NaCl as a Simple Yet Unique Activator of Kaolin: Surface Engineering for Enhanced Heavy Metal Adsorption
Abstract
1. Introduction
2. Materials and Methods
- Activation of Kaolinite: 20 g of kaolin was introduced into 150 mL NaCl solutions at varying concentrations of 0.5, 1.0, 2.0, and 4.0 M in separate beakers. Each beaker was covered and ultrasonicated at 60 Hz for 2 h at a controlled temperature of 80 °C. The ultrasonicated kaolin samples were then washed several times with deionized water and filtered. The washed clay was oven-dried at 70 °C for 24 h to obtain the activated kaolin. Furthermore, in this bench-scale study, the liquor was not recycled after each activation step. The suspension was rather centrifuged and filtered. The brine was then amply diluted with tap water, giving TDS ≈ 9 g L−1, and discharged safely through a drain line for non-hazardous effluents. The clarified brine was characterized using ICP-OES (Thermo iCAP 7400 Duo, Qtegra ISDS V.2.11, Thermo Fisher Scientific Inc., Waltham, MA, USA; λ = 213.856 nm for Zn; MDLs: Zn 0.003, Al 0.010, Si 0.020 mg L−1) and ion chromatography (Metrohm 930 Compact IC Flex v 4.1, Metrohm, Herisau, Switzerland; MDL 0.115 mg L−1).
- Characterization of Activated Kaolin: The physicochemical properties of the NaCl-activated kaolin samples were characterized using a combination of analytical techniques: SEM to analyze morphological changes (using a JEOL JSM-7610F, Tokyo, Japan; with an accelerating voltage of 5.0 kV. The specimen was imaged in secondary electron (SE) mode and under low energy imaging (LEI) function); XRD was used to examine crystallinity and potential phase transformations (Bruker D2 Phaser diffractometer with DIFFRAC.EVA V.6.2, Bruker Corporation, Billerica, MA, USA; operating at 30 kV and 10 mA with a Cu Kα source (λ = 1.5406 Å). The scan was performed, beginning at ~5° 2θ with a 0.083° step size across 900 steps, FTIR for functional group analysis (using a Nicolet iS10 spectrometer, OMNIC Spectra, Thermo Fisher Scientific Inc., Waltham, MA, USA). The spectra were recorded in the range of 4000–400 cm−1 at a resolution of 4 cm−1, with 32 scans averaged per sample); XPS to determine surface elemental composition (using the Escalab Xi+ system, Avantage V.6, Thermo Fisher Scientific Inc., Waltham, MA, USA). The device was equipped with a monochromatic Al Kα source and operating under ultrahigh vacuum of ~10−9 Torr, a spot size of 650 µm, pass energy of 20 to 30 eV, and energy step size of 0.10 eV); Zeta Potential measurements to assess surface charge; and DLS for hydrodynamic particle size distribution analysis (using a Litesizer DLS 500, Kalliope, Anton Paar GmbH, Graz, Austria), with water as the dispersing medium. The cumulant model was applied for size analysis, and the Smoluchowski approximation was applied with automatic voltage adjustment (up to 200 V) and a Henry factor of 1.5 for the zeta potential.
- Adsorption–Desorption Experiments: For each concentration of NaCl-activated kaolin sample, 0.1 g was added to 20 mL of a Zn(NO3)2 solution (adsorbent dosage from other studies, for example, ranges from 0.5 to 4.0 g/L [42]) with an initial Zn2+ concentration (Co) of 70 mg/L (initial pollutant concentration for such studies typically ranges from 10 to 400 mg/L [42,52]). The mixtures were stirred at 200 rpm for 2 h to allow for equilibrium to be reached. The equilibrium time of about 2 h was determined through preliminary studies. This is in line with many other studies involving kaolin, where equilibrium times were typically between 30 and 120 min [42,53].After each adsorption test the slurry was centrifuged (4000 rpm, 10 min, 20 °C), and the supernatant was passed through a 0.22 µm PES syringe filter. Note that 10 mL aliquots were taken, and the concentration of Zn2+ determined using iCAP 7400 Duo, Qtegra ISDS V.2.11, Thermo Fisher Scientific Inc., Waltham, MA, USA; λ = 213.856 nm for Zn; MDLs: Zn 0.003, Al 0.010, Si 0.020 mg L−1). To ensure data accuracy, adsorption experiments were performed in triplicate, and the average Ce was recorded. The Zn2+ removal was calculated as follows:The average zeta potential and the hydrodynamic particle size of the spent kaolin were determined and compared with the values recorded before the heavy metal adsorption. The settling behavior of the spent kaolin suspension was also observed.For regeneration, spent kaolin (0.10 g) was treated with 20 mL of 0.05 mol L−1 HCl (pH ≈ 1.6) and agitated at 200 rpm and 25 °C for 30 min, which is longer than the 20 min required for desorption equilibrium, providing a safety margin. The dilute acid protonates the surface and displaces Zn2+ from ≡AlO−/≡SiO− sites; a subsequent 0.1 mol L−1 NaCl rinse restores the clay’s Na+-exchange form. Finally, three washes with 20 mL deionized water return the slurry to pH 6.5 ± 0.1 and remove residual Cl−, ensuring the material re-enters the next adsorption cycle under conditions identical to the original test. The percent desorption is calculated as follows:
- Leachate Sampling Protocol: The optimal NaCl concentration, determined from the adsorption experiments, was used for the leaching analysis. A series of four batch suspensions (all 20 mL, 25 ± 1 °C, pH 6.5 ± 0.1) were prepared in triplicate: (i) a blank containing only ultrapure water; (ii) a control containing the NaCl-activated kaolin (0.1 g) but with no Zn2+; (iii) an adsorption test in which the same kaolin dose was contacted with 50 mg L−1 Zn2+ for 2 h; and (iv) an identical reactor held for 24 h to analyze additional uptake. After the designated contact time, each slurry was centrifuged (4000 rpm, 10 min), and the supernatant passed through a 0.22 µm PES syringe filter. Zn, Al, and Si were quantified by ICP-OES (Thermo iCAP 7400 Duo, Qtegra ISDS V.2.11, Thermo Fisher Scientific Inc., Waltham, MA, USA; λ = 213.856 nm for Zn; MDLs: Zn 0.003, Al 0.010, Si 0.020 mg L−1). Sodium was measured by ion chromatography (Metrohm 930 Compact IC Flex, Metrohm, Herisau, Switzerland; MDL 0.115 mg L−1). Solution data are reported in mg L−1; solid-phase loadings were obtained from mass balance, i.e., (C0 − Ce) × V divided by the clay mass and expressed as mg g−1 (Zn) or, after blank subtraction, mg g−1 Na+ released. The results of the leachate analysis are presented in Table 1.
3. Modeling and Statistical Analysis
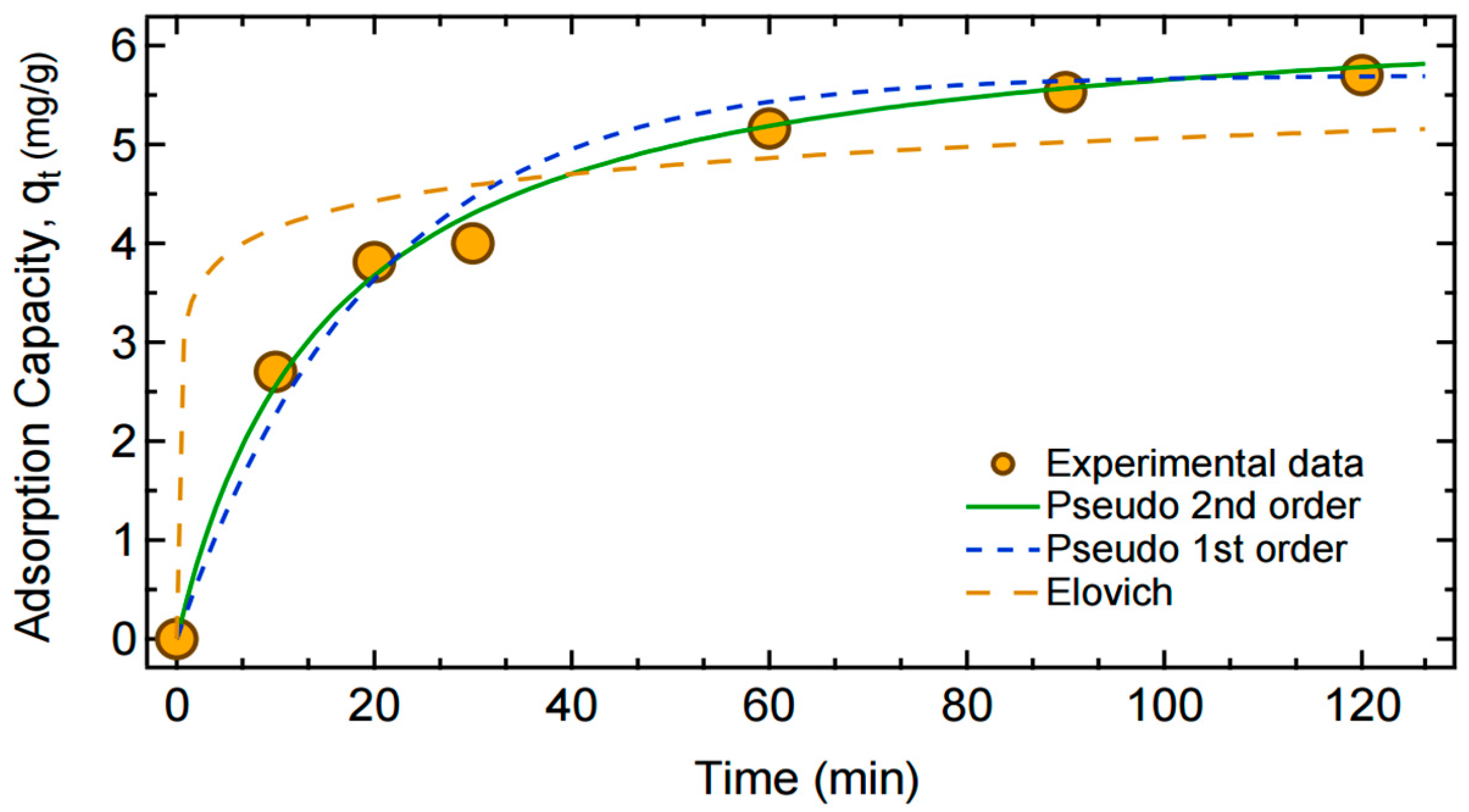
3.1. Kinetic Adsorption Equations
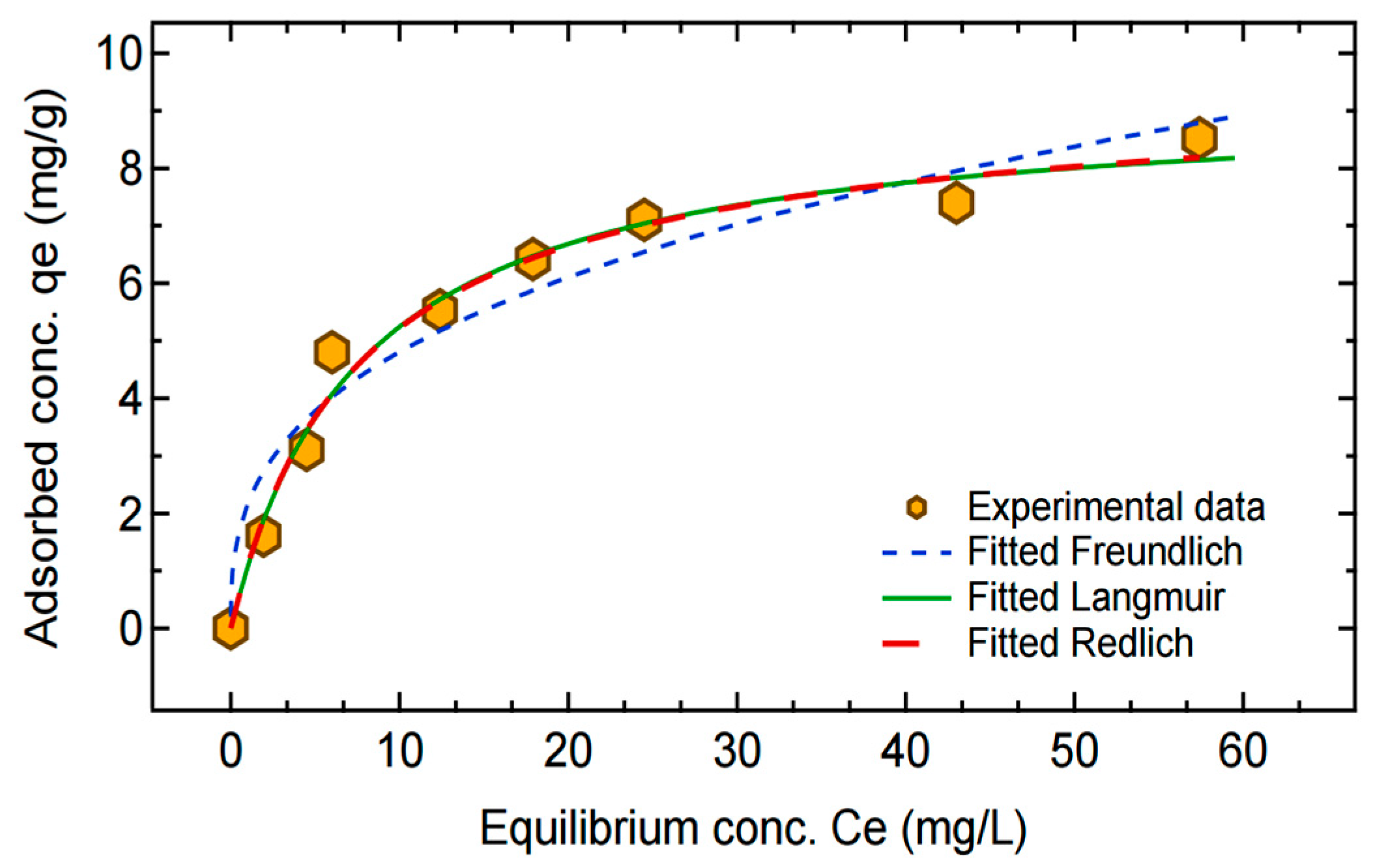
3.2. Adsorption Isotherms
3.3. Thermodynamic Equations
3.4. Statistical Analysis and Comparison Between Models
4. Results and Discussion
4.1. Activation Liquor Analysis
4.2. Characterization
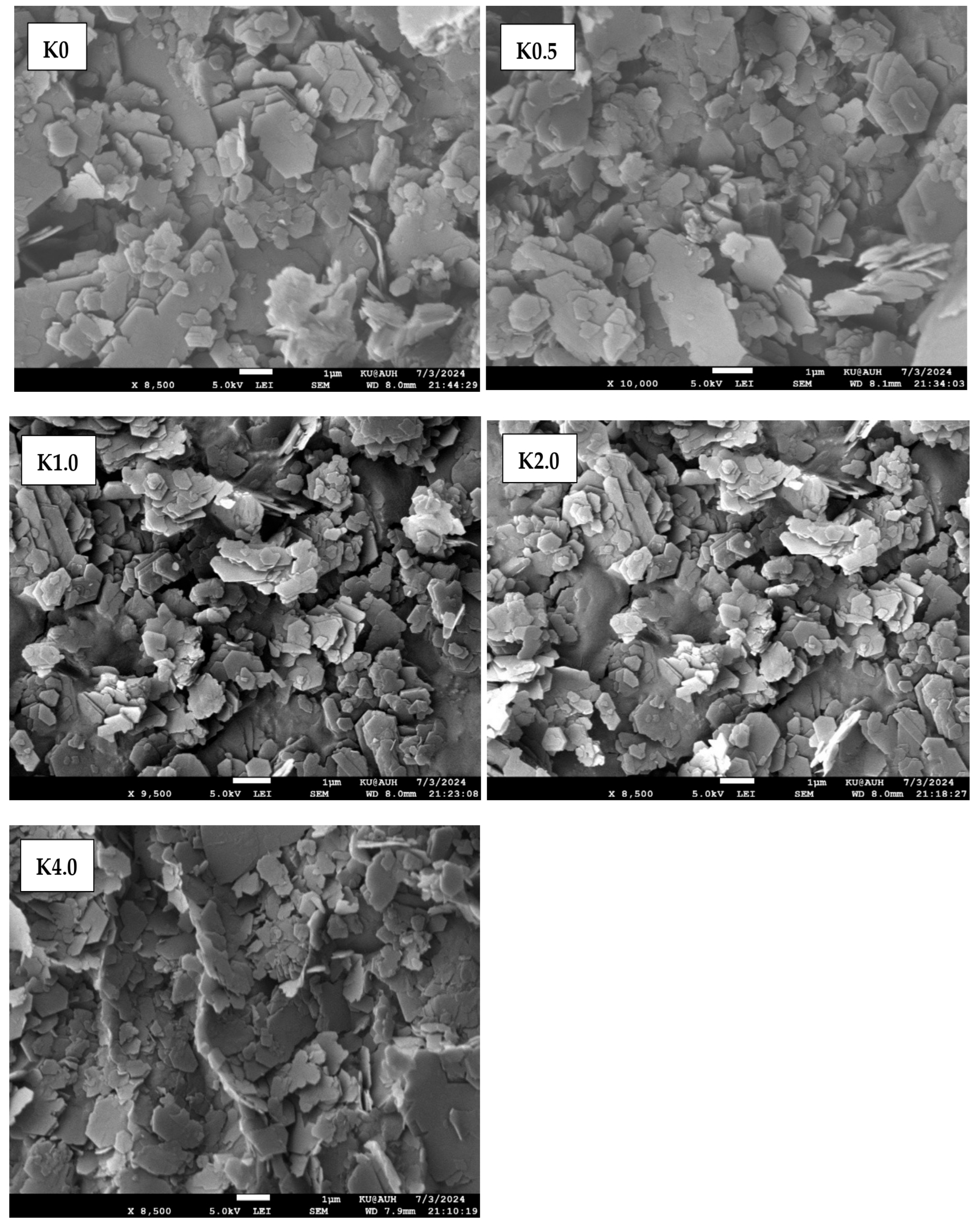
4.2.1. SEM
4.2.2. FTIR
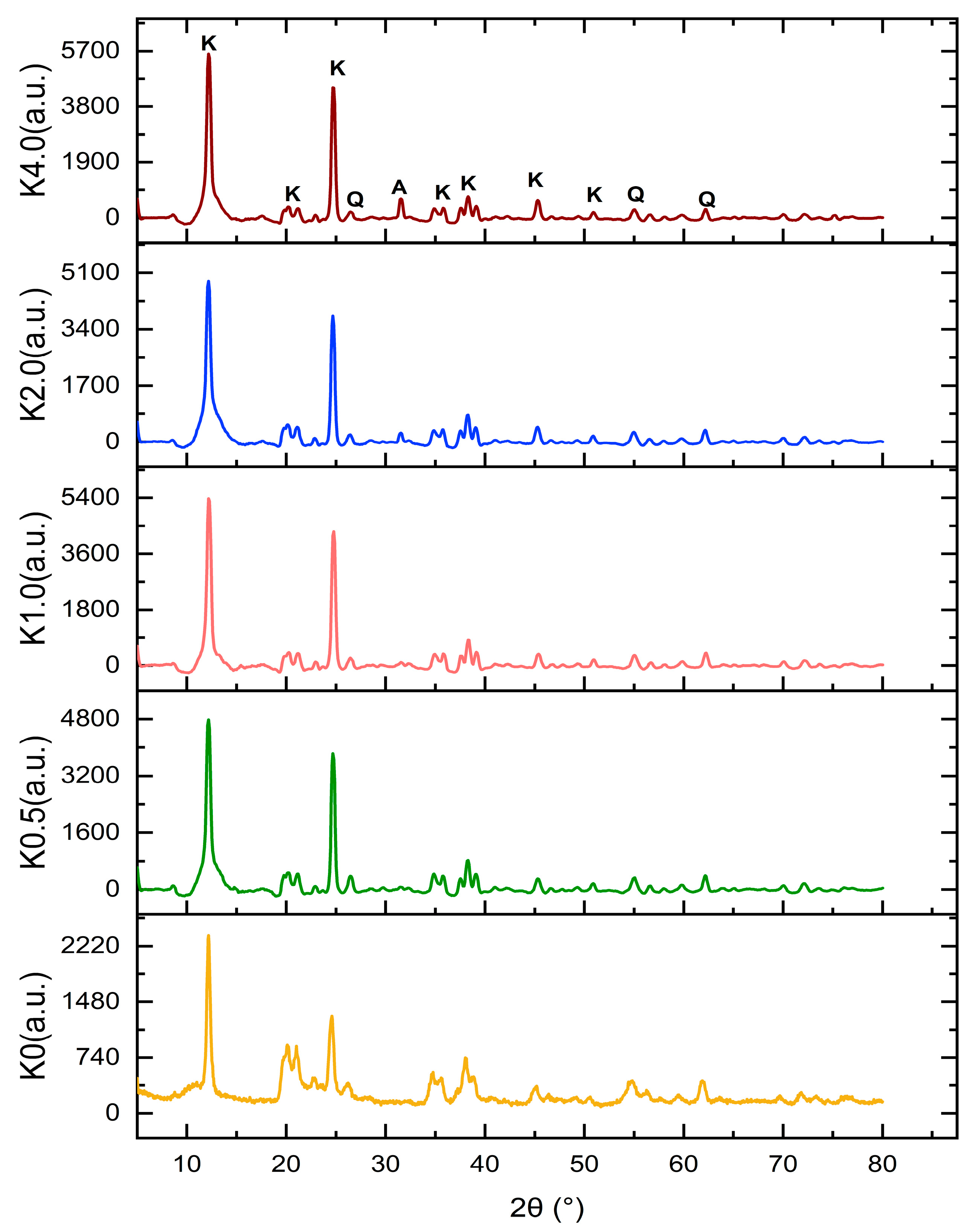
4.2.3. XRD
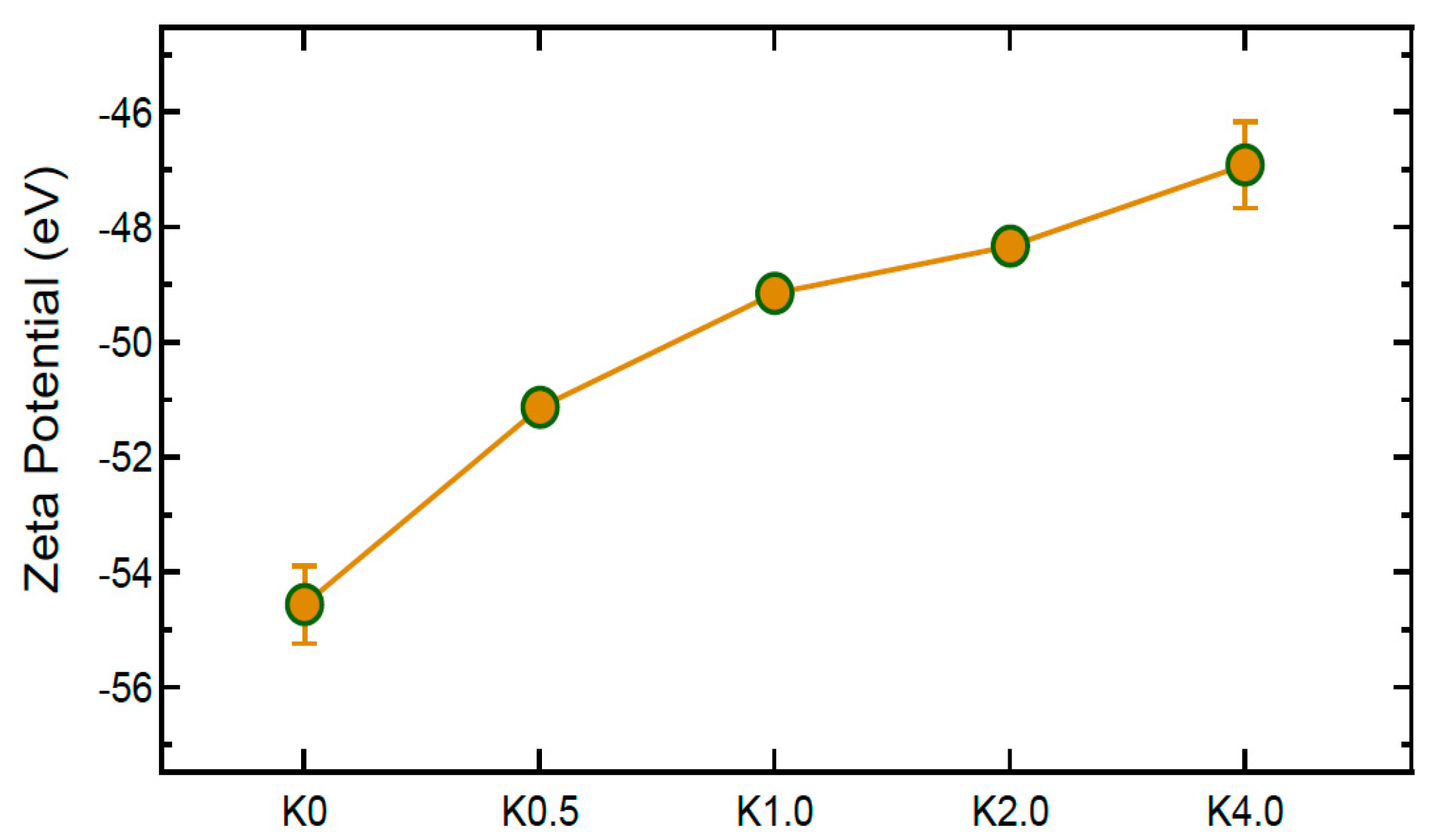
4.2.4. Zeta Potential
4.2.5. Hydrodynamic Particle Size
4.2.6. XPS
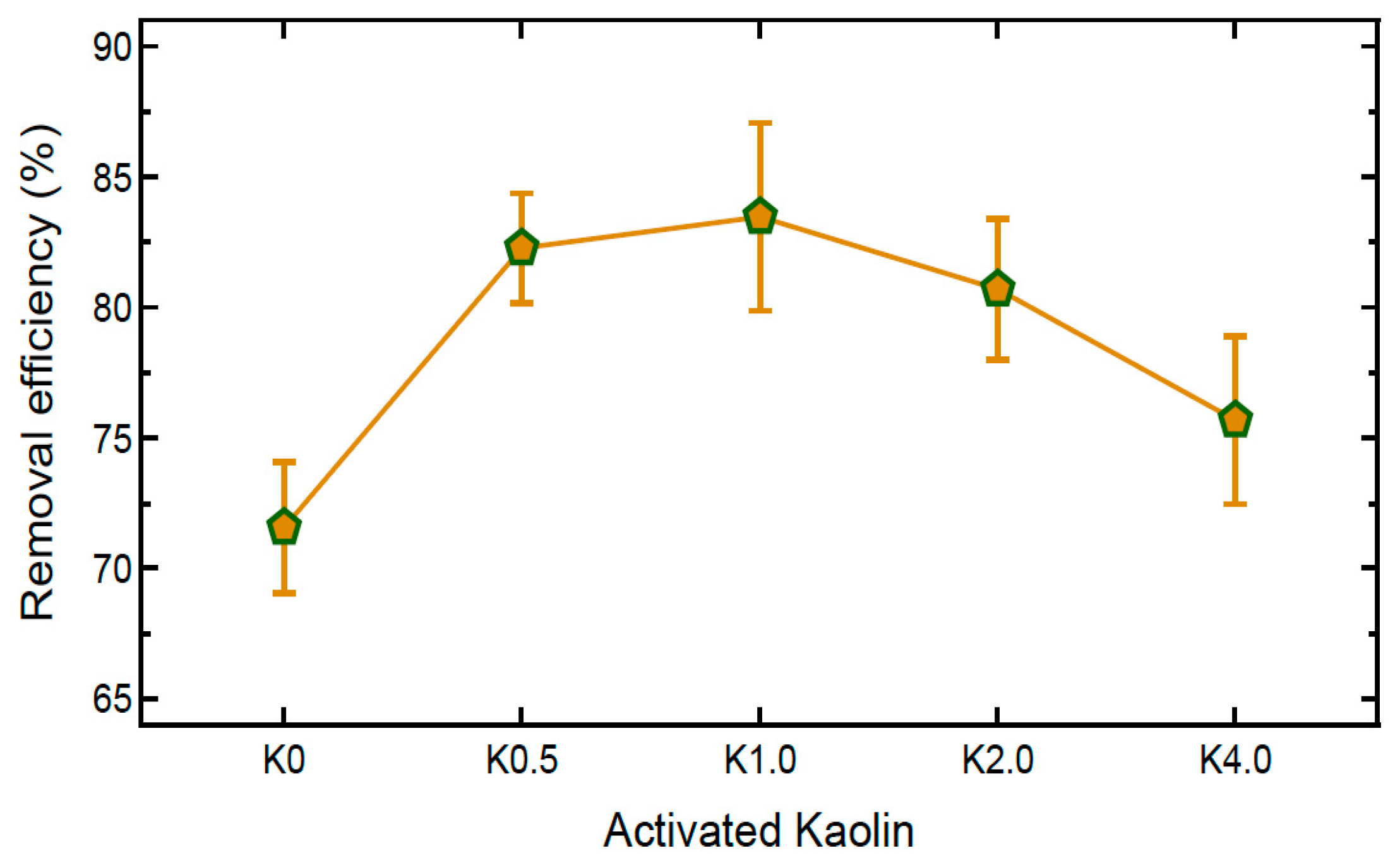
4.3. Heavy Metal Uptake
4.4. Post-Adsorption Analysis
4.5. Adsorption Kinetics, Isotherms, and Thermodynamic Studies
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. NPJ Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Nandi, B.K.; Goswami, A.; Purkait, M.K. Removal of cationic dyes from aqueous solutions by kaolin: Kinetic and equilibrium studies. Appl. Clay Sci. 2009, 42, 583–590. [Google Scholar] [CrossRef]
- Yang, T.; Wang, N.; Gu, H. The adsorption behavior of niobium (V) on kaolin clay and kaolinite. Appl. Clay Sci. 2023, 235, 106866. [Google Scholar] [CrossRef]
- Zewdie, T.M.; Prihatiningtyas, I.; Dutta, A.; Gabbiye Habtu, N.; van der Bruggen, B. Characterization and beneficiation of Ethiopian kaolin for use in fabrication of ceramic membrane. Mater. Res. Express 2021, 8, 115201. [Google Scholar] [CrossRef]
- Worasith, N.; Goodman, B.A. Clay mineral products for improving environmental quality. Appl. Clay Sci. 2023, 242, 106980. [Google Scholar] [CrossRef]
- Belver, C.; Bañares Muñoz, M.A.; Vicente, M.A. Chemical activation of a kaolinite under acid and alkaline conditions. Chem. Mater. 2002, 14, 2033–2043. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Gupta, S. Sen Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: A review. Adv. Colloid Interface Sci. 2008, 140, 114–131. [Google Scholar] [CrossRef]
- David, M.K.; Okoro, U.C.; Akpomie, K.G.; Okey, C.; Oluwasola, H.O. Thermal and hydrothermal alkaline modification of kaolin for the adsorptive removal of lead(II) ions from aqueous solution. SN Appl. Sci. 2020, 2, 1134. [Google Scholar] [CrossRef]
- Ptáček, P.; Frajkorová, F.; Šoukal, F.; Opravil, T. Kinetics and mechanism of three stages of thermal transformation of kaolinite to metakaolinite. Powder Technol. 2014, 264, 439–445. [Google Scholar] [CrossRef]
- Du, H.; Zhong, Z.; Zhang, B.; Zhao, D.; Lai, X.; Wang, N.; Li, J. Comparative study on intercalation-exfoliation and thermal activation modified kaolin for heavy metals immobilization during high-organic solid waste pyrolysis. Chemosphere 2021, 280, 130714. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, J.; Yang, Y.; Yu, Y.; Wu, D. Experimental and theoretical studies on the adsorption performance of lead by thermal pre-activation and phosphate modified kaolin sorbent. Chem. Eng. J. 2023, 451, 138762. [Google Scholar] [CrossRef]
- Yu, M.; Huang, Y.; Xia, W.; Zhu, Z.; Fan, C.; Liu, C.; Dong, L.; Xu, L.; Liu, L.; Zha, J.; et al. PbCl2 Capture by Kaolin and Metakaolin under Different Influencing Factors of Thermal Treatment. Energy Fuels 2020, 34, 2284–2292. [Google Scholar] [CrossRef]
- Cheng, Y.; Xing, J.; Bu, C.; Zhang, J.; Piao, G.; Huang, Y.; Xie, H.; Wang, X. Dehydroxylation and structural distortion of kaolinite as a high-temperature sorbent in the furnace. Minerals 2019, 9, 587. [Google Scholar] [CrossRef]
- Stefanova, R.Y. Metal removal by thermally activated clay marl. J. Environ. Sci. Health—Part A Toxic/Hazardous Subst. Environ. Eng. 2001, 36, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Kumrić, K.R.; Crossed, D.; Signukić, A.D.S.B.; Trtić-Petrović, T.M.; Vukelić, N.S.; Stojanović, Z.; Grbović Novaković, J.D.; Matović, L.L. Simultaneous removal of divalent heavy metals from aqueous solutions using raw and mechanochemically treated interstratified montmorillonite/kaolinite clay. Ind. Eng. Chem. Res. 2013, 52, 7930–7939. [Google Scholar] [CrossRef]
- Dukić, A.B.; Kumrić, K.R.; Vukelić, N.S.; Dimitrijević, M.S.; Baščarević, Z.D.; Kurko, S.V.; Matović, L.L. Simultaneous removal of Pb2+, Cu2+, Zn2+ and Cd2+ from highly acidic solutions using mechanochemically synthesized montmorillonite-kaolinite/TiO2 composite. Appl. Clay Sci. 2015, 103, 20–27. [Google Scholar] [CrossRef]
- Myasnikov, S.K.; Tikhonov, A.Y.; Chipryakova, A.P.; Kulov, N.N. Removal of heavy metal ions from water by an combined sorption–crystallization process using activated clays. Theor. Found. Chem. Eng. 2016, 50, 366–382. [Google Scholar] [CrossRef]
- Korichi, S.; Elias, A.; Mefti, A. Characterization of smectite after acid activation with microwave irradiation. Appl. Clay Sci. 2009, 42, 432–438. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Gupta, S. Sen Adsorption of chromium(VI) from water by clays. Ind. Eng. Chem. Res. 2006, 45, 7232–7240. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Gupta, S. Sen Adsorption of Co(II) from aqueous medium on natural and acid activated kaolinite and montmorillonite. Sep. Sci. Technol. 2007, 42, 3391–3418. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Gupta, S. Sen Adsorptive accumulation of Cd(II), Co(II), Cu(II), Pb(II) and Ni(II) ions from water onto Kaolinite: Influence of acid activation. Adsorpt. Sci. Technol. 2009, 27, 47–68. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Sen Gupta, S. Pb(II) uptake by kaolinite and montmorillonite in aqueous medium: Influence of acid activation of the clays. Colloids Surf. A Physicochem. Eng. Asp. 2006, 277, 191–200. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Gupta, S. Sen Uptake of Ni(II) ions from aqueous solution by kaolinite and montmorillonite: Influence of acid activation of the clays. Sep. Sci. Technol. 2008, 43, 3221–3250. [Google Scholar] [CrossRef]
- Dim, P.E.; Mustapha, L.S.; Termtanun, M.; Okafor, J.O. Adsorption of chromium (VI) and iron (III) ions onto acid-modified kaolinite: Isotherm, kinetics and thermodynamics studies. Arab. J. Chem. 2021, 14, 103064. [Google Scholar] [CrossRef]
- Mustapha, L.S.; Yusuff, A.S.; Dim, P.E. RSM optimization studies for cadmium ions adsorption onto pristine and acid-modified kaolinite clay. Heliyon 2023, 9, e18634. [Google Scholar] [CrossRef]
- Malima, N.M.; Owonubi, S.J.; Lugwisha, E.H.; Mwakaboko, A.S. Thermodynamic, isothermal and kinetic studies of heavy metals adsorption by chemically modified Tanzanian Malangali kaolin clay. Int. J. Environ. Sci. Technol. 2021, 18, 3153–3168. [Google Scholar] [CrossRef]
- Abu, T.O.; Adegoke, H.I.; Odebunmi, E.O. Optimization of Adsorption Potentials of Nigerian Kaolinite Mineral Through Acid Activation. Fountain J. Nat. Appl. Sci. 2023, 12, 55–74. [Google Scholar] [CrossRef]
- Hounsi, A.D.; Lecomte-Nana, G.; Djétéli, G.; Blanchart, P.; Alowanou, D.; Kpelou, P.; Napo, K.; Tchangbédji, G.; Praisler, M. How does Na, K alkali metal concentration change the early age structural characteristic of kaolin-based geopolymers. Ceram. Int. 2014, 40, 8953–8962. [Google Scholar] [CrossRef]
- Esaifan, M.; Rahier, H.; Barhoum, A.; Khoury, H.; Hourani, M.; Wastiels, J. Development of inorganic polymer by alkali-activation of untreated kaolinitic clay: Reaction stoichiometry, strength and dimensional stability. Constr. Build. Mater. 2015, 91, 251–259. [Google Scholar] [CrossRef]
- Shaqour, F.; Ismeik, M.; Esaifan, M. Alkali activation of natural clay using a Ca(OH)2/Na2CO3 alkaline mixture. Clay Miner. 2017, 52, 485–496. [Google Scholar] [CrossRef]
- Alkan, M.; Hopa, Ç.; Yilmaz, Z.; Güler, H. The effect of alkali concentration and solid/liquid ratio on the hydrothermal synthesis of zeolite NaA from natural kaolinite. Microporous Mesoporous Mater. 2005, 86, 176–184. [Google Scholar] [CrossRef]
- Luo, J.; Jiang, T.; Li, G.; Peng, Z.; Rao, M.; Zhang, Y. Porous materials from thermally activated kaolinite: Preparation, characterization and application. Materials 2017, 10, 647. [Google Scholar] [CrossRef]
- Abdallah, S. Remediation of Copper and Zinc from wastewater by modified clay in Asir region southwest of Saudi Arabia. Open Geosci. 2019, 11, 505–512. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Gupta, S. Sen Influence of acid activation of kaolinite and montmorillonite on adsorptive removal of Cd(II) from water. Ind. Eng. Chem. Res. 2007, 46, 3734–3742. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Gupta, S. Sen Removal of Cu(II) by natural and acid-activated clays: An insight of adsorption isotherm, kinetic and thermodynamics. Desalination 2011, 272, 66–75. [Google Scholar] [CrossRef]
- Suraj, G.; Iyer, C.S.P.; Lalithambika, M. Adsorption of cadmium and copper by modified kaolinites. Appl. Clay Sci. 1998, 13, 293–306. [Google Scholar] [CrossRef]
- Cheng, H.; Huang, Y.; Zhu, Z.; Dong, L.; Zha, J.; Yu, M. Enhanced PbCl2 adsorption capacity of modified kaolin in the furnace using a combined method of thermal pre-activation and acid impregnation. Chem. Eng. J. 2021, 414, 128672. [Google Scholar] [CrossRef]
- Lawal, J.; Odebunmi, E.; Adekola, F. Removal of heavy metals from aqueous solutions by adsorption using natural and ammonium carbonate modified kaolinite clays. S. Afr. J. Chem. 2023, 77, 61–73. [Google Scholar] [CrossRef]
- Mammen, M.; Choi, S.K.; Whitesides, G.M. Polyvalent interactions in biological systems: Implications for design and use of multivalent ligands and inhibitors. Angew. Chem.—Int. Ed. 1998, 37, 2754–2794. [Google Scholar] [CrossRef]
- SI, S. Examining the Features of NaCl Activated Kaolinite Nano-layers for Use in Excipients. Bioceram. Dev. Appl. 2018, 8, 1000106. [Google Scholar] [CrossRef]
- Liu, C.; Huang, Y.; Zhu, Z.; Yu, M.; Meng, J.; Bu, C.; Zhang, J. Study on Capture Performance on PbCl2 and CdCl2 using Kaolin Modified by Sodium Ions. ChemistrySelect 2022, 7, e202103554. [Google Scholar] [CrossRef]
- Feng, L.; Qiu, T.; Liu, C. Study on adsorption of ammonia nitrogen by sodium-modified kaolin at calcination temperature. Environ. Sci. Pollut. Res. 2023, 30, 97063–97077. [Google Scholar] [CrossRef]
- Matłok, M.; Petrus, R.; Warchoł, J.K. Equilibrium Study of Heavy Metals Adsorption on Kaolin. Ind. Eng. Chem. Res. 2015, 54, 6975–6984. [Google Scholar] [CrossRef]
- Al-Essa, E.M.; Al-Essa, K.; Halalsheh, N.; Lagum, A.A.; Al-Ma’abreh, A.M.; Saraireh, H.; Shatnawi, K. Removal of Total Phenolic Compounds and Heavy Metal Ions from Olive Mill Wastewater Using Sodium-Activated Jordanian Kaolinite. Sustainability 2025, 17, 4627. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, H.; Xing, H.; Li, H.; Hu, H.; Li, A.; Yao, H. Improved sodium adsorption by modified kaolinite at high temperature using intercalation-exfoliation method. Fuel 2017, 191, 198–203. [Google Scholar] [CrossRef]
- Prabawa, I.D.G.P.; Lestari, R.Y.; Hamdi, S. Modified physical properties of kaolin by intercalation and exfoliation method. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; Volume 980. [Google Scholar] [CrossRef]
- Pham, T.D.; Shrestha, R.A.; Virkutyte, J.; Sillanpää, M. Combined ultrasonication and electrokinetic remediation for persistent organic removal from contaminated kaolin. Electrochim. Acta 2009, 54, 1403–1407. [Google Scholar] [CrossRef]
- Qu, M.; Ma, L.; Zhou, Y.; Zhao, Y.; Wang, J.; Zhang, Y.; Zhu, X.; Liu, X.; He, J. Durable and Recyclable Superhydrophilic-Superoleophobic Materials for Efficient Oil/Water Separation and Water-Soluble Dyes Removal. ACS Appl. Nano Mater. 2018, 1, 5197–5209. [Google Scholar] [CrossRef]
- Dohnalová, Ž.; Svoboda, L.; Šulcová, P. Characterization of kaolin dispersion using acoustic and electroacoustic spectroscopy. J. Min. Metall. Sect. B Metall. 2008, 44, 63–72. [Google Scholar] [CrossRef]
- Wu, J.; Trubyanov, M.; Prvački, D.; Lim, K.; Andreeva, D.V.; Novoselov, K.S. Art and Science of Reinforcing Ceramics with Graphene via Ultrasonication Mixing. ACS Omega 2024, 9, 42944–42949. [Google Scholar] [CrossRef]
- Bhatti, Q.A.; Baloch, M.K.; Schwarz, S.; Ishaq, M. Impact of mechanochemical treatment on surface chemistry and flocculation of kaolinite dispersion. Asia-Pac. J. Chem. Eng. 2023, 18, e2886. [Google Scholar] [CrossRef]
- Ibrahim, H.S.; Jamil, T.S.; Hegazy, E.Z. Application of zeolite prepared from Egyptian kaolin for the removal of heavy metals: II. Isotherm models. J. Hazard. Mater. 2010, 182, 842–847. [Google Scholar] [CrossRef]
- Chai, J.B.; Au, P.I.; Mubarak, N.M.; Khalid, M.; Ng, W.P.Q.; Jagadish, P.; Walvekar, R.; Abdullah, E.C. Adsorption of heavy metal from industrial wastewater onto low-cost Malaysian kaolin clay–based adsorbent. Environ. Sci. Pollut. Res. 2020, 27, 13949–13962. [Google Scholar] [CrossRef]
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: A review. Clean. Eng. Technol. 2020, 1, 100032. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Sotirelis, N.P.; Chrysikopoulos, C.V. Interaction Between Graphene Oxide Nanoparticles and Quartz Sand. Environ. Sci. Technol. 2015, 49, 13413–13421. [Google Scholar] [CrossRef] [PubMed]
- Febrianto, J.; Kosasih, A.N.; Sunarso, J.; Ju, Y.H.; Indraswati, N.; Ismadji, S. Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: A summary of recent studies. J. Hazard. Mater. 2009, 162, 616–645. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. Characteristics of Elovich equation used for the analysis of adsorption kinetics in dye-chitosan systems. Chem. Eng. J. 2009, 150, 366–373. [Google Scholar] [CrossRef]
- Sharafee Shamsudin, M.; Taufik Mohd Din, A.; Sellaoui, L.; Badawi, M.; Bonilla-Petriciolet, A.; Ismail, S. Characterization, evaluation, and mechanism analysis of the functionalization of kaolin with a surfactant for the removal of diclofenac from aqueous solution. Chem. Eng. J. 2023, 465, 142833. [Google Scholar] [CrossRef]
- Spiess, A.N.; Neumeyer, N. An evaluation of R2 as an inadequate measure for nonlinear models in pharmacological and biochemical research: A Monte Carlo approach. BMC Pharmacol. 2010, 10, 6. [Google Scholar] [CrossRef]
- Akaike, H. A New Look at the Statistical Model Identification. IEEE Trans. Automat. Contr. 1974, 17, 716–723. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Multimodel inference: Understanding AIC and BIC in model selection. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Wit, E.; van den Heuvel, E.; Romeijn, J.W. ‘All models are wrong…’: An introduction to model uncertainty. Stat. Neerl. 2012, 66, 217–236. [Google Scholar] [CrossRef]
- Avornyo, A.K.; Hasan, S.W.; Banat, F.; Chrysikopoulos, C.V. Preparation, characterization, and applications of kaolin/kaolin-based composite membranes in oily wastewater treatment: Recent developments, challenges, and opportunities. J. Environ. Manag. 2024, 370, 122800. [Google Scholar] [CrossRef] [PubMed]
- Sachan, A.; Penumadu, D. Identification of microfabric of kaolinite clay mineral using X-ray diffraction technique. Geotech. Geol. Eng. 2007, 25, 603–616. [Google Scholar] [CrossRef]
- Liew, Y.M.; Kamarudin, H.; Mustafa Al Bakri, A.M.; Luqman, M.; Khairul Nizar, I.; Ruzaidi, C.M.; Heah, C.Y. Processing and characterization of calcined kaolin cement powder. Constr. Build. Mater. 2012, 30, 794–802. [Google Scholar] [CrossRef]
- Hernández-Chávez, M.; Vargas-Ramírez, M.; Herrera-González, A.M.; García-Serrano, J.; Hernández-Chávez, M.; Ramírez, A.C.; Romero-Serrano, R.G.; Sánchez-Alvarado, J.A. Thermodynamic analysis of the influence of potassium on the thermal behavior of kaolin raw material. Physicochem. Probl. Miner. Process. 2020, 57, 39–52. [Google Scholar] [CrossRef]
- Yang, K.J.; Sim, J.H.; Jeon, B.; Son, D.H.; Kim, D.H.; Sung, S.J.; Hwang, D.K.; Song, S.; Khadka, D.B.; Kim, J.; et al. Effects of Na and MoS2 on Cu2ZnSnS4 thin-film solar cell. Prog. Photovolt. Res. Appl. 2015, 23, 862–873. [Google Scholar] [CrossRef]
- You, L.; Tan, X.; Liu, Q.; Zhao, L. Ammonium removal from aqueous solutions using an ammonium ion-exchange material, the equilibrium study. Adv. Mater. Res. 2011, 322, 102–107. [Google Scholar] [CrossRef]
- Schoonheydt, R.A.; Johnston, C.T. Surface and interface chemistry of clay minerals. Dev. Clay Sci. 2013, 5, 139–172. [Google Scholar] [CrossRef]
- Allagui, A.; Benaoum, H.; Olendski, O. On the Gouy–Chapman–Stern model of the electrical double-layer structure with a generalized Boltzmann factor. Phys. A Stat. Mech. Its Appl. 2021, 582, 126252. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Li, S.; Zhu, M.; Zhang, J.; Li, Y.; He, Y.; Li, W. Zinc interstitial and oxygen vacancy mediated high Curie-temperature ferromagnetism in Ag-doped ZnO. Ceram. Int. 2020, 46, 18639–18647. [Google Scholar] [CrossRef]
- Seals, R.D.; Alexander, R.; Taylor, L.T.; Dillard, J.G. Core Electron Binding Energy Study of Group IIb-VIIa Compounds. Inorg. Chem. 1973, 12, 2485–2487. [Google Scholar] [CrossRef]
- Katzourakis, V.E.; Chrysikopoulos, C.V. Fitting the Transport and Attachment of Dense Biocolloids in One-Dimensional Porous Media: ColloidFit. Groundwater 2017, 55, 156–159. [Google Scholar] [CrossRef]
- Lankathilaka, K.P.W.; de Silva, R.M.; Mantilaka, M.M.M.G.P.G.; de Silva, K.M.N. Magnetite nanoparticles incorporated porous kaolin as a superior heavy metal sorbent for water purification. Groundw. Sustain. Dev. 2021, 14, 100606. [Google Scholar] [CrossRef]







| Blank DI Water (No Kaolin) | NaCl-Kaolin, No Zn2+ | After Zn2+ Adsorption (2 h) | After Zn2+ Adsorption (24 h Test) | Notes on Results | |
|---|---|---|---|---|---|
| Zn (mg L−1) | <0.003 | <0.003 | 5.1 ± 0.3 | 4.8 ± 0.4 | The blank confirms zero Zn contamination. Kaolin alone does not release Zn. After dosing with 50 mg L−1, only ~5 mg L−1 remains in 2 h and just a further small loss after 24 h. |
| Zn adsorbed (mg g−1) | – | – | 13.0 ± 0.7 | 13.3 ± 1.1 | Mass balance converts the drop in solution Zn to loading on the clay (0.10 g). The value exceeds the Langmuir monolayer (9 mg g−1), showing multilayer uptake/early Zn–Al LDH nucleation at high concentration. The 24-h figure matches the 2-h figure within error. |
| Na+ (mg/L) | 0.046 | 0.276 ± 0.023 | 0.644 ± 0.046 | 0.690 ± 0.046 | The blank gives background Na+ from water and, perhaps, labware. Contact with Na-activated kaolin (no Zn2+) raises Na+ nine-fold because some exchangeable K+/Ca2+/Mg2+ are replaced by Na+. When Zn2+ is present, Na+ rises a further 0.37 mg L−1 owing to Na+ ↔ Zn2+ ion exchange. The plateau at 24 h confirms the exchange is finished in 2 h. |
| Na+ released (mg g−1) | – | 0.055 ± 0.005 | 0.129 ± 0.009 | 0.138 ± 0.011 | Blank-corrected and normalized to clay mass. After Zn2+ uptake, 0.129 mg g−1 Na+ ≈ 5.6 µmol g−1 is released, close to the amount needed to balance the Zn2+ adsorbed on a 2 Na+: Zn2+ basis. |
| Al (mg L−1) | <0.01 | 0.018 ± 0.002 | 0.031 ± 0.003 | 0.034 ± 0.004 | Framework Al dissolution is <0.05 mg L−1 at all times, indicating the kaolinite lattice remains intact during activation and adsorption. |
| Si (mg L−1) | <0.02 | 0.024 ± 0.003 | 0.043 ± 0.005 | 0.044 ± 0.006 | Similarly, low Si release confirms negligible layer dissolution; Zn removal is therefore by surface exchange/complexation, not by clay breakdown and re-precipitation. |
| Cycle | Zn Adsorbed (mg/g) | Zn Desorbed (mg/g) | Efficiency |
|---|---|---|---|
| 1 | 9.22 ± 0.34 | 8.72 ± 0.30 | 94.6 ± 2.3 |
| 2 | 9.06 ± 0.28 | 8.45 ± 0.29 | 93.3 ± 2.5 |
| 3 | 8.89 ± 0.31 | 8.01 ± 0.27 | 90.1 ± 3.0 |
| 4 | 8.54 ± 0.36 | 7.38 ± 0.25 | 86.4 ± 3.5 |
| 5 | 7.97 ± 0.41 | 6.23 ± 0.22 | 78.1 ± 4.1 |
| Isotherm Studies | Kinetic Studies | ||||||
|---|---|---|---|---|---|---|---|
| Model/Parameter | Langmuir | Freundlich | Redlich–Peterson | Model/Parameter | Pseudo-First Order | Pseudo-Second Order | Elovich |
| qm | 9.218 ±0.11 | - | - | K1 | 0.0508 ± 0.006 | - | - |
| KL | 0.132 ±0.03 | - | - | K2 | - | 0.0098 ± 0.002 | - |
| KF | - | 2.169 ± 0.42 | - | qe | 5.699 ± 0.19 | 6.529 ± 0.23 | - |
| N | - | 2.895 ± 0.53 | - | α | - | - | 1485.4 ± 1025.9 |
| KR | - | - | 1.268 ± 0.86 | β | - | - | 2.535 ± 1.08 |
| αR | - | - | 0.150 ± 0.027 | - | - | - | - |
| β | - | - | 0.978 ± 0.28 | - | - | - | - |
| R2 | 0.983 | 0.950 | 0.983 | R2 | 0.905 | 0.993 | 0.843 |
| SSE | 1.076 | 3.228 | 1.069 | SSE | 0.697 | 0.184 | 4.483 |
| χ2 | 0.245 | 0.937 | 0.251 | χ2 | 0.185 | 0.043 | 0.575 |
| AIC | −15.113 | −5.2262 | −13.170 | AIC | −15.520 | −26.177 | −0.632 |
| AICc | −13.113 | −3.2262 | −8.3703 | AICc | −13.120 | −23.777 | 1.767 |
| BIC | −14.718 | −4.8317 | −12.578 | BIC | −15.361 | −26.018 | −0.473 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avornyo, A.K.; Katzourakis, V.E.; Hasan, S.W.; Banat, F.; Chrysikopoulos, C.V. NaCl as a Simple Yet Unique Activator of Kaolin: Surface Engineering for Enhanced Heavy Metal Adsorption. Water 2025, 17, 2120. https://doi.org/10.3390/w17142120
Avornyo AK, Katzourakis VE, Hasan SW, Banat F, Chrysikopoulos CV. NaCl as a Simple Yet Unique Activator of Kaolin: Surface Engineering for Enhanced Heavy Metal Adsorption. Water. 2025; 17(14):2120. https://doi.org/10.3390/w17142120
Chicago/Turabian StyleAvornyo, Amos K., Vasileios E. Katzourakis, Shadi W. Hasan, Fawzi Banat, and Constantinos V. Chrysikopoulos. 2025. "NaCl as a Simple Yet Unique Activator of Kaolin: Surface Engineering for Enhanced Heavy Metal Adsorption" Water 17, no. 14: 2120. https://doi.org/10.3390/w17142120
APA StyleAvornyo, A. K., Katzourakis, V. E., Hasan, S. W., Banat, F., & Chrysikopoulos, C. V. (2025). NaCl as a Simple Yet Unique Activator of Kaolin: Surface Engineering for Enhanced Heavy Metal Adsorption. Water, 17(14), 2120. https://doi.org/10.3390/w17142120








