macrophytes of Gehu Lake. Environ. Sci. Technol. 2010, 23, 64–68. [Google Scholar]
- Chen, Z.N.; Zhang, H.G.; Zhou, W.; Shen, L.J. Analysis on the Relationship between the Distribution of Macrobenthos Community and Nitrogen-Phosphorus Factor in the Gehu Lake. Environ. Monit. Forew. 2016, 8, 45–50, 59. [Google Scholar]
- Luo, J.; Pu, R.; Duan, H.; Ma, R.; Mao, Z.; Zeng, Y.; Huang, L.; Xiao, Q. Evaluating the influences of harvesting activity and eutrophication on loss of aquatic vegetations in Taihu Lake, China. Int. J. Appl. Earth Obs. Geoinf. 2020, 87, 102038. [Google Scholar] [CrossRef]
- Chen, Y.F.; Shi, Q.F.; Qu, J.Y.; He, M.X.; Liu, Q. A pollution risk assessment and source analysis of heavy metals in sediments: A case study of Lake Gehu, China. Chin. J. Anal. Chem. 2022, 50, 100077. [Google Scholar] [CrossRef]
- Bao, X.M.; Yao, J.Y.; Yin, H.B. Occurrence characteristics and bioavailability of heavy metals in surface sediments of Lake Gehu, Taihu Basin. J. Lake Sci. 2016, 28, 1010–1017. [Google Scholar] [CrossRef][Green Version]
- Kim, H.G.; Kwak, I.S. Evaluating the necessity of geographical locality for patterning biological integrity and its responses to multiple stressors in river systems. Ecol. Indic. 2022, 142, 109285. [Google Scholar] [CrossRef]
- Liu, Z.H.; Qin, H.; Wu, X.J.; Zhong, L. Study on the Optimization of Water Resources Regulation in Ecological Restoration of Gehu Lake in the New Period. Water Power 2023, 49, 12–16+28. [Google Scholar]
- Xiong, C.H.; Zhang, R.L.; Wu, X.D.; Feng, L.H.; Wang, L.Q. Distribution and Pollution Assessment of Nutrient and Heavy Metals in Surface Sediments from Lake Gehu in Southern Jiangsu Province, China. Environ. Sci. 2016, 37, 925–934. [Google Scholar]
- Gong, R.; Hu, M.; Xu, Y.X.; Xu, L.G.; Jiang, M.L. Modeling the inhibition effect of recreating submerged plants habitat on sediment resuspension in Lake Gehu. J. Lake Sci. 2024, 36, 1403–1415. [Google Scholar] [CrossRef]
- Zhao, J.; Han, Y.; Liu, J.; Li, B.; Li, J.; Li, W.; Peng, S.; Pan, Y.; Li, A. Occurrence, distribution and potential environmental risks of pollutants in aquaculture ponds during pond cleaning in Taihu Lake Basin, China. Sci. Total Environ. 2024, 939, 173610. [Google Scholar] [CrossRef]
- Vu, H.P.; Nguyen, L.N.; Zdarta, J.; Nga, T.T.V.; Nghiem, L.D. Blue-green algae in surface water: Problems and opportunities. Curr. Pollut. Rep. 2020, 6, 105–122. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, J.; Ding, L.; Li, Y.; Liu, H.X.; Zhao, Y.F.; Fu, G. Eutrophication evolution trajectory influenced by human activities and climate in the shallow Lake Gehu, China. Ecol. Indic. 2022, 138, 108821. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment of the People’s Republic of China. Technical Specifications for Surface Water Environmental Quality Monitoring: HJ 91.2-2022; Ministry of Ecology and Environment: Beijing, China, 2022. [Google Scholar]
- Ministry of Environmental Protection of the People’s Republic of China; Editorial Board of Water and Wastewater Monitoring and Analysis Methods (Eds.) Water and Wastewater Monitoring and Analysis Methods, 4th ed.; Environmental Science Press: Beijing, China, 2002. [Google Scholar]
- Ministry of Ecology and Environment of the People’s Republic of China. Technical Guidelines for Water Ecological Monitoring-Aquatic Organism Monitoring and Evaluation of Lakes and Reservoirs (On Trial): HJ 1296-2023; Ministry of Ecology and Environment: Beijing, China, 2023. [Google Scholar]
- Hu, H.J.; Wei, Y.X. (Eds.) Chinese Freshwater Algae—System, Classification and Ecology; Science Press: Beijing, China, 2006. [Google Scholar]
- Zhou, F.X.; Chen, J.H. Atlas of Microbiology in Freshwater and Zoomacroinvertebrate, 2nd ed.; Chemical Industry Press: Beijing, China, 2011. [Google Scholar]
- Wang, Y.Y. Atlas of Common Aquatic Organisms in Chinese River Basins; Science Press: Beijing, China, 2020. [Google Scholar]
- Stevenson, R.J.; Bahls, L.L. Rapid bioassessment protocols for use in streams and wadeable rivers: Periphyton, benthic macroinvertebrates and fish. In Periphyton Protocols, 2nd ed.; US EPA: Washington, DC, USA, 2002; Volume 123. [Google Scholar]
- Wang, M.C.; Liu, X.Q. Evaluate method and Gradeification standard on lake eutrophication. Environ. Monit. China 2002, 18, 47–49. [Google Scholar]
- Ministry of Ecology and Environment of the People’s Republic of China. Surface Water Environmental Quality Assessment Measures; Ministry of Ecology and Environment: Beijing, China, 2011. [Google Scholar]
- Yang, W.H.; Li, Y.F.; Zhang, M.Y.; Gao, J.T.; Li, W.P. Variation characteristics and trend analysis of water quality of typical lakes in Inner Mongolia based on long time series. J. Lake Sci. 2025, 37, 1249–1266. [Google Scholar]
- Bilgin, A. Trophic state and limiting nutrient evaluations using trophic state/level index methods: A case study of Borçka Dam Lake. Environ. Monit. Assess. 2020, 192, 794. [Google Scholar] [CrossRef] [PubMed]
- Shannon, C.E. A mathematical theory of communications. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Pielou, E.C. Shannon’s formula as a measure of specific diversity: Its use and misuse. Am. Nat. 1966, 100, 463–465. [Google Scholar] [CrossRef]
- Margalef, R. Temporal succession and spatial heterogeneity in phytoplankton. In Perspectives in Marine Biology; Buzzati-Traverson, A.A., Ed.; University of California Press: Berkeley, CA, USA, 1958; pp. 323–349. [Google Scholar]
- Mcnaughton, S.J. Relationships among functional properties of Californian Grassland. Nature 1967, 216, 168–169. [Google Scholar] [CrossRef]
- Liang, D.; Wang, Q.; Wei, N.; Tang, C.; Sun, X.; Yang, Y. Biological indicators of ecological quality in typical urban river-lake ecosystems: The planktonicrotifer community and its response to environmental factors. Ecol. Indic. 2020, 112, 106127. [Google Scholar] [CrossRef]
- Zhang, Q.; Xie, Z.; Li, C.; Ye, C.; Wang, Y.; Ye, Z.; Wei, W.; Wang, H. Environmental factors affecting the phytoplankton composition in the lake of Tibetan Plateau. Diversity 2025, 17, 47. [Google Scholar] [CrossRef]
- Peres-Neto, P.; Legendre, P.; Dray, S.; Borcard, D. Variation partitioning of species data matrices: Estimation and comparison of fractions. Ecology 2006, 87, 2614–2625. [Google Scholar] [CrossRef]
- Lepš, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003; Volume 5. [Google Scholar]
- Smith, V.H.; Tilman, G.D.; Nekola, J.C. Eutrophication: Impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environ. Pollut. 1999, 100, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Lakshmikandan, M.; Li, M.; Pan, B. Cyanobacterial blooms in environmental water: Causes and solutions. Curr. Pollut. Rep. 2024, 10, 606–627. [Google Scholar] [CrossRef]
- Smith, V.H.; Schindler, D.W. Eutrophication science: Where do we go from here? Trends Ecol. Evol. 2009, 24, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Liao, R.H.; Wu, X.G.; Wang, Z.D.; Wang, B.Y.; Ke, F.; Han, C.; Zhou, Q.; Ren, J.H. Spatial distribution and pollution assessment on the main nutrients and heavy metals in sediments of Lake Gehu, Taihu Basin after removing the aquaculture net. J. Lake Sci. 2021, 33, 1436–1447. [Google Scholar] [CrossRef]
- Gao, Y.X.; Zhu, G.W.; Qin, B.Q.; Pang, Y.; Gong, Z.J.; Zhang, Y.L. Effect of ecological engineering on the nutrient content of surface sediments in Lake Taihu, China. Ecol. Eng. 2009, 35, 1624–1630. [Google Scholar] [CrossRef]
- Zhou, B.; Cai, X.; Wang, S.; Yang, X. Analysis of the causes of cyanobacteria bloom: A review. J. Resour. Ecol. 2020, 11, 405–413. [Google Scholar] [CrossRef]
- Paerl, H.W.; Otten, T.G. Harmful cyanobacterial blooms: Causes, consequences, and controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef]
- Ho, J.C.; Michalak, A.M. Challenges in tracking harmful algal blooms: A synthesis of evidence from Lake Erie. J. Great Lakes Res. 2015, 41, 317–325. [Google Scholar] [CrossRef]
- Ger, K.A.; Urrutia-Cordero, P.; Frost, P.C.; Hansson, L.A.; Sarnelle, O.; Wilson, A.E.; Lürling, M. The interaction between cyanobacteria and zooplankton in a more eutrophic world. Harm. Alg. 2016, 54, 128–144. [Google Scholar] [CrossRef]
- Whatley, M.H.; Van Loon, E.E.; Cerli, C.; Arie Vonk, J.; Van Der Geest, H.G.; Admiraal, W. Linkages between benthic microbial and freshwater insect communities in degraded peatland ditches. Ecol. Indic. 2014, 46, 415–424. [Google Scholar] [CrossRef]
- Josefson, A.B.; Hansen, J.L.S.; Asmund, G.; Johansen, P. Threshold response of benthic macrofauna integrity to metal contamination in West Greenland. Mar. Pollut. Bull. 2008, 56, 1265–1274. [Google Scholar] [CrossRef]
- Liu, X.; Sun, T.; Yang, W.; Li, X.; Ding, J.; Fu, X. Meta-analysis to identify inhibition mechanisms for the effects of submerged plants on algae. J. Environ. Manag. 2024, 355, 120480. [Google Scholar] [CrossRef]
- Castro, B.B.; Antunes, S.C.; Pereira, R.; Soares, A.M.V.M.; Gonçalves, F. Rotifer community structure in three shallow lakes: Seasonal fluctuations and explanatory factors. Hydrobiologia 2005, 543, 221–232. [Google Scholar] [CrossRef]
- Scheffer, M.; Rinaldi, S.; Gragnani, A.; Mur, L.R.; van Nes, E.H. On the dominance of filamentous cyanobacteria in shallow, turbid lakes. Ecology 1997, 78, 272–282. [Google Scholar] [CrossRef]
- Klausmeier, C.A.; Litchman, E.; Daufresne, T.; Mur, L.R.; Van Nes, E.H. Optimal nitrogen-to-phosphorus stoichiometry of phytoplankton. Nature 2004, 429, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Ejsmont-Karabin, J. The usefulness of zooplankton as lake ecosystem indicators: Rotifer trophic state index. Pol. J. Ecol. 2012, 60, 339–350. [Google Scholar]
- Su, Y.; Lundholm, N.; Friis, S.M.M.; Elledaard, M. Implications for photonic applications of Bacillariophyta growth and frustule nanostructure changes in response to different light wavelengths. Nano Res. 2015, 8, 2363–2372. [Google Scholar] [CrossRef]
- Zaikova, E.; Walsh, D.A.; Stilwell, C.P.; Mohn, W.W.; Tortell, P.D.; Hallam, S.J. Microbial community dynamics in a seasonally anoxic fjord: Saanich Inlet, British Columbia. Environ. Microbiol. 2010, 12, 172–191. [Google Scholar] [CrossRef]
- Jilbert, T.; Couture, R.M.; Huser, B.J.; Salonen, K. Preface: Restoration of eutrophic lakes: Current practices and future challenges. Hydrobiologia 2020, 847, 4343–4357. [Google Scholar] [CrossRef]
- Land, M.; Granéli, W.; Grimvall, A.; Hoffmann, C.C.; Mitsch, W.J.; Tonderski, K.S.; Verhoeven, J.T.A. How effective are created or restored freshwater wetlands for nitrogen and phosphorus removal? A systematic review. Environ. Evid. 2016, 5, 9. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).