Abstract
The marine habitats of the world’s oceans are being driven beyond their resilience. The ongoing biodiversity crisis is happening fast, within the lifespan of researchers trying to produce the information necessary for the conservation of habitats and marine ecosystems. Here, we report on the destruction of sciaphilic sessile communities and coralligenous concretions produced by the anchoring of a high-tonnage vessel inside a Marine Protected Area in Cyprus. The damage from the anchors and the chains consisted of the dislodgement of large boulders that were dragged or rolled over the seafloor, increasing the breakage and further dislodgement of more boulders; many were left upside-down. The biological communities that thrived in the dark environments below the boulders were directly exposed to high irradiance levels and went through a slow mortality and decaying process, most probably due to a combination of several deterioration agents, such as exposure to direct sunlight, predation, mucilage aggregates, and cyanobacterial blooms. The enforcement of regulatory measures for anchoring and transit in the MPA is necessary to prevent similar destruction. Given the extent of the irreversible damage to these sciaphilic communities, our study is, unfortunately, another environmental post-mortem contribution.
1. Introduction
The marine habitats of the world’s oceans are being driven beyond their resilience with the rapid progress of the ongoing biodiversity crisis within the lifespan of researchers trying to produce the information necessary for the conservation of habitats and marine ecosystems. Mass mortality events among marine organisms, for example, are worsened by human-made disturbances [1] that affect not only coastal areas but also the deep sea [2,3], posing a challenge to management and restoration efforts [4]. In this context of global change, if we aim for realistic evidence-based management, it is imperative to document ecological disturbances and their nature and effects on biological communities [5,6].
One of the human-made activities that creates well-known problems in the marine realm is boat traffic, particularly the impact of vessels anchoring on benthic ecosystems. Anchoring in general (not only within the shipping industry) is known to produce damage to the seascape, benthic habitats, and ecosystems, ranging from physical damage by crushing, for example, to alterations in the influx of sediments with consequences for biogeochemical processes [7,8,9,10,11,12]. It has only been since the 1970s that the first published reports about negative effects from anchors and chains (hereafter anchoring gear) have appeared, and most of those observations were from coral reefs [13,14]. Another type of damage from those early studies is related to the grounding of large vessels (e.g., [15,16]). Soon after, other habitats, such as seagrasses, were also reported in the literature to be affected by anchor damage [17].
Anchoring gear leaves tell-tale damage scars on the seafloor, such as abrasion and scouring when the vessel moves or swings [10,18]. Dramatic changes in the bottom topography, such as the crushing and flattening of benthic communities, the scouring of the sea bottom, and the fragmentation of rocky substrates, can be caused by high-tonnage vessels with their enormous anchors and chains [10,19]. The damage from the anchoring gear of these large vessels to the structural integrity of the seafloor can be compared to erosional changes that operate on a scale of decades, centuries, and even millennia; such is the extreme case of damage on the deep-sea floor from bottom supertrawlers [20]. Even though the comparison between bottom trawlers and anchoring presumes similar depths, anchoring usually takes place in shallower areas, and there are two other important factors to consider: the frequency and the intensity of the anchoring [19]. Both factors make anchoring one of the most common human activities in coastal waters with negative and long-lasting effects. Where the recovery of benthic habitats has been observed, it is linked in the first instance to the characteristics of the physical damage of the anchoring gear and the resulting changes in the seascape topography after the impact, as well as the habitat type and its vulnerability, the recruitment rates of affected organisms, the seasonality of the environmental conditions, and the composition of the benthic assemblage of species before and after the impact [17,21,22,23,24,25]. However, human activities are increasing the intensity, as well as the frequency, of disturbances [26], thus changing the background conditions that otherwise might have allowed for the recovery of any given habitat [5]
In the present study, the impact of a high-tonnage vessel on seafloor topography and the widespread mortality of sciaphilic organisms was evaluated at a boulder field (23–27 m depth) in the Marine Protected Area of Cape Greco (hereafter MPA Cape Greco) in Cyprus. The vessel’s anchoring gear produced devastation on the seafloor and affected an area of the boulder field that was previously studied in 2016. Following the same protocol to assess benthic substrate cover, we revisited the area on several occasions starting one week after the event and compared the changes in cover due to the fracture and dislodgment of large boulders on several substrate categories. We postulated that the sciaphilic and coralligenous communities, which were originally located in semi-dark conditions found under the boulders, would eventually decline due to widespread mortality associated with unnatural exposure to high irradiance levels.
2. Materials and Methods
2.1. Study Site
MPA Cape Greco (Natura 2000, CY3000005; 1875.44 ha, of which around 51% is marine area) is located on the eastern coast of Cyprus, Levantine Sea (Figure 1a). MPA Cape Greco harbors important marine habitats, such as sandbanks, extensive Posidonia oceanica meadows, rocky reefs, submerged and partially submerged caves, Cymodocea seagrass on sandy bottoms, Cystoseira forests on rocky platforms, vermetid reefs, and coralligenous concretions [27]. The zoning of the marine area (Figure 1b) consists of one Core Zone (no-take zone), two Buffer Zones (only professional fishermen are allowed to fish), and two Wider Zones without restrictions to fishing.
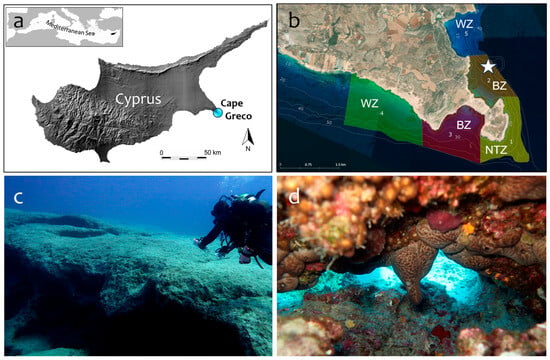
Figure 1.
(a) Location of MPA Cape Greco in Cyprus (eastern Mediterranean). (b) Zoning of the protected marine area: NTZ = No Take Zone (number 1), BZ = Buffer Zone (2, 3), and WZ = Wider Zone (4, 5). The impacted site is indicated with a white star (34.973183° Latitude, 34.082233° Longitude). (c) Boulder field in 2016: exposed surface (sunlit) with few benthic species. (d) Sciaphilic communities dominated by sponges, calcareous algae, bryozoans, and corals underneath the boulders in 2016.
The impacted site is at 23–27 m depth in a boulder field within the northern Buffer Zone (Figure 1b) that has been monitored sporadically (photo-frames) since 2016 and with seasonal frequency since 2021. While the lit upper surface of the boulders was typically a low-diversity benthic community in 2016 (Figure 1c), the shaded sections of the undersides provided dim-light to semi-dark conditions and hosted diverse sciaphilic communities, including coralligenous concretions (Figure 1d). During several days of heavy winds and very large swells between the 27th of January and the 5th of February 2021, a high-tonnage vessel over 100 m in length was sheltering and anchoring at the study site. The low-pressure system affected almost the entire island with gale winds and strong precipitation. In the area of MPA Cape Greco, daily average wind speeds fluctuated between 4.9 m/s and 8.8 m/s, with gusts between 14.2 m/s and 30.4 m/s (Figure A1). The vessel remained in the area until the weather conditions improved.
2.2. Boulder Field Destruction
One week after the departure of the large vessel, the impacted area (ca. 2500 m2) was inspected by divers along compass-oriented transects, taking notes of the unmistakable signs of damage left by the anchors and chains. The survey lines crisscrossed the area, and details of the scarring, scouring, detachment, and breakage of the boulders, as well as sockets (empty spaces left on the boulders by fragmentation [28]), were recorded, including damage to P. oceanica meadows at the deeper margin of the boulder field. To provide an estimate of the boulders’ sizes, the volume was determined (maximum length × width × height) using a flexible measuring tape.
2.3. Substrate Cover
The percentage of cover in the epibenthic communities in the boulder field was previously evaluated in 2016 before the impact and one time in 2021 (February), a few weeks after the event [29]. In that publication, the initial event is examined, but the present study follows the anchor destruction until February 2024. The 2016/2021 data set [29] is complemented with additional surveys from 2021 (April and July) and 2024 (February), and the same protocol was followed for substrate benthic cover determination as in other similar surveys in Cyprus [30,31]. Between three and five photo-frames (13 cm × 19 cm) per boulder (n = 8) were taken of the communities of organisms exposed on the upper (lit) surface. From the pool of photos, the ones with less distortion were selected and aggregated per survey for the analysis (April 2021 = 30 photos, July 2021 = 21, and February 2024 = 25). The percentage of benthic cover was calculated using the photoQuad image processing software [32] with a systematic layout of 200 points.
Sessile organisms (>1 mm) were identified as belonging to the lowest taxonomic group or as morphospecies, and percentage cover was classified into ten main categories: Porifera, Polychaetes (serpulids), Mollusca, Macroalgae, Foraminifera, Cyanobacteria, Corals (Scleractinia), Calcareous Algae, Bryozoa, and Brachiopoda. Diversity among the surveys (2016, 2021, and 2024) was examined using the Shannon–Wiener diversity index (H’), species richness (S), and Pielou’s evenness (P). Statistical analysis for the comparison of the community structures across the five surveys was conducted using the “vegan” package in the R software version 2.5-6 [33]. Phyla and species compositions were clustered into single matrices and plotted using Non-Metric Multi-Dimensional Scaling (nMDS) based on Bray–Curtis dissimilarity (α = 0.05), with a stress value of 0.1 (k = 3, trymax = 500). To test whether the centroids of the clusters from the nMDS differed, a Permutational Multivariate Analysis of Variance using Distance Matrices known as “Adonis” was performed (permutations = 1000, method = Bray, and α = 0.05). To identify which of the nine benthic categories contributed to the separation of the clusters, a MANOVA and an Analysis of Variance (AOV) were run (α = 0.05). Tukey post hoc tests were run to examine which of the surveys were significantly different from the others, both for phyla and species composition.
3. Results
3.1. Anchor and Chain Destruction
Evidence of the anchor and chain damage caused by the high-tonnage vessel was observed within an area of about 2500 m2. Numerous (>40) detached, broken, and overturned boulders were found, some showing sciaphilic communities and coralligenous concretions on the overturned side (Figure 2a). The communities from a few of the massive boulders (from 5 m3 to 11 m3) that were not overturned after being dragged along the bottom (Figure 2b) were crushed or heavily damaged during the displacement of the boulders. Other detached boulders (from 1 m3 to 3 m3) were found resting upside-down near a scarp on the rocky bottom (Figure 2c). The scarring of the rocky seafloor was ubiquitous in the impacted area (Figure 2d), as well as in the fragmentation, which produced slabs and left sockets on other rocks and the platforms (Figure 2e). The damage reached the P. oceanica meadows at the periphery of the boulder field (25–30 m depth), where deep scouring (Figure 2f) uprooted sections of the living mat of P. oceanica and extended further into the sandy bottom.
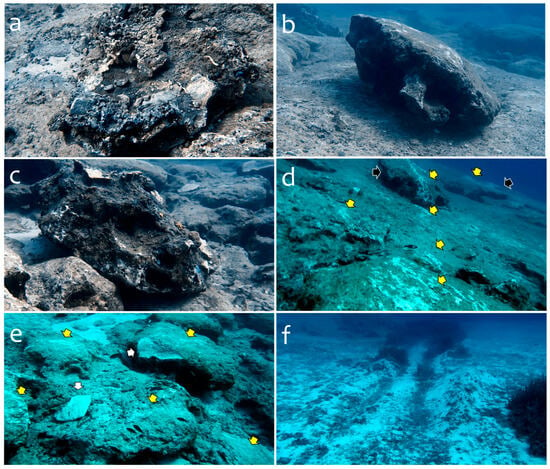
Figure 2.
Examples of detached and broken boulders, scarring, and scouring from anchor and chain damage; 2021 surveys. (a) A broken-off boulder (ca. 2 m3) resting upside-down with sciaphilic communities exposed directly to the sunlight (high irradiance levels). (b) Detached large boulder (ca. 11 m3) that was dragged ca. 20 m from its original position, showing scars on the upper surface from an anchor chain; coralligenous concretions of this boulder were not exposed but crushed and heavily damaged by the dragging. (c) Detached boulder (ca. 3.2 m3) resting upside-down about 10 m from its original position on a section of the bottom with a scarp. (d) General view at the periphery of the boulder field; detached boulders (black arrows); scarring (yellow arrows); distance between the two boulders is about 3 m; scale varies in this perspective. (e) Scars (yellow arrows) and socket and slab (white arrows) from the deepest sections of the boulder field. (f) Extended anchor scouring of sandy bottom and Posidonia oceanica meadows at the deep end of the boulder field.
3.2. Substrate Cover
The indices revealed that the community composition for the 2021 and 2024 surveys had a greater overall diversity compared to 2016 (H′ = 1.603, S = 12, and p = 0.645). The survey with the highest diversity was February 2021 (H′ = 2.717, S = 41, and p = 0.732), which was conducted within one week after the destruction, indicating that after the impact, there was an increase in the total number of species present in the area, as well as a more equally distributed abundance.
Moreover, the nMDS and Adonis for both the phyla (R2 = 0.31, p = 0.001) and species (R2 = 0.24; p = 0.001) categories show that there was a significant difference in the community composition among the surveys (Figure 3). In both cases, the differences were between the 2016 survey, which was conducted before the destruction, and the rest of the surveys, which were conducted afterward, at different timeframes (post hoc p < 0.05).
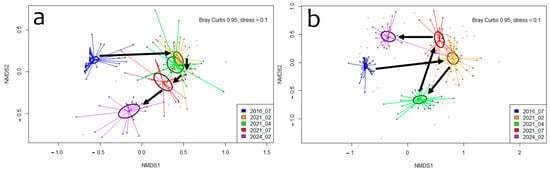
Figure 3.
Two-dimensional representations of a three-dimensional metaMDS ordination with a 95% Bray–Curtis dissimilarity comparing the community composition of the surveyed communities in the phyla (a) and species (b) categories. Arrows show the temporal sequence of the surveys (YYYY_MM).
All substrate categories contributed to the distinction of the community composition of the surveys before and after the destruction (analysis of variance): macroalgae (F = 100.65, df = 4, p < 0.001), calcareous algae (F = 61.48, df = 4, p < 0.001), Cyanobacteria (F = 45.51, df = 4, p < 0.001), Mollusca (F = 12.32, df = 4, p < 0.001), Foraminifera (F = 66.42, df = 4, p < 0.001), Porifera (F = 59.29, df = 4, p < 0.001), Polychaete (F = 24.62, df = 4, p < 0.001), Bryozoan (F = 20.57, df = 4, p < 0.001), corals (F = 127.72, df = 4, p < 0.001), and Brachiopoda (F = 3.03, df = 4, p = 0.02).
The percentage of cover in the substrate shows before- and after-impact trends according to the category (Figure 4, Table A1), for example, the dramatic reduction in calcareous algae that were abundant before the impact, which went from 46 ± 16.6% (unidentified crustose coralline algae thriving on exposed-well-lit surfaces) to 13.1 ± 13.3% (Mesophyllum spp. and Peyssonnelia sp.; dim-environment); the reduction in macroalgae shortly after the impact (from 49 ± 18.1% to 5.5 ± 9.4%); and the subsequent rapid bloom during the third survey, five months after the impact (44 ± 15.5%), reaching the highest percentage of cover, on average, in the last survey in 2024 (>70%). Corals appeared (Madracis pharensis; dim environment) one week after the impact—from 0% in 2016 to 28.1 ± 11%—on the overturned boulders and their subsequent decline over the next sampling surveys, followed by their total disappearance due to mortality in 2024. Sponges showed a similar trend with an increase in cover after the impact of the vessel, from 3.2 ± 10.3% (e.g., Ircinia sp.) in 2016 to between 25% and 28% (both corresponding to species mainly from the dim environment: Spirastrella cunctatrix, for example), as well as 6.3% in 2024 (Cliona sp., exposed environments). Foraminifera also followed the same trend shown by sponges. The disc-shaped Amphistegina sp. (exposed environments) was the only species in 2016 and 2024, whereas Miniacina miniacea (dim environment) was the only one a week after the destruction. Lastly, the cyanobacterial bloom that appeared during the second sampling after the destruction (from 0% in 2016) reached its highest percentage five months later (24.9 ± 17.3%) and coincided with necrosis and organism mortality.
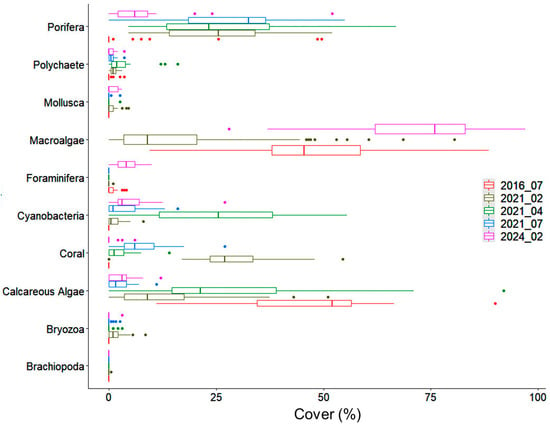
Figure 4.
Box plot of percentages of cover in ten substrate categories according to survey dates (YYYY_MM). Displayed are the minimum and maximum points (ends of whiskers), the interquartile range (boxes), the median (horizontal line), and the outliers (points). The earliest survey is indicated at the bottom of each of the categories in red, and the last survey is indicated at the top of each of the substrate categories in pink.
The necrosis mentioned above was widespread after the first survey in February 2021 (Figure 5); the most affected groups, in decreasing order, were sponges, corals, and calcareous algae (mainly Mesophyllum spp.). Interestingly, Chondrosia reniformis, a sponge species that showed small patches of necrosis, started disappearing from several boulders within a few months, leaving behind empty spaces strikingly similar to feeding or bite scars left by fish (Figure 5b) or displaying missing sections or bite wounds. Similar damage and scars were observed where corals and calcareous algae occupied the substrate. The spread of mortality was fast until the third sampling (June 2021); afterward, dense mats of macroalgae and mucilage aggregates (Figure 5c,d) prevented the inspection and evaluation of the substratum categories.
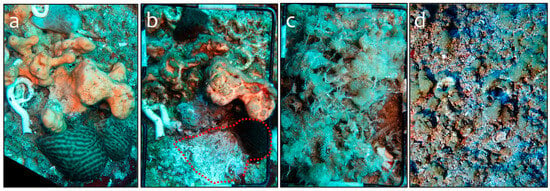
Figure 5.
Examples of the evolution of the substrate cover on the exposed (lit, high irradiance levels) surface of the overturned boulders. (a) Distinctive sciaphilic community; February 2021. (b) Widespread necrosis and cyanobacterial blooms; the absence of a sponge (Chondrosia reniformis) in panel (a) is indicated by a dotted line; April 2021. (c) Bloom of macroalgae including mucilaginous species; June 2021. (d) Heavy colonization by foraminifera, probably Amphistegina sp. (miniscule pinkish dots in the photo), and mucilaginous aggregates, probably Phaeocystis giraudii; February 2024.
4. Discussion
We have documented the dramatic changes in the seascape topography and mortality of sciaphilic communities caused by the anchoring gear of a high-tonnage vessel. Given the current state of global changes and fast local turnover of ecosystems and species, the need to document and understand the role of disturbances is essential [34]. Dramatic ecological changes are happening within the lifespans of researchers. Now more than ever, pre-disturbance data, such as the data in this study, is essential. Because we had benthic information for the boulder field before it was wrecked, we can assess the extent of changes indicated by the increase in diversity and species cover after the impact. In this case, the temporal or short-lived increase was due to the exposure of prosperous sciaphilic communities established in the dim environments below the boulders. Species not adapted to high irradiance levels may experience cellular damage and disruption to metabolic processes, such as photosynthesis [7,35,36,37]. The anthropogenic stress and its consequences were easily recognized. However, this is not the norm; in most cases, there is a juxtaposition of stressors, making the separation of each one’s role difficult [38]. For example, acute (pulse) and chronic (press) stressors [39] are no longer identifiable (if they ever were easy to separate) as “natural” and “anthropogenic”, respectively.
The scale of the acute and catastrophic disturbance to the seascape rearrangement and the destruction observed in this study is comparable to geological changes, such as those produced by earthquakes that have deteriorated and devastated benthic communities (coral reefs). The detachment and collapse of reef framework sections and blocks with massive coral colonies attached were documented in 1991 after a Mw 7.6 earthquake in Central America [40,41], and even now, more than 30 years later, the reef structure still shows the effect of the impacts. In the present case study, there has not been a major loss of habitat complexity as observed when storms and anchoring gear destroy biogenic frameworks such as coral reefs, by crushing and flattening the substrate. The majority of the boulders in MPA Cape Greco were uprooted and dragged over the bottom until they reached a new resting place; fragmentation and breakage on the rocky platform and smaller boulders also happened but on a smaller scale. We propose that, due to the large dimensions of many of the overturned boulders (between 5 and 11 m3), their position will not change; the boulders seem to have stabilized thanks to their own weights. If there are no other similar disturbances, such as the one documented here, sciaphilic communities and coralligenous assemblages may start to recolonize and develop in new spaces and crannies below boulders. Nevertheless, given the slow growth rate of coralligenous species [7,42,43], changes in the community structure are expected, at least during the initial phases of recruitment when opportunistic species colonize the substrate rapidly [7,44], as documented in this study.
There are other significantly harmful stressors that are very likely to interact and affect the recruitment and development of those communities. For example, Marine Heat Waves (MHWs) and Alien Invasive Species (AISs) are two significant forces of ecological change [1,45] that are already affecting the marine habitats of the Levantine Sea in the eastern Mediterranean, a region considered a significant climate change and AIS hotspot [46,47]. During consecutive or prolonged MHWs, mass mortalities of coralligenous and numerous other species have been recorded in Cyprus [1,48] and are associated with long-term changes in the community structure of benthic organisms [1]. On the other hand, Cyprus’ marine habitats are teeming with AISs, established and new, which compete with other Mediterranean species and affect ecological processes [49].
The sciaphilic communities and coralligenous concretions of MPA Cape Greco that survived the crushing caused by the breaking and turning over of massive boulders were unnaturally directly exposed to sunlight (high irradiance levels). These communities, which are adapted to and thrive in dim environments of low irradiance levels [7,38], underwent rapid degradation (necrosis) with widespread mortality and were also affected by the depredation of sponges, corals, calcareous algae, and other calcifying species. Feeding scars suggest that it was fish that scraped off the biogenic crusts and organisms and left the bite wounds. One particular sponge, C. reniformis, was intensively targeted, and this sponge has been reported among the species consumed by the common Mediterranean white seabream Diplodus sargus [50].
The recovery of benthic communities after anchor damage seems to be a generally slow process. Even in tropical areas, coral recovery may take years or decades after such damage [21], and those are species adapted to high-irradiance levels, in contrast to our study case. Three years after the high-tonnage vessel dropped the anchor gear inside MPA Cape Greco, the communities on the well-lit surface of the boulders appear to be dominated by mucilaginous aggregates, macroalgae, and cyanobacteria. The ultimate fate of the sciaphilic communities, as recorded during the last survey in 2024, was total disappearance (i.e., 100% mortality), leaving the space free for a new benthic community.
The level of damage in the studied area surpassed the expected impact of any other ecological or biological agent of change that would have interacted with and affected the sciaphilic community of the boulder field at more than 25 m of depth. This type of anthropogenic disturbance can be averted with measures aiming to manage boat trafficking and anchoring within MPA Cape Greco and with designated sheltering areas according to a risk assessment evaluation on the eventuality of the presence of sensitive habitats [51,52]; for that purpose, high-resolution mapping of benthic habitats is fundamental [53]. Given that the benthic habitats in MPA Cape Greco have been mapped [27], identifying sheltering areas that can be used under “unexpected circumstances” [53], such as severe storms, should be implemented as a management action to reduce the likelihood of anchoring damage. However, the anchoring process in these designated areas must be policed by MPA managers to ensure adherence to regulations [53].
5. Conclusions
This study presents an extreme case of mechanical disturbance that constituted an unprecedented mortality agent in the study area. The anchoring of a high-tonnage vessel inside an MPA during a very strong storm produced dramatic changes in the seafloor topography. The damage consisted of the dislodgement of large boulders, the fragmentation of the rocky substrate, and scouring. A number of boulders were left upside-down, exposing the biological communities that thrive in the dark environments below the boulders to high irradiance levels. Starting a few days after the event (February 2021), repeated assessments of the percentage of cover in these communities tracked their slow decline due to several agents of mortality, including depredation, necrosis, smothering by mucilage aggregates, and cyanobacterial blooms. The total disappearance of the original community was documented during the last survey in February 2024, suggesting that sciaphilic communities are extremely vulnerable to this type of disturbance, which exposes them to unnatural conditions. With this information, we aim to contribute to the protection of the marine habitats of Cyprus by informing the relevant stakeholders of the consequences of this type of damage, which could be prevented with the enforcement of designated areas for anchoring under unexpected conditions. It is in our best interest to ensure that this study will not end up becoming another ecological post-mortem report without lessons to learn.
Author Contributions
Conceptualization, C.J. and A.P.; methodology, C.J.; formal analysis, C.J., M.P. and V.R.; investigation, C.J., A.P., M.P. and V.R.; data curation, C.J. and M.P.; writing—original draft preparation, C.J. and M.P.; writing—review and editing, C.J., A.P., M.P. and V.R.; supervision, C.J.; project administration, C.J. and A.P.; funding acquisition, A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This study received funding from ProtoMedea (MARE/2014/41 [SI2.721917]) and LIFE-IP (Physis LIFE18 IPE/CY/000006).
Data Availability Statement
The data presented in this study are available upon request from the corresponding authors because they are part of an ongoing study about mass mortalities associated with Marine Heat Waves in Cyprus and the Levantine Sea.
Acknowledgments
We thank the funders for supporting this study and Pantelis Patsalou, Marios Papageorgiou and Christiana Tourapi for their contributions to the field and administrative work, respectively.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript.
| MPA | Marine Protected Area |
Appendix A
Meteorological information (daily surface air pressure and wind speed) from January to February 2021 at MPA Cape Greco is shown in Figure A1, and the substrate percentage of cover in the main categories discussed in the text is shown in Table A1.
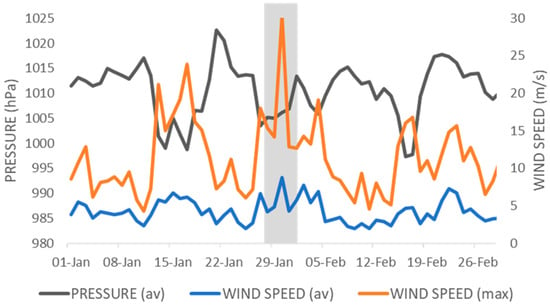
Figure A1.
Daily surface air pressure and wind speed in January and February 2021 (average = av; maximum = max). Shaded area indicates the low-pressure period when the highest wind gusts were recorded and the high-tonnage vessel was sheltering and anchoring inside the study area. Data were taken from an automatic weather station inside MPA Cape Greco (Department of Meteorology, Republic of Cyprus).

Table A1.
Percentage of cover (±standard deviation) of nine major categories of substrate that were present in more than one survey. Brachiopoda is excluded (one survey; <0.1%).
Table A1.
Percentage of cover (±standard deviation) of nine major categories of substrate that were present in more than one survey. Brachiopoda is excluded (one survey; <0.1%).
| Category | 2016 July | 2021 February | 2021 April | 2021 June | 2024 February |
|---|---|---|---|---|---|
| Macroalgae | 49.0 ± 18.1 | 9.2 ± 4.9 | 5.5 ± 9.4 | 44.0 ± 15.5 | 74.1 ± 1.8 |
| Calcareous Algae | 46.0 ± 16.7 | 13.1 ± 13.3 | 27.9 ± 19.7 | 2.4 ± 2.9 | 3.3 ± 1.4 |
| Cyanobacteria | 0.0 | 1.3 ± 1.7 | 24.9 ± 17.3 | 3.5 ± 4.5 | 5.6 ± 4.6 |
| Mollusca | 0.0 | 0.7 ± 1.2 | 0.1 ± 0.4 | 0.1 ± 0.5 | 0.9 ± 0 |
| Foraminifera | 0.6 ± 1 | 0.1 ± 0.2 | 0.0 | 0.0 | 4.3 ± 0 |
| Porifera | 3.2 ± 10.3 | 26.2 ± 14 | 25.6 ± 15.8 | 27.9 ± 16.8 | 6.3 ± 0 |
| Polychaeta | 0.2 ± 0.6 | 1.1 ± 0.8 | 3.0 ± 3.9 | 0.8 ± 1 | 0.4 ± 0 |
| Bryozoa | 0.0 | 1.5 ± 2 | 0.3 ± 0.8 | 0.3 ± 0.7 | 0.1 ± 0 |
| Coral | 0.0 | 28.1 ± 11 | 2.2 ± 2.9 | 7.5 ± 6.1 | 0.2 ± 0 |
References
- Garrabou, J.; Gómez-Gras, D.; Medrano, A.; Cerrano, C.; Ponti, M.; Schlegel, R.; Bensoussan, N.; Turicchia, E.; Sini, M.; Gerovasileiou, V.; et al. Marine heatwaves drive recurrent mass mortalities in the Mediterranean Sea. Glob. Change Biol. 2022, 28, 5708–5725. [Google Scholar] [CrossRef]
- Danovaro, R.; Fanelli, E.; Aguzzi, J.; Billett, D.; Carugati, L.; Corinaldesi, C.; Dell’Anno, A.; Gjerde, K.M.; Jamieson, A.J.; Kark, S.; et al. Ecological variables for developing a global deep-ocean monitoring and conservation strategy. Nat. Ecol. Evol. 2020, 4, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Paulus, E. Shedding Light on Deep-Sea Biodiversity-A Highly Vulnerable Habitat in the Face of Anthropogenic Change. Font. Mar. Sci. 2021, 8, 667048. [Google Scholar] [CrossRef]
- Danovaro, R.; Aronson, J.; Cimino, R.; Gambi, C.; Snelgrove, P.V.; Van Dover, C. Marine ecosystem restoration in a changing ocean. Restor. Ecol. 2021, 29, e13432. [Google Scholar] [CrossRef]
- Newman, E.A. Disturbance Ecology in the Anthropocene. Front. Ecol. Evol. 2019, 7, 147. [Google Scholar] [CrossRef]
- Edmunds, P.J. Decadal-scale time series highlight the role of chronic disturbances in driving ecosystem collapse in the Anthropocene. Ecology 2024, 105, e4360. [Google Scholar] [CrossRef]
- Ballesteros, E. Mediterranean coralligenous assemblages: A synthesis of present knowledge. Oceanogr. Mar. Biol. Annu. Rev. 2006, 44, 123–195. [Google Scholar] [CrossRef]
- Puig, P.; Canals, M.; Company, J.; Martín, J.; Amblas, D.; Lastras, G.; Palanques, A.; Calafat, A.M. Ploughing the deep sea floor. Nature 2012, 489, 286–289. [Google Scholar] [CrossRef]
- Forrester, G.E.; Flynn, R.L.; Forrester, L.M.; Jarecki, L.L. Episodic disturbance from boat anchoring is a major contributor to, but does not alter the trajectory of long-term coral reef decline. PLoS ONE 2015, 10, e0144498. [Google Scholar] [CrossRef]
- Broad, A.; Rees, M.J.; Davis, A.R. Anchor and chain scour as disturbance agents in benthic environments: Trends in the literature and charting a course to more sustainable boating and shipping. Mar. Poll. Bull. 2020, 161, 111683. [Google Scholar] [CrossRef]
- European Commission. Commission Decision 2010/477/EU of 1 September 2010 on Criteria and Methodological Standards on Good Environmental Status of Marine Waters. Available online: http://data.europa.eu/eli/dec/2010/477(2)/oj (accessed on 30 May 2025).
- Davis, A.R.; Broad, A.; Steele, C.; Woods, C.; Przeslawki, R.; Nicholas, W.A.; Maher, W.; Krikowa, F.; Morris, B.; Ingleton, T.C.; et al. Dragging the chain: Anchor scour impacts from high-tonnage commercial vessels on a soft bottom macrobenthic assemblage. Front. Conserv. Sci. 2025, 6, 1487428. [Google Scholar] [CrossRef]
- Davis, G.E. Anchor damage to a coral reef on the coast of Florida. Biol. Conserv. 1977, 11, 29–34. [Google Scholar] [CrossRef]
- Dustan, P.; Halas, J.C. Changes in the reef-coral community of Carysfort Reef, Key Largo, Florida: 1974 to 1982. Coral Reefs 1987, 6, 91–106. [Google Scholar] [CrossRef]
- Smith, S.R. Reef damage and recovery after ship groundings on Bermuda. In Proceedings of the 5th International Coral Reef Symposium, Tahiti, 27 May–1 June 1985; Volume 6, pp. 497–502. [Google Scholar]
- Gittings, S.R.; Bright, T.J.; Choi, A.; Barnett, R.R. The recovery process in a mechanically damaged coral reef community: Recruitment and growth. In Proceedings of the 6th International Coral Reef Symposium, Townsville, QL, Australia, 8–12 August 1988; Volume 2, pp. 225–230. [Google Scholar]
- Creed, J.C.; Amado Filho, G.M. Disturbance and recovery of the macroflora of a seagrass (Halodule wrightii Ascherson) meadow in the Abrolhos-an experimental evaluation anchor damage. J. Exp. Mar. Biol. Ecol. 1999, 235, 285–306. [Google Scholar] [CrossRef]
- Schulze, I.; Schönke, M.; Feldens, P.; Papenmeier, S. Temporal evolution of anchor tracks on a silty seafloor (Eckernförde Bay/Baltic Sea). Front. Remote Sens. 2025, 6, 1576192. [Google Scholar] [CrossRef]
- Watson, S.J.; Ribó, M.; Seabrook, S.; Strachan, L.J.; Hale, R.; Lamarche, G. The footprint of ship anchoring on the seafloor. Sci. Rep. 2022, 12, 7500–7511. [Google Scholar] [CrossRef]
- Roberts, C.M. The Unnatural History of the Sea; Island Press: Washington, DC, USA, 2007; p. 456. [Google Scholar]
- Rogers, C.; Gerrison, V.H. Ten years after the crime-Lasting effects of damage from a cruise ship anchor on a coral reef in St. John, U.S. Virgin Island. Bull. Mar. Sci. 2001, 69, 793–803. [Google Scholar]
- Kininmonth, S.; Lemm, S.; Malone, C.; Hatley, T. Spatial vulnerability assessment of anchor damage within the Great Barrier Reef World Heritage Area, Australia. Ocean. Coast. Manag. 2014, 100, 20–31. [Google Scholar] [CrossRef]
- Davis, A.R.; Broad, A.; Gullett, W.; Reveley, J.; Steele, C.; Schofield, C. Anchors away? The impacts of anchor scour by ocean-going vessels and potential response options. Mar. Policy 2016, 73, 1–7. [Google Scholar] [CrossRef]
- Flynn, R.L.; Forrester, G.E. Boat anchoring contributes substantially to coral reef degradation in the British Virgin Islands. PeerJ 2019, 7, e7010. [Google Scholar] [CrossRef]
- Hernández-Delgado, E.A. Long-Term Persistence of propeller and anchor damage to seagrass canopy and demersal biodiversity in Puerto Rico. Open J. Ecol. 2023, 13, 671–710. [Google Scholar] [CrossRef]
- Boero, F.; Danovaro, R.; Orombelli, G. Introduction-Changes and Crises in the Mediterranean Sea: Current problems. Rend. Fis. Acc. Lincei 2018, 29, 511–513. [Google Scholar] [CrossRef]
- BISE–Biodiversity Information System for Europe. Available online: https://biodiversity.europa.eu/sites/natura2000/CY5000005 (accessed on 3 February 2025).
- Fichaut, B.; Suanez, S. Quarrying, transport and deposition of cliff-top storm deposits during extreme events: Banneg Island, Brittany. Mar. Geol. 2011, 283, 36–55. [Google Scholar] [CrossRef]
- Jimenez, C.; Petrou, A.; Papatheodoulou, M.; Resaikos, V. No one is safe: Destruction of coralligenous concretions and other benthic sciaphilic communities in the MPA Cape Greco (Cyprus). In Proceedings of the 4th Mediterranean Symposium on the conservation of Coralligenous & other Calcareous Bio-Concretions, Genoa, Italy, 20–21 September 2022; Available online: https://spa-rac.org/en/publication/1664/proceedings-of-the-4th-mediterranean-symposium-on-the-conservation-of-the-coralligenous-and-other-calcareous-bio-concretions (accessed on 7 June 2025).
- Jimenez, C.; Andreou, V.; Evriviadou, M.; Munkes, B.; Hadjioannou, L.; Petrou, A.; Abu Alhaija, R. Epibenthic communities associated with unintentional artificial reefs (modern shipwrecks) under contrasting regimes of nutrients in the Levantine Sea (Cyprus and Lebanon). PLoS ONE 2017, 12, e0182486. [Google Scholar] [CrossRef]
- Nardi, A.; Resaikos, V.; Papatheodoulou, M.; Di Carlo, M.; Vedhanarayanan, H.; Regoli, F.; Gorbi, S.; Jimenez, C. Cellular adaptations of the scleractinian coral Madracis pharensis to chronic oil pollution in a Mediterranean shipwreck. Front. Mar. Sci. 2024, 11, 1330894. [Google Scholar] [CrossRef]
- Trygonis, V.; Sini, M. PhotoQuad: A dedicated seabed image processing software, and a comparative error analysis of four photoquadrat methods. J. Exp. Mar. Biol. Ecol. 2012, 424–425, 99–108. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package, R Package Version 2.5-6. Available online: https://CRAN.R-project.org/package=vegan (accessed on 15 May 2025).
- Turner, M.G.; Calder, W.J.; Cumming, G.S.; Hughes, T.P.; Jentsch, A.; LaDeau, S.L.; Lenton, T.M.; Shuman, B.N.; Tu-retsky, M.R.; Ratajczak, Z.; et al. Climate change, ecosystems and abrupt change: Science priorities. Phil. Trans. R. Soc. 2020, 375, 20190105. [Google Scholar] [CrossRef]
- Häder, D.-P. Effects of solar UV-B radiation on aquatic ecosystems. Adv. Space Res. 2000, 26, 2029–2040. [Google Scholar] [CrossRef]
- Pinna, F.; Caragnano, A.; Piazzi, L.; Ragazzola, F.; Stipcich, P.; Rindi, F.; Ceccherelli, G. The Mediterranean bioconstructor Lithophyllum stictiforme shows adaptability to future warming. Front. Mar. Sci. 2022, 9, 930750. [Google Scholar] [CrossRef]
- Krieger, E.C.; Nelson, W.A.; Grand, J.; Le Ru, E.C.; Bury, S.J.; Cossais, A.; Davy, S.K.; Cornwall, C.E. The role of irradiance in controlling coralline algal calcification. Limnol. Oceanogr. 2023, 68, 1269–1284. [Google Scholar] [CrossRef]
- Bevilacqua, S.; Airoldi, L.; Ballesteros, E.; Benedetti-Cecchi, L.; Boero, F.; Bulleri, F.; Cebrian, E.; Cerrano, C.; Claudet, J.; Colloca, F.; et al. Chapter One—Mediterranean rocky reefs in the Anthropocene: Present status and future concerns. In Advances in Marine Biology; Sheppard, C., Ed.; Academic Press: Cambridge, MA, USA, 2021; Volume 89, pp. 1–51. [Google Scholar] [CrossRef]
- Bender, E.A.; Case, T.J.; Gilpin, M.E. Perturbation experiments in community ecology: Theory and practice. Ecology 1984, 65, 1–13. [Google Scholar] [CrossRef]
- Cortés, J.; Soto, R.; Jimenez, C.; Astorga, A. Earthquake associated mortality of intertidal and coral reef organisms (Caribbean of Costa Rica). In Proceedings of the 7th International Coral Reef Symposium, Guam, Micronesia, 22–27 June 1992; Volume 1, pp. 239–244. [Google Scholar]
- Cortés, J.; Soto, R.; Jimenez, C. Efectos ecológicos del terremoto de Limón. Rev. Geol. Amer. Cent. 1994, 17, 187–192. [Google Scholar] [CrossRef][Green Version]
- Clark, M.R.; Rowden, A.A.; Schlacher, T.; Williams, A.; Consalvey, M.; Stocks, K.I.; Rogers, A.D.; O’Hara, T.D.; White, M.; Shank, T.M.; et al. The ecology of seamounts: Structure, function, and human impacts. Ann. Rev. Mar. Sci. 2010, 2, 253–278. [Google Scholar] [CrossRef]
- Martin, C.S.; Giannoulaki, M.; De Leo, F.; Scardi, M.; Salomidi, M.; Knittweis, L.; Pace, M.L.; Garofalo, G.; Gristina, M.; Ballesteros, E.; et al. Coralligenous and maërl habitats: Predictive modelling to identify their spatial distributions across the Mediterranean Sea. Sci. Rep. 2014, 4, 5073. [Google Scholar] [CrossRef]
- Teixidó, N.; Casas, E.; Cebrian, E.; Linares, C.; Garrabou, J. Impacts on coralligenous outcrop biodiversity of a dramatic coastal storm. PLoS ONE 2013, 8, e53742. [Google Scholar] [CrossRef] [PubMed]
- Azzuro, E.; Smeraldo, S.; D’Amen, M. Spatio-Temporal Dynamics of Exotic Fish Species in the Mediterranean Sea: Over a Century of Invasion Reconstructed. Glob. Change Biol. 2022, 28, 6268–6279. [Google Scholar] [CrossRef] [PubMed]
- Giakoumi, S.; Guilhaumon, F.; Kark, S.; Terlizzi, A.; Claudet, J.; Felline, S.; Cerrano, C.; Coll, M.; Danovaro, R.; Fraschetti, S.; et al. Space invaders; biological invasions in marine conservation planning. Divers. Distrib. 2016, 22, 1220–1231. [Google Scholar] [CrossRef]
- Zittis, G.; Almazroui, M.; Alpert, P.; Ciais, P.; Cramer, W.; Dahdal, Y.; Fnais, M.; Francis, D.; Hadjinicolaou, P.; Howari, F.; et al. Climate change and weather extremes in the Eastern Mediterranean and Middle East. Rev. Geophys. 2022, 60, e2021RG000762. [Google Scholar] [CrossRef]
- Jiménez, C.; Hadjioannou, L.; Petrou, A.; Nikolaidis, A.; Evriviadou, M.; Lange, M.A. Mortality of the scleractinian coral Cladocora caespitosa during a warming event in the Levantine Sea (Cyprus). Reg. Environ. Change 2016, 16, 1963–1973. [Google Scholar] [CrossRef]
- Huseyinoglu, M.F.; Arda, Y.; Jiménez, C. Manual of Invasive Alien Species in the Eastern Mediterranean; IUCN: Gland, Switzerland, 2023; 53p, Available online: https://portals.iucn.org/library/node/50729 (accessed on 17 May 2025).
- Bertolino, M.; Reboa, A.; Armenio, C.; Castellano, M.; Felline, S.; Terlizzi, A.; Bavestrello, G. Sponges as feeding resource for the white seabream Diplodus sargus (Linnaeus, 1758) from the Mediterranean Sea. Eur. Zool. J. 2024, 91, 1192–1198. [Google Scholar] [CrossRef]
- Byrnes, T.A.; Dunn, R.J.K. Boating- and Shipping-Related Environmental Impacts and Example Management Measures: A Review. J. Mar. Sci. Eng. 2020, 8, 908. [Google Scholar] [CrossRef]
- Mizzi, M.; Deidun, A.; Gauci, A.; Gauci, R. The Impact of Anchoring on Seafloor Integrity: An Integrated Assessment within a Major Bunkering Area of the Maltese Islands. Geographies 2024, 4, 612–629. [Google Scholar] [CrossRef]
- Davis, A.R.; Broad, A.; Small, M.; Oxenford, H.A.; Morris, B.; Ingleton, T.C. Mapping of benthic ecosystems: Key to improving the management and sustainability of anchoring practices for ocean-going vessels. Cont. Shelf Res. 2022, 247, 104834. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).