Contribution to Distribution and Toxicity Prediction of Organic Pollutants in Receiving Waters from Wastewater Plant Tailwater: A Case Study of the Yitong River, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection
2.3. Water Quality Parameter Analysis
2.4. Instrumental Analysis of Organic Pollution
2.5. Prediction of Toxicity
2.6. Biodiversity Assessment
2.7. Data Processing and Statistical Analysis
3. Results and Discussion
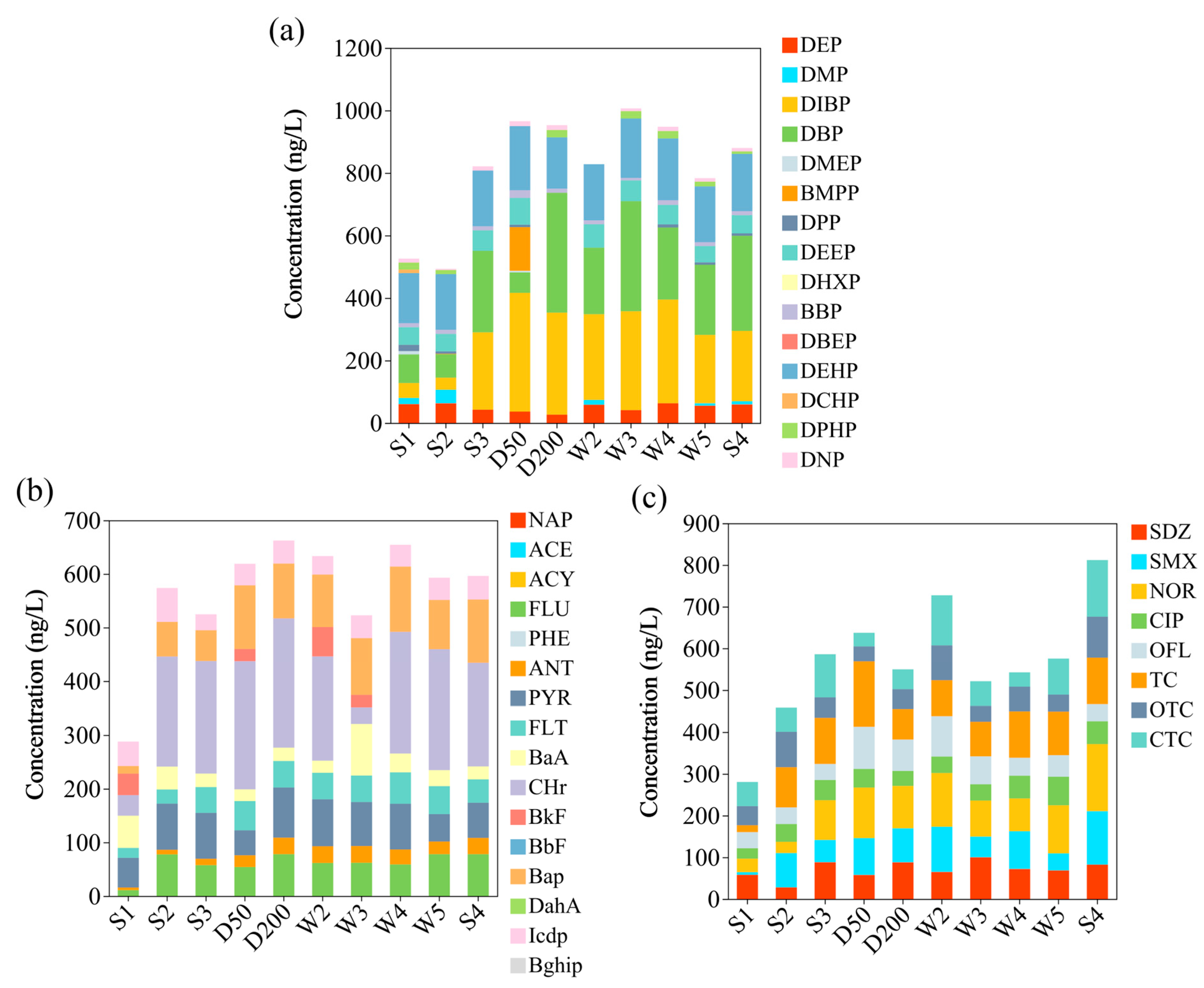
3.1. Organic Pollutant Composition in Urban Rivers
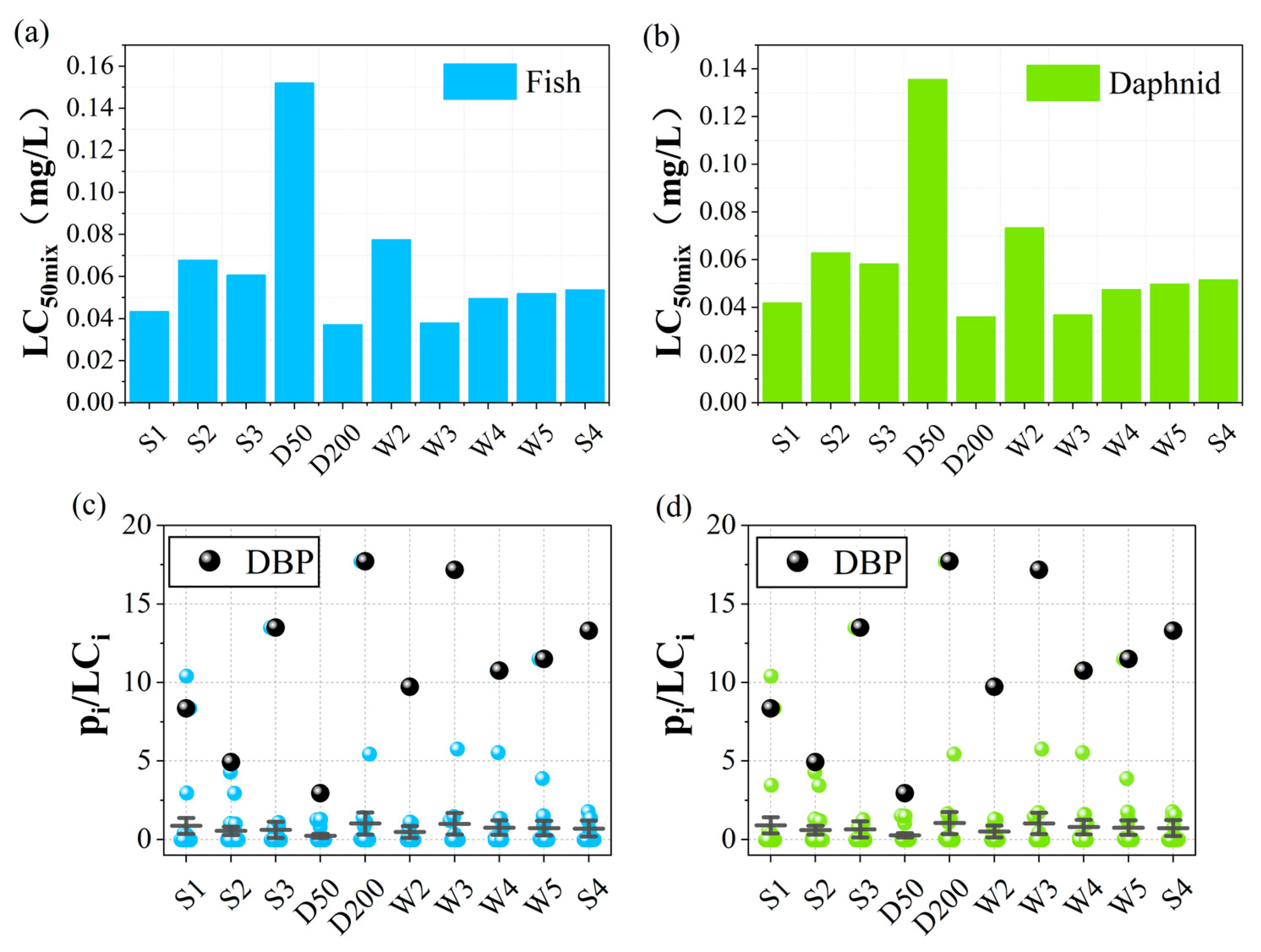
3.2. Mixed Toxicity Prediction Analysis
3.3. Toxicity Contribution
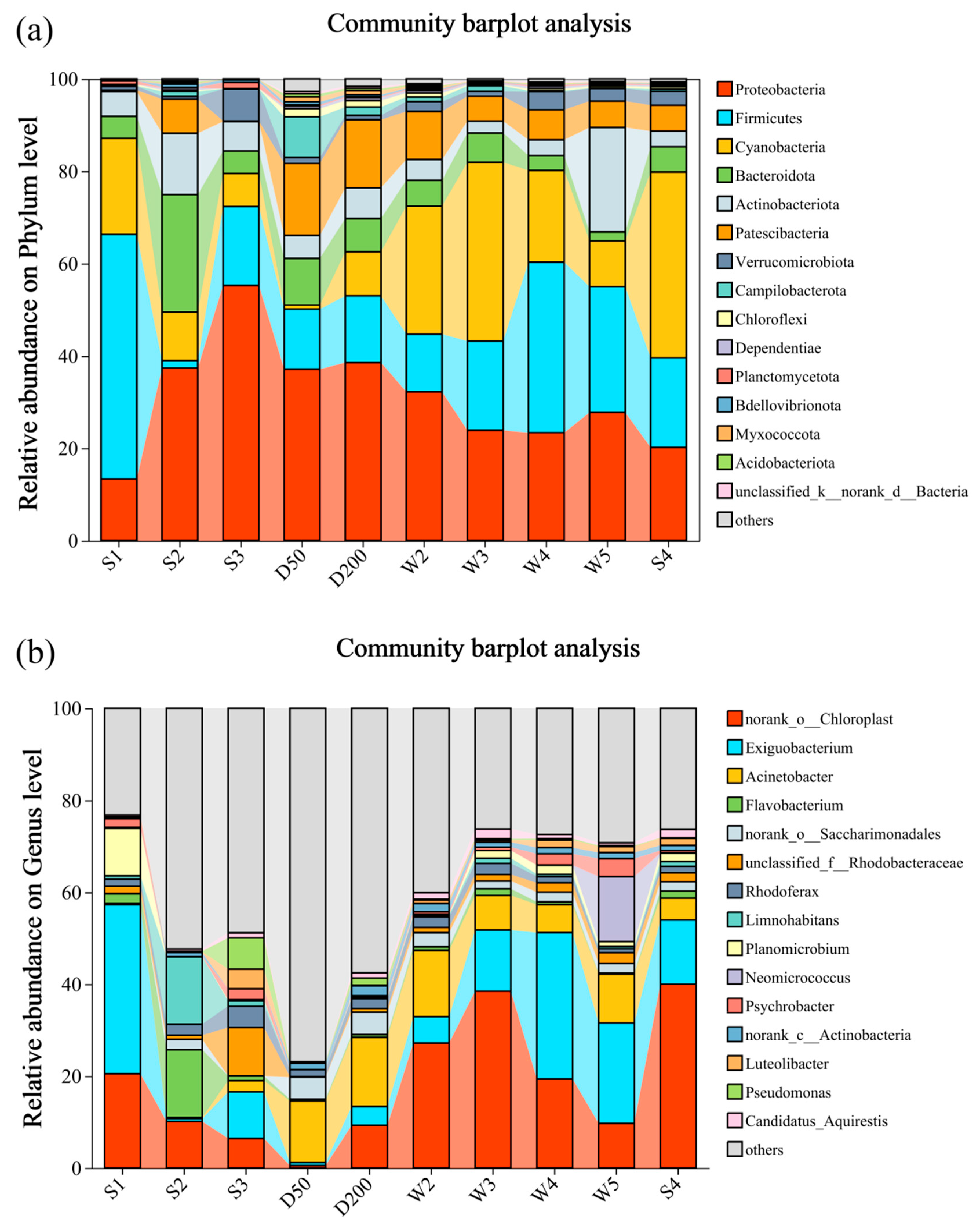
3.4. Effect of Pollutant Composition and Toxicity on Bacterial Community
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yan, Z.; Chen, Y.; Bao, X.; Zhang, X.; Ling, X.; Lu, G. Microplastic pollution in an urbanized river affected by water diversion: Combining with active biomonitoring. J. Hazard. Mater. 2021, 417, 126058. [Google Scholar] [CrossRef] [PubMed]
- Abdi, R.; Endreny, T.; Nowak, D. A model to integrate urban river thermal cooling in river restoration. J. Environ. Manag. 2020, 258, 110023. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Xu, R.; Li, D.; Jiang, S.; Cai, M.; Xiong, J. Source apportionment and ecological risk assessment of antibiotics in Dafeng River Basin using PMF and Monte-Carlo simulation. Environ. Geochem. Health 2024, 46, 479. [Google Scholar] [CrossRef]
- Cooper, R.J.; Warren, R.J.; Clarke, S.J.; Hiscock, K.M. Evaluating the impacts of contrasting sewage treatment methods on nutrient dynamics across the River Wensum catchment, UK. Sci. Total Environ. 2022, 804, 150146. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Santos, M.; Lanzén, A.; Unda-Calvo, J.; Martín, I.; Garbisu, C.; Ruiz-Romera, E. Treated and untreated wastewater effluents alter river sediment bacterial communities involved in nitrogen and sulphur cycling. Sci. Total Environ. 2018, 633, 1051–1061. [Google Scholar] [CrossRef]
- Chen, X.; Wang, M.; Kroeze, C.; Chen, X.; Ma, L.; Chen, X.; Shi, X.; Strokal, M. Nitrogen in the Yangtze River Basin: Pollution Reduction through Coupling Crop and Livestock Production. Environ. Sci. Technol. 2022, 56, 17591–17603. [Google Scholar] [CrossRef]
- He, Y.; Yuan, Q.; Mathieu, J.; Stadler, L.; Senehi, N.; Sun, R.; Alvarez, P.J. Antibiotic resistance genes from livestock waste: Occurrence, dissemination, and treatment. NPJ Clean Water 2020, 3, 4. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, R.; Guan, Y.; Zhuang, T.; Wang, Y.; Tan, R.; Wang, J.; Zhou, R.; Wang, B.; Xu, J.; et al. The profiling of elements and pesticides in surface water in Nanjing, China with global comparisons. Sci. Total Environ. 2021, 774, 145749. [Google Scholar] [CrossRef]
- Tian, X.; Qin, B.; Yang, L.; Li, H.; Zhou, W. Association of phthalate exposure with reproductive outcomes among infertile couples undergoing in vitro fertilization: A systematic review. Environ. Res. 2024, 252, 118825. [Google Scholar] [CrossRef]
- Gong, X.; Xiong, L.; Xing, J.; Deng, Y.; Qihui, S.; Sun, J.; Qin, Y.; Zhao, Z.; Zhang, L. Implications on freshwater lake-river ecosystem protection suggested by organic micropollutant (OMP) priority list. J. Hazard. Mater. 2024, 461, 132580. [Google Scholar] [CrossRef]
- Paluselli, A.; Aminot, Y.; Galgani, F.; Net, S.; Sempéré, R. Occurrence of phthalate acid esters (PAEs) in the northwestern Mediterranean Sea and the Rhone River. Prog. Oceanogr. 2018, 163, 221–231. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiao, Y.; Li, Z.; Tao, Y.; Yang, Y. Hazards of phthalates (PAEs) exposure: A review of aquatic animal toxicology studies. Sci. Total Environ. 2021, 771, 145418. [Google Scholar] [CrossRef] [PubMed]
- Machtinger, R.; Gaskins, A.J.; Racowsky, C.; Mansur, A.; Adir, M.; Baccarelli, A.A.; Hauser, R. Urinary concentrations of biomarkers of phthalates and phthalate alternatives and IVF outcomes. Environ. Int. 2018, 111, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Froger, C.; Quantin, C.; Gasperi, J.; Caupos, E.; Monvoisin, G.; Evrard, O.; Ayrault, S. Impact of urban pressure on the spatial and temporal dynamics of PAH fluxes in an urban tributary of the Seine River (France). Chemosphere 2019, 219, 1002–1013. [Google Scholar] [CrossRef]
- Countway, R.E.; Dickhut, R.M.; Canuel, E.A. Polycyclic aromatic hydrocarbon (PAH) distributions and associations with organic matter in surface waters of the York River, VA Estuary. Org. Geochem. 2003, 34, 209–224. [Google Scholar] [CrossRef]
- Liu, Y.; Beckingham, B.; Ruegner, H.; Li, Z.; Ma, L.; Schwientek, M.; Xie, H.; Zhao, J.; Grathwohl, P. Comparison of Sedimentary PAHs in the Rivers of Ammer (Germany) and Liangtan (China): Differences between Early- and Newly-Industrialized Countries. Environ. Sci. Technol. 2013, 47, 701–709. [Google Scholar] [CrossRef]
- Qi, P.; Qu, C.; Albanese, S.; Lima, A.; Cicchella, D.; Hope, D.; Cerino, P.; Pizzolante, A.; Zheng, H.; Li, J.; et al. Investigation of polycyclic aromatic hydrocarbons in soils from Caserta provincial territory, southern Italy: Spatial distribution, source apportionment, and risk assessment. J. Hazard. Mater. 2020, 383, 121158. [Google Scholar] [CrossRef]
- Cai, M.; Liu, M.; Hong, Q.; Lin, J.; Huang, P.; Hong, J.; Wang, J.; Zhao, W.; Chen, M.; Cai, M.; et al. Fate of Polycyclic Aromatic Hydrocarbons in Seawater from the Western Pacific to the Southern Ocean (17.5° N to 69.2° S) and Their Inventories on the Antarctic Shelf. Environ. Sci. Technol. 2016, 50, 9161–9168. [Google Scholar] [CrossRef]
- Osińska, A.; Korzeniewska, E.; Harnisz, M.; Felis, E.; Bajkacz, S.; Jachimowicz, P.; Niestępski, S.; Konopka, I. Small-scale wastewater treatment plants as a source of the dissemination of antibiotic resistance genes in the aquatic environment. J. Hazard. Mater. 2020, 381, 121221. [Google Scholar] [CrossRef]
- Chen, B.; Liang, X.; Huang, X.; Zhang, T.; Li, X. Differentiating anthropogenic impacts on ARGs in the Pearl River Estuary by using suitable gene indicators. Water Res. 2013, 47, 2811–2820. [Google Scholar] [CrossRef]
- Lekunberri, I.; Villagrasa, M.; Balcázar, J.L.; Borrego, C.M. Contribution of bacteriophage and plasmid DNA to the mobilization of antibiotic resistance genes in a river receiving treated wastewater discharges. Sci. Total Environ. 2017, 601–602, 206–209. [Google Scholar] [CrossRef]
- Li, F.; Zhang, Y.; Altermatt, F.; Zhang, X. Consideration of Multitrophic Biodiversity and Ecosystem Functions Improves Indices on River Ecological Status. Environ. Sci. Technol. 2021, 55, 16434–16444. [Google Scholar] [CrossRef] [PubMed]
- Fanaei, F.; Shahryari, T.; Mortazavi, M.; Nasseh, N.; Pourakbar, M.; Barikbin, B. Hazard identification and integrated risk assessment of drinking water supply system from catchment to consumer based on the World Health Organization’s Water Safety Plan. Desalin. Water Treat. 2023, 286, 257–273. [Google Scholar] [CrossRef]
- Song, L.; Xie, K. Engineering CRISPR/Cas9 to mitigate abundant host contamination for 16S rRNA gene-based amplicon sequencing. Microbiome 2020, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Barber, L.B.; Faunce, K.E.; Bertolatus, D.W.; Hladik, M.L.; Jasmann, J.R.; Keefe, S.H.; Kolpin, D.W.; Meyer, M.T.; Rapp, J.L.; Roth, D.A.; et al. Watershed-Scale Risk to Aquatic Organisms from Complex Chemical Mixtures in the Shenandoah River. Environ. Sci. Technol. 2022, 56, 845–861. [Google Scholar] [CrossRef]
- Kumar, M.; Saggu, S.K.; Pratibha, P.; Singh, S.K.; Kumar, S. Exploring the role of microbes for the management of persistent organic pollutants. J. Environ. Manag. 2023, 344, 118492. [Google Scholar] [CrossRef]
- Zhu, Q.; Xu, L.; Wang, W.; Liu, W.; Liao, C.; Jiang, G. Occurrence, spatial distribution and ecological risk assessment of phthalate esters in water, soil and sediment from Yangtze River Delta, China. Sci. Total Environ. 2022, 806, 150966. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.L.; Ohashi, A.; Ozaki, N.; Kindaichi, T. Comprehensive review of polycyclic aromatic hydrocarbons in water sources, their effects and treatments. Sci. Total Environ. 2019, 696, 133971. [Google Scholar] [CrossRef]
- Fabregat-Safont, D.; Gracia-Marín, E.; Ibáñez, M.; Pitarch, E.; Hernández, F. Analytical key issues and challenges in the LC-MS/MS determination of antibiotics in wastewater. Anal. Chim. Acta 2023, 1239, 340739. [Google Scholar] [CrossRef]
- Liu, Y.; Su, B.; Mu, H.; Zhang, Y.; Chen, L.; Wu, B. Effects of point and nonpoint source pollution on urban rivers: From the perspective of pollutant composition and toxicity. J. Hazard. Mater. 2023, 460, 132441. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, S.; Bai, M.; Wang, S.; Li, F. Distribution, seasonal variation and ecological risk assessment of phthalates in the Yitong River, a typical urban watercourse located in Northeast China. Sci. Total Environ. 2024, 930, 172696. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, T.; Liu, J.; Lu, G.; Wang, H.; Li, Y.; Zheng, F.; Gao, P. Pipeline misconnections in separated sewer systems worsen urban river ecological status through stormwater discharge. J. Environ. Manag. 2025, 389, 126100. [Google Scholar] [CrossRef] [PubMed]
- Ibekwe, A.M.; Ma, J.; Murinda, S.E. Bacterial community composition and structure in an Urban River impacted by different pollutant sources. Sci. Total Environ. 2016, 566–567, 1176–1185. [Google Scholar] [CrossRef]
- Hallas, J.F.; Mackowiak, C.L.; Wilkie, A.C. Mitigating rural WWTP impacts: System dynamics modeling of downstream nutrient outputs. Sci. Total Environ. 2020, 744, 140809. [Google Scholar] [CrossRef]
- Han, T.; Gao, L.; Chen, J.; He, X.; Wang, B. Spatiotemporal variations, sources and health risk assessment of perfluoroalkyl substances in a temperate bay adjacent to metropolis, North China. Environ. Pollut. 2020, 265, 115011. [Google Scholar] [CrossRef]
- Marizzi del Olmo, A.; López-Doval, J.C.; Hidalgo, M.; Serra, T.; Colomer, J.; Salvadó, V.; Escolà Casas, M.; Medina, J.S.; Matamoros, V. Holistic assessment of chemical and biological pollutants in a Mediterranean wastewater effluent-dominated stream: Interactions and ecological impacts. Environ. Pollut. 2025, 370, 125833. [Google Scholar] [CrossRef]
- Tao, H.-Y.; Shi, J.; Zhang, J.; Ge, H.; Zhang, M.; Li, X.-Y. Developmental toxicity and mechanism of dibutyl phthalate and alternative diisobutyl phthalate in the early life stages of zebrafish (Danio rerio). Aquat. Toxicol. 2024, 272, 106962. [Google Scholar] [CrossRef]
- Pei, Y.; Luo, K.; Zeng, J.; Gao, J.; Zhang, H. Review of the zebrafish (Danio rerio) model in disinfection by-products (DBPs) toxicity research: Opportunities and challenges. J. Hazard. Mater. 2025, 495, 138976. [Google Scholar] [CrossRef]
- Li, Z.; Gong, Y.; Okeke, E.S.; Li, D.; Chen, Y.; Feng, W.; Zhao, T.; Yang, L.; Mao, G.; Wu, X. Novel insights into DBP-induced zebrafish liver inflammatory damage: Ferroptosis activating the HMGB1-TLR4-NF-κB signaling pathway. Environ. Pollut. 2025, 376, 126395. [Google Scholar] [CrossRef]
- Gao, M.; Xu, Y.; Liu, Y.; Wang, S.; Wang, C.; Dong, Y.; Song, Z. Effect of polystyrene on di-butyl phthalate (DBP) bioavailability and DBP-induced phytotoxicity in lettuce. Environ. Pollut. 2021, 268, 115870. [Google Scholar] [CrossRef]
- Gaur, N.; Narasimhulu, K.; Y, P. Recent advances in the bio-remediation of persistent organic pollutants and its effect on environment. J. Clean. Prod. 2018, 198, 1602–1631. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, J.; Sun, Z.; Orem, W.H.; Tatu, C.A.; Radulović, N.S.; Milovanović, D.; Pavlović, N.M.; Chan, W. Analysis of Polycyclic Aromatic Hydrocarbons and Phthalate Esters in Soil and Food Grains from the Balkan Peninsula: Implication on DNA Adduct Formation by Aristolochic Acid I and Balkan Endemic Nephropathy. Environ. Sci. Technol. 2021, 55, 9024–9032. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Zhang, X.J.; Yang, Y.; Huang, T.; Li, C.; Su, L.; Zhao, Y.H.; Cronin, M.T.D. Development of thresholds of excess toxicity for environmental species and their application to identification of modes of acute toxic action. Sci. Total Environ. 2018, 616–617, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Sigurnjak Bureš, M.; Ukić, Š.; Cvetnić, M.; Prevarić, V.; Markić, M.; Rogošić, M.; Kušić, H.; Bolanča, T. Toxicity of binary mixtures of pesticides and pharmaceuticals toward Vibrio fischeri: Assessment by quantitative structure-activity relationships. Environ. Pollut. 2021, 275, 115885. [Google Scholar] [CrossRef]
- Severiano, J.d.S.; de Lima, E.R.P.; de Lucena-Silva, D.; Rocha, D.K.G.; Veríssimo, M.E.S.; Figueiredo, B.R.S.; de Lucena Barbosa, J.E.; Molozzi, J. The role of bioturbation triggered by benthic macroinvertebrates in the effectiveness of the Floc & Lock technique in mitigating eutrophication. Water Res. 2023, 246, 120691. [Google Scholar] [CrossRef]
- Cleary, D.W.; Bishop, A.H.; Zhang, L.; Topp, E.; Wellington, E.M.H.; Gaze, W.H. Long-term antibiotic exposure in soil is associated with changes in microbial community structure and prevalence of class 1 integrons. FEMS Microbiol. Ecol. 2016, 92, fiw159. [Google Scholar] [CrossRef]
- Pascual-Benito, M.; Ballesté, E.; Monleón-Getino, T.; Urmeneta, J.; Blanch, A.R.; García-Aljaro, C.; Lucena, F. Impact of treated sewage effluent on the bacterial community composition in an intermittent mediterranean stream. Environ. Pollut. 2020, 266, 115254. [Google Scholar] [CrossRef]
- Wang, Q.; Liang, J.; Zhao, C.; Bai, Y.; Liu, R.; Liu, H.; Qu, J. Wastewater treatment plant upgrade induces the receiving river retaining bioavailable nitrogen sources. Environ. Pollut. 2020, 263, 114478. [Google Scholar] [CrossRef]
- Romero, F.; Sabater, S.; Font, C.; Balcázar, J.L.; Acuña, V. Desiccation events change the microbial response to gradients of wastewater effluent pollution. Water Res. 2019, 151, 371–380. [Google Scholar] [CrossRef]
- Nam, K.; Rodriguez, W.; Kukor, J.J. Enhanced degradation of polycyclic aromatic hydrocarbons by biodegradation combined with a modified Fenton reaction. Chemosphere. 2001, 45, 11–20. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, D.; Ma, W.; Guo, Y.; Wang, A.; Wang, Q.; Lee, D.J. Denitrifying sulfide removal process on high-salinity wastewaters in the presence of Halomonas sp. Appl. Microbiol. Biotechnol. 2016, 100, 1421–1426. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Mago, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods. 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Stackebrandt, E.; Goebel, B.M. Taxonomic Note: A Place for DNA-DNA Reassociation and 16S rRNA Sequence Analysis in the Present Species Definition in Bacteriology. Int. J. Syst. Evol. Microbiol. 1994, 44, 846–849. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Nave Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]



| Target Compound | Sample Preparation | Instrumental Analysis | References |
|---|---|---|---|
| PAEs | Solid-phase extraction | Analysis via gas chromatography–mass spectrometry (GC–MS) | [27] |
| PAHs | Solid-phase extraction | Analysis via gas chromatography–mass spectrometry (GC–MS) | [28] |
| Antibiotic | Solid-phase extraction | Liquid chromatography–tandem mass spectrometry(LC-MS/MS) | [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Bai, M.; Dong, A.; Du, X.; Ding, Y.; Zhao, K. Contribution to Distribution and Toxicity Prediction of Organic Pollutants in Receiving Waters from Wastewater Plant Tailwater: A Case Study of the Yitong River, China. Water 2025, 17, 2061. https://doi.org/10.3390/w17142061
Zhang X, Bai M, Dong A, Du X, Ding Y, Zhao K. Contribution to Distribution and Toxicity Prediction of Organic Pollutants in Receiving Waters from Wastewater Plant Tailwater: A Case Study of the Yitong River, China. Water. 2025; 17(14):2061. https://doi.org/10.3390/w17142061
Chicago/Turabian StyleZhang, Xiaoyu, Mingxuan Bai, Ang Dong, Xinrong Du, Yuzhu Ding, and Ke Zhao. 2025. "Contribution to Distribution and Toxicity Prediction of Organic Pollutants in Receiving Waters from Wastewater Plant Tailwater: A Case Study of the Yitong River, China" Water 17, no. 14: 2061. https://doi.org/10.3390/w17142061
APA StyleZhang, X., Bai, M., Dong, A., Du, X., Ding, Y., & Zhao, K. (2025). Contribution to Distribution and Toxicity Prediction of Organic Pollutants in Receiving Waters from Wastewater Plant Tailwater: A Case Study of the Yitong River, China. Water, 17(14), 2061. https://doi.org/10.3390/w17142061





