Spatiotemporal Dynamics and Driving Factors of Phytoplankton Community Structure in the Liaoning Section of the Liao River Basin in 2010, 2015, and 2020
Abstract
1. Introduction
2. Materials and Methods
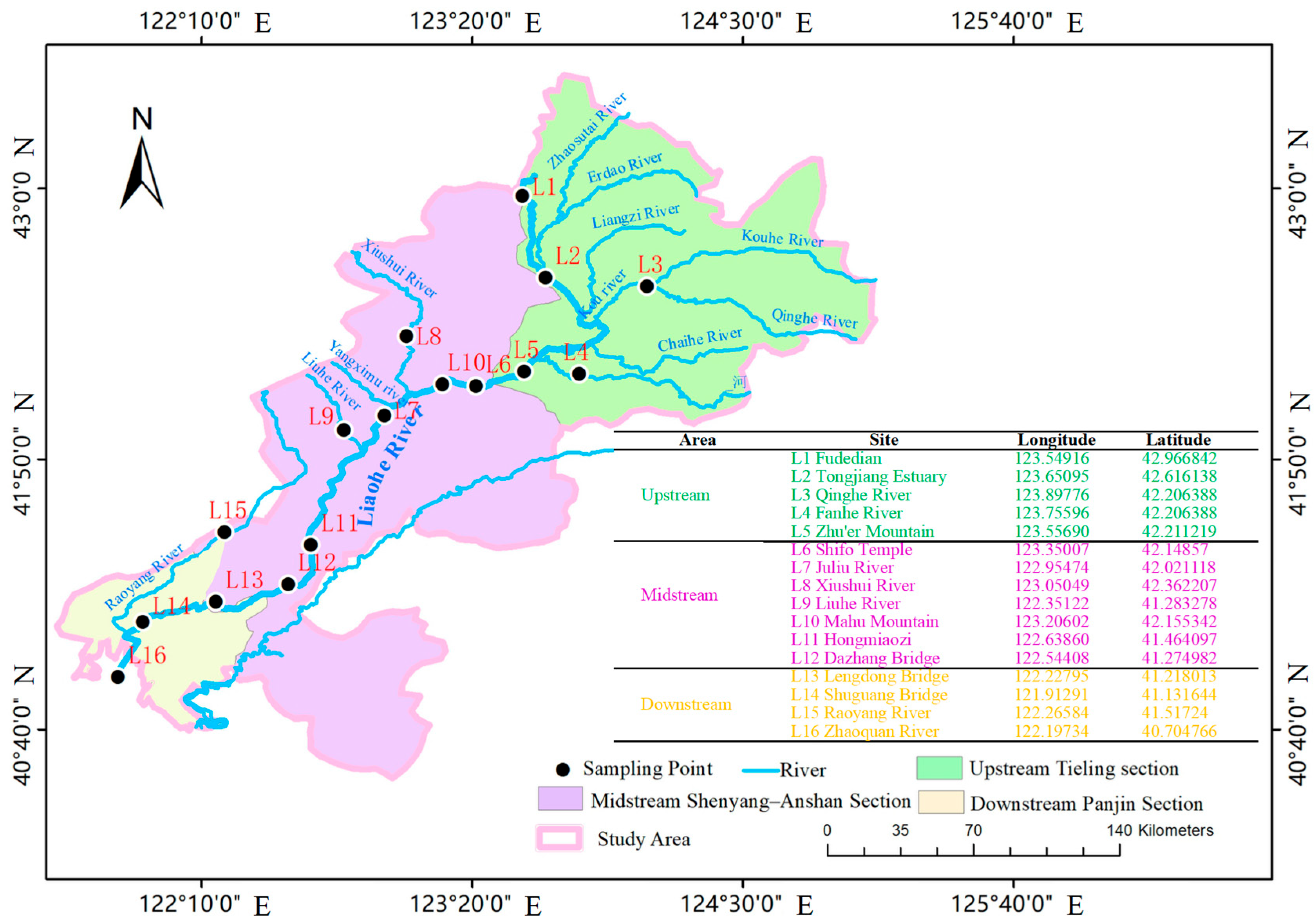
2.1. Study Area
2.2. Sample Collection and Analytical Methods
2.2.1. Collection and Identification of Phytoplankton
2.2.2. Determination of Physical and Chemical Indicators in Surface Water
2.3. Data Processing and Analysis
2.3.1. Analysis of Community Diversity
2.3.2. Statistical Analysis
2.3.3. Evaluation Methodology
3. Results
3.1. Physical and Chemical Indicators of Surface Water Quality
3.2. Changes in Phytoplankton Community Structure
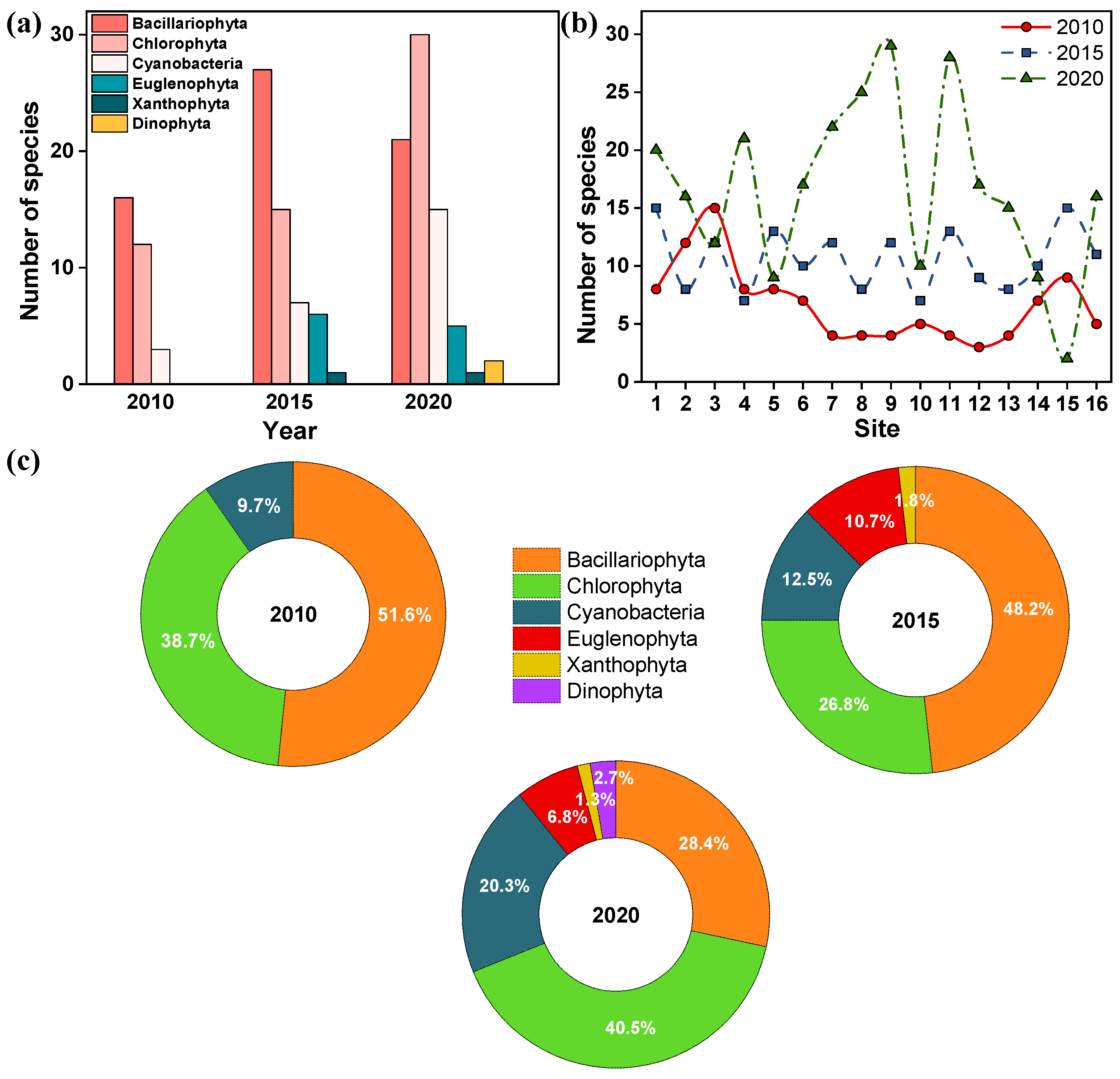
3.2.1. Species Composition
3.2.2. Advantageous Species Change
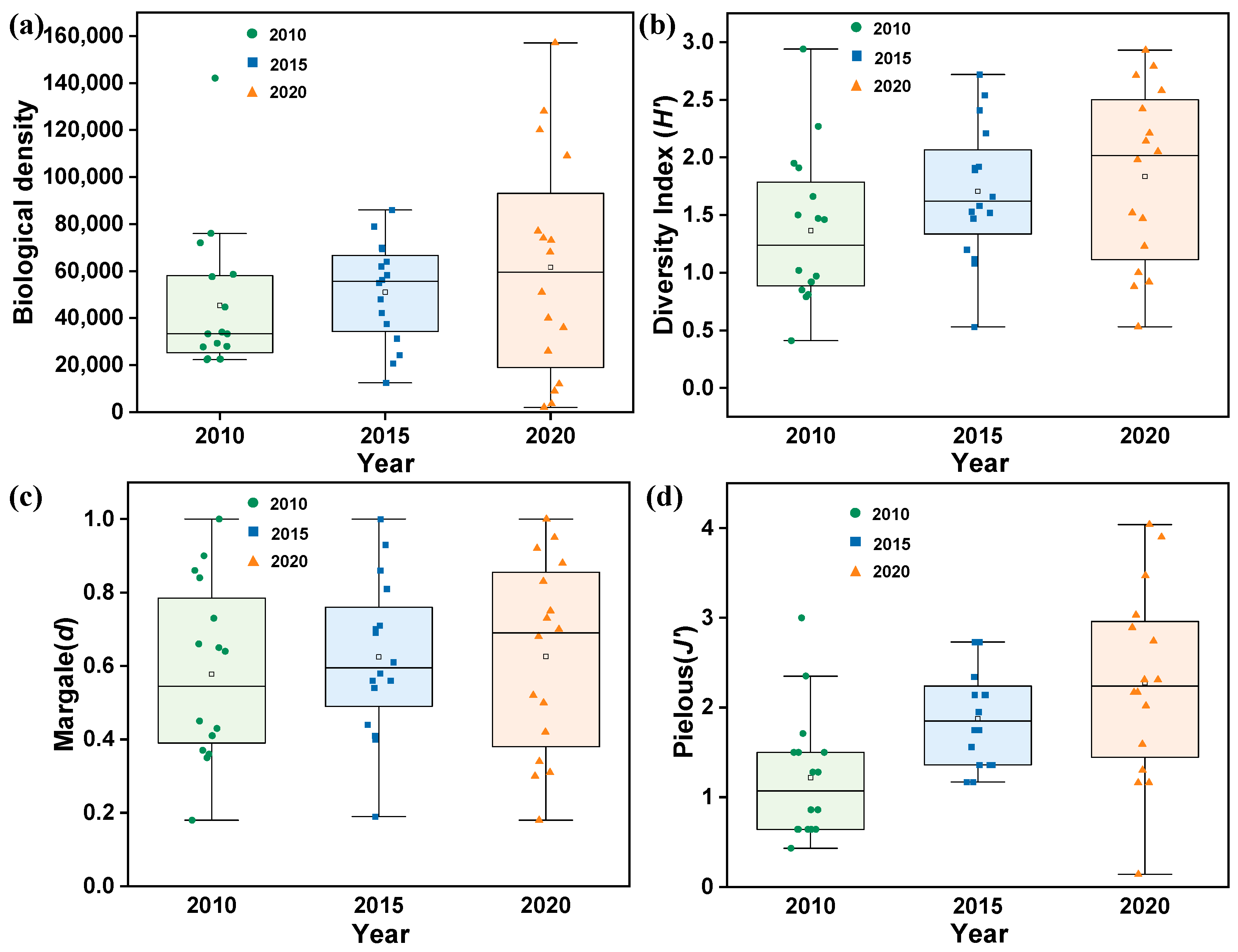
3.3. Changes in Biological Density and Diversity Indices
3.4. Biodiversity Index Evaluation Analysis
4. Discussion
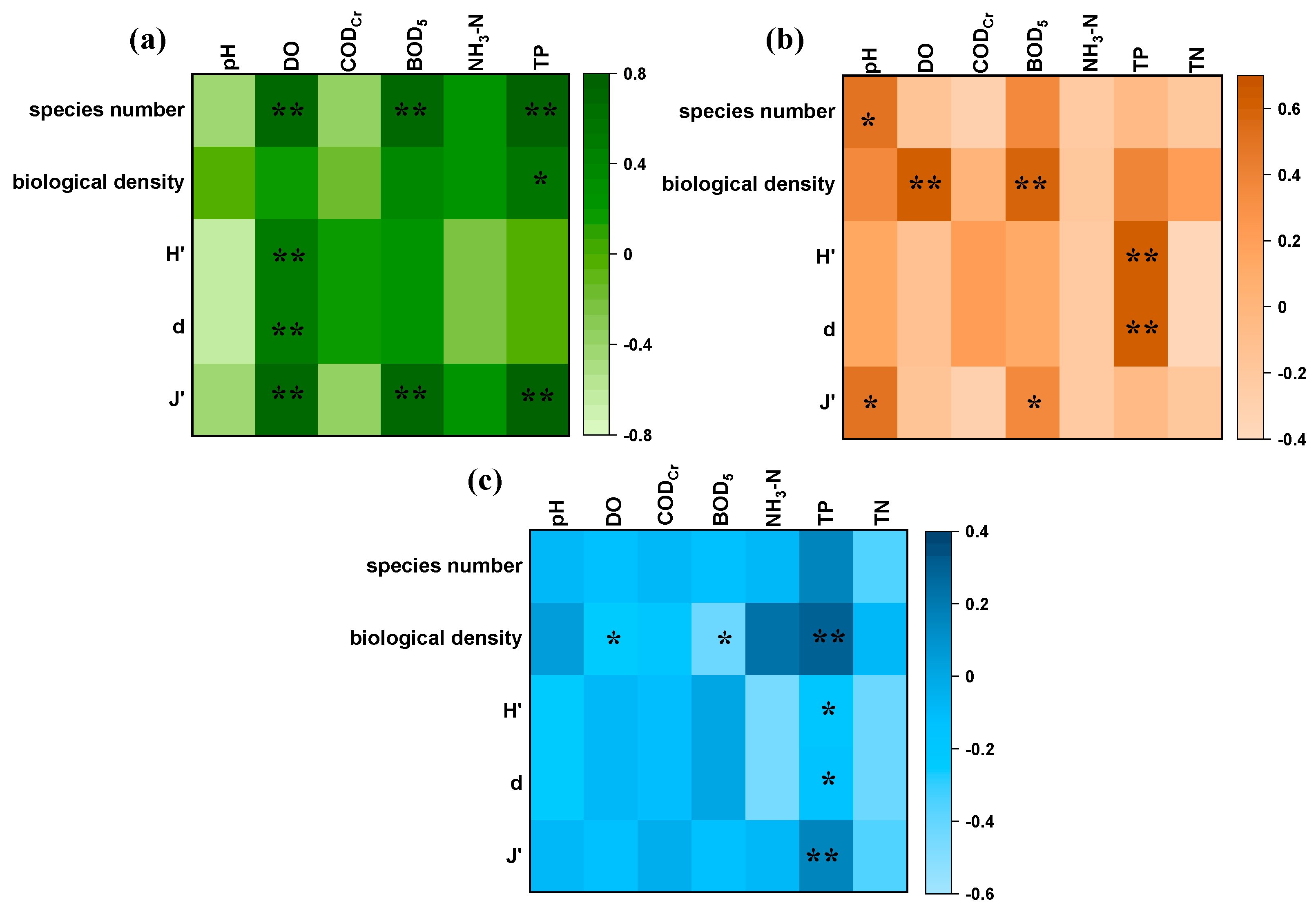
4.1. Phytoplankton Community Structure and Its Drivers
4.2. Phytoplankton Diversity and Its Drivers
4.2.1. Driving Effects of Aquatic Environmental Factors
4.2.2. Driving Effects of Hydrological Factors
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, M.; Li, Z.; Zhang, X. Analysis on Biological Evaluation of Regional Water Environment. Environ. Prot. Sci. 1996, 54–57, 7–78. [Google Scholar]
- Cardinale, B.J.; Palmer, M.A.; Collins, S.L. Species diversity enhances ecosystem functioning through interspecific facilitation. Nat. Chem. 2002, 415, 426–429. [Google Scholar] [CrossRef]
- Li, X.; Xu, Z.; Zhang, S.; Gao, W.; Dong, Q.; Guo, F.; Zhu, Z.; Yang, W.; Yang, Z. Eutrophication-Driven Changes in Plankton Trophic Interactions: Insights from Trade-Offs in Functional Traits. Environ. Sci. Technol. 2024, 59, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Long, S.; Zhang, T.; Fan, J.; Li, C.; Xiong, K. Responses of phytoplankton functional groups to environmental factors in the Pearl River, South China. Environ. Sci. Pollut. Res. 2020, 27, 42242–42253. [Google Scholar] [CrossRef] [PubMed]
- Voulvoulis, N.; Arpon, K.D.; Giakoumis, T. The EU Water Framework Directive: From great expectations to problems with implementation. Sci. Total Environ. 2017, 575, 358–366. [Google Scholar] [CrossRef]
- Guo, Y.; Ding, Z. Research on Prediction of Main Water Quality Indicators in Liao River Basin Based on ARIMA Model. J. Green Sci. Technol. 2021, 23, 34–36. [Google Scholar]
- Li, Q.; Zhao, W.; Yin, X.; Zhang, W.; Meng, W. Study on the diversity of phytoplankton species in the Taizi River system of Liao River. J. Dalian Ocean. Univ. 2011, 26, 322–327. [Google Scholar]
- Zhao, C.; Zhu, Z.; Zhou, D. Rivers and Dams of the World; China Water & Power Press: Beijing, China, 2000. [Google Scholar]
- Reynolds, C.S.; Irish, A.E.; Elliott, J.A. The ecological basis for simulating phytoplankton responses to environmental change (PROTECH). Ecol. Model. 2001, 140, 271–291. [Google Scholar] [CrossRef]
- Hui, T.; Xie, P.; Guo, L.; Chu, Z.; Liu, M. Study on the Composition and Succession of Dominant Species of Phytoplankton in Yanghe Reservoir. Acta Hydrobiol. Hydrobiol. Sin. 2015, 39, 524–532. [Google Scholar]
- Huang, H.; Yang, Y.; Yang, L.; Zuo, G.; Zeng, R. Study on the Changes of Phytoplankton in the Ecological Monitoring Area of Bohai Bay from 2004 to 2015. Haiyang Xuebao 2018, 40, 14. [Google Scholar]
- Liu, D.; Morrison, R.J.; West, R.J. Phytoplankton Assemblages as an Indicator of Water Quality in Seven Temperate Estuarine Lakes in South-East Australia. In Eutrophication: Causes, Consequences and Control; Springer: Dordrecht, The Netherlands, 2014; Volume 2, pp. 191–202. [Google Scholar]
- Li, L.; Xia, R.; Xia, X.; Dou, M.; Ling, M.; Chen, Y.; Dou, J.; Zhang, K.; Jia, R.; Yang, C. Hydrological variability significantly influences aquatic biological integrity at the spatial scale in semi-arid watersheds. J. Hydrol. 2025, 660, 133359. [Google Scholar] [CrossRef]
- Bowes, M.J.; Gozzard, E.; Johnson, A.C.; Scarlett, P.M.; Roberts, C.; Read, D.S.; Armstrong, L.K.; Harman, S.A.; Wickhami, H.D. Spatial and temporal changes in chlorophyll-a concentrations in the River Thames basin, UK: Are phosphorus concentrations beginning to limit phytoplankton biomass? Sci. Total Environ. 2012, 426, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, P.; Feng, C.; Wen, R. Ecological study on phytoplankton in Liaohe Estuary. Mar. Environ. Sci. 2013, 32, 679–682. [Google Scholar]
- Han, M.; Shu, Y. Atlas of Freshwater Organisms in China; Ocean Press: Beijing, China, 1995. [Google Scholar]
- Hu, H.; Wei, Y. Freshwater Algae of China: Systematics, Taxonomy, and Ecology; Science Press: Beijing, China, 2006. [Google Scholar]
- Lampitt, R.S.; Wishner, K.F.; Turley, C.M.; Angel, M.V. Marine snow studies in the Northeast Atlantic Ocean: Distribution, composition and role as a food source for migrating plankton. Mar. Biol. 1993, 116, 689–702. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, F. Investigation and protection strategy of plankton in the Harbin section of the Songhua River, China. Appl. Ecol. Environ. Res. 2024, 22, 5791. [Google Scholar] [CrossRef]
- Reynolds, C.S. The Ecology of Phytoplankton; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Yang, D.; Zhou, X.; Chen, J.; Luo, H.; Li, J.; An, M.; Jiang, H.; Shao, J. Investigation and diversity analysis of plankton resources in the Beipanjiang Grand Canyon Basin. Genom. Appl. Biol. 2021, 40, 639–648. [Google Scholar]
- Lan, K.; Liu, B.; Peng, D.; Ren, X.; Yang, D. Investigation and diversity analysis of plankton resources in spring in Dumu River. Chin. J. Mt. Agric. Biol. 2021, 40, 15–22. [Google Scholar]
- Li, D.; Lu, W.; Zhang, L.; Zhang, Z. Phytoplankton Community in the East Liao River Basin: A Canonical Correspondence Analysis with Aquatic Environmental Factors. Water Resour. Hydropower Northeast. China 2013, 31, 2. [Google Scholar]
- Li, X.; Yu, H.; Wang, H.; Ma, C. Phytoplankton community structure in relation to environmental factors and ecological assessment of water quality in the upper reaches of the Genhe River in the Greater Hinggan Mountains. Environ. Sci. Pollut. Res. 2019, 26, 17512–17519. [Google Scholar] [CrossRef]
- Arhonditsis, G.B.; Winder, M.; Brett, M.T.; Schindler, D.E. Patterns and mechanisms of phytoplankton variability in Lake Washington (USA). Water Res. 2004, 38, 4013–4027. [Google Scholar] [CrossRef]
- Zhao, K.; Bai, X.; Dong, A.; Li, C.; Lu, H. A Study on the Correlation between the Spatial and Temporal Distribution of Phytoplankton Community and Environmental Factors in Changchun Section of the Yitong River. Pol. J. Environ. Stud. 2022, 31, 3955–3969. [Google Scholar] [CrossRef]
- GB 3838-2002; Environmental Quality Standards for Surface Water. Standards Press of China: Beijing, China, 2002.
- Li, F.; Dong, F.; Duan, M.; Wang, G.; Zhu, L. Characteristics of Phytoplankton Community Structure and Water Quality Assessment in the Daliao River System in Summer. Chin. J. Ecol. 2011, 30, 8. [Google Scholar]
- Cloern, J.E.; Foster, S.Q.; Kleckner, A.E. Phytoplankton primary production in the world’s estuarine-coastal ecosystems. Biogeosciences 2014, 11, 2477–2501. [Google Scholar] [CrossRef]
- Haque, M.A.; Jewel, M.A.S.; Akhi, M.M.; Atique, U.; Paul, A.K.; Iqbal, S.; Islam, M.S.; Das, S.K.; Alam, M.M. Seasonal dynamics of phytoplankton community and functional groups in a tropical river. Environ. Monit. Assess. 2021, 193, 1–16. [Google Scholar] [CrossRef]
- Wen, G.; Wang, R.; Wen, C.; Bai, Y.; Cap, R.; Si, F.; Li, K.; Huang, T. Research Progress on the Formation Causes and In-situ Control of Freshwater Dinoflagellate Blooms. China Environ. Sci. 2023, 43, 2239–2253. [Google Scholar]
- Song, G.; Zhang, Y.; Qiu, P.; Ruan, G.; Bi, Y. Structural Characteristics of the Phytoplankton Community and Its Influencing Factors in Lushui Reservoir. J. Hydroecol. 2024, 45, 49–57. [Google Scholar]
- Chen, F.; Wang, W.; He, T.; Zhou, H.; Qian, B. Analysis of the New Situation of Water Bloom Development and Governance Countermeasures in the Middle and Lower Reaches of the Hanjiang River. Yangtze River 2023, 54, 7–17. [Google Scholar]
- Wang, G.; Wang, L.; Wu, W. Evolutionary Trends of Ecological Security of Drinking Water Sources in the Main Stream of the Liao River Based on GIS. Chin. J. Appl. Ecol. 2007, 18, 6. [Google Scholar]
- Xing, Y.; Lu, Z.; Wang, T.; Xu, X.; Wang, L.; Zhang, W. Community Assembly Mechanisms and Driving Factors of Phytoplankton Functional Groups in the Urban River Network of Shanghai during Winter. J. Shanghai Ocean. Univ. 2025, 34, 56–68. [Google Scholar]
- Gu, X.; Li, H.; Mao, Z.; Chen, H.; Shen, R.; Zeng, Q. Research progress on ecological interaction between cyanobacteria bloom and freshwater fish. Chin. Sci. Bull. 2021, 66, 2649–2662. [Google Scholar] [CrossRef]
- Zhou, G.; Kuan, Q.; Hu, Z.; Cai, Q. Algae diversity evaluation and ‘bloom’ control of four tributaries in the Three Gorges Reservoir Area. China Environ. Sci. 2006, 3, 337–341. [Google Scholar]
- He, Y.; Zheng, Y.; Li, W.; Zhang, Z.; Ma, Y.; Zhao, T.; Ren, Y. Community Structure Characteristics of Eukaryotic Phytoplankton in Danjiangkou Reservoir in Early Spring and Their Relationships with Environmental Factors. Acta Sci. Circumstantiae 2021, 41, 9. [Google Scholar]
- Kuang, Q.; Ma, P.; Hu, Z.; Zhou, G. Research progress on algae biological evaluation and management of lake eutrophication. J. Saf. Environ. 2005, 5, 87–91. [Google Scholar]
- Yang, M.; Bi, Y.; Ai, Y.; Hu, J.; Zhu, K.; Hu, Z. Preliminary Study on the Impact of Water Flow Velocity on Phytoplankton in the Xiangxi River Bay under Artificial Control Conditions. Resour. Environ. Yangtze Basin 2012, 21, 220–224. [Google Scholar]
- Dong, Y.; Zhang, P.; Li, X.; Zheng, B. The Spatiotemporal Distribution of Macrophytes in Qionghai Lake and Causes of Degradation. J. Lake Sci. 2024, 36, 236–246. [Google Scholar]




| Water Quality Evaluation Standard | Shannon–Wiener Diversity (H′) | Pielou’s Evenness (J) | Margalef’s Richness (d) |
|---|---|---|---|
| Clean | >3.0 | >0.8 | >5.0 |
| Light pollution | 2.0–3.0 | 0.5–0.8 | 3.0–5.0 |
| Moderate pollution | 1.0–2.0 | 0.3–0.5 | 1.0–3.0 |
| Heavy pollution | 0–1.0 | 0–0.3 | 0–1.0 |
| Year | H′ Evaluation Results | d Evaluation Results | J′ Evaluation Results |
|---|---|---|---|
| 2010 | Moderate pollution | Moderate pollution | Light pollution |
| 2015 | Moderate pollution | Moderate pollution | Light pollution |
| 2020 | Moderate pollution | Moderate pollution | Light pollution |
| Hydrological Factors | Year | H′ (P) | d (P) | J′ (P) |
|---|---|---|---|---|
| Flow velocity | 2010 | 0.618 | 0.047 | 0.640 |
| 2015 | 0.071 | 0.754 | 0.071 | |
| 2020 | 0.529 | 0.449 | 0.529 | |
| Flow rate | 2010 | 0.160 | 0.292 | 0.172 |
| 2015 | 0.365 | 0.753 | 0.376 | |
| 2020 | 0.348 | 0.793 | 0.348 | |
| Water depth | 2010 | 0.592 | 0.127 | 0.835 |
| 2015 | 0.079 | 0.765 | 0.077 | |
| 2020 | 0.346 | 0.364 | 0.346 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, K.; Hu, Z.; Pang, R.; Li, M.; Liu, L. Spatiotemporal Dynamics and Driving Factors of Phytoplankton Community Structure in the Liaoning Section of the Liao River Basin in 2010, 2015, and 2020. Water 2025, 17, 2182. https://doi.org/10.3390/w17152182
Peng K, Hu Z, Pang R, Li M, Liu L. Spatiotemporal Dynamics and Driving Factors of Phytoplankton Community Structure in the Liaoning Section of the Liao River Basin in 2010, 2015, and 2020. Water. 2025; 17(15):2182. https://doi.org/10.3390/w17152182
Chicago/Turabian StylePeng, Kang, Zhixiong Hu, Rui Pang, Mingyue Li, and Li Liu. 2025. "Spatiotemporal Dynamics and Driving Factors of Phytoplankton Community Structure in the Liaoning Section of the Liao River Basin in 2010, 2015, and 2020" Water 17, no. 15: 2182. https://doi.org/10.3390/w17152182
APA StylePeng, K., Hu, Z., Pang, R., Li, M., & Liu, L. (2025). Spatiotemporal Dynamics and Driving Factors of Phytoplankton Community Structure in the Liaoning Section of the Liao River Basin in 2010, 2015, and 2020. Water, 17(15), 2182. https://doi.org/10.3390/w17152182






