Optical, Photocatalytic, and Antibacterial Properties of Sol-Gel Derived Fe Doped SrTiO3 Powders
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis SrFe1−XTiXO3 (X = 1, 0.85) Catalysts
2.3. Catalysts Characterization
2.4. Photocatalytic Activity Tests
2.5. Antibacterial Testing
3. Results and Discussion
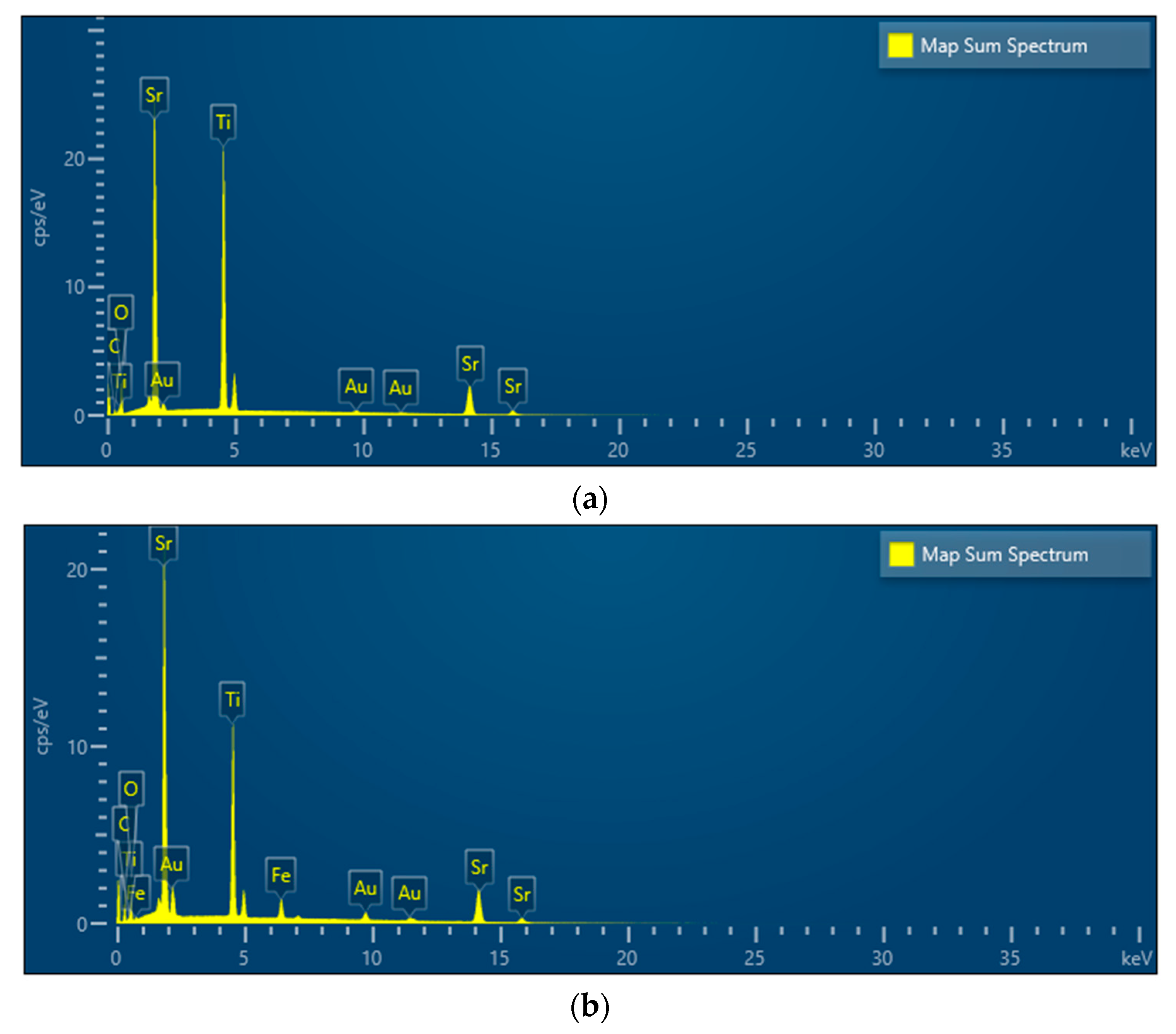
3.1. XRD and SEM Morphology of the Prepared Catalysts
3.2. Low-Temperature Nitrogen Adsorption
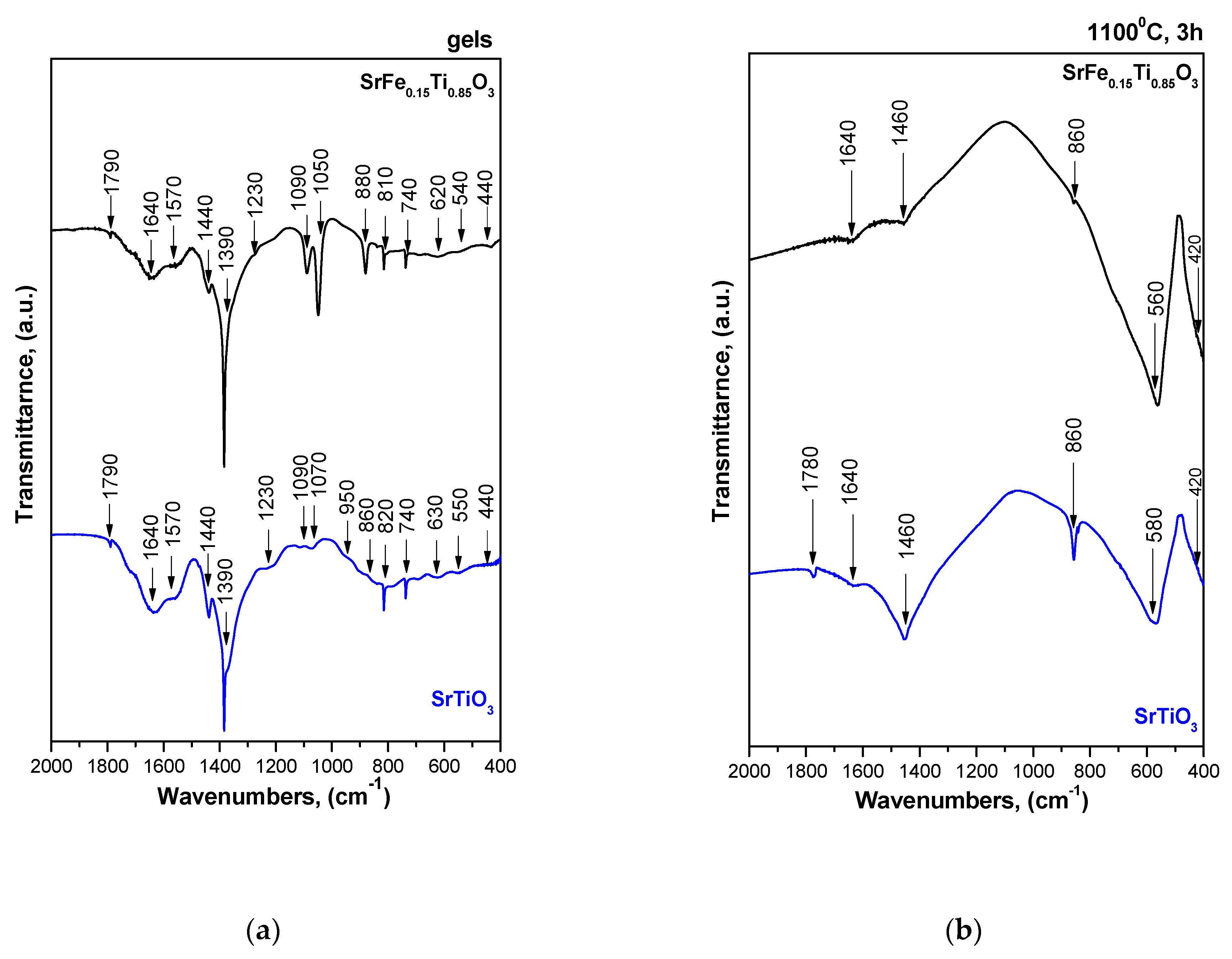
3.3. IR Structural Investigations
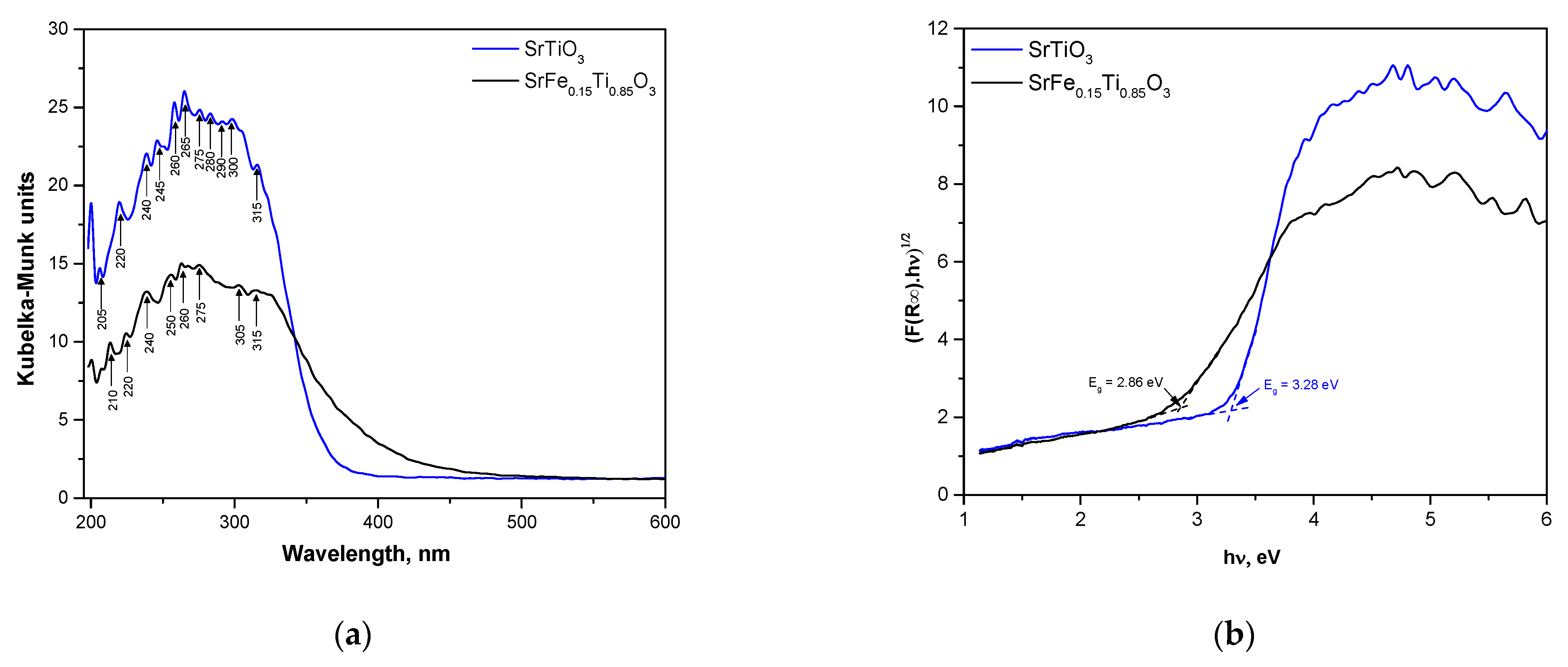
3.4. UV-Vis Spectroscopy
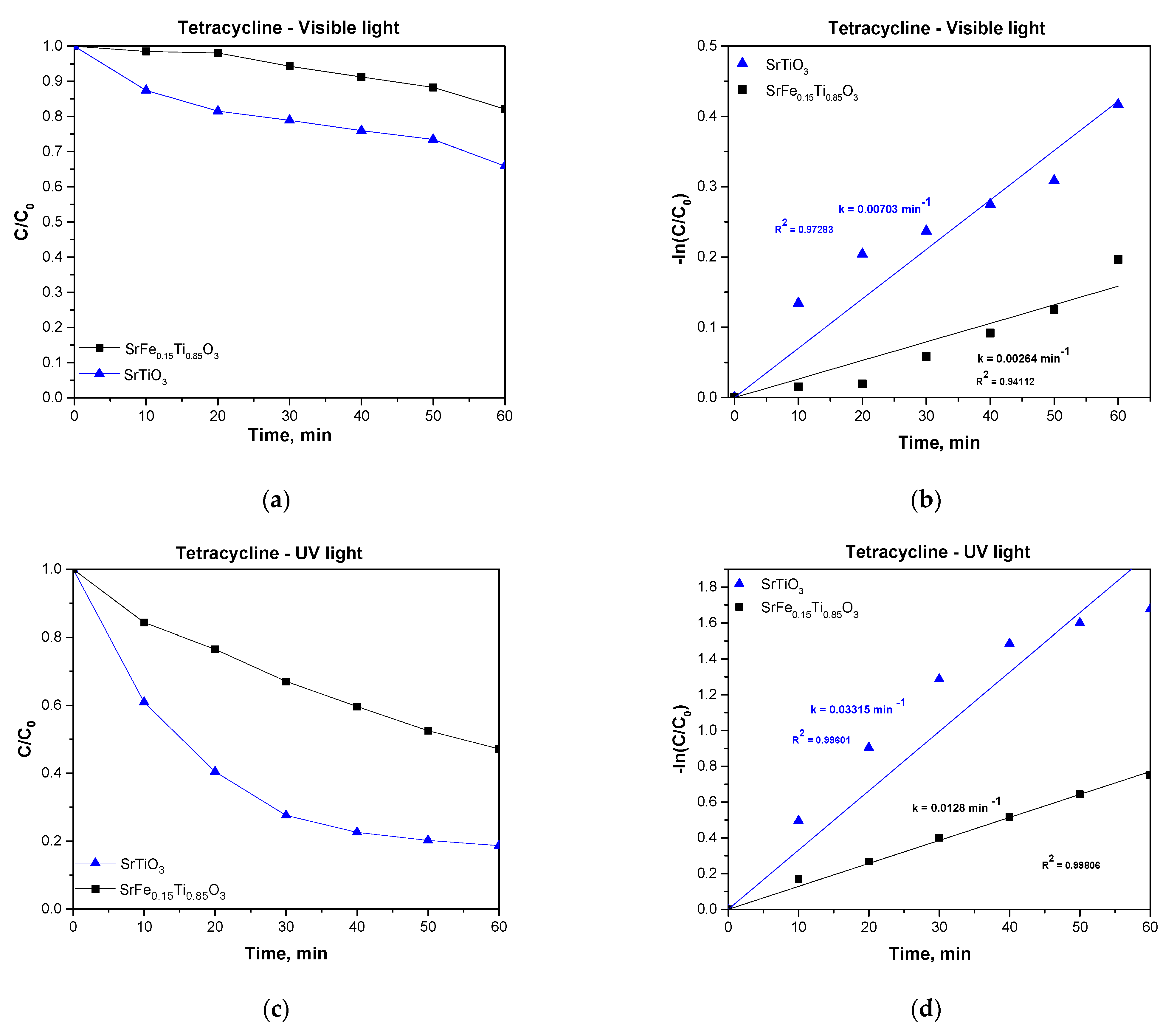
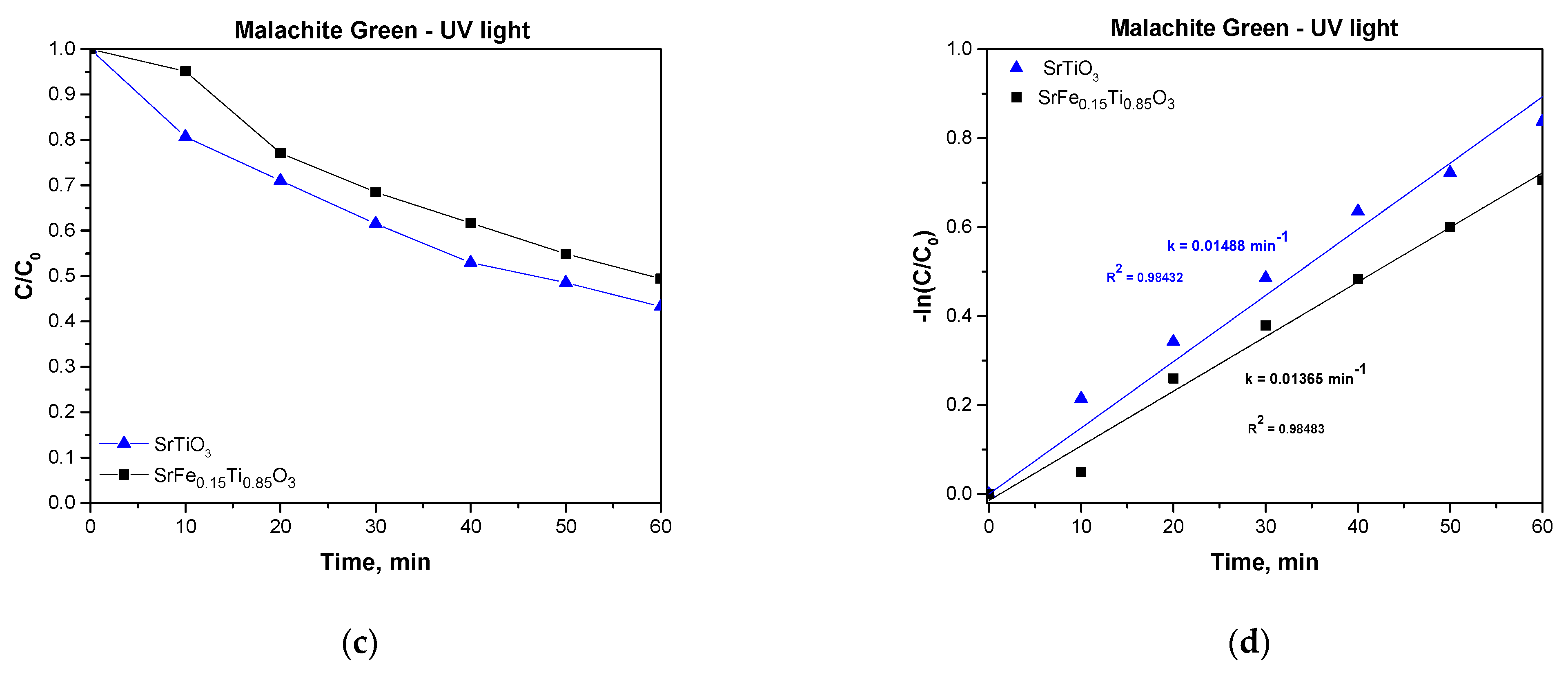
3.5. Photocatalytic Properties
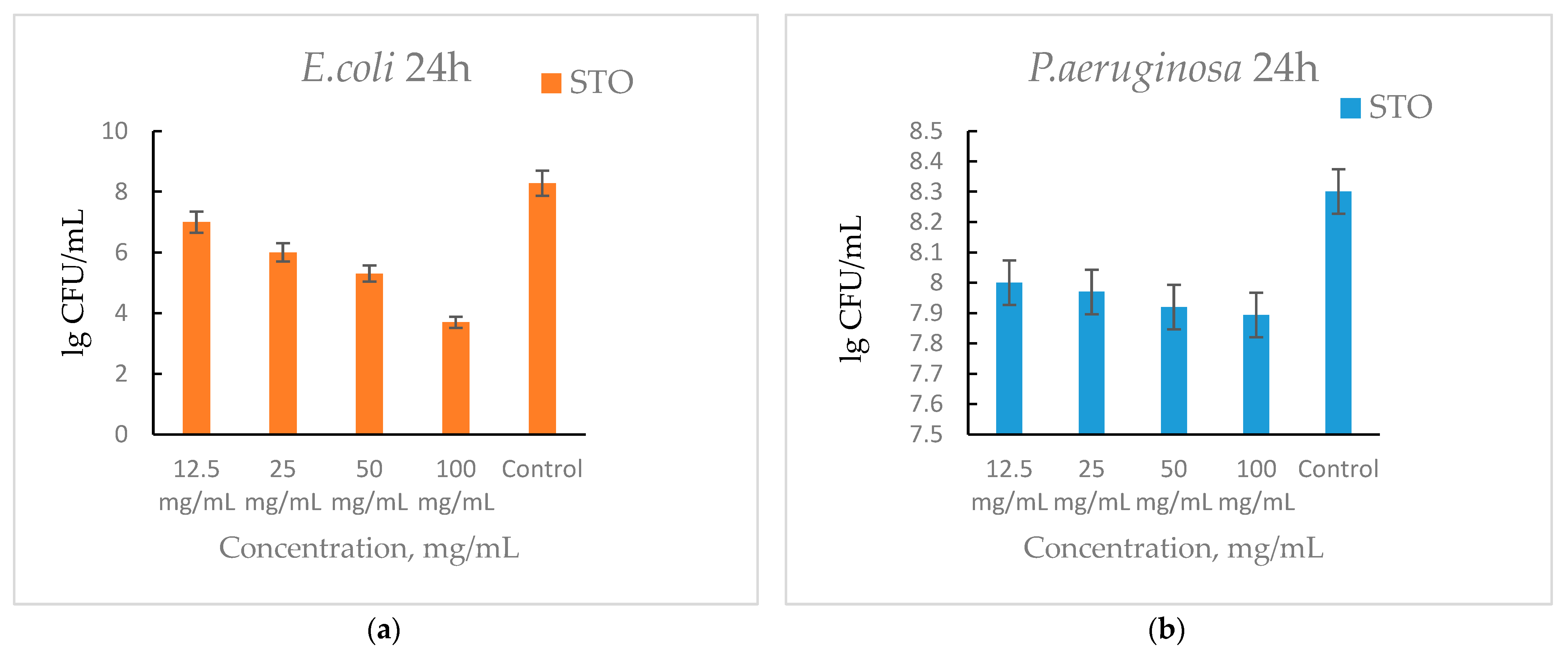
3.6. Antibacterial Activity Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Ollis, D.F.; Pelizzetti, E.; Serpone, N. Photocatalyzed Destruction of Water Contaminants. Environ. Sci. Technol. 1991, 25, 1522–1529. [Google Scholar] [CrossRef]
- Abdi, M.; Mahdikhah, V.; Sheibani, S. Visible light photocatalytic performance of La-Fe co-doped SrTiO3 perovskite powder. Opt. Mater. 2020, 102, 109803. [Google Scholar] [CrossRef]
- Temerov, F.; Baghdadi, Y.; Rattner, E.; Eslava, S. A Review on Halide Perovskite-Based Photocatalysts: Key Factors and Challenges. ACS Appl. Energy Mater. 2022, 5, 14605–14637. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liang, Y.; He, Q.; Xu, R.; Chen, D.; Xu, X.; Hu, H. Review of Doping SrTiO3 for Photocatalytic Applications. Bull. Mater. Sci. 2022, 46, 6. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Jorge, M.E.M.; Ciríaco, L.; Pacheco, M.J.; Lopes, A. Perovskites (La,Ba)(Fe,Ti)O3:AO7 Photocatalysis under Visible Light. Rev. Adv. Mater. Sci. 2020, 59, 151–159. [Google Scholar] [CrossRef]
- Schalli, M.; Romero-González, L.E. Microbiological Behavior of Waterborne Pathogens. Heliyon 2023, 9, e21634. [Google Scholar] [CrossRef]
- Mondal, S.K.; Chakraborty, S.; Manna, S.; Mandal, S.M. Antimicrobial Nanoparticles: Current Landscape and Future Challenges. RSC Pharm. 2024, 1, 388–402. [Google Scholar] [CrossRef]
- Franco, D.; Calabrese, G.; Guglielmino, S.P.P.; Conoci, S. Metal-Based Nanoparticles: Antibacterial Mechanisms and Biomedical Application. Microorganisms 2022, 10, 1778. [Google Scholar] [CrossRef]
- Barylyak, A.; Wojnarowska-Nowak, R.; Kus-Liśkiewicz, M.; Krzemiński, P.; Płoch, D.; Cieniek, B.; Bobitski, Y.; Kisała, J. Photocatalytic and Antibacterial Activity Properties of Ti Surface Treated by Femtosecond Laser—A Prospective Solution to Peri-Implant Disease. Sci. Rep. 2024, 14, 20926. [Google Scholar] [CrossRef]
- Ubaldi, F.; Valeriani, F.; Volpini, V.; Lofrano, G.; Romano Spica, V. Antimicrobial Activity of Photocatalytic Coatings on Surfaces: A Systematic Review and Meta-Analysis. Coatings 2024, 14, 92. [Google Scholar] [CrossRef]
- Da Silva, L.F.; Bernardi, M.I.B.; Maia, L.J.Q.; Frigo, G.J.M.; Mastelaro, V.R. Synthesis and Thermal Decomposition of SrTi1−xFexO3 (0.0 ≤ x ≤ 0.1) Powders Obtained by the Polymeric Precursor Method. J. Therm. Anal. Calorim. 2009, 97, 173–177. [Google Scholar] [CrossRef]
- Lobacheva, O.; Yiu, Y.M.; Chen, N.; Sham, T.K.; Goncharova, L.V. Changes in Local Surface Structure and Sr Depletion in Fe-Implanted SrTiO3 (001). Appl. Surf. Sci. 2017, 393, 74–81. [Google Scholar] [CrossRef]
- Yu, J.G.; Dai, G.P.; Huang, B. Effects of Calcination Temperature on Photocatalytic Activity of Hydrothermal Synthesized Nano-Sized SrTiO3 Powders. J. Phys. Chem. Solids 2008, 69, 2357–2362. [Google Scholar]
- Mostafa, E.M.; Amdeha, E. Enhanced Photocatalytic Degradation of Malachite Green Dye by Highly Stable Visible-Light-Responsive Fe-Based Tri-Composite Photocatalysts. Environ. Sci. Pollut. Res. Int. 2022, 29, 69861–69874. [Google Scholar] [CrossRef]
- Xiang, Q.; Cheng, B.; Yu, J. TiO2-Based Photocatalysts for Hydrogen Production. J. Phys. Chem. Lett. 2018, 9, 3452–3461. [Google Scholar]
- Zou, Z.; Ye, J.; Sayama, K.; Arakawa, H. Direct Splitting of Water under Visible Light Irradiation with an Oxide Semiconductor Photocatalyst. Nature 2001, 414, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Siebenhofer, M.; Viernstein, A.; Morgenbesser, M.; Fleig, J.; Kubicek, M. Photoinduced Electronic and Ionic Effects in Strontium Titanate. Mater. Adv. 2021, 2, 7583–7619. [Google Scholar] [CrossRef] [PubMed]
- Jiang-Ni, Y.; Zhi-Yong, Z.; Jun-Feng, Y.; Wu, Z. Electronic Structure and Optical Properties of La-Doped SrTiO3 and Sr2TiO4 by Density Function Theory. Chin. Phys. Lett. 2009, 26, 017107. [Google Scholar] [CrossRef]
- Karczewski, J.; Riegel, B.; Gazda, M.; Jasinski, P.; Kusz, B. Electrical and Structural Properties of Nb-Doped SrTiO3 Ceramics. J. Electroceram. 2009, 24, 326–330. [Google Scholar] [CrossRef]
- Wang, D.; Ye, J.; Kako, T.; Kimura, T. Photophysical and Photocatalytic Properties of SrTiO3 Doped with Cr Cations on Different Sites. J. Phys. Chem. B 2006, 110, 15824–15830. [Google Scholar] [CrossRef] [PubMed]
- Villa-Bracamonte, M.F.; Montes-Bojorquez, J.R.; Ayon, A.A. Optical properties study of a perovskite solar cell film stack by spectroscopic ellipsometry and spectrophotometry. Results Opt. 2024, 15, 100640. [Google Scholar] [CrossRef]
- Li, X.; Zhao, H.; Xu, N.; Zhou, X.; Zhang, C.; Chen, N. Electrical Conduction Behavior of La, Co Co-Doped SrTiO3 Perovskite as Anode Material for Solid Oxide Fuel Cells. Int. J. Hydrogen Energy 2009, 34, 6407–6414. [Google Scholar] [CrossRef]
- Da Silva, L.F.; M’Peko, J.; Andrés, J.; Beltrán, A.; Gracia, L.; Bernardi, M.I.B.; Mesquita, A.; Antonelli, E.; Moreira, M.L.; Mastelaro, V.R. Insight into the Effects of Fe Addition on the Local Structure and Electronic Properties of SrTiO3. J. Phys. Chem. C 2014, 118, 4930–4940. [Google Scholar] [CrossRef]
- El-Sadek, M.; Farahat, M.; Ali, H.; Zaki, Z. Synthesis of SrTiO3 from Celestite and Rutile by Mechanical Activation Assisted Solid-State Reaction. Adv. Powder Technol. 2022, 33, 103548. [Google Scholar] [CrossRef]
- Kalyani, V.; Vasile, B.S.; Ianculescu, A.; Testino, A.; Carino, A.; Buscaglia, M.T.; Buscaglia, V.; Nanni, P. Hydrothermal Synthesis of SrTiO3: Role of Interfaces. Cryst. Growth Des. 2015, 15, 5712–5725. [Google Scholar] [CrossRef]
- Luxová, J.; Dohnalová, Ž.; Šulcová, P.; Reinders, N. Mn-Doped SrTiO3 Perovskite: Synthesis and Characterisation of a Visible Light-Active Semiconductor. Mater. Sci. Eng. B 2024, 313, 117889. [Google Scholar] [CrossRef]
- Sharma, N.; Hernadi, K. The Emerging Career of Strontium Titanates in Photocatalytic Applications: A Review. Catalysts 2022, 12, 1619. [Google Scholar] [CrossRef]
- Feldhoff, A.; Arnold, M.; Martynczuk, J.; Gesing, T.M.; Wang, H. The Sol–Gel Synthesis of Perovskites by an EDTA/Citrate Complexing Method Involves Nanoscale Solid State Reactions. Solid State Sci. 2008, 10, 689–701. [Google Scholar] [CrossRef]
- Navas, D.; Fuentes, S.; Castro-Alvarez, A.; Chavez-Angel, E. Review on Sol-Gel Synthesis of Perovskite and Oxide Nanomaterials. Gels 2021, 7, 275. [Google Scholar] [CrossRef]
- Tsvetkov, M.; Encheva, E.; Petrova, S.; Spassova, I.; Milanova, M. Mechanochemically Synthesized Solid Solutions La1−xCexFeO3+x/2 for Activation of Peroxydisulfate in Catalytic Reaction for Tetracycline Degradation. Crystals 2023, 13, 769. [Google Scholar] [CrossRef]
- Zhang, L.; Tan, P.Y.; Chow, C.L.; Lim, C.K.; Tan, O.K.; Tse, M.S.; Sze, C.C. Antibacterial activities of mechanochemically synthesized perovskite strontium titanate ferrite metal oxide. Colloids Surf. A Physicochem. Eng. Asp. 2014, 456, 169–175. [Google Scholar] [CrossRef]
- Hench, L.L.; West, J.K. The sol-gel process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Jose, S.; Joshy, D.; Narendranath, S.B.; Periyat, P. Recent Advances in Infrared Reflective Inorganic Pigments. Sol. Energy Mater. Sol. Cells 2019, 194, 7–27. [Google Scholar] [CrossRef]
- Koli, V.B.; Delekar, S.D.; Pawar, S.H. Photoinactivation of bacteria by using Fe-doped TiO2-MWCNTs nanocomposites. J. Mater. Sci. Mater. Med. 2016, 27, 1–10. [Google Scholar] [CrossRef]
- Li, Y.; Niu, S.; Hao, Y.; Zhou, W.; Wang, J.; Liu, J. Role of Oxygen Vacancy on Activity of Fe-Doped SrTiO3 Perovskite Bifunctional Catalysts for Biodiesel Production. Renew. Energy 2022, 199, 1258–1271. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties And Electronic Structure of Amorphous Germanium. Phys. Status Solidi B 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Reller, L.B.; Weinstein, M.; Jorgensen, J.H.; Ferraro, M.J. Antimicrobial Susceptibility Testing: A Review of General Principles and Contemporary Practices. Clin. Infect. Dis. 2009, 49, 1749–1755. [Google Scholar]
- Long, Y. A-Site Ordered Quadruple Perovskite Oxides AA3B4O12. Chin. Phys. B 2016, 25, 078108. [Google Scholar] [CrossRef]
- Vegard, L. Die Konstitution der Mischkristalle und die Raumfüllung der Atome. Z. Phys. 1921, 5, 17–26. [Google Scholar] [CrossRef]
- Goodenough, J.B. Electronic and Ionic Transport Properties and Other Physical Aspects of Perovskites. Rep. Prog. Phys. 2004, 67, 1915–1993. [Google Scholar] [CrossRef]
- Ghadiri, M.; Amini, M.M. The Effect of Metal Dopants on Structural, Optical and Photocatalytic Properties of Perovskite-Type Oxides: A Review. Mater. Sci. Semicond. Process. 2020, 107, 104849. [Google Scholar] [CrossRef]
- Ohtomo, A.; Hwang, H.Y. A High-Mobility Electron Gas at the LaAlO3/SrTiO3 Heterointerface. Nature 2004, 427, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Singh, R.P.; Pandey, A.; Pandey, A. Photocatalytic antibacterial performance of TiO2 and Ag-doped TiO2 against S. aureus. P. aeruginosa and E. coli. Beilstein J. Nanotechnol. 2013, 4, 345–351. [Google Scholar] [CrossRef]
- Goldschmidt, V.M. Die Gesetze der Krystallochemie. Naturwissenschaften 1926, 14, 477–485. [Google Scholar] [CrossRef]
- Łańcucki, Ł.; Mizera, A.; Łącz, A.; Drożdż, E.; Bućko, M.M.; Pasierb, P. Development and Chemical Stability Evaluation of Enhanced Surface LaFe1–xTixO3 (LFT) Perovskites Using Polystyrene Nanospheres as Templating Agent. J. Alloys Compd. 2017, 727, 863–870. [Google Scholar] [CrossRef]
- Arman, M.M.; El-Dek, S.I. Enhancement in Dielectric and Optical Properties of La1–xCexFeO3 Nanoparticles. J. Mater. Sci. Appl. 2019, 8, 5396–5404. [Google Scholar] [CrossRef]
- Fuentes, S.; Muñoz, P.; Barraza, N.; Chávez-Angel, E.; Sotomayor Torres, C.M. Structural Characterisation of Slightly Fe-Doped SrTiO3 Grown via a Sol–Gel Hydrothermal Synthesis. J. Sol-Gel Sci. Technol. 2015, 75, 593–601. [Google Scholar] [CrossRef]
- Sahner, K.; Gouma, P.; Moos, R. Electrodeposited and Sol-Gel Precipitated p-Type SrTi1−xFexO3−δ Semiconductors for Gas Sensing. Sensors 2007, 7, 1871–1886. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Beattie, I.R.; Gilson, T. Single Crystal Laser Raman Spectroscopy. Proc. R. Soc. A 1968, 307, 407–429. [Google Scholar]
- Henry, M.; Leavage, J.; Sanchez, C. Sol-Gel Chemistry of Transition Metal Oxides. Prog. Solid State Chem. 1988, 18, 259–341. [Google Scholar]
- Velasco, M.J.; Rubio, F.; Rubio, J.; Oteo, J. Hydrolysis of Titanium Tetrabutoxide. Study by FT-IR Spectroscopy. Spectrosc. Lett. 1999, 32, 289–304. [Google Scholar] [CrossRef]
- Busca, G.; Saussey, H.; Saur, O.; Lavalley, J.C.; Lorenzelli, V. FT-IR characterization of the surface acidity of different titanium dioxide anatase preparations. Appl. Catal. 1985, 14, 245–260. [Google Scholar] [CrossRef]
- Iordanova, R.; Bachvarova-Nedelcheva, A.; Gegova, R.; Kostov, K.L.; Dimitriev, Y. Sol–Gel Synthesis of Composite Powders in the TiO2–TeO2–SeO2 System. J. Sol-Gel Sci. Technol. 2016, 79, 12–28. [Google Scholar] [CrossRef]
- Rocha-Rangel, E.; Pech-Rodríguez, W.J.; Lopez Hernandez, J.; Calles-Arriaga, C.; Armendáriz-Mireles, E.N.; Castillo-Robles, J.A. Synthesis of SrTiO3 by the Calcination of SrCO3 And TiO2 Mixtures Intensively Ground by Means of High Energy Milling. Arch. Metall. Mater. 2020, 52, 621–626. [Google Scholar] [CrossRef]
- Iordanova, R.; Dimitriev, Y.; Dimitrov, V.; Kassabov, S.; Klissurski, D. Glass Formation and Structure in the System MoO3–Bi2O3–Fe2O3. J. Non-Cryst. Solids 1998, 231, 227–233. [Google Scholar] [CrossRef]
- Bachvarova-Nedelcheva, A.; Iordanova, R.; Naydenov, A.; Stoyanova, A.; Georgieva, N.; Nemska, V.; Foteva, T. Sol–Gel Obtaining of TiO2/TeO2 Nanopowders with Biocidal and Environmental Applications. Catalysts 2023, 13, 257. [Google Scholar] [CrossRef]
- Abbas, N.; Shao, G.N.; Haider, M.S.; Imran, S.M.; Park, S.S.; Kim, H.T. Sol–Gel Synthesis of TiO2-Fe2O3 Systems: Effects of Fe2O3 Content and Their Photocatalytic Properties. J. Ind. Eng. Chem. 2016, 39, 112–120. [Google Scholar] [CrossRef]
- Atma, R.; Awalendra, K.T. Tunability of dielectric, optical and magnetic property by simultaneous co-substitution in LaFeO3. Mater. Sci. Eng. B 2017, 224, 139–149. [Google Scholar]
- Hasan, T.; Saha, A.; Khan, M.N.I.; Rashid, R.; Basith, M.A.; Bashar, M.S.; Ahmed, I. Structural, Electrical, and Magnetic Properties of Ce and Fe Doped SrTiO3. AIP Adv. 2022, 12, 095003. [Google Scholar] [CrossRef]
- Nagaveni, K.; Hegde, M.S.; Ravishankar, N.; Subbanna, G.N.; Madras, G. Synthesis and Structure of Nanocrystalline TiO2 with Lower Band Gap Showing High Photocatalytic Activity. Langmuir 2004, 20, 2900–2907. [Google Scholar] [CrossRef]
- Ngyen, V.V.H.; Beydoun, D.; Amal, R.J. Photocatalytic reduction of selenite and selenate using TiO2 photocatalyst. Photochem. Photobiol. A Chem. 2005, 171, 113–120. [Google Scholar] [CrossRef]
- Meng, Z.D.; Zhu, L.; Ullah, K.; Ye, S.; Oh, W.-C. Detection of oxygen species generated by WO3 modification fullerene/TiO2 in the degradation of 1,5-diphenyl carbazide. Mater. Res. Bull. 2014, 56, 45–53. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, D.; Huang, Q.; Jin, X.; Liu, H. UV–Visible Spectra of Perovskite Iron-Doped Ba0.72Sr0.28TiO3. Mater. Lett. 2008, 62, 407–409. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, C.-L.; Li, J.-C.; Yang, K. Structural and Electronic Properties of Fe-Doped BaTiO3 and SrTiO3. Chin. Phys. 2007, 16, 1422. [Google Scholar]
- Thomas, R.; Dube, D.C. Optical Properties of Sol–Gel Processed Amorphous and Crystalline SrTiO3 Thin Films. Jpn. J. Appl. Phys. 2000, 39, 1771. [Google Scholar] [CrossRef]
- Bhoyar, D.N.; Somvanshi, S.B.; Nalle, P.B.; Mande, V.K.; Pandit, A.A.; Jadhavet, K.M. Multiferroic Fe3+ ion doped BaTiO3 Perovskite Nanoceramics: Structural, Optical, Electrical and Dielectric Investigations. J. Phys. Conf. Ser. 2020, 1644, 012058. [Google Scholar] [CrossRef]
- Ross-Medgaarden, E.I.; Wachs, I.E. Structural Determination of Bulk and Surface Tungsten Oxides with UV−Vis Diffuse Reflectance Spectroscopy and Raman Spectroscopy. J. Phys. Chem. C 2007, 111, 15089–15099. [Google Scholar] [CrossRef]
- Niu, J.; Ding, S.; Zhang, L.; Zhao, J.; Feng, C. Visible-light-mediated Sr-Bi2O3 photocatalysis of tetracycline: Kinetics, mechanisms and toxicity assessment. Chemosphere 2013, 93, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ibhadon, A.O.; Fitzpatrick, P. Heterogeneous Photocatalysis: Recent Advances and Applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef]
- Li, P.; Liu, C.; Wu, G.; Heng, Y.; Lin, S.; Ren, A.; Lv, K.; Xiao, L.; Shi, W. Solvothermal synthesis and visible light-driven photocatalytic degradation for tetracycline of Fe-doped SrTiO3. RSC Adv. 2014, 4, 47615. [Google Scholar] [CrossRef]
- Modirshahla, N.; Behnajady, M.A. Decolorization of Malachite Green by UV/H2O2: Influence of Operational Parameters and Kinetic Modeling. J. Hazard. Mater. 2006, 137, 226–232. [Google Scholar] [CrossRef]
- Figueroa, R.A.; MacKay, A.A. Sorption of Oxytetracycline to Iron Oxides and Iron Oxide-Rich Soils. Environ. Sci. Technol. 2005, 39, 6664–6671. [Google Scholar] [CrossRef]
- Gu, C.; Karthikeyan, K.G. Interaction of Tetracycline with Aluminum and Iron Hydrous Oxides. Environ. Sci. Technol. 2005, 39, 2660–2667. [Google Scholar] [CrossRef] [PubMed]
- Rosa, D.; D’Agostino, F.; Bavasso, I.; Di Palma, L. An Innovative and Easy Method for Iron-Doped Titania Synthesis. Chem. Eng. Trans. 2023, 101, 13–18. [Google Scholar]
- El-Salamony, R.A.; Amdeha, E.; Ghoneim, S.A.; Badawy, N.A.; Salem, K.M.; Al-Sabagh, A.M. Titania Modified Activated Carbon Prepared from Sugarcane Bagasse: Adsorption and Photocatalytic Degradation of Methylene Blue under Visible Light Irradiation. Environ. Technol. 2017, 38, 3122–3136. [Google Scholar] [CrossRef] [PubMed]
- Shymanovska, V.; Kernazhitsky, L.; Puchkovska, G.; Naumov, V.; Khalyavka, T.; Kshnyakin, V.; Kshnyakina, S.; Chernyak, V. The Impurity Ion Influence on the Optical and Photocatalytic Properties of Anatase and Rutile. J. Nanoelectron. Phys. 2011, 3, 63–73. [Google Scholar]
- Musah, A.M.I.; Or, S.; Awodugba, A.O. Precursor Impurity-Mediated Effect in the Photocatalytic Activity of Precipitated Zinc Oxide. J. Am. Ceram. Soc. 2024, 107, 8269–8280. [Google Scholar]
- Slavin, Y.N.; Asnis, J.; Hńfeli, U.O.; Bach, H. Metal Nanoparticles: Understanding the Mechanisms Behind Antibacterial Activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Doolan, J.A.; Williams, G.T.; Hilton, K.L.F.; Chaudhari, R.; Fossey, J.S.; Goult, B.T.; Hiscock, J.R. Advancements in Antimicrobial Nanoscale Materials and Self-Assembling Systems. Chem. Soc. Rev. 2022, 51, 8696–8755. [Google Scholar] [CrossRef] [PubMed]

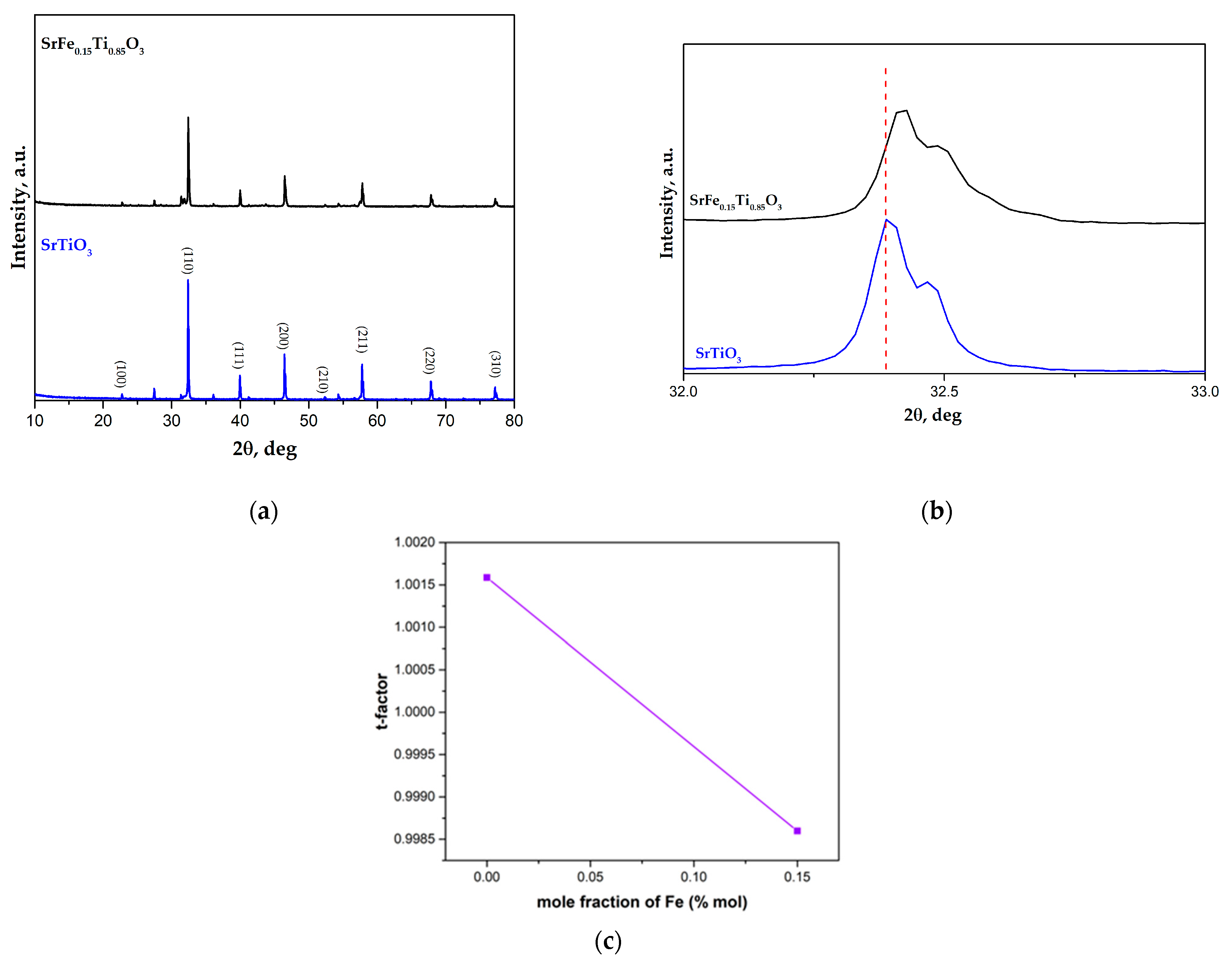
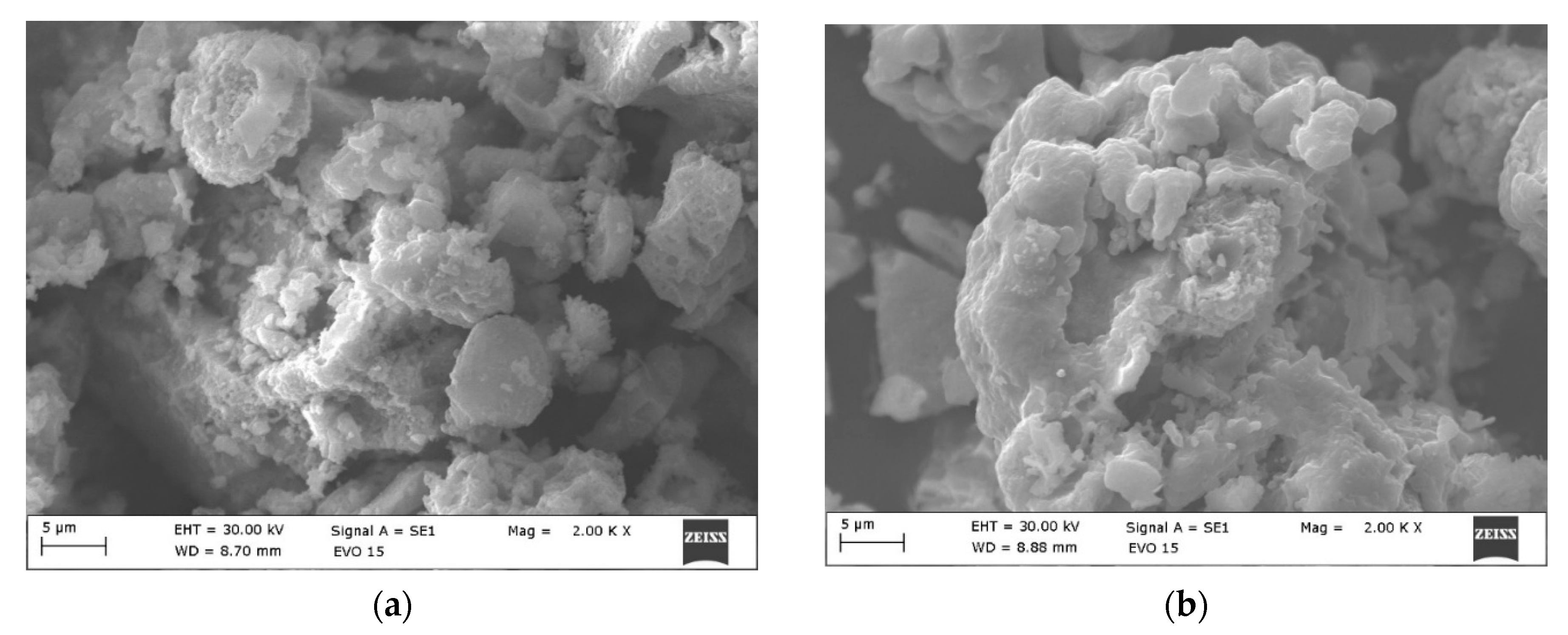
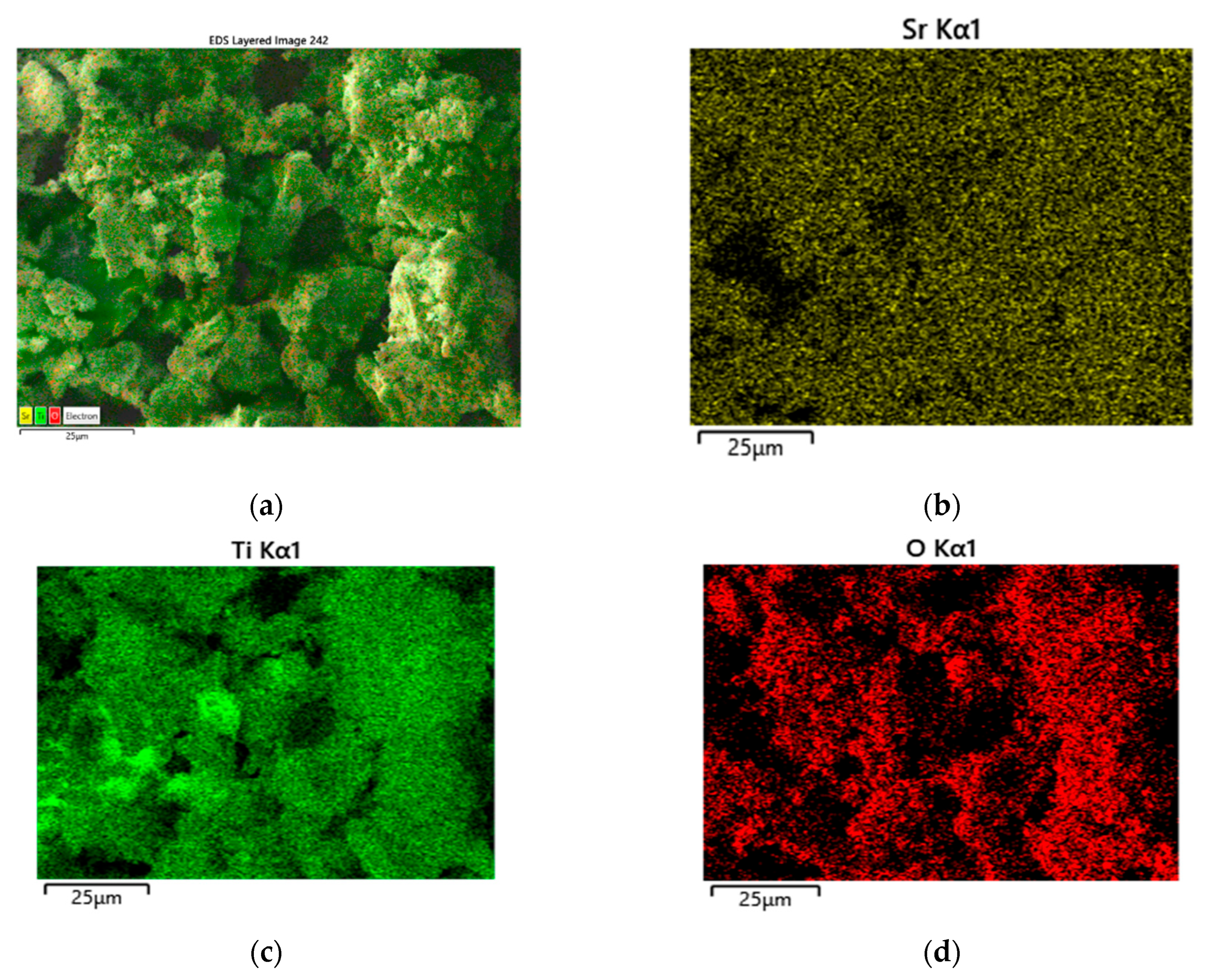
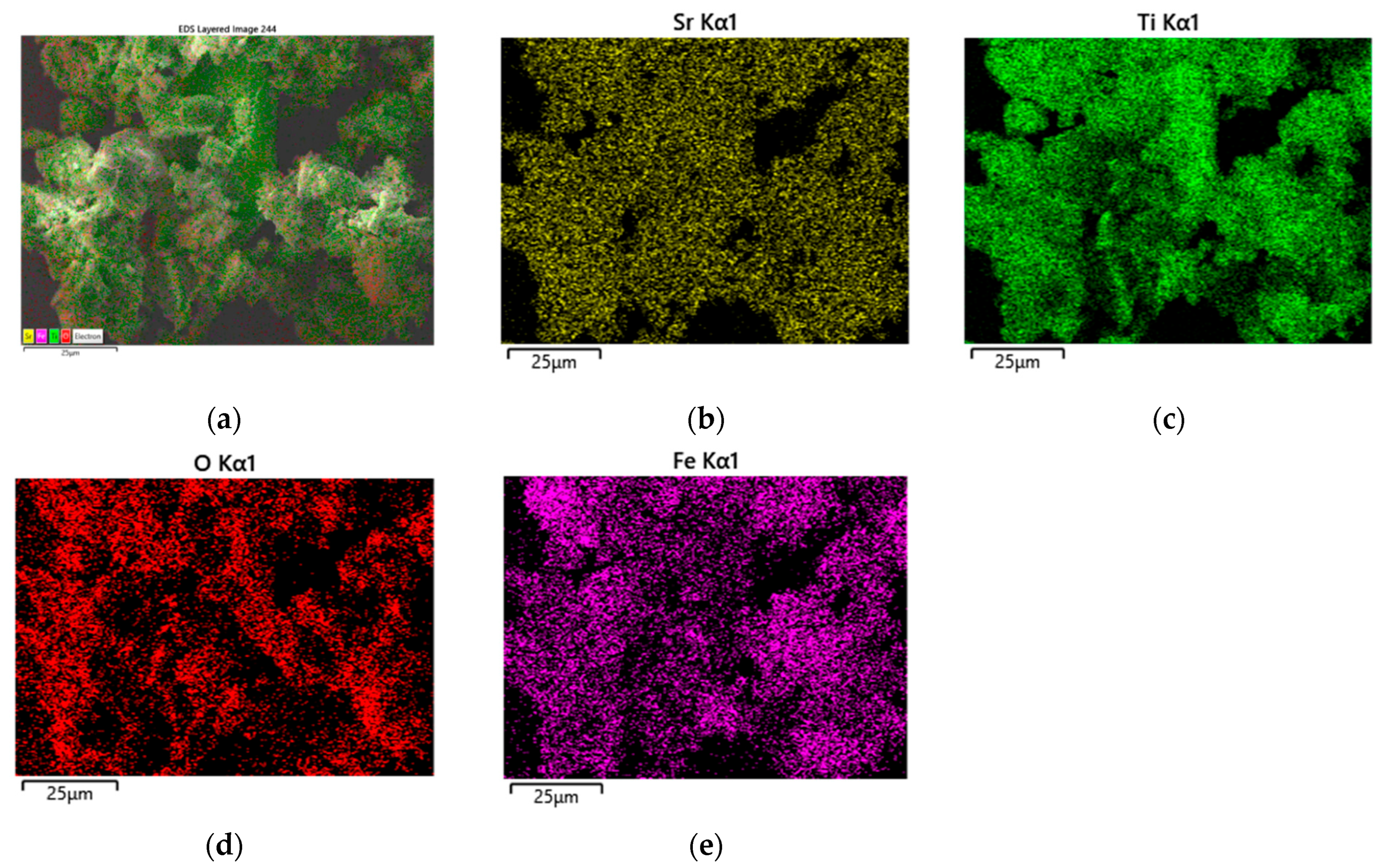



| Sample | SBET, m2/g | Vt, cm3/g | Dav, nm |
|---|---|---|---|
| SrFe0.15Ti0.85O3 | 3 | 0.020 | 21 |
| SrTiO3 | 4 | 0.024 | 24 |
| Sample | Eg, eV | ECB, eV | EVB, eV | Cut-Off, nm | Refractive Index, n |
|---|---|---|---|---|---|
| SrTiO3 | 3.28 | −0.822 | 2.46 | 378 | 2.32 |
| SrFe0.15Ti0.85O3 | 2.86 | −0.797 | 2.48 | 433 | 2.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrova, S.; Ivanova, K.; Ivanova, I.; Bachvarova-Nedelcheva, A. Optical, Photocatalytic, and Antibacterial Properties of Sol-Gel Derived Fe Doped SrTiO3 Powders. Water 2025, 17, 2072. https://doi.org/10.3390/w17142072
Petrova S, Ivanova K, Ivanova I, Bachvarova-Nedelcheva A. Optical, Photocatalytic, and Antibacterial Properties of Sol-Gel Derived Fe Doped SrTiO3 Powders. Water. 2025; 17(14):2072. https://doi.org/10.3390/w17142072
Chicago/Turabian StylePetrova, Stefani, Kalina Ivanova, Iliana Ivanova, and Albena Bachvarova-Nedelcheva. 2025. "Optical, Photocatalytic, and Antibacterial Properties of Sol-Gel Derived Fe Doped SrTiO3 Powders" Water 17, no. 14: 2072. https://doi.org/10.3390/w17142072
APA StylePetrova, S., Ivanova, K., Ivanova, I., & Bachvarova-Nedelcheva, A. (2025). Optical, Photocatalytic, and Antibacterial Properties of Sol-Gel Derived Fe Doped SrTiO3 Powders. Water, 17(14), 2072. https://doi.org/10.3390/w17142072







