Efficient Chromium(VI) Removal Through In Situ Nano-Iron Sulfide Formation at the Cathode of Microbial Fuel Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. MFC Configuration and Operation
2.2. Experimental Design and Setup
2.2.1. In Situ Self-Assembly of Nano-FeS Hybridized Biocathode
2.2.2. Cr(VI) Removal Experiment by Nano-FeS Hybridized Biocathode
2.3. Measurements and Analyses
2.4. Analysis and Testing of Biocathodes
2.5. Data Analysis Methods
3. Results and Discussion
3.1. Effect of Different Conditions on the In Situ Synthesis of Nano-FeS by Biocathode
3.1.1. Synthesis Quantity of Nano-FeS
3.1.2. Physicochemical Analysis of Nano-FeS
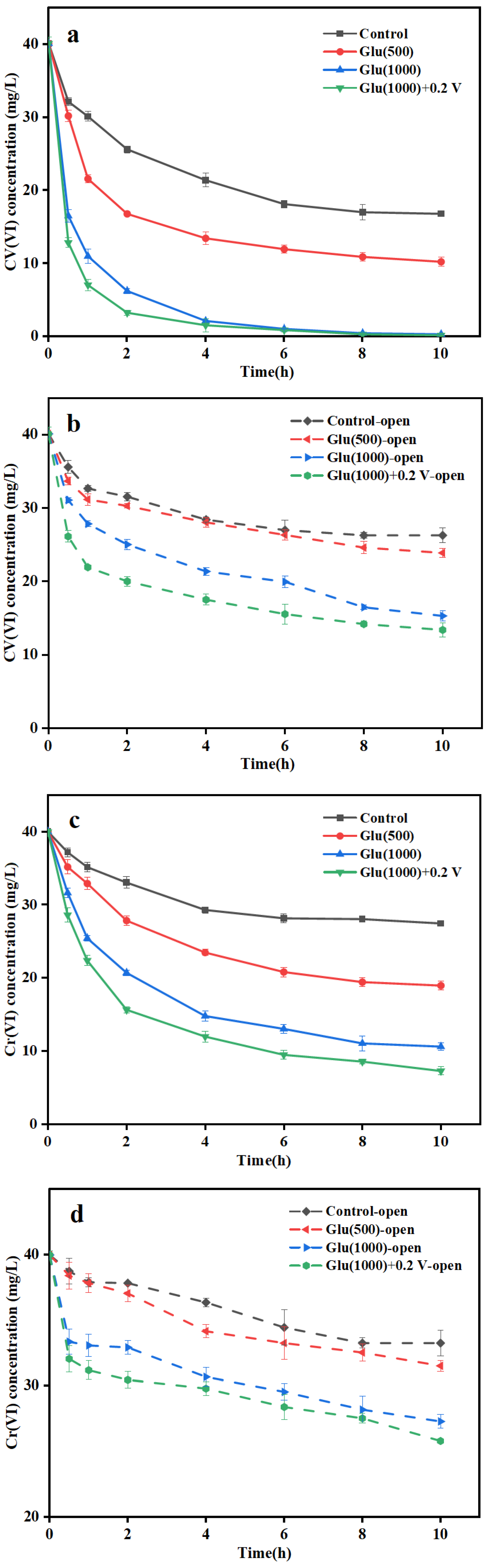
3.2. Cr(VI) Removal by Biocathode MFC
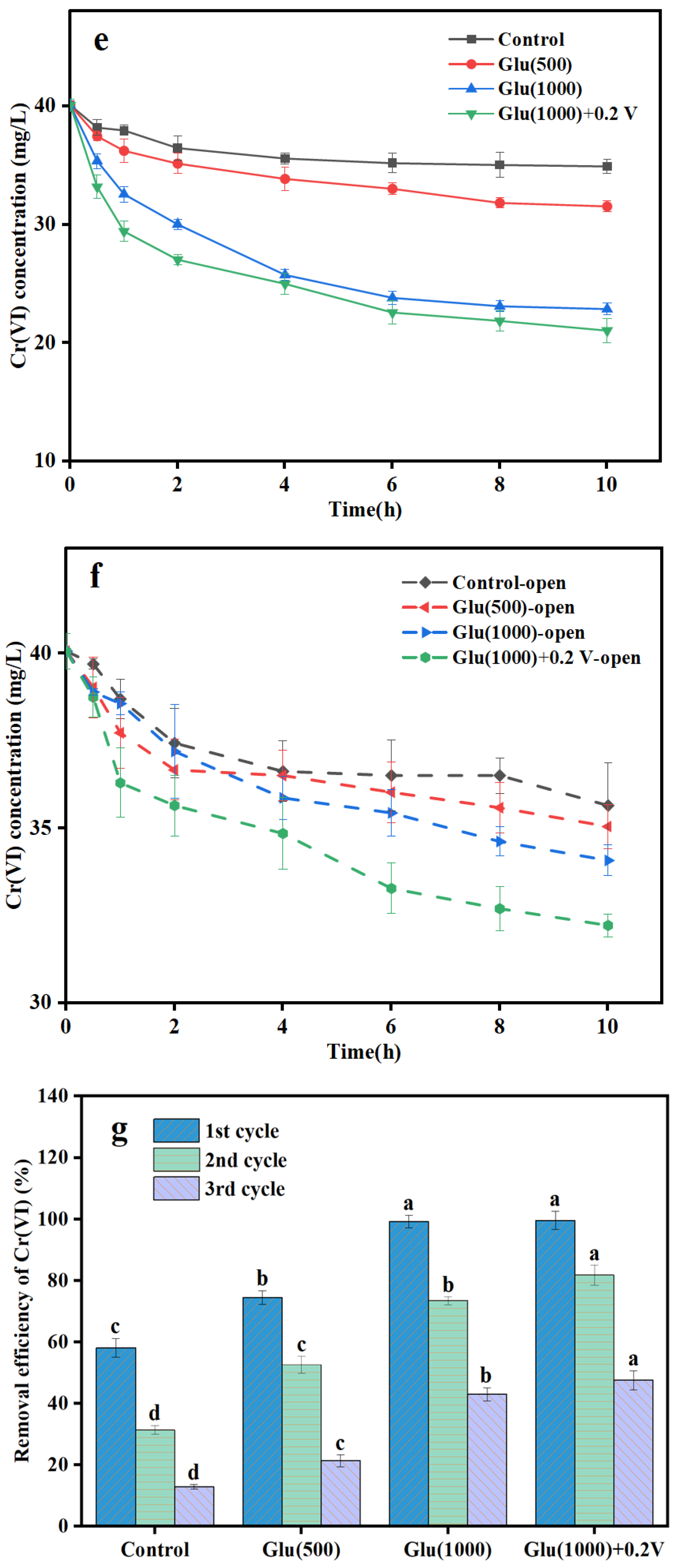
3.2.1. Cr(VI) Removal Performance
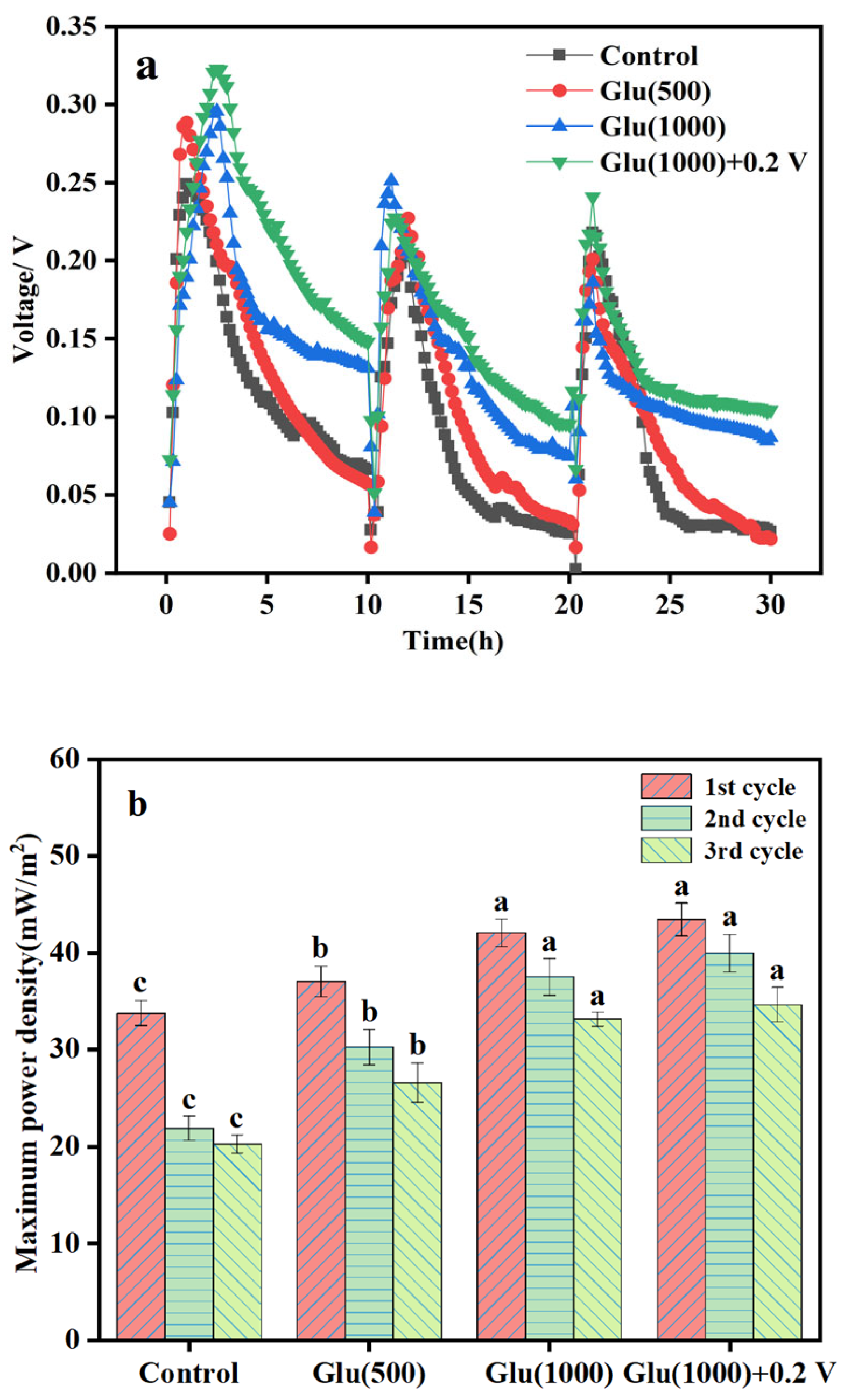
3.2.2. Electricity Generation in Biocathode MFC
3.3. Analysis of Nano-FeS Biocathode Before and After Cr(VI) Removal
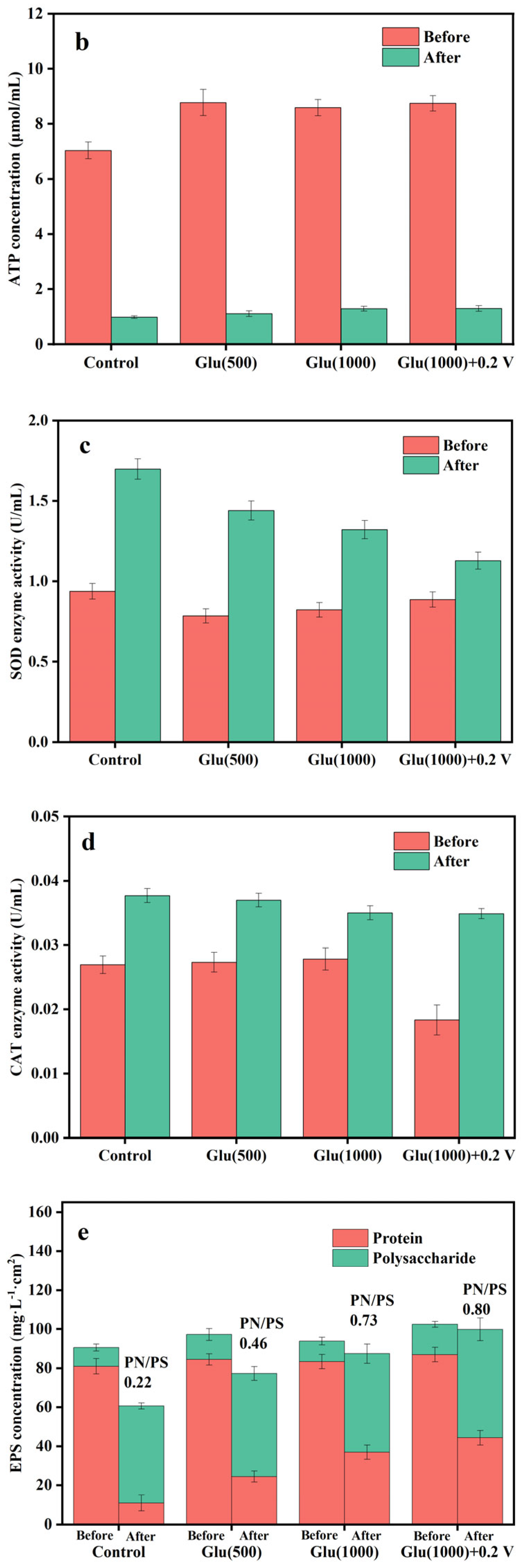
3.3.1. Physiological and Biochemical Analysis of Nano-FeS Biocathodes
3.3.2. Physicochemical Analysis of Nano-FeS Biocathode
3.3.3. Microbial Community Analysis of Nano-FeS Biocathodes
3.4. Enhancement Mechanism of Nano-FeS Biocathode and Practical Implicatons
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, D.; Zhang, B.; Shi, S.; Tang, R.; Qiao, C.; Li, T.; Jia, J.; Yang, M.; Si, X.; Wang, Y.; et al. Engineering extracellular electron transfer to promote simultaneous brewing wastewater treatment and chromium reduction. J. Hazard. Mater. 2024, 465, 133171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Y.; Li, C. Influence of Cr(VI) concentration on Cr(VI) reduction and electricity production in microbial fuel cell. Environ. Sci. Pollut. Res. 2021, 28, 54170–54176. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.; Wang, L.; Waseem, H.; Djellabi, R.; Oladoja, N.A.; Pan, G. FeS@rGO nanocomposites as electrocatalysts for enhanced chromium removal and clean energy generation by microbial fuel cell. Chem. Eng. J. 2020, 384, 123335. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, X.; Wang, M.; Yao, M.; Xue, J. Fabrication of a long-term reductive material by ball milling of mackinawite with sodium bisulfate to remove Cr(VI) in groundwater. J. Environ. Chem. Eng. 2024, 12, 114949. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, S.; Sumpradit, T.; Zhou, J.; Qu, J. Magnetic nanoparticles doped biochar cathode in a two-chamber microbial fuel cell for the adsorption-reduction of hexavalent chromium. Int. J. Hydrogen Energy 2024, 63, 163–172. [Google Scholar] [CrossRef]
- Kumar, A.; Guo, C.; Sharma, G.; Pathania, D.; Naushad, M.; Kalia, S.; Dhiman, P. Magnetically recoverable ZrO2/Fe3O4/chitosan nanomaterials for enhanced sunlight driven photoreduction of carcinogenic Cr(VI) and dechlorination & mineralization of 4-chlorophenol from simulated waste water. RSC Adv. 2016, 6, 13251–13263. [Google Scholar] [CrossRef]
- Huang, L.; Chai, X.; Cheng, S.; Chen, G. Evaluation of carbon-based materials in tubular biocathode microbial fuel cells in terms of hexavalent chromium reduction and electricity generation. Chem. Eng. J. 2011, 166, 652–661. [Google Scholar] [CrossRef]
- Tandukar, M.; Huber, S.J.; Onodera, T.; Pavlostathis, S.G. Biological chromium(VI) reduction in the cathode of a microbial fuel cell. Environ. Sci. Technol. 2009, 43, 8159–8165. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, X.; Song, T.; Zhang, L.; Jia, H.; Wei, P. Effect of acclimatization on hexavalent chromium reduction in a biocathode microbial fuel cell. Bioresour. Technol. 2015, 180, 185–191. [Google Scholar] [CrossRef]
- Cheng, Z.; Xiong, J.; Min, D.; Cheng, L.; Liu, D.; Li, W.; Jin, F.; Yang, M.; Yu, H. Promoting bidirectional extracellular electron transfer of Shewanella oneidensis MR-1 for hexavalent chromium reduction via elevating intracellular cAMP level. Biotechnol. Bioeng. 2020, 117, 1294–1303. [Google Scholar] [CrossRef]
- Yu, X.; Guo, T.; Liu, X.; Zhou, B.; Zhai, X.; Yang, J.; Wang, X.; Hou, Y.; Yang, Q. Improving surface properties of cathode and increasing abundance of autotrophic bacteria for chromium reduction with amino functionalized carbon nanotubes. J. Environ. Chem. Eng. 2022, 10, 108005. [Google Scholar] [CrossRef]
- Kamali, M.; Aminabhavi, T.M.; Abbassi, R.; Dewil, R.; Appels, L. Engineered nanomaterials in microbial fuel cells—Recent developments, sustainability aspects, and future outlook. Fuel 2022, 310, 122347. [Google Scholar] [CrossRef]
- Cheng, C.; Hu, Y.; Shao, S.; Yu, J.; Zhou, W.; Cheng, J.; Chen, Y.; Chen, S.; Chen, J.; Zhang, L. Simultaneous Cr(VI) reduction and electricity generation in Plant-Sediment Microbial Fuel Cells (P-SMFCs): Synthesis of non-bonding Co3O4 nanowires onto cathodes. Environ. Pollut. 2019, 247, 647–657. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Li, Y.; Ni, L.; Zhu, W. Effect of stabilizer EDTA on the thermal hazard of green synthesis process of adipic acid and development of microchannel continuous flow process. Emerg. Manag. Sci. Technol. 2023, 3, 22. [Google Scholar] [CrossRef]
- Chen, Y.; Liang, W.; Li, Y.; Wu, Y.; Chen, Y.; Xiao, W.; Zhao, L.; Zhang, J.; Li, H. Modification, application and reaction mechanisms of nano-sized iron sulfide particles for pollutant removal from soil and water: A review. Chem. Eng. J. 2019, 362, 144–159. [Google Scholar] [CrossRef]
- Huo, Y.; Li, W.; Chen, C.; Li, C.; Zeng, R.; Lau, T.; Huang, T. Biogenic FeS accelerates reductive dechlorination of carbon tetrachloride by Shewanella putrefaciens CN32. Enzym. Microb. Technol. 2016, 95, 236–241. [Google Scholar] [CrossRef]
- Yu, Y.; Cheng, Q.; Sha, C.; Chen, Y.; Naraginti, S.; Yong, Y. Size-controlled biosynthesis of FeS nanoparticles for efficient removal of aqueous Cr(VI). Chem. Eng. J. 2020, 379, 122404. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, X.; Pan, Z.; Wang, Y.; Xu, Q.; Bai, J.; Jia, H.; Zhou, J.; Yong, X.; Wu, X. Biosynthesized iron sulfide nanoparticles by mixed consortia for enhanced extracellular electron transfer in a microbial fuel cell. Bioresour. Technol. 2020, 318, 124095. [Google Scholar] [CrossRef]
- Fan, M.; Zhuang, X.; Gao, Z.; Lv, Z.; Dong, W.; Xin, F.; Chen, Y.; Jia, H.; Wu, X. Electroactive microorganisms synthesizing iron sulfide nanoparticles for enhanced hexavalent chromium removal in microbial fuel cells. Sci. Total Environ. 2023, 889, 164311. [Google Scholar] [CrossRef]
- Berner, R.A. Sedimentary pyrite formation: An update. Geochim. Cosmochim. Acta 1984, 48, 605–615. [Google Scholar] [CrossRef]
- Liang, B.; Cheng, H.; Kong, D.; Gao, S.; Sun, F.; Cui, D.; Kong, F.; Zhou, A.; Liu, W.; Ren, N.; et al. Accelerated reduction of chlorinated nitroaromatic antibiotic chloramphenicol by biocathode. Environ. Sci. Technol. 2013, 47, 5353–5361. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xiong, X.; Owens, G.; Brunetti, G.; Zhou, J.; Yong, X.; Xie, X.; Zhang, L.; Wei, P.; Jia, H. Anode modification by biogenic gold nanoparticles for the improved performance of microbial fuel cells and microbial community shift. Bioresour. Technol. 2018, 270, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Tong, F.; Yong, X.; Zhou, J.; Zhang, L.; Jia, H.; Wei, P. Effect of NaX zeolite-modified graphite felts on hexavalent chromium removal in biocathode microbial fuel cells. J. Hazard. Mater. 2016, 308, 303–311. [Google Scholar] [CrossRef]
- Zhuang, X.; Tang, S.; Dong, W.; Xin, F.; Jia, H.; Wu, X. Improved performance of Cr(VI)-reducing microbial fuel cells by nano-FeS hybridized biocathodes. RSC Adv. 2023, 13, 6768–6778. [Google Scholar] [CrossRef] [PubMed]
- Karamanev, D.G.; Nikolov, L.N.; Mamatarkova, V. Rapid simultaneous quantitative determination of ferric and ferrous ions in drainage waters and similar solutions. Miner. Eng. 2002, 15, 341–346. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Y.; Fang, Z.; Shi, Y.; Cheng, Q.; Chen, Y.; Shi, W.; Yang, C. Single cell electron collectors for highly efficient wiring-up electronic abiotic/biotic interfaces. Nat. Commun. 2020, 11, 4087. [Google Scholar] [CrossRef]
- Hao, L.; Zhang, B.; Cheng, M.; Feng, C. Effects of various organic carbon sources on simultaneous V(V) reduction and bioelectricity generation in single chamber microbial fuel cells. Bioresour. Technol. 2016, 201, 105–110. [Google Scholar] [CrossRef]
- Huang, L.; Chai, X.; Chen, G.; Logan, B.E. Effect of set potential on hexavalent chromium reduction and electricity generation from biocathode microbial fuel cells. Environ. Sci. Technol. 2011, 45, 5025–5031. [Google Scholar] [CrossRef]
- Hansel, C.M.; Lentini, C.J.; Tang, Y.; Johnston, D.T.; Wankel, S.D.; Jardine, P.M. Dominance of sulfur-fueled iron oxide reduction in low-sulfate freshwater sediments. ISME J. 2015, 9, 2400–2412. [Google Scholar] [CrossRef]
- Xia, D.; Yi, X.; Lu, Y.; Huang, W.; Xie, Y.; Ye, H.; Dang, Z.; Tao, X.; Li, L.; Lu, G. Dissimilatory iron and sulfate reduction by native microbial communities using lactate and citrate as carbon sources and electron donors. Ecotoxicol. Environ. Saf. 2019, 174, 524–531. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Huang, Y.; Zhang, K.; Lu, P. Simultaneous removal of organic matter and iron from hydraulic fracturing flowback water through sulfur cycling in a microbial fuel cell. Water Res. 2018, 147, 461–471. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Fu, Q.; Li, J.; Zhang, L.; Zhu, X.; Liao, Q. In situ biosynthesis of FeS nanoparticles boosts current generation in bioelectrochemical systems through efficient electron transfer. Small 2024, 20, e2309648. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Wu, J.; Cui, S.; Wang, X.; Liu, H.; He, R.; Yang, C.; Deng, X.; Tan, Z.; Li, W. Self-regenerable bio-hybrid with biogenic ferrous sulfide nanoparticles for treating high-concentration chromium-containing wastewater. Water Res. 2021, 206, 117731. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Xing, D.; Lu, L.; Wei, M.; Liu, B.; Ren, N. Ferric iron enhances electricity generation by Shewanella oneidensis MR-1 in MFCs. Bioresour. Technol. 2013, 135, 630–634. [Google Scholar] [CrossRef]
- Murugan, M.; Miran, W.; Masuda, T.; Lee, D.S.; Okamoto, A. Biosynthesized iron sulfide nanocluster enhanced anodic current generation by sulfate reducing bacteria in microbial fuel cells. ChemElectroChem. 2018, 5, 4015–40220. [Google Scholar] [CrossRef]
- Deng, X.; Dohmae, N.; Kaksonen, A.H.; Okamoto, A. Biogenic iron sulfide nanoparticles to enable extracellular electron uptake in sulfate-reducing bacteria. Angew. Chem. Int. Ed. 2020, 59, 5995–5999. [Google Scholar] [CrossRef]
- Liu, X.; Chen, M.; Wang, D.; Du, F.; Xu, N.; Sun, W.; Han, Z. Cr(VI) removal during cotransport of nano-iron-particles combined with iron sulfides in groundwater: Effects of D. Vulgaris S. putrefaciens. J. Hazard. Mater. 2024, 472, 134583. [Google Scholar] [CrossRef]
- Hou, X.; Huang, L. Synergetic magnetic field and loaded Fe3O4 for simultaneous efficient acetate production and Cr(VI) removal in microbial electrosynthesis systems. Chem. Eng. J. Adv. 2020, 2, 100019. [Google Scholar] [CrossRef]
- Ma, L.; Chen, N.; Feng, C.; Yao, Y.; Wang, S.; Wang, G.; Su, Y.; Zhang, Y. Enhanced Cr(VI) reduction in biocathode microbial electrolysis cell using Fenton-derived ferric sludge. Water Res. 2022, 212, 118144. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, J.; Mo, W.; Li, Q.; Wan, R.; Peng, S. Simultaneous treatment of chromium-containing wastewater and electricity generation using a plant cathode-sediment microbial fuel cell: Investigation of associated mechanism and influencing factors. Environ. Sci. Pollut. Res. 2023, 30, 41159–41171. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Mamathaxim, N.; Habibul, N.; Hu, Y. Simultaneous sulfamethazine and Cr(VI) removal in lab-scale microbial fuel cell-constructed wetland. J. Environ. Sci. 2025, 154, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Fei, K.; Song, T.; Wang, H.; Zhang, D.; Tao, R.; Xie, J. Electrophoretic deposition of carbon nanotube on reticulated vitreous carbon for hexavalent chromium removal in a biocathode microbial fuel cell. R. Soc. Open Sci. 2017, 4, 170798. [Google Scholar] [CrossRef]
- Du, J.; Bao, J.; Lu, C.; Werner, D. Reductive sequestration of chromate by hierarchical FeS@Fe0 particles. Water Res. 2016, 102, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Li, Y.; Li, J.; Wang, Y.; Xu, H.; Zhao, Y. Bioreduction of hexavalent chromium using a novel strain CRB-7 immobilized on multiple materials. J. Hazard. Mater. 2019, 368, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhao, Y.; Zhang, C.; Zhao, M.; Jia, X.; Mutabazi, E.; Liu, Y. New insights into hexavalent chromium exposure in electron donor limited denitrification: Bio-electron behavior. Bioresour. Technol. 2023, 380, 129088. [Google Scholar] [CrossRef]
- Feng, H.J.; Chen, L.; Ding, Y.C.; Ma, X.J.; How, S.W.; Wu, D. Mechanism on the microbial salt tolerance enhancement by electrical stimulation. Bioelectrochemistry 2022, 147, 108206. [Google Scholar] [CrossRef]
- Aliko, V.; Qirjo, M.; Sula, E.; Morina, V.; Faggio, C. Antioxidant defense system, immune response and erythron profile modulation in gold fish, Carassius auratus, after acute manganese treatment. Fish Shellfish Immunol. 2018, 76, 101–109. [Google Scholar] [CrossRef]
- Hou, R.; Luo, C.; Zhou, S.; Wang, Y.; Yuan, Y.; Zhou, S. Anode potential-dependent protection of electroactive biofilms against metal ion shock via regulating extracellular polymeric substances. Water Res. 2020, 178, 115845. [Google Scholar] [CrossRef]
- Cai, T.; Zhang, Y.; Wang, N.; Zhang, Z.; Lu, X.; Zhen, G. Electrochemically active microorganisms sense charge transfer resistance for regulating biofilm electroactivity, spatio-temporal distribution, and catabolic pathway. Chem. Eng. J. 2022, 442, 136248. [Google Scholar] [CrossRef]
- Akhzari, F.; Naseri, T.; Mousavi, S.M.; Khosravi-Darani, K. A sustainable solution for alleviating hexavalent chromium from water streams using Lactococcus lactis AM99 as a novel Cr(VI)-reducing bacterium. J. Environ. Manag. 2024, 353, 120190. [Google Scholar] [CrossRef]
- Zhuang, Z.; Yang, G.; Mai, Q.; Guo, J.; Liu, X.; Zhuang, L. Physiological potential of extracellular polysaccharide in promoting Geobacter biofilm formation and extracellular electron transfer. Sci. Total Environ. 2020, 741, 140365. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Jin, Y.; Bao, J.; Kang, D.; Xie, J. Graphene/biofilm composites for enhancement of hexavalent chromium reduction and electricity production in a biocathode microbial fuel cell. J. Hazard. Mater. 2016, 317, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Hu, J.; Lieber, A.M.; Jackan, C.S.; Biffinger, J.C.; Fitzgerald, L.A.; Ringeisen, B.R.; Lieber, C.M. Nanoparticle facilitated extracellular electron transfer in microbial fuel cells. Nano Lett. 2014, 14, 6737–6742. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhang, Y.; Yang, L.; Zhu, K.; Yuan, Y.; Li, G.; Yuan, Y.; Zhang, Q.; Bai, Z. Boosting oxygen reduction reaction kinetics through perturbating electronic structure of single-atom Fe-N3S1 catalyst with sub-nano FeS cluster. J. Colloid Interface Sci. 2023, 650, 924–933. [Google Scholar] [CrossRef]
- Zhang, P.; Xu, X.; Zhang, X.; Zou, K.; Liu, B.; Qing, T.; Feng, B. Nanoparticles-EPS corona increases the accumulation of heavy metals and biotoxicity of nanoparticles. J. Hazard. Mater. 2021, 409, 124526. [Google Scholar] [CrossRef]
- Amanze, C.; Zheng, X.; Anaman, R.; Wu, X.; Fosua, B.A.; Xiao, S.; Xia, M.; Ai, C.; Yu, R.; Wu, X.; et al. Effect of nickel (II) on the performance of anodic electroactive biofilms in bioelectrochemical systems. Water Res. 2022, 222, 118889. [Google Scholar] [CrossRef]
- Fu, X.; Wu, J.; Li, J.; Ding, J.; Cui, S.; Wang, X.; Wang, Y.; Liu, H.; Deng, X.; Liu, D.; et al. Heavy-metal resistant bio-hybrid with biogenic ferrous sulfide nanoparticles: pH-regulated self-assembly and wastewater treatment application. J. Hazard. Mater. 2023, 446, 130667. [Google Scholar] [CrossRef]
- Kumar, S.S.; Kumar, V.; Gude, V.G.; Malyan, S.K.; Pugazhendhi, A. Alkalinity and salinity favor bioelectricity generation potential of Clostridium, Tetrathiobacter and Desulfovibrio consortium in Microbial Fuel Cells (MFC) treating sulfate-laden wastewater. Bioresour. Technol. 2020, 306, 123110. [Google Scholar] [CrossRef]
- Thacker, U.; Parikh, R.; Shouche, Y.; Madamwar, D. Reduction of chromate by cell-free extract of Brucella sp. isolated from Cr (VI) contaminated sites. Bioresour. Technol. 2007, 98, 1541–1547. [Google Scholar] [CrossRef]
- Galai, S.; de los Ríos, A.P.; Hernández-Fernández, F.J.; Kacem, S.H.; Ramírez, F.M.; Quesada-Medina, J. Microbial fuel cell application for azoic dye decolorization with simultaneous bioenergy production using stenotrophomonas sp. Chem. Eng. Technol. 2015, 38, 1511–1518. [Google Scholar] [CrossRef]
- Salvian, A.; Farkas, D.; Ramirez-Moreno, M.; Torruella-Salas, D.; Berna, A.; Avignone-Rossa, C.; Varcoe, J.R.; Esteve-Nunez, A.; Gadkari, S. Resilience of anodic biofilm in microbial fuel cell biosensor for BOD monitoring of urban wastewater. npj Clean Water 2024, 7, 53. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, Y.; Liu, X.; Xu, J.; Liu, Y.; Guo, W.; Liu, X.; Chen, J. Effects of sulfamethoxazole on nitrogen removal and molecular ecological network in integrated vertical-flow constructed wetland. Ecotoxicol. Environ. Saf. 2021, 219, 112292. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xiao, W.; Wang, J.; Mirza, Z.A.; Wang, T. Optimized synthesis of FeS nanoparticles with a high Cr(VI) removal capability. J. Nanomater. 2016, 2016, 7817296. [Google Scholar] [CrossRef]
- Zhang, D.; Wei, Y.; Zhang, M.; Wu, S.; Zhou, L. A collaborative strategy for enhanced anaerobic co-digestion of food waste and waste activated sludge by using zero valent iron and ferrous sulfide. Bioresour. Technol. 2022, 347, 126420. [Google Scholar] [CrossRef]




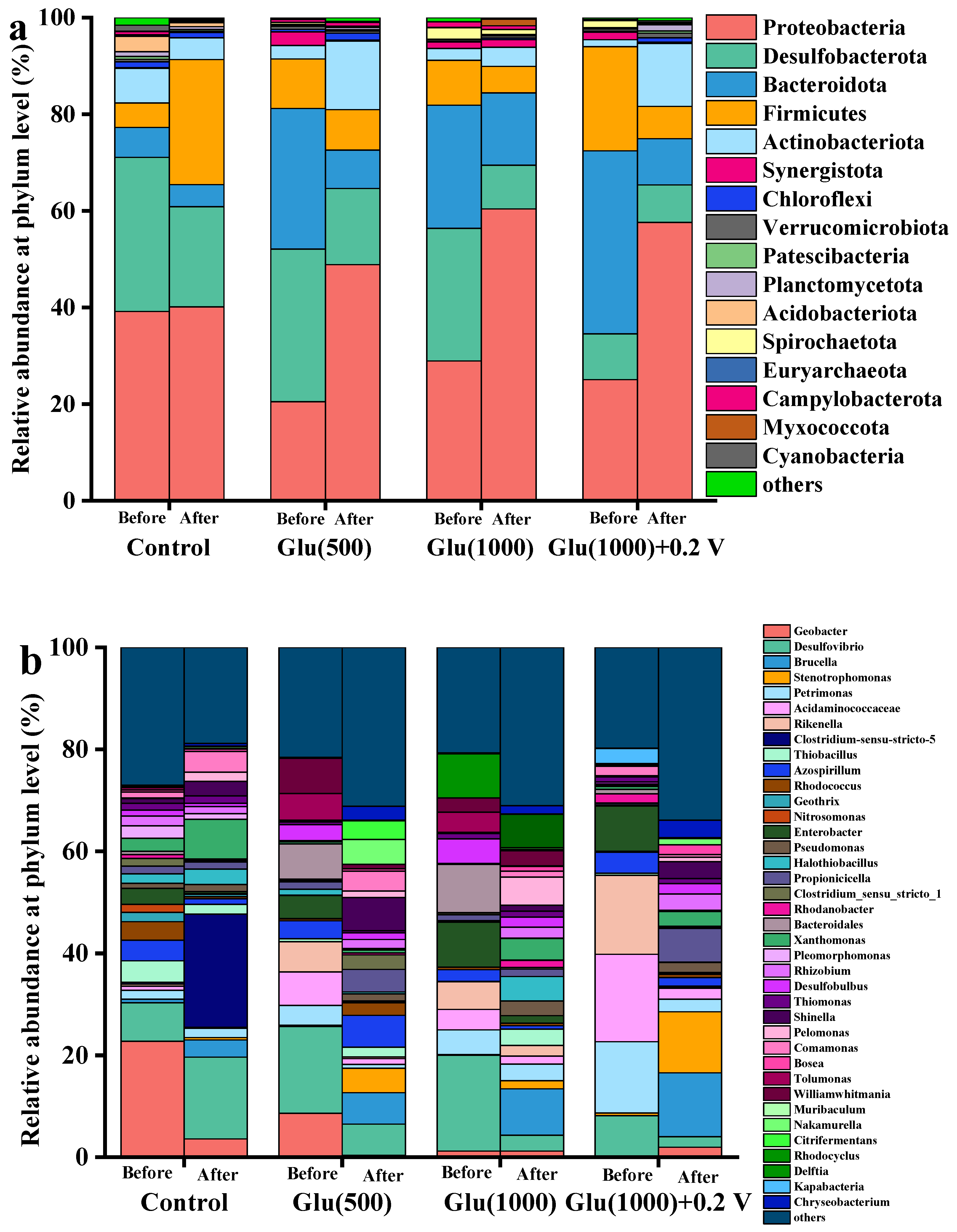
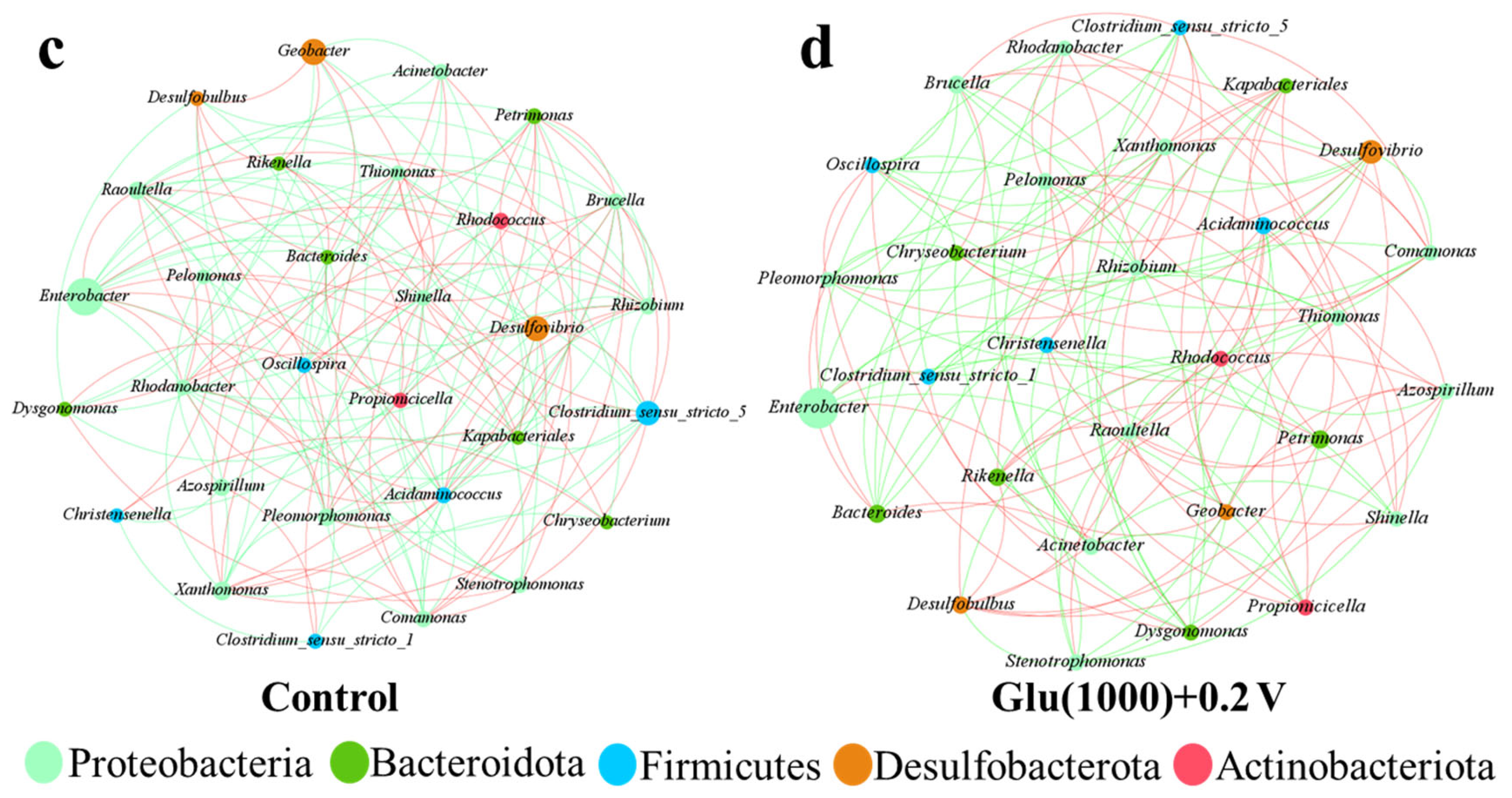
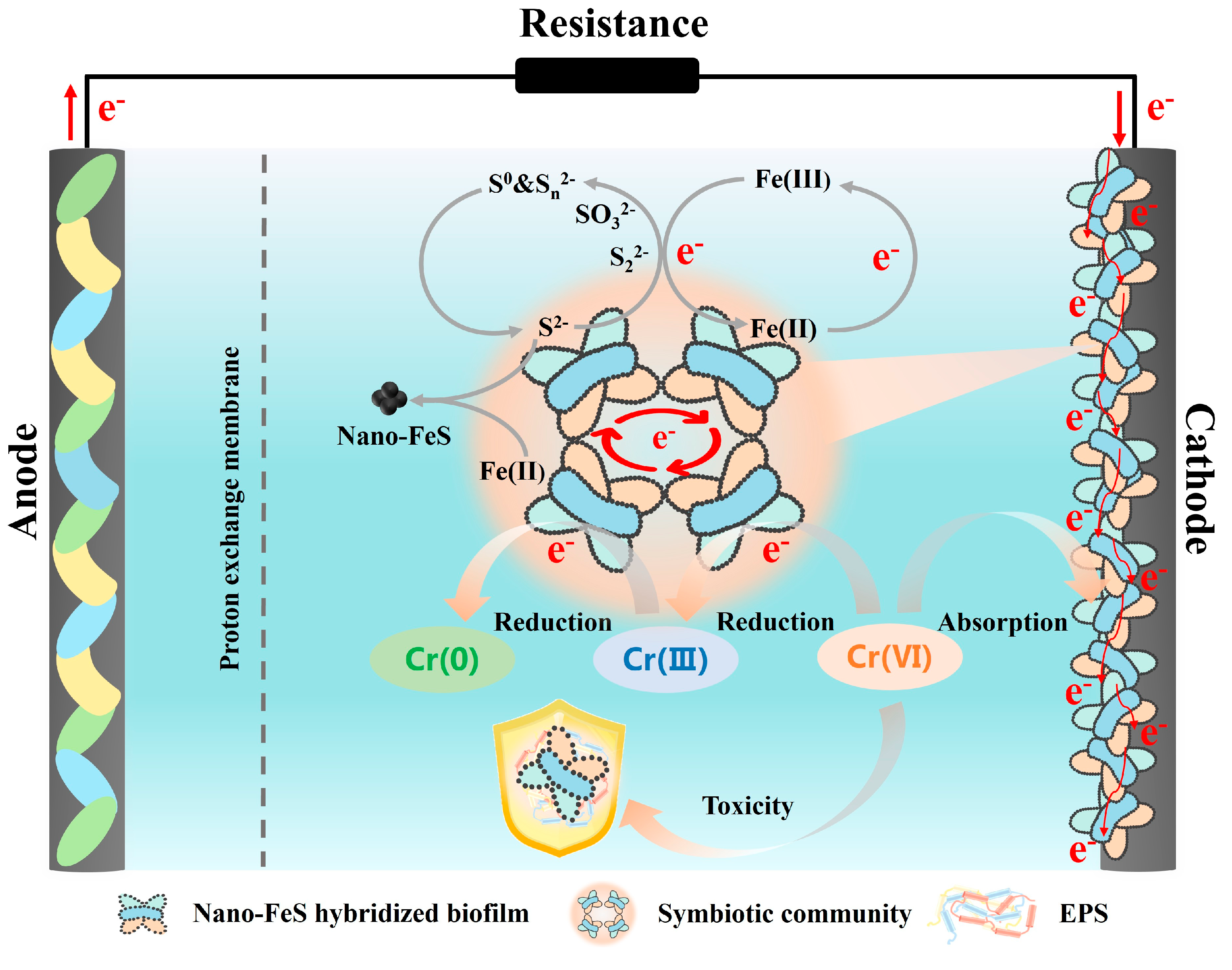
| BES Type | Biocathode Condition | Cr(VI) Concentration (mg/L) | Maximum Cr(VI) Removal Rate (mg/L·h) | Reaction Time (h) | Reference |
|---|---|---|---|---|---|
| Sediment microbial fuel cell (SMFC) | Plant biocathode | 108 | 0.19 | 624 | [40] |
| Microbial electrosynthesis system (MES) | Nano-Fe3O4 hybridized Serratia marcescens Q1-biocathode | 60 | 2.30 | 24 | [38] |
| Microbial electrolysis cell (MEC) | Fenton ferric sludge was incorporated into the mixed culture biocathode | 10 | 2.50 | 4 | [39] |
| Constructed wetland–microbial fuel cell (CW-MFC) | Plant biocathode | 10 | 0.17 | 60 | [41] |
| MFC | Mixed culture biocathode (Carbon nanotubes modified vitreous carbon as cathode electrode) | 20 | 0.78 | 48 | [42] |
| MFC | Graphene hybridized mixed culture–biocathode | 40 | 0.83 | 48 | [43] |
| MFC | Nano-FeS hybridized mixed culture–biocathode | 40 | 3.99 | 10 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Cao, D.; Tang, S.; Hu, Y.; Dong, W.; Wu, X. Efficient Chromium(VI) Removal Through In Situ Nano-Iron Sulfide Formation at the Cathode of Microbial Fuel Cells. Water 2025, 17, 2073. https://doi.org/10.3390/w17142073
Guo Y, Cao D, Tang S, Hu Y, Dong W, Wu X. Efficient Chromium(VI) Removal Through In Situ Nano-Iron Sulfide Formation at the Cathode of Microbial Fuel Cells. Water. 2025; 17(14):2073. https://doi.org/10.3390/w17142073
Chicago/Turabian StyleGuo, Yanyun, Diwen Cao, Shien Tang, Yujing Hu, Weiliang Dong, and Xiayuan Wu. 2025. "Efficient Chromium(VI) Removal Through In Situ Nano-Iron Sulfide Formation at the Cathode of Microbial Fuel Cells" Water 17, no. 14: 2073. https://doi.org/10.3390/w17142073
APA StyleGuo, Y., Cao, D., Tang, S., Hu, Y., Dong, W., & Wu, X. (2025). Efficient Chromium(VI) Removal Through In Situ Nano-Iron Sulfide Formation at the Cathode of Microbial Fuel Cells. Water, 17(14), 2073. https://doi.org/10.3390/w17142073






