Recovery of Nutrients from the Aqueous Phase of Hydrothermal Liquefaction—A Review
Abstract
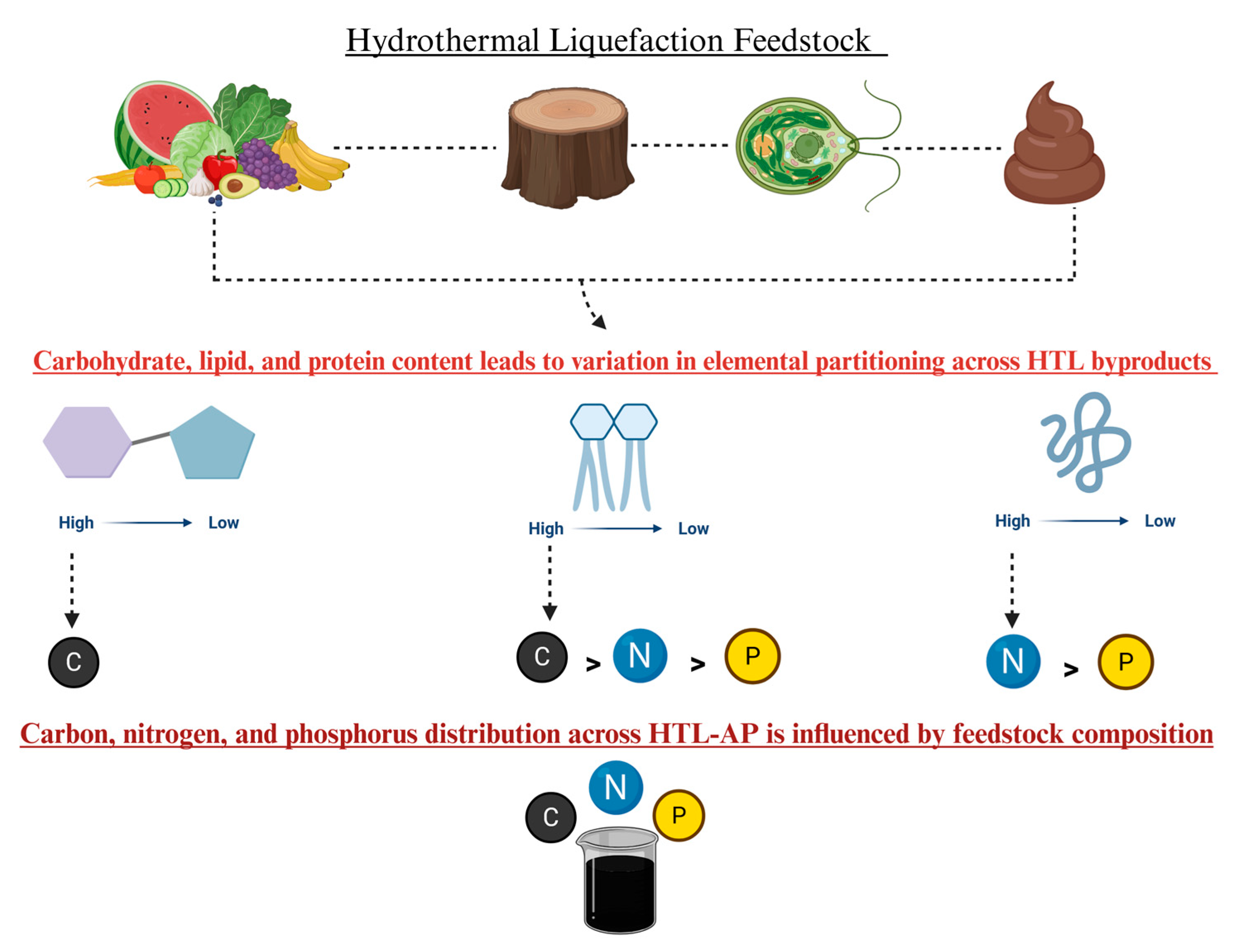
1. Introduction
2. HTL-AP Water Quality by Feedstock
2.1. Food Waste-Derived HTL-AP
2.2. Algae-Derived HTL-AP
2.3. Manure-Derived HTL-AP
2.4. Wood-Derived HTL-AP
3. HTL-AP Treatment Methods
3.1. Physical Treatment Methods
3.2. Sand Filtration
3.3. Reverse Osmosis
3.4. Nanofiltration
3.5. Activated Carbon Filtration
3.6. Chemical Treatment Methods
3.7. Struvite Precipitation
3.8. Ozone
3.9. Electrochemical Oxidation
3.10. Biological Treatment Methods
3.11. Anaerobic Digestion
3.12. Fungal Treatment
3.13. Microalgae Treatment
4. Future Directions and Challenges
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jha, S.; Okolie, J.A.; Nanda, S.; Dalai, A.K. A Review of Biomass Resources and Thermochemical Conversion Technologies. Chem. Eng. Technol. 2022, 45, 791–799. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.J. Thermochemical conversion of biomass: Potential future prospects. Renew. Sustain. Energy Rev. 2023, 187, 113754. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Kishore, N.; Gu, S. A review on hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- Elhassan, M.; Abdullah, R.; Kooh, M.R.R.; Chou Chau, Y.F. Hydrothermal liquefaction: A technological review on reactor design and operating parameters. Bioresour. Technol. Rep. 2023, 21, 101314. [Google Scholar] [CrossRef]
- Aktas, K.; Liu, H.; Eskicioglu, C. Treatment of aqueous phase from hydrothermal liquefaction of municipal sludge by adsorption: Comparison of biochar, hydrochar, and granular activated carbon. J. Environ. Manag. 2024, 356, 120619. [Google Scholar] [CrossRef]
- Couto, E.; Calijuri, M.L.; Assemany, P. Biomass production in high rate ponds and hydrothermal liquefaction: Wastewater treatment and bioenergy integration. Sci. Total Environ. 2020, 724, 138104. [Google Scholar] [CrossRef] [PubMed]
- Jesse, S.D.; Zhang, Y.; Margenot, A.J.; Davidson, P.C. Hydroponic Lettuce Production Using Treated Post-Hydrothermal Liquefaction Wastewater (PHW). Sustainability 2019, 11, 3605. [Google Scholar] [CrossRef]
- Davidson, S.D.; Lopez-Ruiz, J.A.; Zhu, Y.; Cooper, A.R.; Albrecht, K.O.; Dagle, R.A. Strategies to Valorize the Hydrothermal Liquefaction-Derived Aqueous Phase into Fuels and Chemicals. ACS Sustain. Chem. Eng. 2019, 7, 19889–19901. [Google Scholar] [CrossRef]
- Bao, R.; Wang, S.; Feng, J.; Duan, Y.; Liu, K.; Zhao, J.; Liu, H.; Yang, J. A Review of Hydrothermal Biomass Liquefaction: Operating Parameters, Reaction Mechanism, and Bio-Oil Yields and Compositions. Energy Fuels 2024, 38, 8437–8459. [Google Scholar] [CrossRef]
- Mansuri, S.Q.; Shekhawat, V.P.S. Hydrothermal liquefaction: Exploring feedstock for sustainable biofuel production. Environ. Exp. Biol. 2024, 22, 135–147. [Google Scholar] [CrossRef]
- Why Should We Care About Food Waste?|Home. Available online: https://www.usda.gov/about-food/food-safety/food-loss-and-waste/why-should-we-care-about-food-waste (accessed on 29 June 2025).
- Kostyukevich, Y.; Vlaskin, M.; Borisova, L.; Zherebker, A.; Perminova, I.; Kononikhin, A.; Popov, I.; Nikolaev, E. Investigation of bio-oil produced by hydrothermal liquefaction of food waste using ultrahigh resolution Fourier transform ion cyclotron resonance mass spectrometry. Eur. J. Mass Spectrom. 2018, 24, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Bayat, H.; Dehghanizadeh, M.; Jarvis, J.M.; Brewer, C.E.; Jena, U. Hydrothermal Liquefaction of Food Waste: Effect of Process Parameters on Product Yields and Chemistry. Front. Sustain. Food Syst. 2021, 5, 658592. [Google Scholar] [CrossRef]
- Biswas, B.; Rahman, T.; Adhikari, S. Mono-and bi-metal catalytic hydrothermal liquefaction of food waste: Screening the process parameter on product yield and characterizations. J. Clean. Prod. 2024, 471, 143398. [Google Scholar] [CrossRef]
- Summers, S.; Valentine, A.; Wang, Z.; Zhang, Y. Pilot-Scale Continuous Plug-Flow Hydrothermal Liquefaction of Food Waste for Biocrude Production. Ind. Eng. Chem. Res. 2023, 62, 12174–12182. [Google Scholar] [CrossRef]
- Aierzhati, A.; Stablein, M.J.; Wu, N.E.; Kuo, C.T.; Si, B.; Kang, X.; Zhang, Y. Experimental and model enhancement of food waste hydrothermal liquefaction with combined effects of biochemical composition and reaction conditions. Bioresour. Technol. 2019, 284, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Research, B.; Board, D. Federal Activities Report on the Bioeconomy: ALGAE BRD; US Department of Energy (USDOE): Washington DC, USA, 2020.
- Barreiro, D.L.; Samorì, C.; Terranella, G.; Hornung, U.; Kruse, A.; Prins, W. Assessing microalgae biorefinery routes for the production of biofuels via hydrothermal liquefaction. Bioresour. Technol. 2014, 174, 256–265. [Google Scholar] [CrossRef]
- Ramírez-Romero, A.; Martin, M.; Boyer, A.; Bolzoni, R.; Matricon, L.; Sassi, J.F.; Steyer, J.P.; Delrue, F. Microalgae adaptation as a strategy to recycle the aqueous phase from hydrothermal liquefaction. Bioresour. Technol. 2023, 371, 128631. [Google Scholar] [CrossRef]
- Yuan, C.; Zhao, S.; Ni, J.; He, Y.; Cao, B.; Hu, Y.; Wang, S.; Qian, L.; Abomohra, A. Integrated route of fast hydrothermal liquefaction of microalgae and sludge by recycling the waste aqueous phase for microalgal growth. Fuel 2023, 334, 126488. [Google Scholar] [CrossRef]
- Yin, S.; Shao, Y.; Bao, T.; Zhu, J. Review on Nitrogen Transformation during Microalgae Thermochemical Liquefaction: Recent Advances and Future Perspectives. Energy Fuels 2023, 37, 1525–1544. [Google Scholar] [CrossRef]
- Constantinescu, M.; Bucura, F.; Ionete, E.I.; Spiridon, Ş.I.; Ionete, R.E.; Zaharioiu, A.; Marin, F.; Ion-Ebrasu, D.; Botoran, O.R.; Roman, A. Cattle manure thermochemical conversion to hydrogen-rich syngas, through pyrolysis and gasification. Int. J. Hydrogen Energy 2024, 79, 1058–1070. [Google Scholar] [CrossRef]
- Lu, J.; Li, H.; Zhang, Y.; Liu, Z. Nitrogen Migration and Transformation during Hydrothermal Liquefaction of Livestock Manures. ACS Sustain. Chem. Eng. 2018, 6, 13570–13578. [Google Scholar] [CrossRef]
- Vadlamudi, D.P.; Pecchi, M.; Sudibyo, H.; Tester, J.W. Direct and Two-Stage Hydrothermal Liquefaction of Chicken Manure: Impact of Reaction Parameters on Biocrude Oil Upgradation. ACS Sustain. Chem. Eng. 2024, 12, 4300–4313. [Google Scholar] [CrossRef]
- Liu, Q.; Kong, G.; Zhang, G.; Cao, T.; Wang, K.; Zhang, X.; Han, L. Recent advances in hydrothermal liquefaction of manure wastes into value-added products. Energy Convers. Manag. 2023, 292, 117392. [Google Scholar] [CrossRef]
- USDA. USDA Implementation Framework for a Plan to Enable the Bioeconomy in America: Building a Resilient Biomass Supply A Message from Secretary Vilsack; USDA: Washington, DC, USA, 2022.
- Moreira, G.D.O.; Costa, G.F.; Cavalcante, R.M.; Young, A.F. Process simulation and economic evaluation of pyrolysis and hydrothermal liquefaction as alternatives for the valorization of wood waste from the pulp and paper industry. Energy Convers. Manag. 2025, 325, 119387. [Google Scholar] [CrossRef]
- Wörner, M.; Hornung, U.; Karagöz, S.; Zevaco, T.; Dahmen, N. Focus on hydrochars produced from hydrothermal liquefaction of beech wood, soda lignin and black liquor. Eur. J. Wood Wood Prod. 2025, 83, 61. [Google Scholar] [CrossRef]
- Seehar, T.H.; Toor, S.S.; Shah, A.A.; Nielsen, A.H.; Pedersen, T.H.; Rosendahl, L.A. Catalytic hydrothermal liquefaction of contaminated construction wood waste for biocrude production and investigation of fate of heavy metals. Fuel Process. Technol. 2021, 212, 106621. [Google Scholar] [CrossRef]
- Hamoda, M.F.; Al-Ghusain, I.; AL-Mutairi, N.Z. Sand filtration of wastewater for tertiary treatment and water reuse. Desalination 2004, 164, 203–211. [Google Scholar] [CrossRef]
- Jesse, S.D.; Davidson, P.C. Treatment of Post-Hydrothermal Liquefaction Wastewater (PHWW) for Heavy Metals, Nutrients, and Indicator Pathogens. Water 2019, 11, 854. [Google Scholar] [CrossRef]
- Ibrahim, G.P.S.; Isloor, A.M.; Farnood, R. Fundamentals and basics of reverse osmosis. In Current Trends and Future Developments on (Bio-) Membranes: Reverse and Forward Osmosis: Principles, Applications, Advances; Elsevier: Amsterdam, The Netherlands, 2020; pp. 141–163. [Google Scholar] [CrossRef]
- Tufty, B. Reverse Osmosis Purifies. Sci. News-Lett. 1965, 88, 247. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Teow, Y.H.; Ang, W.L.; Chung, Y.T.; Oatley-Radcliffe, D.L.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Lyu, H.; Fang, Y.; Ren, S.; Chen, K.; Luo, G.; Zhang, S.; Chen, J. Monophenols separation from monosaccharides and acids by two-stage nanofiltration and reverse osmosis in hydrothermal liquefaction hydrolysates. J. Memb. Sci. 2016, 504, 141–152. [Google Scholar] [CrossRef]
- Kizza, R.; Eskicioglu, C. Ultrafiltration fractionation of potentially inhibitory substances of hydrothermal liquefaction aqueous phase derived from municipal sludge. Water Res. 2024, 257, 121703. [Google Scholar] [CrossRef]
- Sayegh, A.; Prakash, N.S.; Pedersen, T.H.; Horn, H.; Saravia, F. Treatment of hydrothermal liquefaction wastewater with ultrafiltration and air stripping for oil and particle removal and ammonia recovery. J. Water Process Eng. 2021, 44, 102427. [Google Scholar] [CrossRef]
- Zhang, X.; Scott, J.; Sharma, B.K.; Rajagopalan, N. Advanced treatment of hydrothermal liquefaction wastewater with nanofiltration to recover carboxylic acids. Environ. Sci. 2018, 4, 520–528. [Google Scholar] [CrossRef]
- Erkelens, M.; Ball, A.S.; Lewis, D.M. The application of activated carbon for the treatment and reuse of the aqueous phase derived from the hydrothermal liquefaction of a halophytic Tetraselmis sp. Bioresour. Technol. 2015, 182, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Huang, J.; Chen, H.; He, Z. Reducing nitrogen content in bio-oil from hydrothermal liquefaction of microalgae by using activated carbon-pretreated aqueous phase as the solvent. Biomass Bioenergy 2022, 167, 106638. [Google Scholar] [CrossRef]
- Pastor, L.; Mangin, D.; Barat, R.; Seco, A. A pilot-scale study of struvite precipitation in a stirred tank reactor: Conditions influencing the process. Bioresour. Technol. 2008, 99, 6285–6291. [Google Scholar] [CrossRef]
- Kumar, R.; Pal, P. Assessing the feasibility of N and P recovery by struvite precipitation from nutrient-rich wastewater: A review. Environ. Sci. Pollut. Res. 2015, 22, 17453–17464. [Google Scholar] [CrossRef]
- Shanmugam, S.R.; Adhikari, S.; Shakya, R. Nutrient removal and energy production from aqueous phase of bio-oil generated via hydrothermal liquefaction of algae. Bioresour. Technol. 2017, 230, 43–48. [Google Scholar] [CrossRef]
- Ovsyannikova, E.; Kruse, A.; Becker, G.C. Feedstock-Dependent Phosphate Recovery in a Pilot-Scale Hydrothermal Liquefaction Bio-Crude Production. Energies 2020, 13, 379. [Google Scholar] [CrossRef]
- Bauer, S.K.; Cheng, F.; Colosi, L.M. Evaluating the Impacts of ACP Management on the Energy Performance of Hydrothermal Liquefaction via Nutrient Recovery. Energies 2019, 12, 729. [Google Scholar] [CrossRef]
- Rekhate, C.V.; Srivastava, J.K. Recent advances in ozone-based advanced oxidation processes for treatment of wastewater—A review. Chem. Eng. J. Adv. 2020, 3, 100031. [Google Scholar] [CrossRef]
- Rodríguez, A.; Rosal, R.; Perdigón-Melón, J.A.; Mezcua, M.; Agüera, A.; Hernando, M.D.; Letón, P.; Fernández-Alba, A.R.; García-Calvo, E. Ozone-Based Technologies in Water and Wastewater Treatment. Handb. Environ. Chem. 2008, 5, 127–175. [Google Scholar] [CrossRef]
- Yang, L.; Si, B.; Martins, M.A.; Watson, J.; Chu, H.; Zhang, Y.; Tan, X.; Zhou, X.; Zhang, Y. Improve the biodegradability of post-hydrothermal liquefaction wastewater with ozone: Conversion of phenols and N-heterocyclic compounds. Water Sci. Technol. 2018, 2017, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Si, B.; Tan, X.; Chu, H.; Zhou, X.; Zhang, Y.; Zhang, Y.; Zhao, F. Integrated anaerobic digestion and algae cultivation for energy recovery and nutrient supply from post-hydrothermal liquefaction wastewater. Bioresour. Technol. 2018, 266, 349–356. [Google Scholar] [CrossRef]
- Si, B.; Yang, L.; Zhou, X.; Watson, J.; Tommaso, G.; Chen, W.T.; Liao, Q.; Duan, N.; Liu, Z.; Zhang, Y. Anaerobic conversion of the hydrothermal liquefaction aqueous phase: Fate of organics and intensification with granule activated carbon/ozone pretreatment. Green Chem. 2019, 21, 1305–1318. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Panizza, M. Electrochemical oxidation of organic pollutants for wastewater treatment. Curr. Opin. Electrochem. 2018, 11, 62–71. [Google Scholar] [CrossRef]
- Matayeva, A.; Biller, P. Hydrothermal liquefaction aqueous phase treatment and hydrogen production using electro-oxidation. Energy Convers. Manag. 2021, 244, 114462. [Google Scholar] [CrossRef]
- Ciarlini, J.; Alves, L.; Rajarathnam, G.P.; Haynes, B.S.; Montoya, A. Electrochemical oxidation of nitrogen-rich post-hydrothermal liquefaction wastewater. Algal Res. 2020, 48, 101919. [Google Scholar] [CrossRef]
- Cantero, B.C.B.; Zhang, Y.; Davidson, P.C. Electrolysis of HTL-AP for nutrient recovery by converting cyclic nitrogen to nitrate-N fertilizer. Environ. Pollut. 2024, 363, 125069. [Google Scholar] [CrossRef]
- Uddin, M.M.; Wright, M.M. Anaerobic digestion fundamentals, challenges, and technological advances. Phys. Sci. Rev. 2023, 8, 2819–2837. [Google Scholar] [CrossRef]
- Tatla, H.K.; Ismail, S.; Khan, M.A.; Dhar, B.R.; Gupta, R. Coupling hydrothermal liquefaction and anaerobic digestion for waste biomass valorization: A review in context of circular economy. Chemosphere 2024, 361, 142419. [Google Scholar] [CrossRef]
- Swetha, A.; ShriVigneshwar, S.; Gopinath, K.P.; Sivaramakrishnan, R.; Shanmuganathan, R.; Arun, J. Review on hydrothermal liquefaction aqueous phase as a valuable resource for biofuels, bio-hydrogen and valuable bio-chemicals recovery. Chemosphere 2021, 283, 131248. [Google Scholar] [CrossRef]
- Sankaran, S.; Khanal, S.K.; Jasti, N.; Jin, B.; Pometto, A.L.; Van Leeuwen, J.H. Use of Filamentous Fungi for Wastewater Treatment and Production of High Value Fungal Byproducts: A Review. Crit. Rev. Environ. Sci. Technol. 2010, 40, 400–449. [Google Scholar] [CrossRef]
- Liu, M.; Wang, J.; Umeda, I.; Wang, Z.; Kumar, S.; Zheng, Y. Harnessing filamentous fungi and fungal-bacterial co-culture for biological treatment and valorization of hydrothermal liquefaction aqueous phase from corn stover. Bioresour. Technol. 2024, 409, 131240. [Google Scholar] [CrossRef]
- Leme, V.F.C.; Lopez, K.; Costa, T.; Conerty, B.; Leonelli, L.B.; Zhang, Y.; Davidson, P.C. Hydrothermal liquefaction aqueous phase mycoremediation to increase inorganic nitrogen availability. Heliyon 2024, 10, e31992. [Google Scholar] [CrossRef]
- Lopez, K.; Leme, V.F.C.; Warzecha, M.; Davidson, P.C. Wastewater Nutrient Recovery via Fungal and Nitrifying Bacteria Treatment. Agriculture 2024, 14, 580. [Google Scholar] [CrossRef]
- Wang, J.; Li, L.; Liu, Y.; Li, W. A review of partial nitrification in biological nitrogen removal processes: From development to application. Biodegradation 2021, 32, 229–249. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Raouf, N.; Al-Homaidan, A.A.; Ibraheem, I.B.M. Microalgae and wastewater treatment. Saudi J. Biol. Sci. 2012, 19, 257–275. [Google Scholar] [CrossRef]
- Rout, P.R.; Goel, M.; Mohanty, A.; Pandey, D.S.; Halder, N.; Mukherjee, S.; Bhatia, S.K.; Sahoo, N.K.; Varjani, S. Recent Advancements in Microalgal Mediated Valorisation of Wastewater from Hydrothermal Liquefaction of Biomass. BioEnergy Res. 2022, 16, 45–60. [Google Scholar] [CrossRef]
- Barreiro, D.L.; Bauer, M.; Hornung, U.; Posten, C.; Kruse, A.; Prins, W. Cultivation of microalgae with recovered nutrients after hydrothermal liquefaction. Algal Res. 2015, 9, 99–106. [Google Scholar] [CrossRef]


| HTL-AP Feedstock | Target | pH | Recovery | Advantages | Disadvantages | References |
|---|---|---|---|---|---|---|
| Mixed Sludge | Recovery of carboxylic acids | 6.42 | 96% | High recovery for all organics with 97.2% retention efficiency for volatile fatty acids and 82.4% for sugars | Adsorption fouling and sensitivity to transmembrane pressure and pH on the transport of organics through the membrane | [36] |
| Sewage sludge | Recovery of ammonia | 9.00 | 88% | High recovery of ammonia can be achieved in less than 5 h | The stability of the small droplets close to the membrane surface decreases due to the decrease in available surfactants; hence, the small droplets coalesce into larger ones, subsequently enhancing the rejection | [37] |
| Synthetic HTL-AP | Recovery of carboxylic acids, phenols, nitrogen-containing compounds, and ketones | 2.00–11.00 | Approximately 27.5% of carboxylic acids were retained with the pH adjusted to 8. Approximately 92% of phenols were retained, and 95% of nitrogen cyclic compounds were retained at pH 8. | Coupled carboxylic acid and phenols are retained more efficiently in the membrane | The pH causes rejections due to low molecular weight neutral organic species incorporated into the solution matrix | [38] |
| HTL-AP Feedstock | Target | pH | Recovery | Advantages | Disadvantages | References |
|---|---|---|---|---|---|---|
| Slurry | Recovery of carboxylic acids | NA | A total of10% wet acetic acid and 1.7% wet weight propionic acid were recovered | A total of 62% of carboxylic acids were retained with activated carbon | A total of 34% of carbon is lost during treatment, since retention of the liquid in the porous structure of the filter is a limitation with batch filtration | [8] |
| Spirulina platensis | Recovery of nitrogen cyclic compounds and carboxylic acids | NA | Carboxylic acids dropped slightly from 18.14% to 15.82% after treatment. Acetic acid, one of the organic acids, displayed a high concentration in the AP. | Selectivity of nitrogen-containing compounds for absorption in the filter | Negligible adsorption of organic acids | [40] |
| Slurry | Removal of organic contaminants | NA | Approximately 88% removal of total nitrogen from the HTL-AP. A total of 40% removal of hydrocarbons was achieved through the treatment. A total of 31% of organic carbon was removed from the HTL-AP. A total of 9% increased ammoniacal nitrogen was observed after treatment | Removal of organic nitrogen and organic carbon after treatment. Selectivity of absorption of nitrogen over organic carbon with the filter. | Dilution of HTL-AP is needed after treatment to enable microalgae growth. | [39] |
| HTL-AP Feedstock | Target | pH | Recovery | Advantages | Disadvantages | References |
|---|---|---|---|---|---|---|
| Nannochloropsis sp. | Recovery of phosphorus and nitrogen in the form of struvite | 7.8 ± 0.05 | A 100% removal of ammoniacal nitrogen, 23% removal of COD, and 8 mg/L of phosphate ion recovered after the highest levels of pH, temperature, and reaction times were applied for the struvite synthesis | Phosphate and ammoniacal nitrogen were removed at all experimental conditions through struvite synthesis | The presence of different ions could lead to the formation of parasitic compounds and reduce the removal of ammoniacal nitrogen. This method is most effective for nutrient recovery at higher pH, higher reaction times, and higher temperatures | [43] |
| Slurry of Sewage Sludge and Spirulina | Recovery of nutrients to produce marketable fertilizer with phosphorus precipitation of the solid phase of HTL and addition of HTL-AP | NA | Phosphate ions can be recovered approximately 66% for Spirulina HTL-AP and 99% for Sewage Sludge HTL-AP through struvite precipitation | Excess of ammoniacal nitrogen in the HTL-AP could be beneficial for struvite crystallization. | HTL-AP with excess ammonium ions could cause a low recovery rate of ammoniacal nitrogen through struvite precipitation. Increased chemical consumption is a limiting factor for this method of acquisition | [44] |
| Seven different non-food organic feedstock | Recovery of nutrients through struvite precipitation | 4.4 | Approximately, the recovery range was 50% of nitrogen, and around 100% of phosphorus recovery was obtained through precipitation | Struvite precipitation can produce ammonium and phosphate from HTL-AP with less nitrogen and phosphorus available | NH4+/NH3 volatilization occurs during the precipitation process. Competitive reactions take place in the HTL-AP and limit the recovery of phosphate, ammonium, and magnesium | [45] |
| HTL-AP Feedstock | Target | pH | Recovery | Advantages | Disadvantages | References |
|---|---|---|---|---|---|---|
| Swine manure | Decrease in organic compounds | 5.1 | After three hours of treatment with ozone, 6% of the nitrogen cyclic compounds distribution decreased while the distribution of organic acids increased in the HTL-AP. After two hours of treatment 20% increase in ammonium was observed. | The biodegradability of HTL-AP increased after ozonation since an increase in the biological oxygen demand and chemical oxygen demand ratio was observed. | Low removal of chemical oxygen demands due to a lack of hydroxyl radicals under slightly acidic conditions. Ozonolysis is favored under alkaline conditions. | [48] |
| Swine manure | Study the effect of ozone and activated carbon on the HTL-AP for its use in anaerobic digestion and microalgae growth | 4.5 | A 71% removal of chemical oxygen demand was achieved through ozone treatment and anaerobic digestion. A two-times increase in ammonium in the HTL-AP was observed after ozone–anaerobic digestion treatment, as well as a three-times increase in pH after treatment with anaerobic digestion and ozone was observed | A slight increase in phosphorus can be obtained with coupled systems of ozonation and anaerobic digestion. Ozone Sand anaerobic digestion helped to enhance the recovery of methane. | Removal of hydrocarbons is difficult to achieve with ozone treatment compared to activated carbon. | [49] |
| Swine Manure | Reveal the fate of compounds of the HTL-AP through ozone | NA | A total of 60% of the initial ammonia was removed with ozone and anaerobic digestion | Higher removal efficiency was observed with the HTL-AP with a higher initial chemical oxygen demand concentration. | Less than 20% of organic nitrogen was degraded with ozone and anaerobic digestion | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogarin Cantero, B.C.; Li, Y.; Kalita, P.; Zhang, Y.; Davidson, P. Recovery of Nutrients from the Aqueous Phase of Hydrothermal Liquefaction—A Review. Water 2025, 17, 2099. https://doi.org/10.3390/w17142099
Bogarin Cantero BC, Li Y, Kalita P, Zhang Y, Davidson P. Recovery of Nutrients from the Aqueous Phase of Hydrothermal Liquefaction—A Review. Water. 2025; 17(14):2099. https://doi.org/10.3390/w17142099
Chicago/Turabian StyleBogarin Cantero, Barbara Camila, Yalin Li, Prasanta Kalita, Yuanhui Zhang, and Paul Davidson. 2025. "Recovery of Nutrients from the Aqueous Phase of Hydrothermal Liquefaction—A Review" Water 17, no. 14: 2099. https://doi.org/10.3390/w17142099
APA StyleBogarin Cantero, B. C., Li, Y., Kalita, P., Zhang, Y., & Davidson, P. (2025). Recovery of Nutrients from the Aqueous Phase of Hydrothermal Liquefaction—A Review. Water, 17(14), 2099. https://doi.org/10.3390/w17142099






