Photocatalytic Degradation of Oxytetracycline and Imidacloprid Under Visible Light with Sr0.95Bi0.05TiO3: Influence of Aqueous Matrix
Abstract
1. Introduction
2. Materials and Methods
2.1. Bi-Doped SrTiO3 Preparation and Characterization
2.2. Theoretical Method
2.3. River Water Sample Collection and Characterization
2.4. Photocatalytic Experiments
3. Results
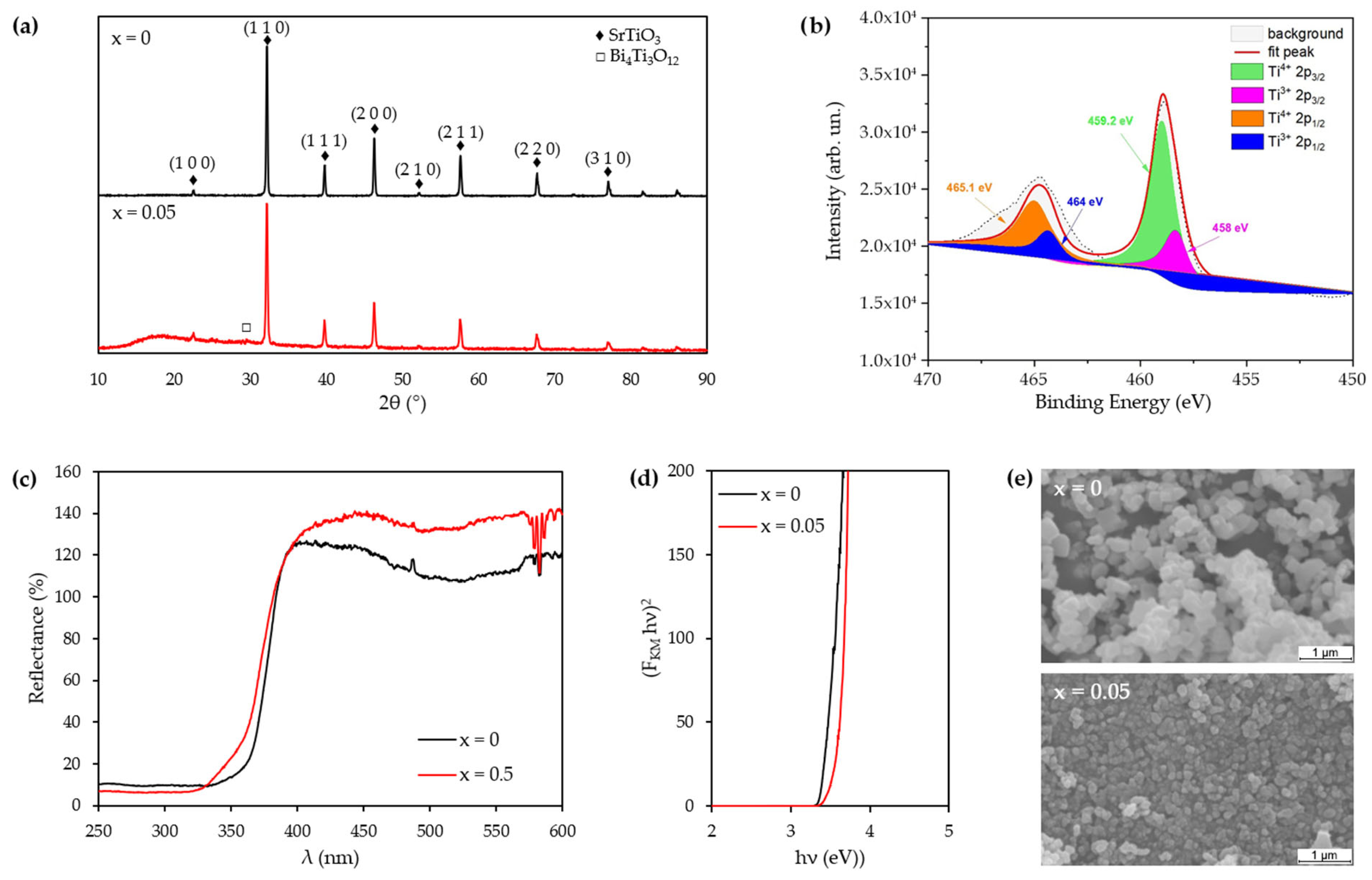
3.1. Perovskite Characterization
3.2. Electronic Structure Calculations
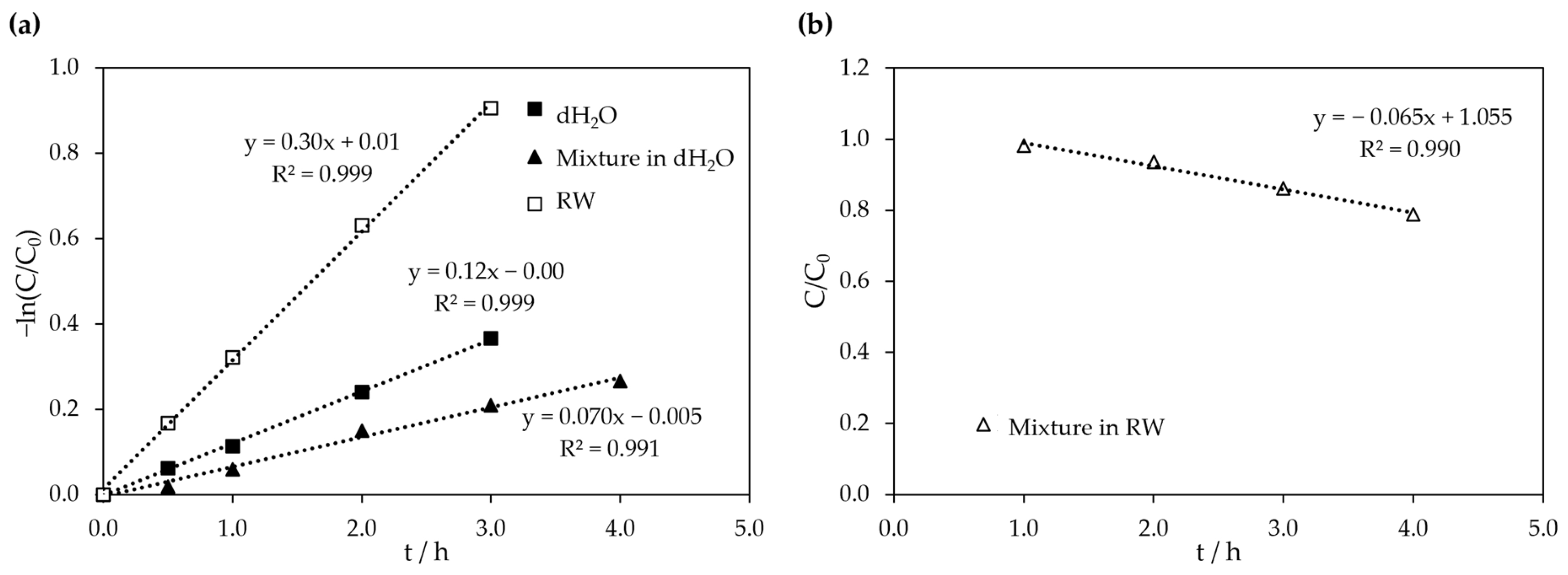
3.3. Photocatalytic Activity of Sr0.95Bi0.05TiO3 Under Visible Light
3.4. COD and TOC Removals
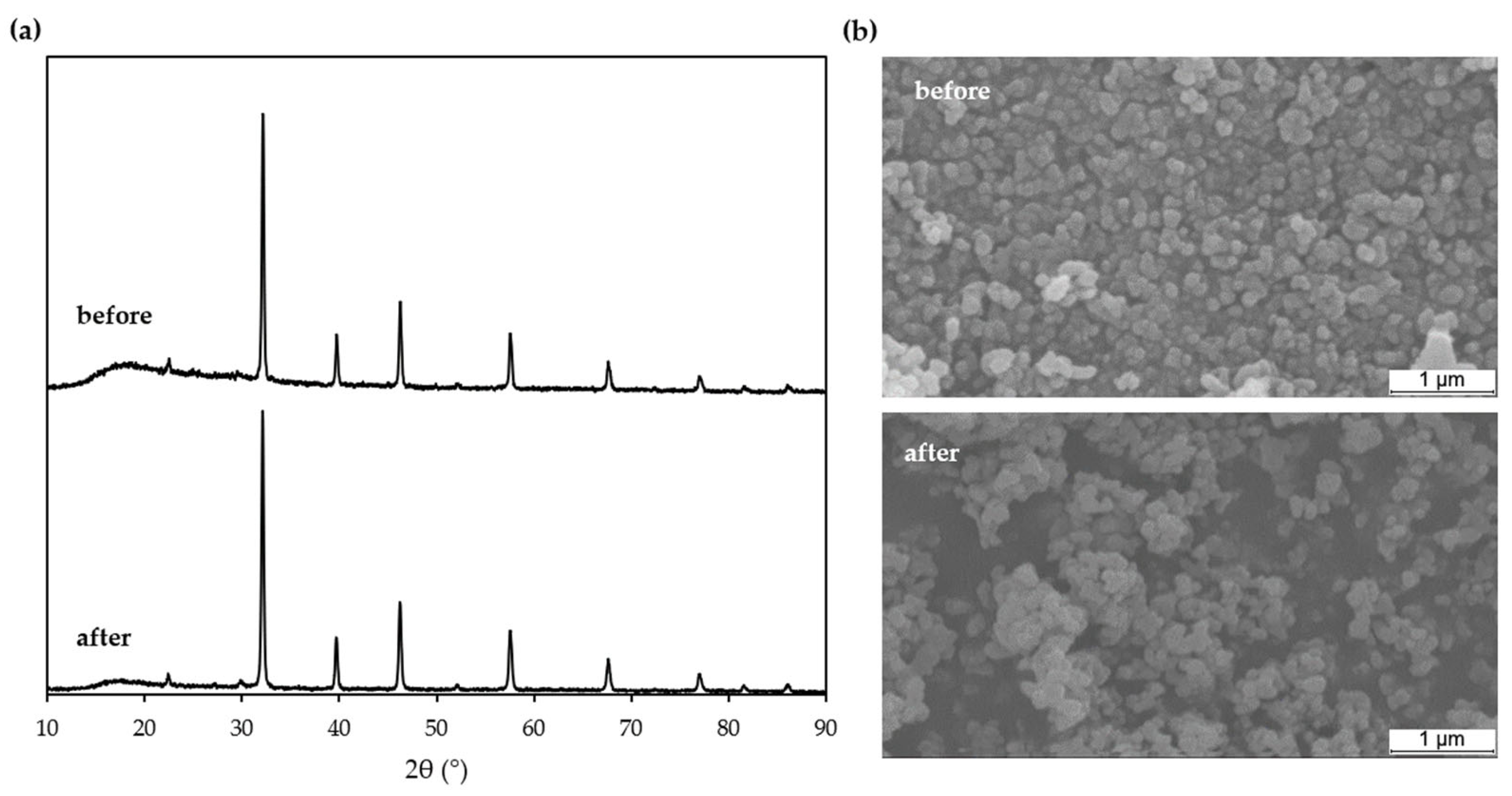
3.5. Reusability
3.6. Toxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wei, K.; Faraj, Y.; Yao, G.; Xie, R.; Lai, B. Strategies for improving perovskite photocatalysts reactivity for organic pollutants degradation: A review on recent progress. Chem. Eng. J. 2021, 414, 128783. [Google Scholar] [CrossRef]
- Madkhali, N.; Prasad, C.; Malkappa, K.; Choi, H.Y.; Govinda, V.; Bahadur, I.; Abumousa, R.A. Recent update on photocatalytic degradation of pollutants in wastewater using TiO2-based heterostructured materials. Results Eng. 2023, 17, 100920. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Wang, G. Photocatalytic advanced oxidation processes for water treatment: Recent advances and perspective. Chem. Asian J. 2020, 15, 3239–3253. [Google Scholar] [CrossRef]
- Pelosato, R.; Bolognimo, I.; Fontana, F.; Sora, I.N. Applications of heterogeneous photocatalysis to the degradation of oxytetracycline in water: A review. Molecules 2022, 27, 2743. [Google Scholar] [CrossRef]
- Zabar, R.; Komel, T.; Fabjan, B.J.; Kralj, B.; Trebse, P. Photocatalytic degradation with immobilised TiO2 of three selected neonicotinoid insecticides: Imidacloprid, thiamethoxam and clothianidin. Chemosphere 2012, 89, 293–301. [Google Scholar] [CrossRef]
- Zeshan, M.; Bhatti, I.A.; Mohsin, M.; Iqbal, M.; Amjed, N.; Nisar, J.; AlMasoud, N.; Alomar, T.S. Remediation of pesticides using TiO2 based photocatalytic strategies: A review. Chemosphere 2022, 30, 134525. [Google Scholar] [CrossRef] [PubMed]
- Kamat, P.V.; Jin, S. Semiconductor photocatalysis: “Tell us the complete story!”. ACS Energy Lett. 2018, 3, 622–623. [Google Scholar] [CrossRef]
- Huang, L.; Huang, X.; Yan, J.; Liu, Y.; Jiang, H.; Zhang, H.; Tang, J.; Liu, Q. Research progresses on the application of perovskite in adsorption and photocatalytic removal of water pollutants. J. Hazard. Mater. 2023, 442, 130024. [Google Scholar] [CrossRef]
- Parwaiz, S.; Khan, M.M. Perovskites and perovskite-based heterostructures for photocatalytic energy and environmental applications. J. Environm. Chem. Eng. 2024, 12, 113175. [Google Scholar] [CrossRef]
- Silva, P.S.; Pereira, D.; Calisto, V.; Martins, M.A.; Otero, M.; Esteves, V.I.; Lima, D.L.D. Biochar-TiO2 magnetic nanocomposites for photocatalytic solar-driven removal of antibiotics from aquaculture effluents. J. Environ. Manage. 2021, 294, 112937. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, S.; Rishikesh; Manna, A.K.; Soni, R.K. Fabrication of ZnO-TiO2 nanohybrids for rapid sunlight driven photodegradation of textile dyes and antibiotic residue molecules. Opt. Mater. 2020, 107, 110138. [Google Scholar] [CrossRef]
- Zhang, S.; Ou, X.; Xiang, Q.; Carabineiro, A.A.C.; Fan, J.; Lv, K. Research progress in metal sulfides for photocatalysis: From activity to stability. Chemosphere 2022, 303, 135085. [Google Scholar] [CrossRef]
- Ng, Y.H.; Ikeda, S.; Matsumura, M.; Amal, R. A perspective on fabricating carbon-based nanomaterials by photocatalysis and their applications. Energy Environ. Sci. 2012, 5, 9307–9318. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Oyewo, O.A.; Onwudiwe, D.C. The performance of bismuth-based compounds in photocatalytic applications. Surf. Interfaces 2021, 23, 100927. [Google Scholar] [CrossRef]
- Silveira, J.E.; de Souza, A.S.; Pasini, F.N.N.; Ribeiro, A.R.; Scopel, W.L.; Zazo, J.A.; Casas, J.A.; Paz, W.S. A comprehensive study of the reduction of nitrate on natural FeTiO3: Photocatalysis and DFT calculations. Sep. Purif. Technol. 2023, 306, 122570. [Google Scholar] [CrossRef]
- Silveira, J.E.; Paz, W.S.; Garcia-Muñoz, P.; Zazo, J.A.; Casas, J.A. UV-LED/ilmenite/persulfate for azo dye mineralization: The role of sulfate in the catalyst deactivation. Appl. Catal. B Environ. 2017, 219, 314–321. [Google Scholar] [CrossRef]
- Silveira, J.E.; Garcia-Costa, A.L.; Carbajo, J.; Ribeiro, A.R.; Pliego, G.; Paz, W.S.; Zazo, J.A.; Casas, J.A. Nitrate removal in saline water by photo-reduction using natural FeTiO3 as catalyst. Chem. Eng. J. Adv. 2022, 12, 100387. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, Q.; Xu, K.; Qiu, L.; Song, J.; Wang, L. Study on visible light photocatalytic performance of BiVO4 modified by graphene analogue boron nitride. Chemosphere 2022, 307, 135811. [Google Scholar] [CrossRef]
- Sanei, A.; Dashtian, K.; Seyf, J.Y.; Seidi, F.; Kolvari, E. Biomass derived reduced-graphene-oxide supported α-Fe2O3/ZnO S-scheme heterostructure: Robust photocatalytic wastewater remediation. J. Environ. Manage. 2023, 332, 117377. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Q.; Tian, X.; Hong, Y.; Nie, Y.; Su, N.; Jin, G.; Zhai, Z.; Fu, C. Highly efficient photocatalytic degradation of oil pollutants by oxygen deficient SnO2 quantum dots for water remediation. Chem. Eng. J. 2021, 404, 127146. [Google Scholar] [CrossRef]
- Liu, J.; Qu, X.; Zhang, C.; Dong, W.; Fu, C.; Wangan, J.; Zhang, Q. High-yield aqueous synthesis of partial-oxidized black phosphorus as layered nanodot photocatalysts for efficient visible-light driven degradation of emerging organic contaminants. J. Clean. Prod. 2022, 377, 134228. [Google Scholar] [CrossRef]
- Reddy, K.R.; Reddy, C.; Nadagouda, M.; Shetti, N.; Jaesool, S.; Aminabhavi, T.M. Polymeric graphitic carbon nitride (g-C3N4)-based semiconducting nanostructured materials: Synthesis methods, properties and photocatalytic applications. J. Environ. Manage. 2019, 238, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Mateo, D.; Albero, J.; García, H. Titanium-perovskite-supported RuO2 nanoparticles for photocatalytic CO2 methanation. Joule 2019, 3, 1949–1962. [Google Scholar] [CrossRef]
- Mai, X.T.; Bui, D.N.; Pham, V.K.; Pham, T.H.T.; Nguyen, T.T.L.; Chau, H.D.; Tran, T.K.N. Effect of CuO loading on the photocatalytic activity of SrTiO3/MWCNTs nanocomposites for dye degradation under visible light. Inorganics 2022, 10, 211. [Google Scholar] [CrossRef]
- Phoon, B.L.; Lai, C.W.; Juan, J.C.; Show, P.-L.; Chen, W.-H. A review of synthesis and morphology of SrTiO3 for energy and other applications. Int. J. Energy Res. 2019, 43, 5151–5174. [Google Scholar] [CrossRef]
- Kimijima, T.; Kanie, K.; Nakaya, M.; Muramatsu, A. Solvothermal synthesis of SrTiO3 nanoparticles precisely controlled in surface crystal planes and their photocatalytic activity. Appl. Catal. B Environ. 2014, 144, 462–467. [Google Scholar] [CrossRef]
- Li, F.; Yu, K.; Lou, L.-L.; Su, Z.; Liu, S. Theoretical and experimental study of La/Ni co-doped SrTiO3 photocatalyst. Mater. Sci. Eng. B 2010, 172, 136–141. [Google Scholar] [CrossRef]
- Jia, A.; Su, Z.; Lou, L.-L.; Liu, S. Synthesis and characterization of highly-active nickel and lanthanum co-doped SrTiO3. Solid State Sci. 2010, 12, 1140–1145. [Google Scholar] [CrossRef]
- Huang, S.-T.; Lee, W.W.; Chang, J.-L.; Huang, W.-S.; Chou, S.-Y.; Chen, C.-C. Hydrothermal synthesis of SrTiO3 nanocubes: Characterization, photocatalytic activities, and degradation pathway. J. Taiwan Inst. Chem. Eng. 2014, 45, 1927–1936. [Google Scholar] [CrossRef]
- Wu, G.; Li, P.; Xu, D.; Luo, B.; Hong, Y.; Shi, W.; Liu, C. Hydrothermal synthesis and visible-light-driven photocatalytic degradation for tetracycline of Mn-doped SrTiO3 nanocubes. Appl. Surf. Sci. 2015, 333, 39–47. [Google Scholar] [CrossRef]
- Hou, D.; Hu, X.; Ho, W.; Hu, P.; Huang, Y. Facile fabrication of porous Cr-doped SrTiO3 nanotubes by electrospinning and their enhanced visible-light-driven photocatalytic properties. J. Mater. Chem. A 2015, 3, 3935–3943. [Google Scholar] [CrossRef]
- Xu, Y.; Liang, Y.; He, Q.; Xu, R.; Chen, D.; Xu, X.; Hu, H. Review of doping SrTiO3 for photocatalytic applications. Bull. Mater. Sci. 2023, 46, 6. [Google Scholar] [CrossRef]
- Nunes, M.J.; Lopes, A.; Pacheco, M.J.; Ciríaco, L.; Fiadeiro, P.T. Photocatalytic degradation of the AO7 under visible light with Bi-doped SrTiO3. In Textiles, Identity and Innovation: In Touch; CRC Press: London, UK, 2020; pp. 349–355. [Google Scholar]
- Nunes, M.J.; Lopes, A.; Pacheco, M.J.; Ciríaco, L. Visible-light-driven AO7 photocatalytic degradation and toxicity removal at Bi-doped SrTiO3. Materials 2022, 15, 2465. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, N.; Liu, J.; Wang, X.; Robertson, P.K.J.; Chen, X.; Chen, C.; Luo, J.; Wang, C. Full-spectrum Bi@SrTiO3 for bi-directional promotion effects on photocatalytic redox reaction: Insight into intermediates and mechanism. J. Rare Earths 2024, 42, 917–929. [Google Scholar] [CrossRef]
- Mehra, S.; Saroha, J.; Rani, E.; Sharma, V.; Goswami, L.; Gupta, G.; Srivastava, A.K.; Sharma, S.N. Development of visible light-driven SrTiO3 photocatalysts for the degradation of organic pollutants for waste-water treatment: Contrasting behavior of MB & MO dyes. Opt. Mater. 2023, 136, 113344. [Google Scholar] [CrossRef]
- EMEA/CVMP (European Agency for the Evaluation of Medicinal Products—Committee for Veterinary Medicinal Products). Oxytetracycline, Tetracycline, Chlortetracycline. Summary Report 3. EMEA/MRL/023/95. 1995. Available online: https://www.ema.europa.eu/en/documents/mrl-report/oxytetracycline-tetracycline-chlortetracycline-summary-report-3-committee-veterinary-medicinal-products_en.pdf (accessed on 10 February 2024).
- Suyamud, B.; Chen, Y.; Quyen, D.T.T.; Dong, Z.; Zhao, C.; Hu, J. Antimicrobial resistance in aquaculture: Occurrence and strategies in Southeast Asia. Sci. Total Environ. 2024, 907, 167942. [Google Scholar] [CrossRef] [PubMed]
- European Commission (E.U.). Commission Implementing Regulation (EU) 2018/783 of 29 May 2018 amending Implementing Regulation (EU) No 540/2011 as regards the conditions of approval of the active substance imidacloprid. Off. J. Eur. Union 2018, 132, 31–34. [Google Scholar]
- Náfrádi, M.; Hlogyik, T.; Farkas, L.; Alapi, T. Comparison of the heterogeneous photocatalysis of imidacloprid and thiacloprid-reaction mechanism, ecotoxicity, and the effect of matrices. J. Environ. Chem. Eng. 2021, 9, 106684. [Google Scholar] [CrossRef]
- Silveira, J.E.; Inacio, G.J.; Batista, N.N.; Morais, W.P.; Menezes, M.G.; Zazo, J.A.; Casas, J.A.; Paz, W.P. Franckeite-derived van der Waals heterostructure with highly efficient photocatalytic NOx abatement: Theoretical insights and experimental evidences. J. Environ. Chem. Eng. 2024, 12, 111998. [Google Scholar] [CrossRef]
- Nunes, M.J.; Lopes, A.; Pacheco, M.J.; Ciríaco, L. Preparation, characterization and environmental applications of Sr1-x(La,Bi)xTiO3 perovskites immobilized on Ni-foam: Photodegradation of the Acid Orange 7. Environ. Sci. Pollut. Res. 2017, 24, 11102–11110. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter. 2009, 21, 395502. [Google Scholar] [CrossRef]
- Piskunov, S.; Heifets, E.; Eglitis, R.I.; Borstel, G. Bulk properties and electronic structure of SrTiO3, BaTiO3, PbTiO3 perovskites: An ab initio HF/DFT study. Comput. Mater. Sci. 2004, 29, 165–178. [Google Scholar] [CrossRef]
- Zhou, E.; Raulot, J.-M.; Xu, H.; Hao, H.; Shen, Z.; Liu, H. Structural, electronic, and optical properties of rare-earth-doped SrTiO3 perovskite: A first-principles study. Phys. B Condens. Matter. 2022, 643, 414160. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, A.; Usman, Z.; Khalid, N.R.; Jin, H.B.; Cao, C.B. Structural, electronic and optical properties of copper-doped SrTiO3 perovskite: A DFT study. Phys. B Condens. Matter. 2019, 552, 52–57. [Google Scholar] [CrossRef]
- Tao, J.; Perdew, J.P.; Tang, H.; Shahi, C. Origin of the size-dependence of the equilibrium van der Waals binding between nanostructures. J. Chem. Phys. 2018, 148, 074110. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association, American Water Works Association, Water Environment Federation. Standard Methods for the Examination of Water and Wastewater, 24th ed.; Lipps, W.C., Braun-Howland, E.B., Baxter, T.E., Eds.; APHA Press: Washington, DC, USA, 2023. [Google Scholar]
- OCDE. Test No. 202: Daphnia sp. Acute Immobilisation Test, OECD Guidelines for testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2004. [Google Scholar]
- ISO 6341; Water Quality—Determination of the Inhibition of the Mobility of Daphnia magna Straus (Cladocera, Crustacea)—Acute Toxicity Test. International Organization for Standardization: Geneva, Switzerland, 2012.
- Sakamoto, K.; Hayashi, F.; Sato, K.; Hirano, M.; Ohtsu, N. XPS spectral analysis for a multiple oxide comprising NiO, TiO2, and NiTiO3. Appl. Surf. Sci. 2020, 526, 146729. [Google Scholar] [CrossRef]
- Yu, J.; Li, C.; Li, B.; Zhu, X.; Zhang, R.; Ji, L.; Tang, D.; Asiri, A.M.; Sun, X.; Li, Q.; et al. A perovskite La2Ti2O7 nanosheet as an efficient electrocatalyst for artificial N2 fixation to NH3 in acidic media. Chem. Commun. 2019, 55, 6401–6404. [Google Scholar] [CrossRef] [PubMed]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Zhai, C. Construction of a novel p-n heterojunction CdS QDs/LaMnO3 composite for photodegradation of oxytetracycline. Mater. Sci. Semicond. Process. 2022, 144, 106568. [Google Scholar] [CrossRef]
- Wessels, J.M.; Ford, W.E.; Szymczak, W.; Schneider, S. The complexation of tetracycline and anhydrotetracycline with Mg2+ and Ca2+: A spectroscopic study. J. Phys. Chem. B 1998, 102, 9323–9331. [Google Scholar] [CrossRef]
- Xuan, R.; Arisi, L.; Wang, Q.; Yates, S.R.; Biswas, K.C. Hydrolysis and photolysis of oxytetracycline in aqueous solution. J. Environ. Sci. Health B 2010, 45, 73–81. [Google Scholar] [CrossRef]
- Kudlek, E.; Dudziak, M.; Bohdziewicz, J. Influence of inorganic ions and organic substances on the degradation of pharmaceutical compound in water matrix. Water 2016, 8, 532. [Google Scholar] [CrossRef]
- Qiang, Z.; Adams, C. Potentiometric determination of acid dissociation constants (pKa) for human and veterinary antibiotics. Water Res. 2004, 38, 2874–2890. [Google Scholar] [CrossRef]
- Jin, X.; Xu, H.; Qiu, S.; Jia, M.; Wang, F.; Zhang, A.; Jiang, X. Direct photolysis of oxytetracycline: Influence of initial concentration, pH and temperature. J. Photochem. Photobiol. A Chem. 2017, 332, 224–231. [Google Scholar] [CrossRef]
- Liu, Y.; He, X.; Fu, Y.; Dionysiou, D.D. Degradation kinetics and mechanism of oxytetracycline by hydroxyl radical-based advanced oxidation processes. Chem. Eng. J. 2016, 284, 1317–1327. [Google Scholar] [CrossRef]
- Pereira, J.H.O.S.; Reis, A.C.; Queirós, D.; Nunes, O.C.; Borges, M.T.; Vilar, V.J.P.; Boaventura, R.A.R. Insights into solar TiO2-assisted photocatalytic oxidation of two antibiotics employed in aquatic animal production, oxolinic acid and oxytetracycline. Sci. Total Environ. 2013, 463–464, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Acero, J.L.; Real, F.J.; Benitez, F.J.; Matamoros, E. Degradation of neonicotinoids by UV irradiation: Kinetics and effect of real water constituents. Sep. Purif. Technol. 2019, 211, 218–226. [Google Scholar] [CrossRef]
- Banić, N.D.; Šojić, D.V.; Krstić, J.B.; Abramović, B.F. Photodegradation of neonicotinoid active ingredients and their commercial formulations in water by different advanced oxidation processes. Water Air Soil Pollut. 2014, 225, 1954. [Google Scholar] [CrossRef]
- Liang, F.; Zhang, Y.-H.; He, B.; Yang, J.; Shi, Q.; Shi, F.-N. Enhanced photocatalytic degradation of imidacloprid and RhB by the precursor derived Bi12.7Co0.3O19.35 under different pH value. J. Phys. Chem. Solids 2022, 164, 110638. [Google Scholar] [CrossRef]
- Garcia, C.R.; Oliva, J.; Chávez, D.; Esquivel, B.; Gómez-Solís, C.; Martínez-Sánchez, E.; Mtz-Enriquez, A.I. Effect of Bismuth Dopant on the Photocatalytic Properties of SrTiO3 under Solar Irradiation. Top. Catal. 2021, 64, 155–166. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Liu, B.; Zhang, Y.; Zhao, Y.; Wang, S. Directional regulation of reactive oxygen species in titanium dioxide boosting the photocatalytic degradation performance of azo dyes. J. Colloid Interface Sci. 2024, 673, 275–283. [Google Scholar] [CrossRef]
- Targhan, H.; Rezaei, A.; Aliabadi, A.; Zheng, H.; Cheng, H.; Aminabhavi, T.M. Adsorptive and photocatalytic degradation of imidacloprid pesticide from wastewater via the fabrication of ZIF-CdS/Tpy quantum dots. Chem. Eng. J. 2024, 482, 148983. [Google Scholar] [CrossRef]
- Xu, K.; Yang, X.; Ruan, L.; Qi, S.; Liu, J.; Liu, K.; Pan, S.; Feng, G.; Dai, Z.; Yang, X.; et al. Superior adsorption and photocatalytic degradation capability of mesoporous LaFeO3/g-C3N4 for removal of oxytetracycline. Catalysts 2020, 10, 301. [Google Scholar] [CrossRef]
- Hernández-Arellano, D.L.; Durán-Álvarez, J.C.; Zanella, R.; López-Juárez, R. Effect of heat treatment on the structure and photocatalytic properties of BiYO3 and BiY0.995Ni0.005O3 ceramic powders. Ceram. Int. 2020, 46, 20291–20298. [Google Scholar] [CrossRef]
- Espíndola, J.C.; Cristóvão, R.O.; Mendes, A.; Boaventura, R.A.R.; Vilar, V.J.P. Photocatalytic membrane reactor performance towards oxytetracycline removal from synthetic and real matrices: Suspended vs immobilized TiO2-P25. Chem. Eng. J. 2019, 378, 122114. [Google Scholar] [CrossRef]
- Andronic, L.; Vladescu, A.; Enesca, A. Synthesis, characterisation, photocatalytic activity, and aquatic toxicity evaluation of TiO2 nanoparticles. Nanomaterials 2021, 11, 3197. [Google Scholar] [CrossRef]
- Safety Data Sheet, Oxytetracycline, Hydrochloride. Available online: https://www.merckmillipore.com/PT/en/product/msds/EMD_BIO-500105?ReferrerURL=https%3A%2F%2Fwww.google.com%2F (accessed on 14 September 2023).
- Safety Data Sheet, Imidacloprid. Available online: https://labelsds.com/images/user_uploads/Imidacloprid%202F%20TI%20SDS%202-12-21.pdf (accessed on 14 September 2023).






| Parameter | TC | TOC | IC | NO3− | Cl− | SO42− | Na+ | Ca2+ |
|---|---|---|---|---|---|---|---|---|
| (mg L−1) | 5.16 | 2.14 | 3.02 | 2.90 | 3.70 | 2.58 | 4.67 | 2.80 |
| Compound | Metals (wt %) | Ti3+/Ti4+ Ratio (Surface) | Crystallite Size (nm) | Eg (eV) | |||
|---|---|---|---|---|---|---|---|
| Sr | Bi | Ti | O | ||||
| SrTiO3 | 47.3 | - | 25.0 | 27.8 | - | 78 | 3.43 |
| Sr0.95Bi0.05TiO3 | 42.0 | 3.4 | 24.8 | 29.8 | 0.35 | 63 | 3.65 |
| Pollutant | Photocatalyst | Catalyst Dose (g L−1) | Ci Pollutant (mg L−1) | t (min) | Type of Radiation | Degradation Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|---|
| OTC | LaFeO3 | 0.5 | 40 | 120 | Visible | ~50 | [68] |
| 2 wt % LaFeO3/g-C3N4 | 90 | ||||||
| NiY0.995Ni0.005O3 | 0.25 | 30 | 300 | Visible | 97 | [69] | |
| TiO2-P25 | 0.4 | 5 | 30 | UV | 90% | [70] | |
| Sri0.95Bi0.05TiO3 | 0.2 | 25 | 60 | Visible | 96 (dH2O) | [This work] | |
| ~100 (RW) | |||||||
| IMD | TiO2 | 0.6 | 5 | 360 | UV + Visible | 99 | [71] |
| Bi12.7Co0.3O19.35 | 1.0 | 10 | 240 | Visible | 96 | [64] | |
| Sr0.95Bi0.05TiO3 | 0.2 | 5 | 180 | Visible | 31 (dH2O) | [This work] | |
| 60 (RW) |
| Compound | Aqueous Matrix | kapp | R2 |
|---|---|---|---|
| OTC | dH2O | 3.26 h−1 | 0.991 |
| RW | 5.58 h−1 | 0.998 | |
| Mixture in dH2O | 3.26 h−1 | 0.996 | |
| Mixture in RW | 4.62 h−1 | 0.980 | |
| IMD | dH2O | 0.12 h−1 | 0.999 |
| RW | 0.30 h−1 | 0.999 | |
| Mixture in dH2O | 0.07 h−1 | 0.991 | |
| Mixture in RW | 0.06 M h−1 | 0.990 |
| Sample | Removal (%) | ||
|---|---|---|---|
| COD | TOC | ||
| 2 h | 4 h | 4 h | |
| Mixture in dH2O | 15.1 | 34.5 | 31.4 |
| Mixture in RW | 19.5 | 44.8 | 33.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, M.J.; Lopes, A.; Pacheco, M.J.; Fiadeiro, P.T.; Inacio, G.J.; Silveira, J.E.; Ribeiro, A.R.; Paz, W.S.; Ciríaco, L. Photocatalytic Degradation of Oxytetracycline and Imidacloprid Under Visible Light with Sr0.95Bi0.05TiO3: Influence of Aqueous Matrix. Water 2025, 17, 2177. https://doi.org/10.3390/w17152177
Nunes MJ, Lopes A, Pacheco MJ, Fiadeiro PT, Inacio GJ, Silveira JE, Ribeiro AR, Paz WS, Ciríaco L. Photocatalytic Degradation of Oxytetracycline and Imidacloprid Under Visible Light with Sr0.95Bi0.05TiO3: Influence of Aqueous Matrix. Water. 2025; 17(15):2177. https://doi.org/10.3390/w17152177
Chicago/Turabian StyleNunes, Maria J., Ana Lopes, Maria J. Pacheco, Paulo T. Fiadeiro, Guilherme J. Inacio, Jefferson E. Silveira, Alyson R. Ribeiro, Wendel S. Paz, and Lurdes Ciríaco. 2025. "Photocatalytic Degradation of Oxytetracycline and Imidacloprid Under Visible Light with Sr0.95Bi0.05TiO3: Influence of Aqueous Matrix" Water 17, no. 15: 2177. https://doi.org/10.3390/w17152177
APA StyleNunes, M. J., Lopes, A., Pacheco, M. J., Fiadeiro, P. T., Inacio, G. J., Silveira, J. E., Ribeiro, A. R., Paz, W. S., & Ciríaco, L. (2025). Photocatalytic Degradation of Oxytetracycline and Imidacloprid Under Visible Light with Sr0.95Bi0.05TiO3: Influence of Aqueous Matrix. Water, 17(15), 2177. https://doi.org/10.3390/w17152177







