Cu–Co–O-Codoped Graphite Carbon Nitride as an Efficient Peroxymonosulfate Activator for Sulfamethoxazole Degradation: Characterization, Performance, and Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of CN, O-CN and Cu–Co–O-g-C3N4
2.3. Characterization of Catalysts
2.4. SMX Degradation Experiments
3. Results and Discussion
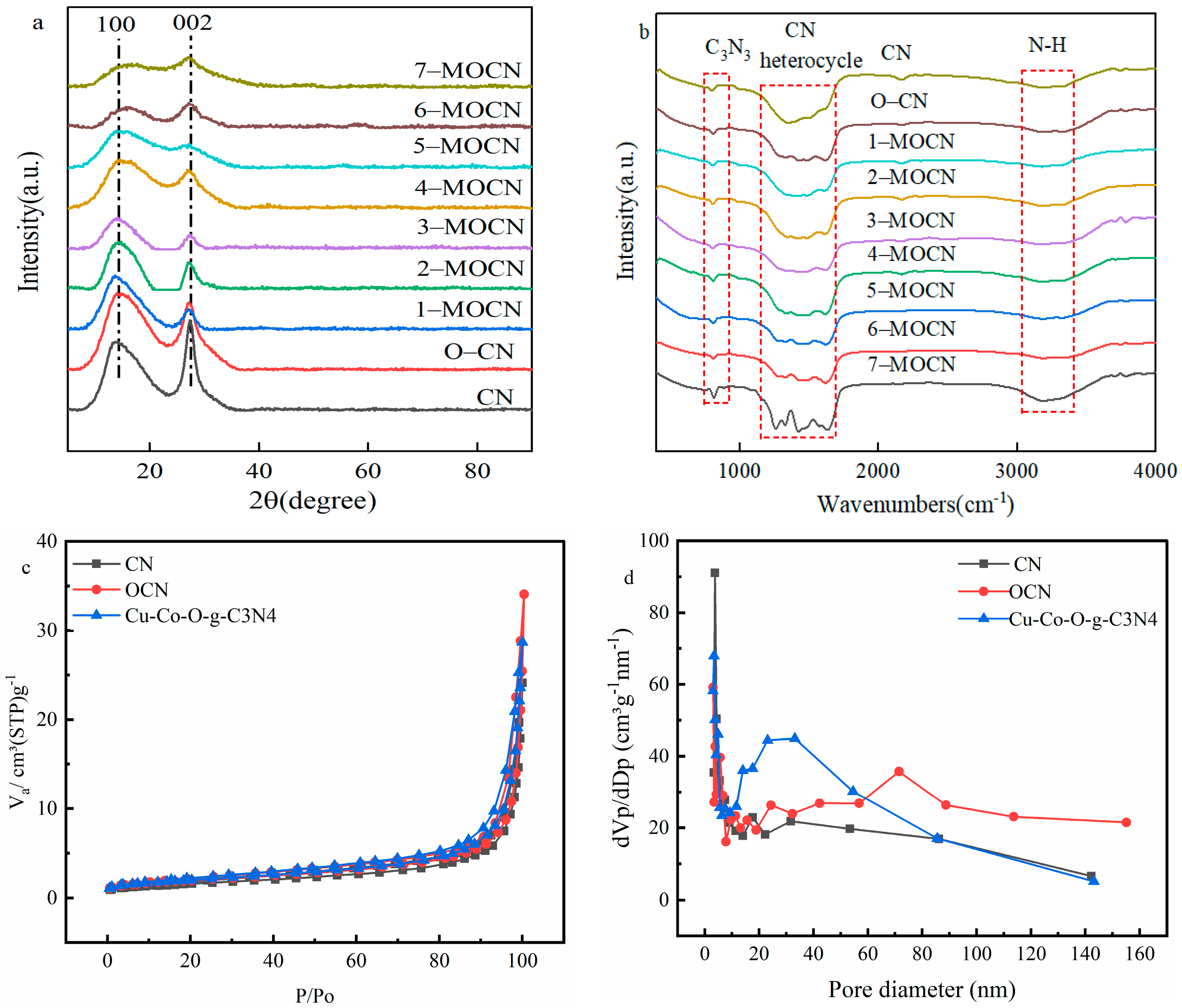
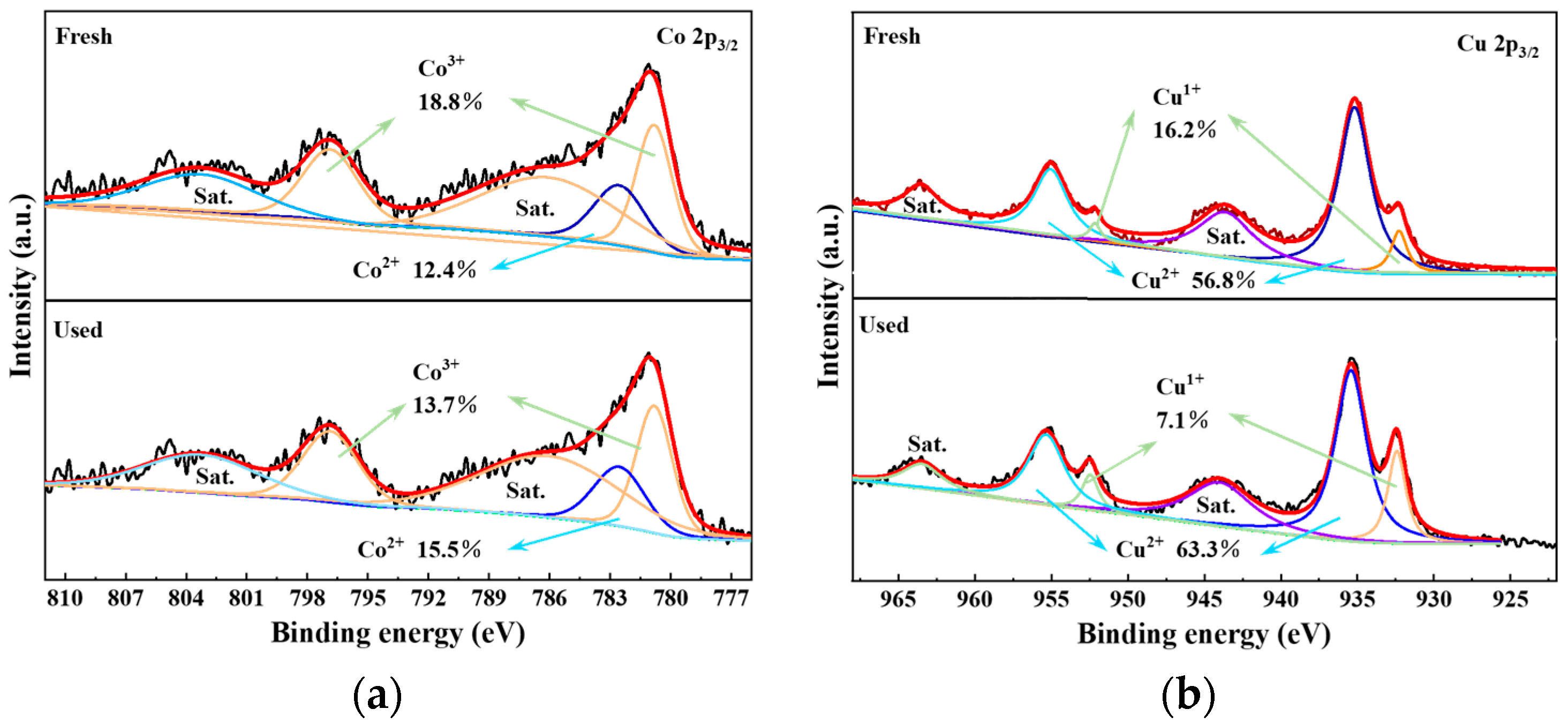
3.1. Characterization Results of Catalysts
3.1.1. Morphological and Structural Analysis
3.1.2. Surface Functional Groups and Pore Size Distribution
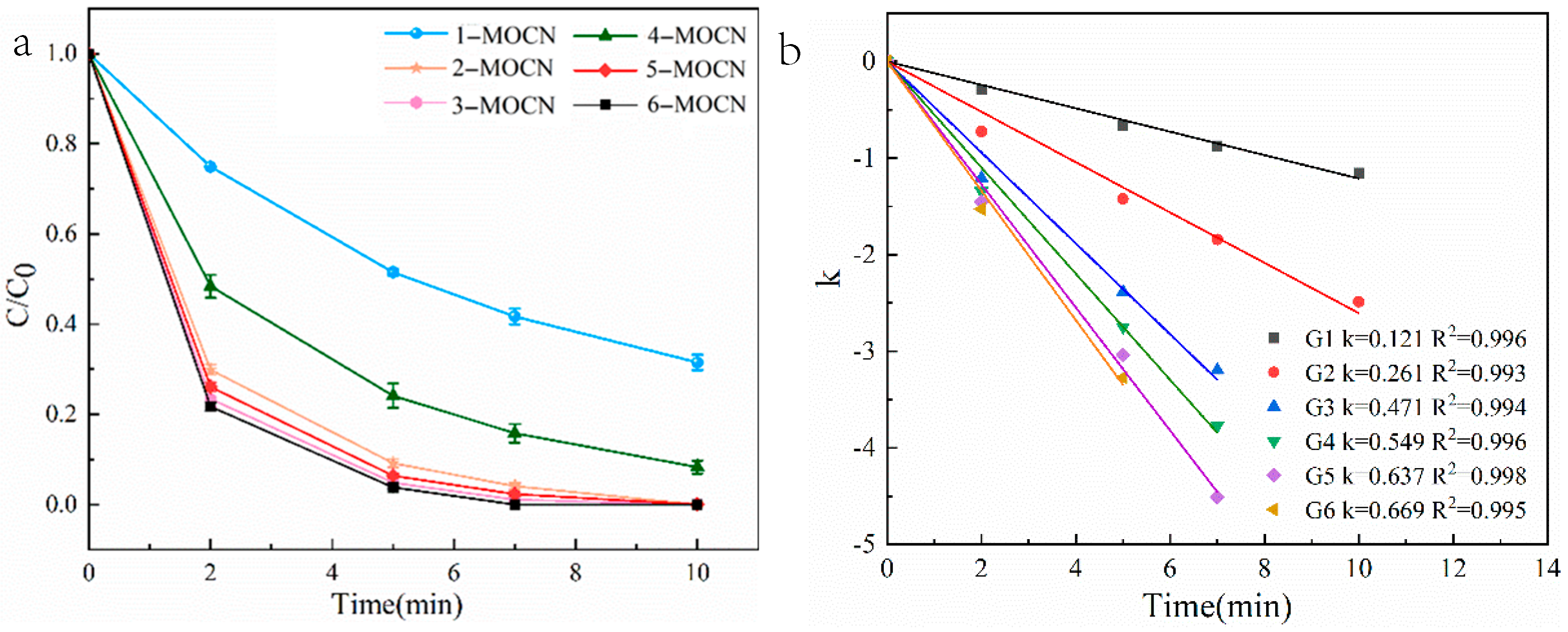
3.2. Degradation of SMX
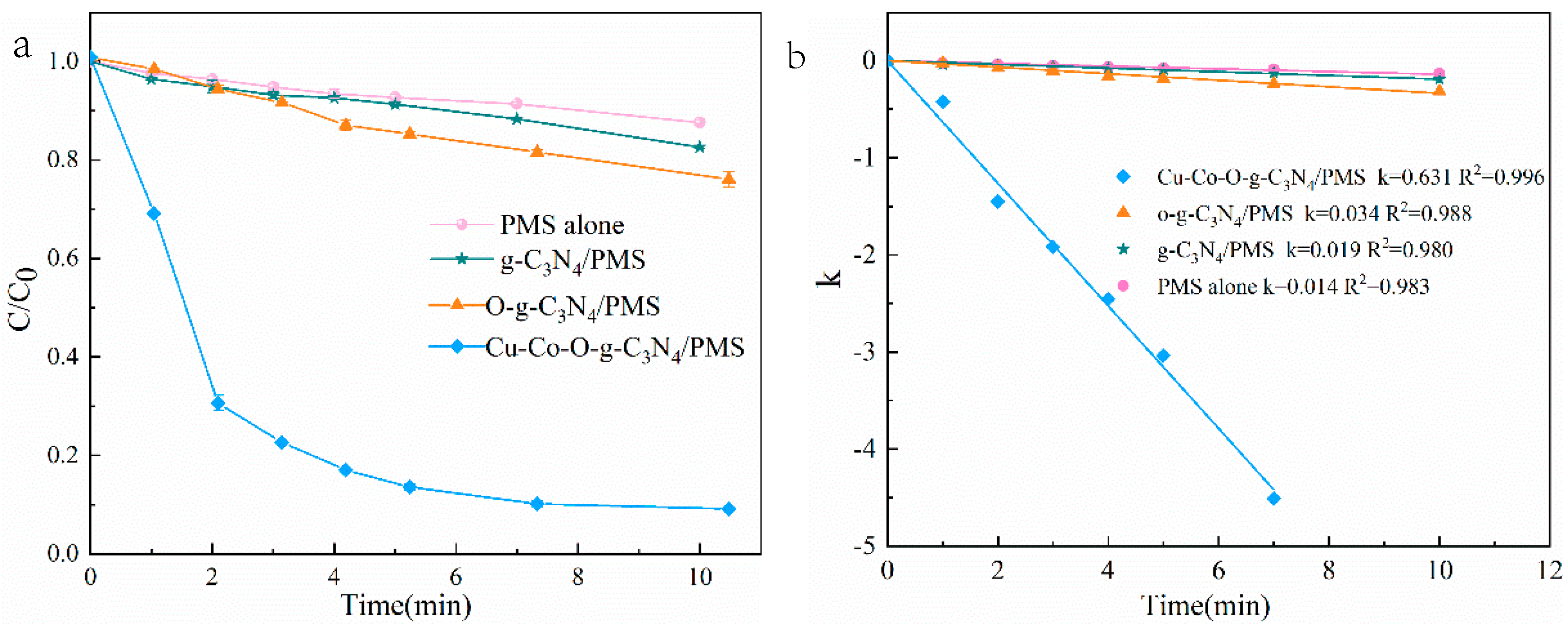
3.2.1. Catalytic Activity of Cu–Co–O-g-C3N4
3.2.2. Reactive Oxygen Species in the 5-MOCN/PMS System
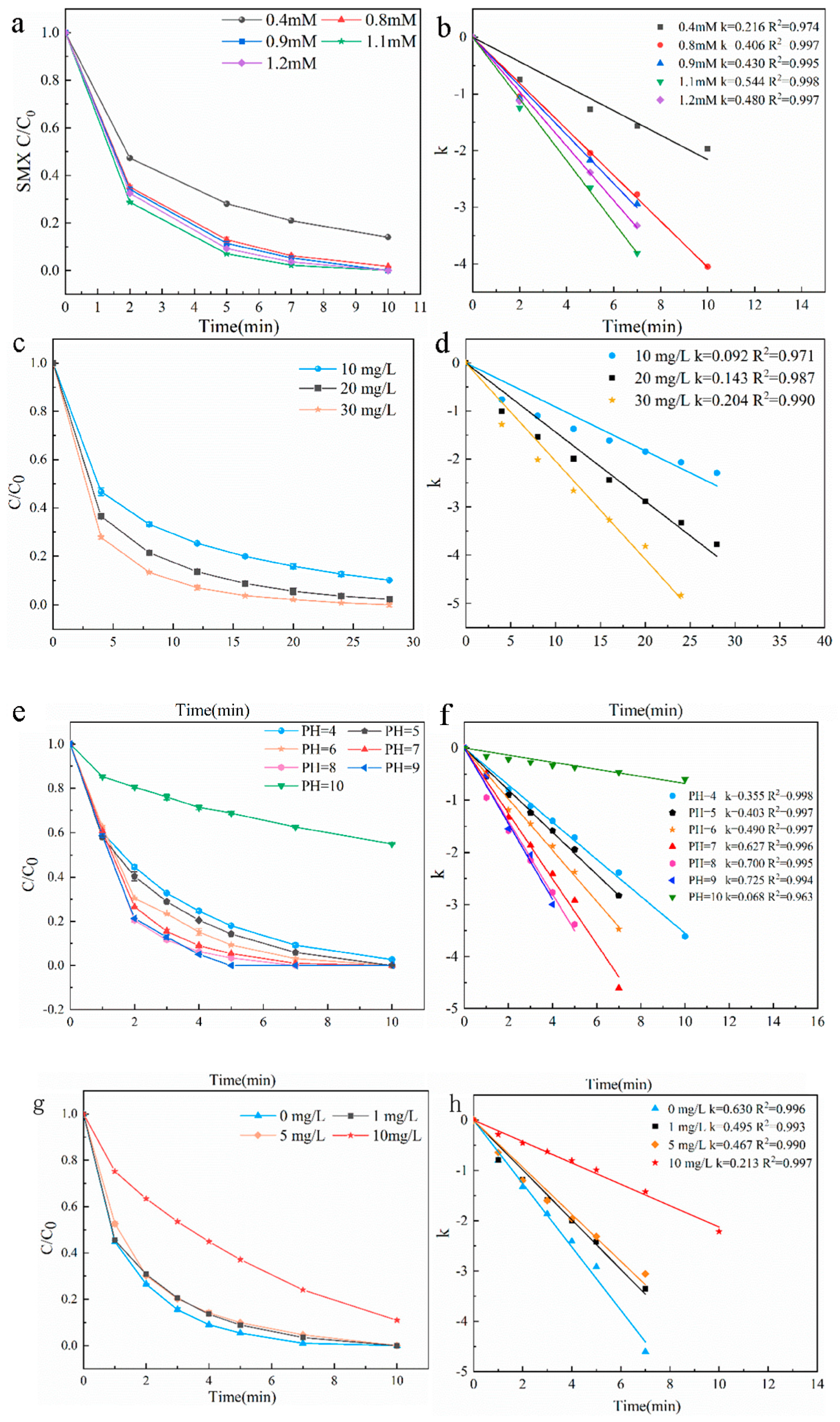
3.2.3. Influences of Various Reaction Conditions
3.3. Reaction Mechanism
4. Conclusions
- Superior Catalytic Performance: The Cu–Co–O-g-C3N4/PMS system achieved 90% SMX removal within 10 min, outperforming pristine g-C3N4 (14%) and O-doped g-C3N4 (22%), with a reaction rate constant (0.63 min−1) 45-fold higher than that of g-C3N4 alone. The synergistic effect of Cu-Co bimetallic doping and oxygen incorporation enhanced the active sites, stabilized metal ions, and minimized leaching, ensuring sustained catalytic activity. The Cu⁺/Cu2⁺ and Co2⁺/Co3⁺ redox cycles facilitated continuous PMS activation while promoting ROS generation.
- Optimized Reaction Conditions: The system exhibited broad pH adaptability, with optimal performance at neutral to alkaline conditions. Excessive PMS (>1.1 mM) led to self-scavenging of SO4•−, highlighting the importance of balanced oxidant dosing.
- Mechanistic Insights: Characterization (XRD, FTIR, XPS) confirmed successful doping and revealed that O incorporation modified the electronic structure of g-C3N4, reducing its bandgap and enhancing charge carrier separation. The dual radical/non-radical mechanism was elucidated through quenching experiments and PMS decomposition studies, with 1O2 identified as the dominant species.
- Environmental Implications: The Cu–Co–O-g-C3N4 catalyst offers a sustainable solution for antibiotic removal, addressing challenges such as metal leaching and pH sensitivity in conventional advanced oxidation processes (AOPs). This work provides a design strategy for multi-heteroatom-doped carbon nitride catalysts, emphasizing the synergy between bimetal doping and nonmetal modification for water purification.
- Future Perspectives: Further research should focus on (1) long-term stability in real wastewater matrices, (2) scalability of synthesis, and (3) applications for other emerging contaminants.
- Significance: This study advances the development of efficient eco-friendly PMS activators for wastewater treatment, contributing to safer water resources and sustainable environmental remediation.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, Y.; Ji, Y.; Lu, J.; Yin, X.; Zhou, Q. Degradation of sulfamethoxazole by Co3O4-palygorskite composites activated peroxymonosulfate oxidation. Chem. Eng. J. 2021, 406, 126759. [Google Scholar] [CrossRef]
- Qin, J.; Wang, Q.; Han, B.; Jin, C.; Luo, C.; Sun, Y.; Dai, Z.; Wang, S.; Liu, H.; Zheng, X.; et al. Heterogeneous Fe-Ni dual-atom catalysts coupled N-vacancy engineering for enhanced activation of peroxymonosulfate. Appl. Catal. B Environ. Energy 2025, 360, 124538. [Google Scholar] [CrossRef]
- Oh, W.-D.; Dong, Z.; Lim, T.-T. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects. Appl. Catal. B Environ. 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Xie, M.; Tang, J.; Kong, L.; Lu, W.; Natarajan, V.; Zhu, F.; Zhan, J. Cobalt doped g-C3N4 activation of peroxymonosulfate for monochlorophenols degradation. Chem. Eng. J. 2019, 360, 1213–1222. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Gao, Q.; Ding, J.; Zhao, G.; Zhao, Q.; Li, L.; Zhao, X.; Bu, L.; Zhou, S.; Qiu, S. Exploring the synergism of sunlight and electrooxidation on persulfate activation for efficient degradation of bisphenol S: Performance, Pathway, and mechanism. Chem. Eng. J. 2022, 437, 135318. [Google Scholar] [CrossRef]
- Peng, J.; Zhou, H.; Liu, W.; Ao, Z.; Ji, H.; Liu, Y.; Su, S.; Yao, G.; Lai, B. Insights into heterogeneous catalytic activation of peroxymonosulfate by natural chalcopyrite: pH-dependent radical generation, degradation pathway and mechanism. Chem. Eng. J. 2020, 397, 125387. [Google Scholar] [CrossRef]
- Guo, S.; Deng, Z.; Li, M.; Jiang, B.; Tian, C.; Pan, Q.; Fu, H. Phosphorus-Doped Carbon Nitride Tubes with a Layered Micro-nanostructure for Enhanced Visible-Light Photocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. 2016, 55, 1830–1834. [Google Scholar] [CrossRef]
- Chai, C.; Fan, C.; Liu, J.; Zhang, X.; Wang, Y.; Li, R.; Duan, D.; Wang, Y. Photoinduced g-C3N4-promoted Mn2+/Mn3+/Mn4+ redox cycles for activation of peroxymonosulfate. J. Solid State Chem. 2019, 277, 466–474. [Google Scholar] [CrossRef]
- Zuo, S.; Guan, Z.; Xia, D.; Yang, F.; Xu, H.; Huang, M.; Li, D. Polarized heterogeneous CuO-CN for peroxymonosulfate nonradical activation: An enhancement mechanism of mediated electron transfer. Chem. Eng. J. 2021, 420, 127619. [Google Scholar] [CrossRef]
- Chen, F.; Liu, L.-L.; Chen, J.-J.; Li, W.W.; Chen, Y.P.; Zhang, Y.J.; Wu, J.H.; Mei, S.C.; Yang, Q.; Yu, H.Q. Efficient decontamination of organic pollutants under high salinity conditions by a nonradical peroxymonosulfate activation system. Water Res. 2021, 191, 116799. [Google Scholar] [CrossRef] [PubMed]
- Che, W.; Cheng, W.; Yao, T.; Tang, F.; Liu, W.; Su, H.; Wei, S. Fast Photoelectron Transfer in (Cring)–C3N4 Plane Heterostructural Nanosheets for Overall Water Splitting. J. Am. Chem. Soc. 2017, 139, 3021–3026. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Shi, Y.; Tao, Y.; Zhang, H. Enhanced persulfate-mediated photocatalytic oxidation of bisphenol A using bioelectricity and a g-C3N4/Fe2O3 heterojunction. Chem. Eng. J. 2019, 359, 933–943. [Google Scholar] [CrossRef]
- Liu, C.; Liu, L.; Tian, X.; Wang, Y.; Li, R.; Zhang, Y.; Song, Z.; Xu, B.; Chu, W.; Qi, F.; et al. Coupling metal–organic frameworks and g-CN to derive Fe@N-doped graphene-like carbon for peroxymonosulfate activation: Upgrading framework stability and performance. Appl. Catal. B Environ. 2019, 255, 117763. [Google Scholar] [CrossRef]
- Liu, Y.; Niu, X.; Yang, X.; Chen, Y.; Li, X.; Liu, J.; Chen, W. Regulating the Co 3d center of Co3O4 by Co3O4/g-C3N4 to enhance electronic activity for sulfamethizole effectively degaradtion by permonosulfate activation. Chem. Eng. J. 2024, 489, 151417. [Google Scholar] [CrossRef]
- Fu, M.; Chai, B.; Zhang, X.; Sun, Y.; Fan, G.; Song, G. Nitrogen self-doped chitosan carbon aerogel integrating with CoAl-LDH for ultra-efficient sulfamethoxazole degradation based on PS-AOPs: From batch to continuous process. J. Environ. Chem. Eng. 2023, 11, 110650. [Google Scholar] [CrossRef]
- Oh, W.D.; Chang, V.W.; Hu, Z.T.; Goei, R.; Lim, T.T. Enhancing the catalytic activity of g-C3N4 through Me doping (Me = Cu, Co and Fe) for selective sulfathiazole degradation via redox-based advanced oxidation process. Chem. Eng. J. 2017, 323, 260–269. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Wang, J. Peroxymonosulfate Activation by Fe–Co–O-Codoped Graphite Carbon Nitride for Degradation of Sulfamethoxazole. Environ. Sci. Technol. 2020, 54, 10361–10369. [Google Scholar] [CrossRef]
- Guan, Z.; Zuo, S.; Yang, F.; Zhang, B.; Xu, H.; Xia, D.; Huang, M.; Li, D. The p and d hybridization interaction in Fe-N-C boosts peroxymonosulfate non-radical activation. Sep. Purif. Technol. 2021, 258, 118025. [Google Scholar] [CrossRef]
- Zhou, X.; Luo, C.; Luo, M.; Wang, Q.; Wang, J.; Liao, Z.; Chen, Z.; Chen, Z. Understanding the synergetic effect from foreign metals in bimetallic oxides for PMS activation: A common strategy to increase the stoichiometric efficiency of oxidants. Chem. Eng. J. 2020, 381, 122587. [Google Scholar] [CrossRef]
- Tian, Y.; Li, Q.; Zhang, M.; Nie, Y.; Tian, X.; Yang, C.; Li, Y. pH-dependent oxidation mechanisms over FeCu doped g-C3N4 for ofloxacin degradation via the efficient peroxymonosulfate activation. J. Clean. Prod. 2021, 315, 128207. [Google Scholar] [CrossRef]
- Peng, G.; You, W.; Zhou, W.; Zhou, G.; Qi, C.; Hu, Y. Activation of peroxymonosulfate by phosphite: Kinetics and mechanism for the removal of organic pollutants. Chemosphere 2021, 266, 129016. [Google Scholar] [CrossRef]
- Wu, Z.; Tong, Z.; Xie, Y.; Sun, H.; Gong, X.; Qin, P.; Liang, Y.; Yuan, X.; Zou, D.; Jiang, L. Efficient degradation of tetracycline by persulfate activation with Fe, Co and O co−doped g−C3N4: Performance, mechanism and toxicity. Chem. Eng. J. 2022, 434, 134732. [Google Scholar] [CrossRef]
- Zhao, H.; Ren, Y.; Liu, C.; Li, L.; Li, N.; Lai, B.; Li, J. In-depth insights into Fe(III)-doped g-C3N4 activated peracetic acid: Intrinsic reactive species, catalytic mechanism and environmental application. J. Hazard. Mater. 2023, 459, 132117. [Google Scholar] [CrossRef]
- You, J.; Li, J.; Zhang, H.; Luo, M.; Xing, B.; Ren, Y.; Liu, Y.; Xiong, Z.; He, C.; Lai, B. Removal of Bisphenol A via peroxymonosulfate activation over graphite carbon nitride supported NiCx nanoclusters catalyst: Synergistic oxidation of high-valent nickel-oxo species and singlet oxygen. J. Hazard. Mater. 2023, 445, 130440. [Google Scholar] [CrossRef]
- Gao, B.; Dou, M.; Wang, J.; Li, S.; Wang, D.; Ci, L.; Fu, Y. Efficient persulfate activation by carbon defects g-C3N4 containing electron traps for the removal of antibiotics, resistant bacteria and genes. Chem. Eng. J. 2021, 426, 131677. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Wang, J. Iron and sulfur co-doped graphite carbon nitride (FeOy/S-g-C3N4) for activating peroxymonosulfate to enhance sulfamethoxazole degradation. Chem. Eng. J. 2020, 382, 122836. [Google Scholar] [CrossRef]
- Feng, X.C.; Xiao, Z.J.; Shi, H.T.; Zhou, B.Q.; Wang, Y.M.; Chi, H.Z.; Ren, N.Q. How Nitrogen and Sulfur Doping Modified Material Structure, Transformed Oxidation Pathways, and Improved Degradation Performance in Peroxymonosulfate Activation. Environ. Sci. Technol. 2022, 56, 14048–14058. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Q.; Nan, J. Cu–Co–O-Codoped Graphite Carbon Nitride as an Efficient Peroxymonosulfate Activator for Sulfamethoxazole Degradation: Characterization, Performance, and Mechanism. Water 2025, 17, 2161. https://doi.org/10.3390/w17142161
Xiao Q, Nan J. Cu–Co–O-Codoped Graphite Carbon Nitride as an Efficient Peroxymonosulfate Activator for Sulfamethoxazole Degradation: Characterization, Performance, and Mechanism. Water. 2025; 17(14):2161. https://doi.org/10.3390/w17142161
Chicago/Turabian StyleXiao, Qiliang, and Jun Nan. 2025. "Cu–Co–O-Codoped Graphite Carbon Nitride as an Efficient Peroxymonosulfate Activator for Sulfamethoxazole Degradation: Characterization, Performance, and Mechanism" Water 17, no. 14: 2161. https://doi.org/10.3390/w17142161
APA StyleXiao, Q., & Nan, J. (2025). Cu–Co–O-Codoped Graphite Carbon Nitride as an Efficient Peroxymonosulfate Activator for Sulfamethoxazole Degradation: Characterization, Performance, and Mechanism. Water, 17(14), 2161. https://doi.org/10.3390/w17142161




