Enrichment Strategies for Enhanced Food Waste Hydrolysis in Acidogenic Leach Bed Reactors
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrates and Inocula
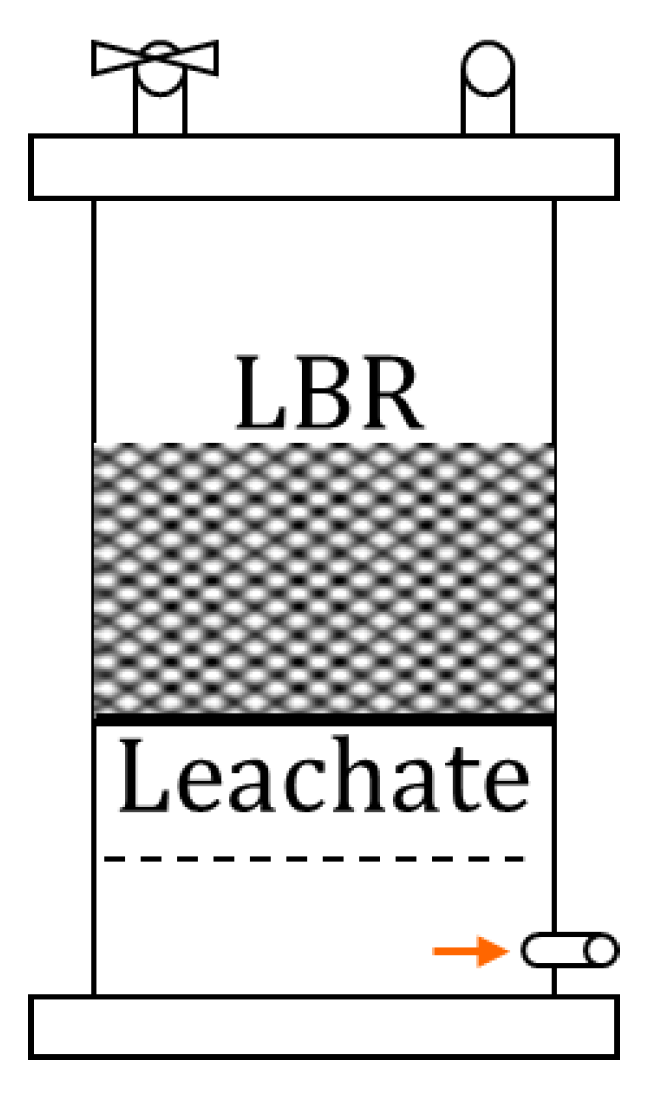
2.2. Experimental Set-Up
2.3. Analytical Methods
2.4. Kinetics Study
2.5. DNA Extraction and High-Throughput 16S rRNA Gene Pyrosequencing
3. Results
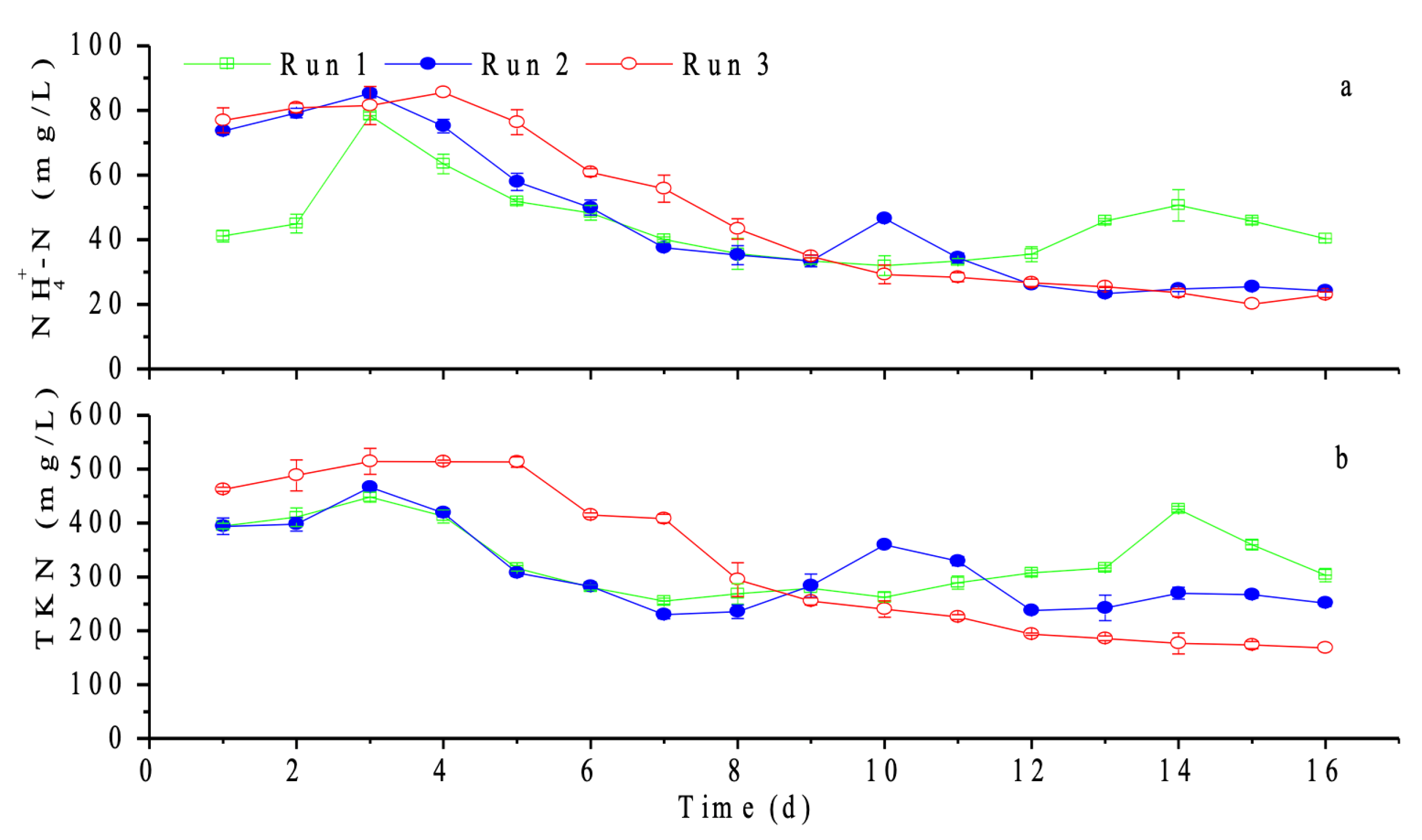
3.1. Performances of the Anaerobic Digestion Process
3.1.1. COD Leaching
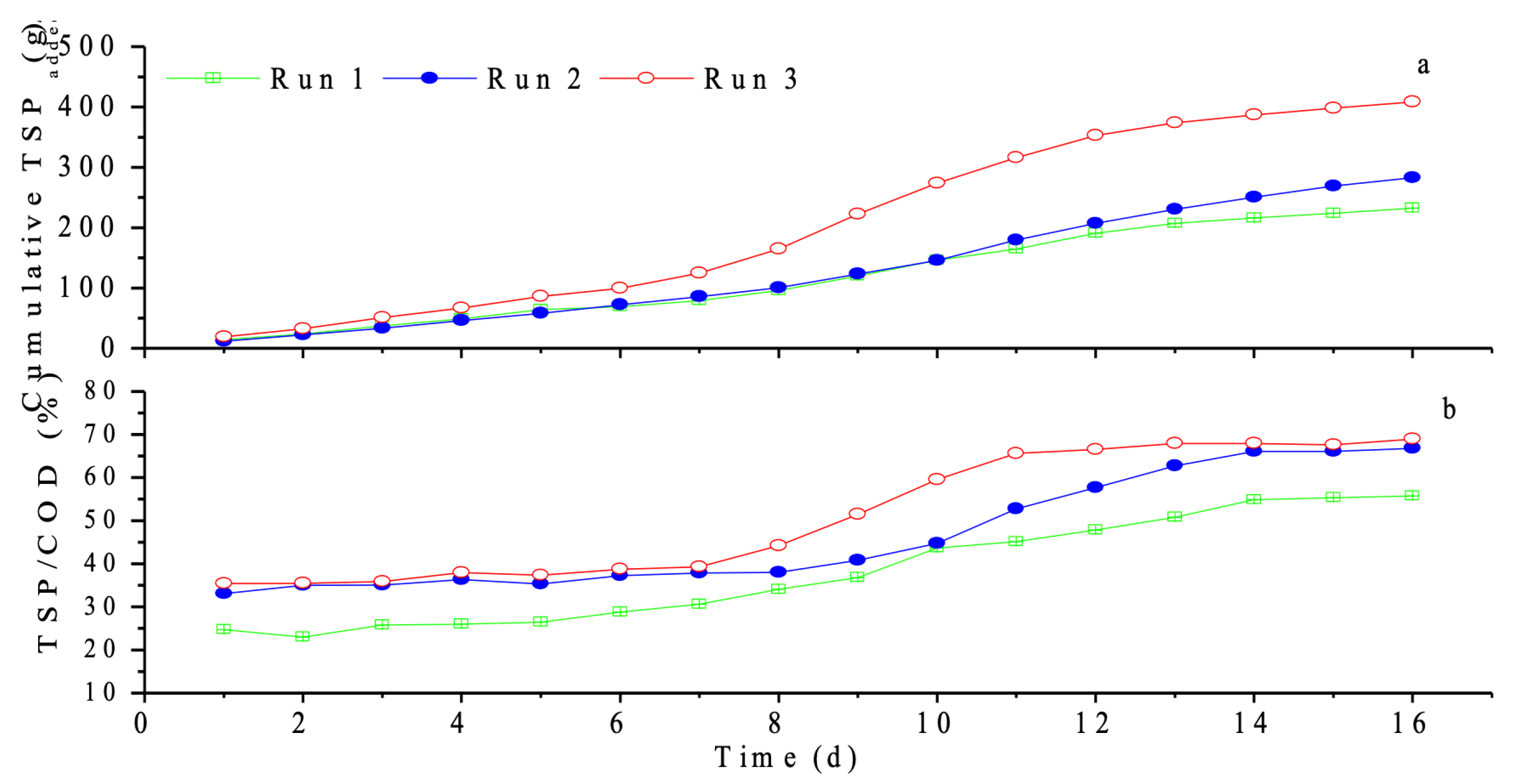
3.1.2. Leaching of Total Soluble Products (TSPs)
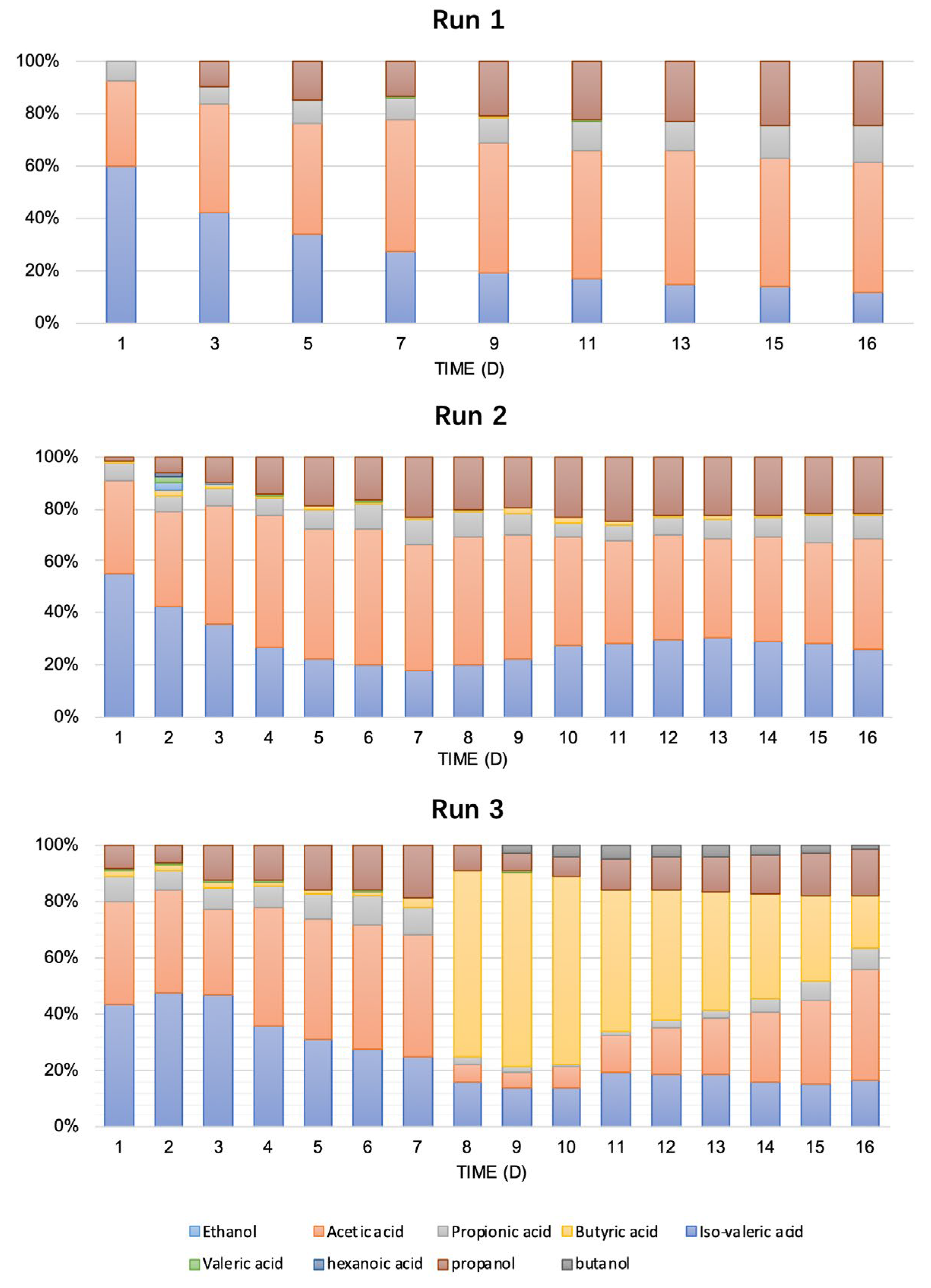
3.1.3. TSP Speciation
3.2. Kinetic Analysis
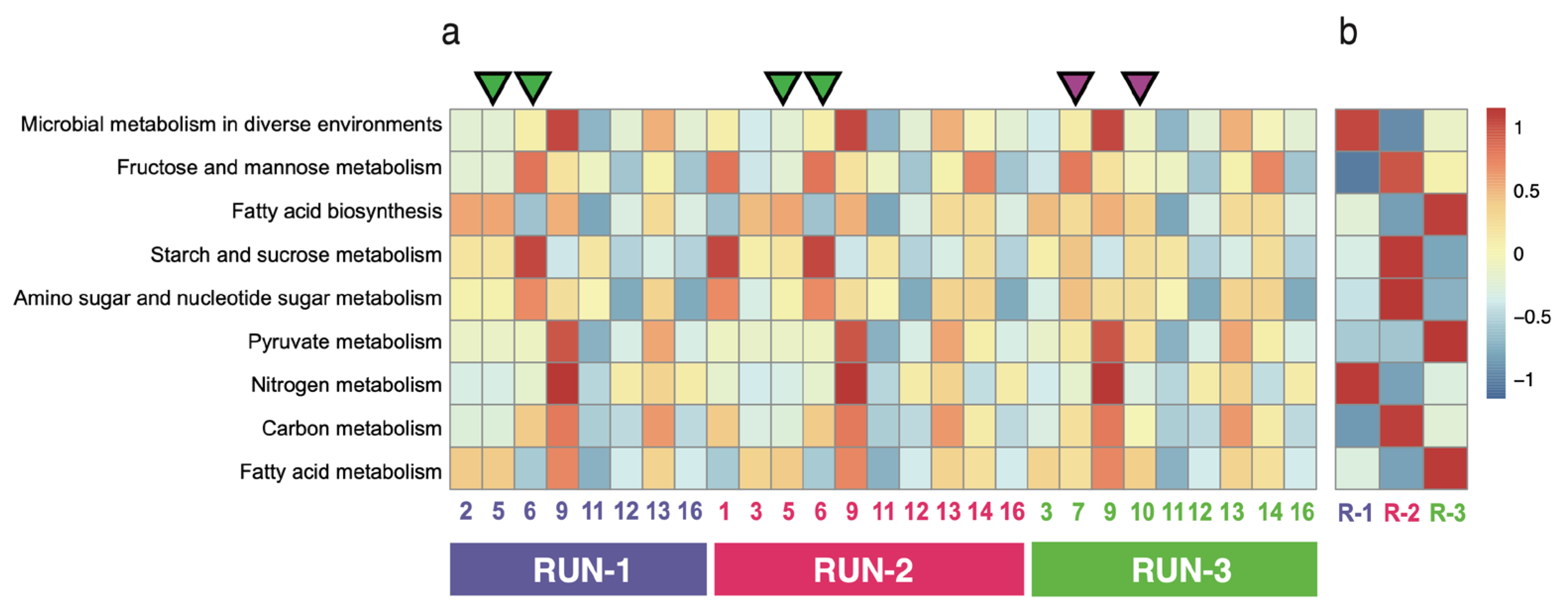
3.3. Bacterial Diversity of Enriched Consortium
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiao, J.; Tao, T.; Shi, Y.; Zhao, J.; Wu, B.; Tai, J.; Xu, M.; Zhang, X.; Peng, Y.; Bi, Z. Megacity’s pathway toward sustainable food waste management and its environmental performance in a developing country: Evidence from Shanghai, China. Sci. Total Environ. 2023, 892, 164706. [Google Scholar] [CrossRef]
- Anand, N.; Palani, S.G. A comprehensive investigation of toxicity and pollution potential of municipal solid waste landfill leachate. Sci. Total Environ. 2022, 838, 155891. [Google Scholar] [CrossRef]
- Hunter, S.M.; Blanco, E.; Borrion, A. Expanding the anaerobic digestion map: A review of intermediates in the digestion of food waste. Sci. Total Environ. 2020, 767, 144265. [Google Scholar] [CrossRef]
- Raketh, M.; Kongjan, P.; Sani, K.; Trably, E.; Cheirsilp, B.; Jariyaboon, R. Biodegradation efficiencies and economic feasibility of single-stage and two-stage anaerobic digestion of desulfated Skim Latex Serum (SLS) by using rubber wood ash. Process Saf. Environ. 2022, 162, 721–732. [Google Scholar] [CrossRef]
- Chen, Z.; Li, D.; Wen, Q. Investigation of hydrolysis acidification process during anaerobic treatment of coal gasification wastewater (CGW): Evolution of dissolved organic matter and biotoxicity. Sci. Total Environ. 2020, 723, 137995. [Google Scholar] [CrossRef]
- Chakraborty, D.; Karthikeyan, O.P.; Selvam, A.; Palani, S.G.; Ghangrekar, M.M.; Wong, J.W. Two-phase anaerobic digestion of food waste: Effect of semi-continuous feeding on acidogenesis and methane production. Bioresour. Technol. 2022, 346, 126–396. [Google Scholar] [CrossRef]
- Lv, Y.; Chang, N.; Li, Y.; Liu, J. Anaerobic co-digestion of food waste with municipal solid waste leachate: A review and prospective application with more benefits. Resour. Conserv. Recycl. 2021, 174, 105832. [Google Scholar] [CrossRef]
- Jiang, M.; Qiao, W.; Wang, Y.; Zou, T.; Lin, M.; Dong, R. Balancing acidogenesis and methanogenesis metabolism in thermophilic anaerobic digestion of food waste under a high loading rate. Sci. Total Environ. 2022, 824, 153867. [Google Scholar] [CrossRef]
- Qi, C.; Zhang, Y.; Jia, S.; Wang, R.; Han, Y.; Luo, W.; Li, G.; Li, Y. Effects of digestion duration on energy efficiency, compost quality, and carbon flow during solid state anaerobic digestion and composting hybrid process. Sci. Total Environ. 2022, 811, 151363. [Google Scholar] [CrossRef]
- Radadiya, P.; Lee, J.; Venkateshwaran, K.; Benn, N.; Lee, H.-S.; Hussain, A. Acidogenic fermentation of food waste in a leachate bed reactor (LBR) at high volumetric organic Loading: Effect of granular activated carbon (GAC) and sequential enrichment of inoculum. Bioresour. Technol. 2022, 361, 127705. [Google Scholar] [CrossRef]
- Rocamora, I.; Wagland, S.T.; Villa, R.; Simpson, E.D.; Fernández, O.; Bajón-Fernández, Y. Dry anaerobic digestion of organic waste: A review of operational parameters and their impact on process performance. Bioresour. Technol. 2020, 299, 122681. [Google Scholar] [CrossRef]
- Jeong, K.; Abbas, A.; Shin, J.; Son, M.; Kim, Y.M.; Cho, K.H. Prediction of biogas production in anaerobic co-digestion of organic wastes using deep learning models. Water Res. 2021, 205, 117697. [Google Scholar] [CrossRef]
- Ruas, G.; López-Serna, R.; Scarcelli, P.G.; Serejo, M.L.; Boncz, M.Á.; Muñoz, R. Influence of the hydraulic retention time on the removal of emerging contaminants in an anoxic-aerobic algal-bacterial photobioreactor coupled with anaerobic digestion. Sci. Total Environ. 2022, 827, 154262. [Google Scholar] [CrossRef]
- Fu, S.F.; Liu, R.; Sun, W.X.; Zhu, R.; Zou, H.; Zheng, Y.; Wang, Z.-Y. Enhancing energy recovery from corn straw via two-stage anaerobic digestion with stepwise microaerobic hydrogen fermentation and methanogenesis. J. Clean. Prod. 2020, 247, 119651. [Google Scholar] [CrossRef]
- Selvam, A.; Xu, S.Y.; Gu, X.Y.; Wong, J.W.C. Food waste decomposition in leachbed reactor: Role of neutralizing solutions on the leachate quality. Bioresour. Technol. 2010, 101, 1707–1714. [Google Scholar] [CrossRef]
- Zhou, H.; Jiang, J.; Zhao, Q.; Wang, Z.; Li, L.; Gao, Q.; Wang, K. Performance of high solids enzymatic hydrolysis and bioethanol fermentation of food waste under the regulation of saponin. Bioresour. Technol. 2023, 387, 129486. [Google Scholar] [CrossRef]
- Wimmer, B.H.; Moraïs, S.; Zalk, R.; Mizrahi, I.; Medalia, O. Phylogenetic diversity of core rumen microbiota as described by cryo-ET. microLife 2023, 4, uqad010. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis; Part 2; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 539–580. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA; American Water Works Association an Water Environment Federation: Denver, CO, USA, 2005. [Google Scholar]
- Vavilin, V.A.; Fernandez, B.; Palatsi, J.; Flotats, X. Hydrolysis kinetics in anaerobic degradation of particulate organic material: An overview. Waste Manag. 2008, 28, 939–951. [Google Scholar] [CrossRef]
- Taheri, E.; Amin, M.M.; Pourzamani, H.; Fatehizadeh, A.; Ghasemian, M.; Bina, B. Comparison of acetate-butyrate and acetate-ethanol metabolic pathway in biohydrogen production. J. Med. Signals Sens. 2018, 8, 101–107. [Google Scholar]
- Yan, B.H.; Selvam, A.; Wong, J.W. Application of rumen microbes to enhance food waste hydrolysis in acidogenic leach-bed reactors. Bioresour. Technol. 2014, 168, 64–71. [Google Scholar] [CrossRef]
- Perman, E.; Schnürer, A.; Björn, A. Serial anaerobic digestion improves protein degradation and biogas production from mixed food waste. Biomass Bioenerg. 2022, 161, 106478. [Google Scholar] [CrossRef]
- Dong, X.; Xin, Y.; Jian, W.; Liu, X.; Ling, D. Bifidobacterium thermacidophilum sp. nov., isolated from an anaerobic digester. Int. J. Syst. Evol. Microbiol. 2000, 50, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Provorov, N.A.; Andronov, E.E.; Kimeklis, A.K.; Onishchuk, O.P.; Igolkina, A.A.; Karasev, E.S. Microevolution, speciation and macroevolution in rhizobia: Genomic mechanisms and selective patterns. Front. Plant Sci. 2022, 13, 1026943. [Google Scholar] [CrossRef] [PubMed]
- Veiga, P.; Bulbarela-Sampieri, C.; Furlan, S.; Maisons, A.; Chapot-Chartier, M.-P.; Erkelenz, M.; Mervelet, P.; Noirot, P.; Frees, D.; Kuipers, O.P. SpxB regulates O-acetylation-dependent resistance of Lactococcus lactis peptidoglycan to hydrolysis. J. Biol. Chem. 2007, 282, 19342–19354. [Google Scholar] [CrossRef]
- Petrov, K.; Urshev, Z.; Petrova, P. L (+)-Lactic acid production from starch by a novel amylolytic Lactococcus lactis subsp. lactis B84. Food Microbiol. 2008, 25, 550–557. [Google Scholar] [CrossRef]
- Huang, M.; Li, X.; Wu, Y.; Meng, X.; Tong, P.; Yuan, J.; Yang, A.; Wu, Z.; Chen, H.; Xiong, C. Potential allergenicity and hydrolysis assessment of bovine casein and β-casein by treatment with lactic acid bacteria. J. Food Biochem. 2022, 46, e14424. [Google Scholar] [CrossRef]

| Parameter | Food Waste | Cow Manure |
|---|---|---|
| Total Solid (TS), % | 39.7 ± 0.01 | 10.2 ± 0.1 |
| Volatile Solid (VS/TS), % | 97.6 ± 0.1 | 89.6 ± 0.01 |
| Total Organic Carbon (TOC), % | 47.0 ± 0.6 | 50.7 ± 0.01 |
| Total Kjeldahl Nitrogen (TKN), g/kg | 20.0 ± 0.1 | 14.0 ± 0.01 |
| COD | Treatment | k (d−1) | SD | R2 |
|---|---|---|---|---|
| Run-1 | 0.30 | 0.001 | 0.99 | |
| Run-2 | 0.36 | 0.001 | 0.99 | |
| Run-3 | 0.56 | 0.001 | 0.99 | |
| TKN | Run-1 | 0.40 | 0.001 | 0.99 |
| Run-2 | 0.43 | 0.002 | 0.96 | |
| Run-3 | 0.46 | 0.002 | 0.96 |
| Phylotype | Closest Classified Relative (% certainty) | Closest Described Bacterium (Accession #) | Identity (%) |
|---|---|---|---|
| Firmicutes | 99 | Clostridium acetobutylicum HP7 (FM994940) | 96 |
| Firmicutes | 100 | Uncultured firmicute bacterium (JF986956) | 97 |
| Proteobacteria | 100 | Uncultured Novosphingobium sp. t301d4 | 99 |
| Proteobacteria | 100 | Sphingomonas sp. AV6C (AF434172) | 98 |
| Firmicutes | 100 | Weissella cibaria (AJ295989) | 99 |
| Firmicutes | 86 | Clostridium acetobutylicum strain HP7(FM994940) | 92 |
| Firmicutes | 99 | Lactobacillus rhamnosus strain NT10 (JN813101) | 91 |
| Firmicutes | 100 | Clostridium sp. BL-22 16S (DQ196626) | 98 |
| Firmicutes | 99 | Clostridium tyrobutyricum (L08062) | 92 |
| Firmicutes | 100 | Lactococcus lactis (AM157424) | 100 |
| Firmicutes | 100 | Lactobacillus jensenii (AF243159) | 99 |
| Firmicutes | 99 | Streptomyces sp. 06-3 (AM889469) | 96 |
| Firmicutes | 100 | Clostridium sporogenes strain CL2 (JF836013) | 97 |
| Firmicutes | 100 | Lactobacillus casei (AJ272201) | 99 |
| Firmicutes | 100 | Uncultured bacterium clone HPR122 (DQ464579) | 95 |
| Actinobacteria | 100 | Bifidobacterium thermacidophilum (AY148470) | 100 |
| Firmicutes | 99 | Clostridium sp. strain Z6 (AY949859) | 97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, L.; Li, Y.; Yang, X.; Zhu, Y.; Yan, B.; Feng, K. Enrichment Strategies for Enhanced Food Waste Hydrolysis in Acidogenic Leach Bed Reactors. Water 2025, 17, 2082. https://doi.org/10.3390/w17142082
Zheng L, Li Y, Yang X, Zhu Y, Yan B, Feng K. Enrichment Strategies for Enhanced Food Waste Hydrolysis in Acidogenic Leach Bed Reactors. Water. 2025; 17(14):2082. https://doi.org/10.3390/w17142082
Chicago/Turabian StyleZheng, Lei, Yuanhua Li, Xiaofang Yang, Yongjuan Zhu, Binghua Yan, and Kejun Feng. 2025. "Enrichment Strategies for Enhanced Food Waste Hydrolysis in Acidogenic Leach Bed Reactors" Water 17, no. 14: 2082. https://doi.org/10.3390/w17142082
APA StyleZheng, L., Li, Y., Yang, X., Zhu, Y., Yan, B., & Feng, K. (2025). Enrichment Strategies for Enhanced Food Waste Hydrolysis in Acidogenic Leach Bed Reactors. Water, 17(14), 2082. https://doi.org/10.3390/w17142082






