Abstract
In this study, the presence of heavy metals (HMs) is determined to assess surface water contamination; biosorbent materials are also used to remove them and thus improve their quality. The objective of this work was to study the spatial distribution of HMs in water samples from Tequesquitengo Lake, Morelos, Mexico; pectin was also used for HM biosorption. For this, fifteen water samples were collected from the central and peripheral zones of the lake; HMs such as Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb, Zn, As, and Hg were identified and quantified by atomic absorption spectroscopy (AAS). The metal evaluation index (HEI) was calculated, as well as the percentage of HM removal with pectin. The water samples presented high concentrations of Pb, Cr, and Mn in contrast to the other HMs studied. Furthermore, these showed high concentrations (161.2, 85.2, and 65.6 µg/L, respectively) in the peripheral zone. Therefore, these values exceed the permissible limit for human consumption, except for Mn. The HEI value indicated that the lake water exhibits low contamination. After the adsorption of HMs with pectin, Cr (100%), Ni (83%) and Cd (37%) were removed, reducing the total concentration of HMs in the water in all samples.
1. Introduction
Heavy metals (HMs) are pollutants and may come from a variety of natural sources such as volcanism, bedrock and weathering [1], as well as anthropogenic sources such as mining, the chemical industry, wastewater, the fertilizers and pesticides used in agriculture, vehicles, and tourism; this is due to urbanization [2,3,4]. HMs such as cadmium (Cd), mercury (Hg) and lead (Pb) are toxic even at low concentrations, and can cause degenerative diseases in humans [5,6]; meanwhile, iron (Fe), magnesium (Mg), and zinc (Zn) are considered essential for living beings, but can affect health at high concentrations [7]. On the other hand, the presence of HMs in the environment and particularly in aquatic ecosystems has caused a decrease in water quality and changes in physicochemical characteristics, thus limiting its consumption and use [8]. Based on the above, various research groups have implemented some HM evaluation indices to estimate the level of contamination in surface waters and sediments. To do this, they have used a scale of values that consider the concentrations of evaluated heavy metals and their concentrations allowed by existing standards [9,10].
On the other hand, several research groups have studied the removal of contaminants such as HMs, dyes, the bacteria present in synthetic and surface waters using natural coagulants (bio-adsorbents), such as moringa seeds, algae and their components, agro-industrial waste, and Opuntia ficus-indica and its pectic components, among others. Several studies highlight the efficiency, low cost, and environmental friendliness of these bioadsorbents [11,12,13,14,15]. Pectin is an anionic polysaccharide obtained from the cell wall of plants, is mainly composed of 1-4-α-D-galactopyranosyl uronic units (α-D-galacturonic acid), and some of its carboxyl groups may be methoxylated (O-CH3); it may also contain L-rhamnose [16,17]. Pectin obtained from orange peel or other citrus fruits has been used in the removal of metal ions and in evaluating the highest removal or adsorption capacity through the effect of various parameters such as the adsorbent dose, pH, and type of metal ion, among others.
Gandhi et al. [18] studied the use of dehydrated Opuntia ficus-indica in the removal of chromium (VI) at pH values ranging from 3 to 9 and obtained better metal removal with an increasing pH, attributed to a greater negative charge on the colloidal surfaces that retain chromium (VI). Shao et al. [19] studied the adsorption of Cu (II), Cd (II), Hg (II) and Pb (II) in aqueous solution (synthetic water) on chitosan–pectin gel beads, evaluating the effect of pH and its adsorption mechanism. The authors found that chitosan–pectin beads had a relatively high capacity to adsorb Cu (II), Cd (II), Hg (II) and Pb (II) (80 mg/g) at pH values ranging from 4 to 9, attributed to the fact that the deprotonation of amino and carboxylate groups is favored in that pH range, promoted by the interaction between the metals and the beads, forming complexes with the functional groups (carboxyl, carbonyl, hydroxyl), in addition to ion exchange by electrostatic attraction. This means that the beads could be used in the removal of heavy metals from various natural waters. Currently, most studies on the use of pectin in the removal of metal ions have been conducted with aqueous solutions made with metal salts (synthetic water), and there are few studies where this material has been applied in natural waters.
It is known that lakes are bodies of surface water made up of biological diversity. They were generated from geological formations and help regulate the climate, as well as maintain a balance in the ecosystem where it is located; they are generally freshwater, but there are also saline lakes [8,20]. Surface waters or bodies of water are of great importance for human beings, since they are necessary for the development of economic activities, particularly in the agricultural, industrial, livestock and aquaculture sectors, and they are mainly used for consumption and energy generation.
Tequesquitengo Lake is in a peri-urban area of the state of Morelos and is considered the main water reservoir in Morelos. Martínez-Rodríguez and Gutiérrez-Ojeda [21] analyzed the water level variations in Tequesquitengo Lake; they assessed the interaction between the lake and the underlying aquifer, as well as the transfer of water from the Colotepec River for the irrigation system and the presence of a deep aquifer. They concluded that the deep aquifer is the cause of an increase in the water level in the lake, while the lower level is related to a decrease in the aquifer by showing a relationship between the water levels of the lake and the levels of the underlying aquifer.
Castellanos-Trujillo and Palacios-Mayorga [22] studied the physical, chemical and biological characteristics of water samples collected in Tequesquitengo Lake in order to contribute knowledge related to the occasional phenomenon of fish mortality. They obtained high electrical conductivity and total residual solid values, a high Mg/Ca ratio, and high concentrations of sodium, sulfates and strontium; thus, with these results, the lake was classified as oligohaline. They also postulated that the convection of waters by surface cooling generates the consumption of dissolved O2 by sulfide ions, leading to the massive asphyxiation of fish and marine fauna.
Various recreational and sporting activities are carried out on Tequesquitengo Lake, which is why it is one of the main tourist destinations in Morelos. However, the waters of the lake, due to the activities carried out there and its location, are exposed to contamination by anthropogenic and natural effects, respectively. Therefore, the objective of this study was to evaluate the physicochemical parameters and the total and dissolved concentration of heavy metals in the waters of Tequesquitengo Lake, as well as the removal of heavy metals by pectin.
2. Materials and Methods
2.1. Study Area and Collection of Water Samples
Tequesquitengo Lake is in the municipality of Jojutla, Morelos, central Mexico (latitude 18°31′13.44′′ to 18°38′58.2′′ N and longitude 99°18′6.84′′ to 99°8′52.44′′ W). It is close to the Amacuzac, Apatlaco and Coatetelco rivers, as well as Rodeo Lake. The Chichinautzin Biological Corridor Park and the city of Cuernavaca are in the north of the lake, and it is in the northeast of the Iztaccihuatl–Popocatepetl National Park. Tequesquitengo Lake has a basin area of 30 km2, a flow rate of 168 hm3, and an average temperature of 24.5 °C; approximately 57,682 people live in the periphery of the lake [23]. Furthermore, the main uses of the lake’s water are related to recreational, agricultural, livestock and fishing activities [24]. In addition, industry has developed near the lake; this is related to the hotel industry, the production of chemical and agrochemical products, as well as the automotive and pharmaceutical industries.
In the area of Tequesquitengo Lake, fifteen sampling stations were selected (Figure 1) to evaluate the presence and concentration of heavy metals, and water samples were collected at equal distances during March 2019 (post-bridge holiday). The sampling stations were divided into two different areas for a better understanding of the results: Peripheral area (samples 1–9) and Central area (samples 10–15). The water samples were collected in high-density polyethylene containers (3.8 L), previously washed with deionized water, and immersed in an ultrapure solution of 2% nitric acid (HNO3, Fermont, Productos Químicos Monterrey, Monterrey, Mexico). After collecting the water samples, they were taken to the laboratory and stored in a cold chamber (4 °C) for further analysis [25].
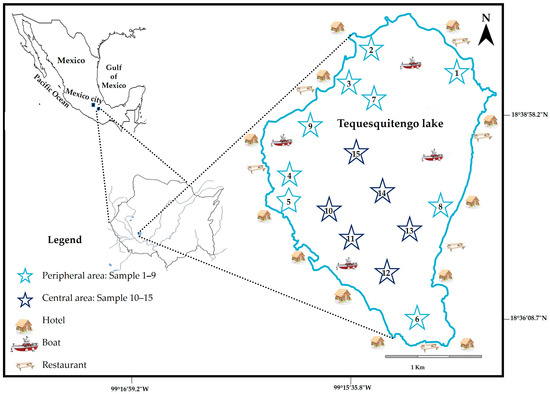
Figure 1.
Map showing the area of the sampling sites along Tequesquitengo Lake, Morelos, Mexico.
2.2. Field and Laboratory Analysis of Water Samples
The physicochemical parameters, namely pH, turbidity, conductivity and temperature, were determined in situ for each water sample; these were determined using an potentiometer (pH Tests 30 model, Oakton Ltd., Melbourne, Australia), a portable turbidimeter (model HI 93703, HANNAH Instruments, Smithfield, RI, USA), and an conductivity meter (aqua model, HM Digital, Carson, CA, USA), respectively. The precision of direct measurements was ±0.5 NTU for turbidity, ±0.01 for pH, ±2% for conductivity, and ±0.5 °C for temperature.
The concentration of total/dissolved HMs in the water samples was determined as follows: The water samples were filtered using 0.45 µm membranes (preconditioned with 1% HNO3) (only for dissolved HMs) and acidified with 2 mL of HNO3 (Fermont, Productos Químicos Monterrey, Monterrey, Mexico); subsequently, each of the samples (500 mL) was digested and analyzed in an atomic absorption spectrophotometer (AAS) (Analyst 100 model, Perkin Elmer, Springfield, IL, USA). The concentration of Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb and Zn was determined using the flame technique, while for As and Hg, the hydride generation technique was used. Milli Q water was used as an internal control and standard reference material (High purity 1605428) to maintain quality control, with a recovery percentage between 88 and 109% for the elements analyzed. Each of the determinations was carried out in triplicate.
2.3. Data Assessment—Heavy Metal Evaluation Index (HEI)
The heavy metal evaluation index (HEI) gives an estimate of the overall quality of the water with respect to HMs and it is expressed as ∑Hc/Hmax, where Hc is the monitored value of the ith parameter and Hmax is the maximum permissible concentration of the ith parameter. The HEI values are classified into three categories, which include Low contamination (HEI > 400), medium contamination (400 < HEI < 800) and high contamination (HEI > 800) [9].
2.4. Removal Experiments
The heavy metal removal experiments using pectin were carried out using the jar method [26] and performed according to the methodology reported by González-Avilez et al. [11], with minor modifications made to the speed of rapid stirring. The study was carried out with 15 water samples at pH 5.5 (using 0.1 N HCl, to promote the removal process) and a temperature of 25 ± 1 °C. For the HM removal studies, pectin from citrus peel (200 mg) (Sigma-Aldrich, Saint Luois, MO, USA) was added to 200 mL of water sample (concentration of 1 g/L); this was followed by rapid stirring (230 rpm) for 1 min, slow stirring (85 rpm) for 30 min and resting for 1 h.
The water samples were then centrifuged at 10,000 rpm for 8 min (Model 323-K model, Hermle-LaborTechnik GmbH–Z, Wehingen, Germany), and the pH, turbidity, and conductivity were determined in the recovered supernatant. Also, the concentration of HMs in the supernatant was determined by AAS. The evaluation of each of the 15 water samples was carried out in triplicate. The initial total concentration of heavy metals (IC) in the different water samples was calculated using Equation (1):
where Ci (µg/mL) is the initial concentration of each of the HMs (Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb, Zn, As and Hg) studied in each water sample. After the removal process, the removal of HMs (crHM) in each sample was calculated using Equation (2):
where Cf (µg/mL) is the final concentration of each of the HM’s present in the water samples after the removal process. The total concentration of the HMs removed (TcrHM) was calculated with the addition of crHM values for each sample (Equation (3)).
Finally, the total concentration of unremoved metals (TcnrHM) was calculated with the addition of Cf values for each metal (Equation (4)). The percentage of HMs removed (%rHM) from the water samples was calculated using Equation (5):
2.5. Analysis by ATR-FTIR
The identification of the functional groups of pectin, before and after the HM removal process, was carried out by Fourier Transform Infrared Spectrophotometry (FTIR) using an IR-Affinity 1 (Shimadzu Co., Kyoto, Japan) with an ATR plate; this was operated in a frequency range of 4000–800 cm−1 and at a resolution of 4 cm−1.
2.6. Statistical Analysis
The statistical analysis of the physicochemical parameters of the studied water samples, the standard deviation, the statistical differences, as well as the correlation analysis between said parameters and the concentrations of total heavy metals, before and after removal, was carried out using Statistica software version 12.0 (2013, StatSoft Inc. Tulsa, OK, USA) with significance levels of p < 0.05, 0.01 and 0.001.
3. Results and Discussion
3.1. Physicochemical Parameters
The spatial variations in the physical parameters of the water samples collected from Tequesquitengo Lake are presented in Figure 2a–d. The temperature ranges of the lake areas studied were 28.3 to 31.5 °C (average 30 °C) due to the topography of the region; this temperature is typical of that recorded in this area of the State of Morelos during the month of March [27]. The minimum and maximum pH values were 8.9 and 9.1 for the peripheral zone (average 9.0) and between 8.9 and 9.0, respectively, for the central zone of the lake (average 8.9). Castellano-Trujillo and Palacios-Mayorga [22] and Hernández and Tapia [28] studied water samples from the same lake in different decades and reported pH values similar to those obtained in the present work. They mentioned that the water was alkaline and reported values between 8.5 and 8.7 and 7.7 and 8.5, respectively. On the other hand, the high pH values presented by the water samples from Tequesquitengo Lake may be related to the geochemical composition of the soil in the region, which is made up of tequesquite [sodium chloride (NaCl) and sodium carbonate (NaCO3)] and calcareous rocks [calcium carbonate (CaCO3)] [29]. These compounds could dissociate in water to generate hydroxyl ions (OH−), which allows the pH of the water to increase (Figure 3) [29,30].
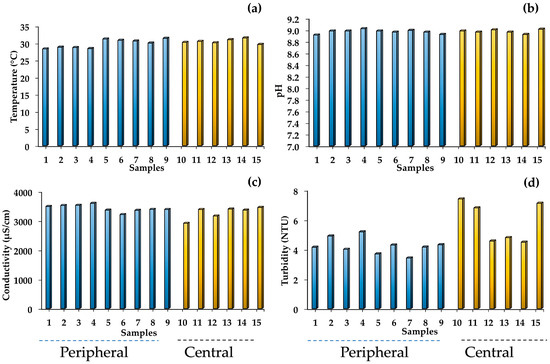
Figure 2.
Physical characteristics of the water in Tequesquitengo Lake, Morelos, Mexico. (a) Temperature, (b) pH, (c) Conductivity, (d) Turbidity.
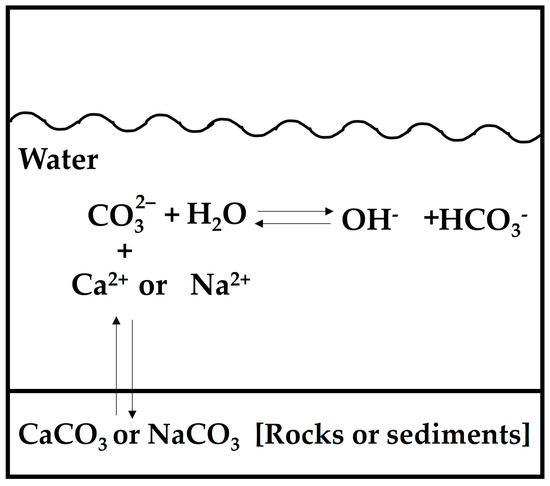
Figure 3.
Dissociation of compounds of the rocks.
The electrical conductivity values were between 3211 and 3594 µS/cm for the peripheral area (average 3426 µS/cm), and between 2911 and 3454 µS/cm for the central area of the lake (average 3279 µS/cm). The conductivity values indicate the presence of dissociated ions (cations and anions) in the water; therefore, higher values may be due to the dissolution of rocks in the lake [31]. Regarding turbidity, the distribution pattern revealed a wide range of variations as follows: between 3.42 and 5.18 NTU for the peripheral area (average 4.23 NTU) and between 4.48 and 7.4 NTU for the central lake area (average 5.86 NTU). The turbidity values in the central zone suggest that they may be due to fine particles of rocks or mud in the lake, while the lower values may be influenced by external factors [3].
3.2. Heavy Metals (Total and Dissolved)
The concentrations of total and dissolved heavy metals (Cd, Cu, Cr, Ni, Pb, Fe and Mn) quantified in the water samples are presented in the graphs shown in Figure 4. These show the variations in the total fractions and those dissolved; it is also seen that the relative order decreased as follows: Peripheral area of the lake: Pb (114 to 231 µg/L and 82 to 225 µg/L, for total and dissolved, respectively) > Cr (58 to 131 µg/L and 46 to 91 µg/L) > Mn (50 to 77 µg/L and 46 to 74 µg/L) > Ni (3 to 34 µg/L and 2 to 30 µg/L) > Cu (0 to 40 µg/L and 0 to 17 µg/L) > Cd (10 to 17 µg/L and 2 to 13 µg/L) > Fe (0 to 21 µg/L and 0 to 18 µg/L). Central area of the lake: Pb (118 to 164 µg/L and 105 to 141 µg/L) > Mn (57 to 74 µg/L and 46 to 74 µg/L) > Cr (17 to 80 µg/L and 0 to 67 µg/L) > Ni (0 to 35 µg/L and 0 to 25 µg/L) > Fe (6 to 42 µg/L and 0 to 20 µg/L) > Cd (7 to 12 µg/L and 2 to 11 µg/L) > Cu (0 to 14 µg/L and 0 to 3 µg/L).
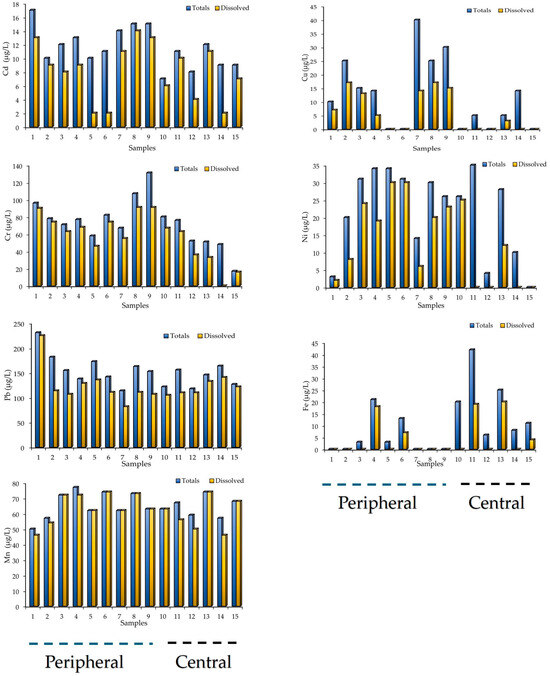
Figure 4.
Total and dissolved heavy metal concentrations in the water in Tequesquitengo Lake, Morelos, Mexico.
The water samples collected in the peripheral zone of the lake presented the highest concentrations of total heavy metals, namely Pb (average 161.2 µg/L), Cr (average 85.2 µg/L) and Mn (average 65.6 µg/L); this result can be attributed to aquatic activities (use of boats) and hotels, which are carried out and used with high frequency during vacations. This result is consistent with what was reported by Retama et al. [31], who reported a high concentration of heavy metals in sediments collected from tourist beaches in Huatulco, Oaxaca, Mexico. Also, this result could be due to domestic and agricultural activities and the dissolution of rocks in the lake water [3]. The quantified concentrations of Pb and Cr in the present study are comparable with the permissible values documented in SSA [32] and WHO standards [33] (Table 1). This result shows that these HMs exceeded the recommended limit values, while the concentration of Mn in the water samples is within the permissible limit in water according to the limit values documented in the SSA [32] and WHO standards [33]. Thus, the water samples collected in the central area of the lake presented the highest concentrations of Fe (average 18.6 µg/L); this result may be due to the type of soil and the town sunk in the lake [21]. The concentration of Fe quantified in these water samples is within the permissible limit values reported in the WHO [33] and SSA standards [32].

Table 1.
Total heavy metal concentration in water of Tequesquitengo Lake, Morelos, Mexico.
The concentrations of Cu and Ni quantified in the water samples of Tequesquitengo Lake were compared with the permissible values according to WHO [33] and SSA [32] standards and were within the permissible limits; meanwhile, the Cd concentration exceeded the value allowed in those standards. Heavy metals such as As, Hg, Zn and Co were not detected in most of the lake water samples (except for some samples) (Table 1). The absence of some HMs (As and Zn) in the lake is a good result, since these HMs are highly toxic even at low concentrations [9,10]. This result is consistent with what was reported by Castellanos-Trujillo and Palacios-Mayorga [22], who related it to its elimination in the liquid phase by the S = of the anoxic in the central zone of the lake. Therefore, the results obtained in the present work indicated that the concentrations of As, Hg, Zn and Co are within the permissible limits according to what is reported in the WHO [33] and SSA [32] standards. The total concentrations of HMs studied in the present work were compared with the concentrations of HMs found in other saline water lakes from other countries in the world, to evaluate their contamination. The average total concentrations of Mn, Cu, Cr, Cd, Ni, Pb, Zn and Fe in the present study were lower than the concentrations reported in Mangla Dam Lake in Pakistan [3] and Tanis Lake in Egypt [34].
3.3. Correlation Matrix
The correlation matrix between the physical characteristics of and total heavy metal concentrations in water samples from the peripheral and central areas of Tequesquitengo Lake is presented in Table 2. In the peripheral area, a significant positive correlation indicates a close relationship between pH and Mn (R2 = 0.73; p < 0.05), Fe and Mn (R2 = 0.72; p < 0.05) and Mn and Ni (R2 = 0.81; p < 0.01), suggesting that the above association of these elements is due to alloys of the Fe-Mn oxides of sunken objects in the lake, such as the bell and cross of the church [35], a wrought iron fence, and a ladder used in the beach area to help people enter the lake to swim, and the possible weathering of rocks [31].

Table 2.
Correlation matrix of the physicochemical characteristics of water samples from Tequesquitengo Lake, Morelos, Mexico.
The significant positive correlation between temperature and Cu (R2 = 0.85; p < 0.05) and Cu and Pb (R2 = 0.86 p < 0.05) in the central area of the lake suggests that the copper and lead ions in the water are due to anthropogenic sources mainly derived from pipes, paint, propeller shafts and aquatic fittings [3]; the significant positive correlation between Cr and Ni (R2 = 0.85; p < 0.05) is due to the weathering of ultramafic rocks [36] and products with these alloys in them, such bronzers, turbine parts, and valve stems [37,38].
3.4. Heavy Metal Evaluation Index (HEI)
The HEI values calculated for the sampling station range from 13.5 to 30.7, with a mean value of 17.35; therefore, the lake is considered less contaminated (values > 400) [9]. These results indicate that the concentration pattern of the lake is influenced by geological formations in the region (naturally enriched).
3.5. Removal
The results obtained from the physicochemical characteristics evaluated in the water studied before and after the removal process with pectin are shown in Table 3. The pH of a solution is a very important factor in the process of removal because of its influence on the surface adsorbent properties and the absorbate speciation [15,39]. The pH of all the samples of water in the peripheral and central areas studied was weakly acidic and showed a decrease compared with the initial pH (pH 5.5 to pH ~ 4). A possible explanation for this result could be that some functional groups (carboxylic acid), due to the pectin (the absorbent material) being in an aqueous medium (water samples) and having the characteristics of a weak acid, are deprotonated in a greater or lesser proportion due to excess of free H+ ions in the water; therefore, the pH decreases [13,40]. Meanwhile, the conductivity of the water of the lake studied after the removal decreased compared to the initial values; this was due to the reduction in water ions caused by removal with pectin. The turbidity of the water samples after the removal process showed two behaviors (increase and decrease of turbidity); an increase was observed in the peripheral area (samples 1, 5 and 6) and central area (samples 12, 13 and 14), while a decrease was observed in the rest of samples and attributed to the capacity of the pectin to adsorb particles in the water [41].

Table 3.
Physicochemical characteristics of Tequesquitengo Lake before and after the removal process.
The total concentration of heavy metals in the water before and after the removal process was determined using Equation (1) for the IC (initial total concentration), Equation (3) for the TcrHM (total concentration of removed metals) and Equation (4) for the TcnrHM (total concentration of unremoved metals); the results are shown in Figure 5. Most of the water samples studied after the process of removal with pectin showed a higher total concentration of heavy metals removed compared to heavy metals not removed.
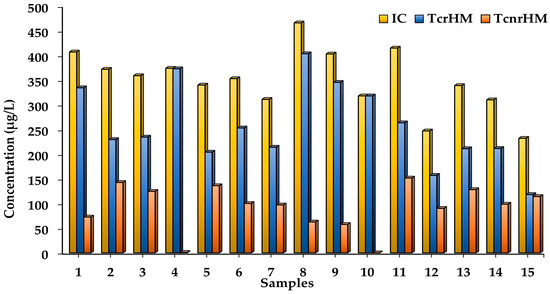
Figure 5.
The total concentration of heavy metals in the water throughout the removal process.
Also, it was observed that the total initial concentration (values of 232 to 466 µg/L) can be a factor that influences the total concentration of metals removed (values of 12 to 4 µg/L) in samples 1–4, 6–9, 11 and 14. The highest TcrHM values were observed in samples 1, 4, 8, 9 and 10, with 33, 37, 4, 35 and 31 µg/L; this corresponds to the 82, 99.7, 86.5, 85.7 and 100% total removal of metals, respectively. It is clear that in samples 4 and 10, more than 99% of the metals studied were removed, with an initial pH of 5.5 and an initial turbidity greater than 5. The results confirm the capacity of the pectin to remove heavy metals from the water samples of Tequesquitengo Lake; this could be through the interactions presented between the positive charges of the metal ions (M+) and the negative charges of the functional groups or active sites of the pectin (OH−, COOH−) [40].
The percentage of heavy metals removed (Mn, Cd, Cr, Ni, Pb and other metals) from the water of Tequesquitengo Lake using pectin is shown in Table 4. The heavy metals studied in all samples varied in their %rHM, for the Cr showed complete removal in all the samples of water (100%). These results could be attributed to the initial pH of samples (pH 5.5), considering that the optimum pH for the removal of chromium in solution is pH 5.5; this is according to the results obtained by Castañeda-Figueredo et al. [42] and Ehsanpour et al. [43] in a study on the removal of chromium (VI) from aqueous solution using an eggshell/poly pyrrole composite and the effect of the particle size of orange, potato, and passion fruit peel (bioadsorbent) on the removal of lead and chromium, respectively. On the contrary, the %rHM for Mn was higher than 70% in only three samples, and most of the samples were between 33 and 60%; these percentages are medium and low, and can be attributed to the initial pH and concentration of metal. This is because the optimum pH for the removal of Mn is between pH 6 and 8 according to the results obtained in a study on rice husk ash as an adsorbent material in synthetic water [44] and on the root of V. zizanoides as an adsorbent in a hydroponic system [45], respectively.

Table 4.
Percentage of heavy metals removed with pectin in water samples from Tequesquitengo Lake.
From the results presented in Table 4, it can be observed that the percentages of heavy metal removal by pectin presented the following order: Cr (100%) > Ni (91.5%) > Cd (83.5%) > Pb (67.4%) > Mn (49%). This result may be related to the affinity of the heavy metals present in the water samples for the functional groups, namely, the hydroxyl and carboxyl groups that make up the pectin molecules; these confer a negative electrical charge to the macromolecules, which allows them to interact with the heavy metals [11]. Furthermore, this affinity is related to the type of chemical interaction and the order of union between the metal ions and the pectin molecules, and is based on Pearson’s theory of Lewis acids and bases [46]. This theory mentions that Lewis’s acids and bases are classified as hard and soft; hard acids prefer to bind to hard bases to form ionic complexes, while soft acids prefer to bind to soft bases to form covalent complexes.
On the other hand, the carboxyl (COO−) and methoxyl (COOCH3) groups present in pectin macromolecules are attributed to the characteristic of hard bases or nuclei; likewise, metal ions such as Na+, Ca2+, Cr2+, and Fe3+ are considered hard acids, while Cd2+, Pb2+, and Ni2+ are considered soft acids. Therefore, the affinity between pectin’s functional groups and the Cr2+ metal ion can be explained by the selectivity between hard bases and acids, suggesting strong ionic interactions [46,47]. On the other hand, in the case of Ni2+, Cd2+ and Pb2+, they present a large ionic radius (0.69, 0.97 and 1.19 Å, respectively) and low electronegativity values (1.91, 1.7 and 2.33 Pauling, respectively); the ionic bonds that can occur between these heavy metals and the pectin molecules could be polar covalent, which suggests moderately strong interactions [47].
Moreover, according to Wang et al. [48], both the adsorption capacity and selectivity of pectin for heavy metals depend on their origin. For example, in a study by Ibarra-Rodríguez et al. [47], they found the following order of affinity for a cactus pectin: Ca2+ > Cu2+ > Zn2+ > Cr3+ > Ni2+ > Pb2+ > Cd2+; this could explain the high removal percentages obtained for Cr and Ni in the present study, suggesting the high affinity of these metal ions for pectin. Also, the main adsorption mechanisms between pectin and HMs are based on ion exchange, complex formation, and surface adsorption [49]. This is because pectin, by containing abundant carboxyl and hydroxyl groups, is allowed to bind to metal ions through these mechanisms [50].
The removal of the metals Cd, Ni and Pb varied in the samples; for example, for Ni and Cd, high values of removal were obtained in most of the samples, at 100% and greater than 80%, respectively. These results are attributed to the initial pH of the water of the lake (pH 5.5), because they are within the optimum value for the adsorption of metals (pH 3.7 to 7 for Cd, pH 5 for Ni) according to what was reported by Onditi et al. [40] and Gutiérrez et al. [51]. Meanwhile, Pb showed lower removal in most of the samples, with values greater than 52%. Similar observations were obtained in [41,42], where the use of polysaccharides derived from Opuntia in the removal of Pb2+ in synthetic water was studied and it was found that the better pH for removal was between 5 and 6, with a removal percentage of 80%, demonstrating that the removal depends on pH and the initial concentration of the metal; the latter is caused by the fact that the active sites of the flocculant (pectin) interact with the metal ion (Pb2+), attracting them via electrostatic charges, and because when the active sites are saturated with metal ions, electrostatic repulsion is generated.
The physicochemical values of the water samples from Tequesquitengo Lake and the Cf of each heavy metal in the water samples after the removal process with pectin as a bio-adsorbent material and their permissible values for drinking water established by the standard are shown in Table 3 and Table 5. In these tables, it can be observed that, after the removal process, the water had a concentration of Cd, Cr, Ni and Mn less than the initial values and that the values obtained are within the permissible ranges for human use and consumption established by the Official National and International Standard. Moreover, the concentration of heavy metals such as Cd and Cr was reduced below the permissible limits; also, the turbidity values are reduced and less than the permissible limits for most of the samples. The results obtained in the present study consider pectin as a valuable material that could be used to remove Cd, Cr, Mn, Cu and Ni in fresh water.

Table 5.
Final concentration of heavy metals in water samples from Tequesquitengo Lake.
3.6. ATR-FTIR
The FTIR spectra of the citrus pectin before and after the heavy metal removal process in water samples from the peripheral and central area of the lake are shown in Figure 6. The transmittance bands were used to determine the vibrational frequency of functional groups and their changes; this helped to identify the functional groups that are related to the bridging process with metal ions in the water samples [13,17]. The pectin sample spectra before the removal process showed bands at around 3371 cm−1 and were due to the bounded O-H stretching of carboxylic acid and alcohols: other bands at 2951 and 2893 cm−1 corresponded to the vibrations of the C-H asymmetric stretching of the methyl group (alkanes/alkyl).
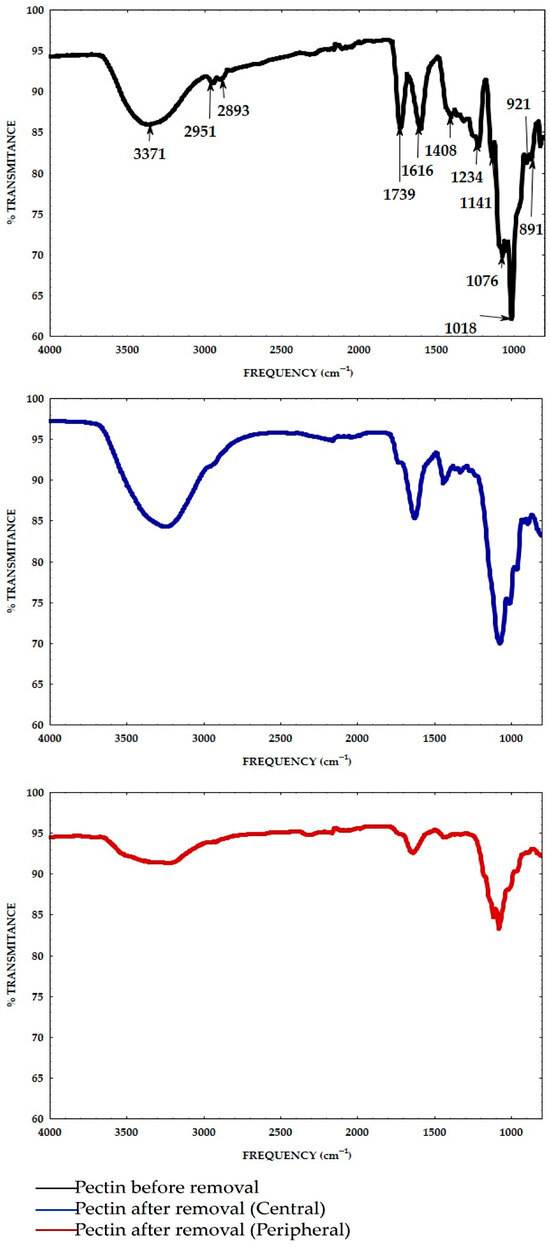
Figure 6.
FTIR spectra of citrus pectin before and after the heavy metal removal process, and after the removal of heavy metals from water samples in the peripheral and central area with pectin.
Bands at 1739 cm−1 corresponded to the C=O stretching vibration of carboxylic acid (COOH groups) in the polysaccharides components; also, the bands at 1616 and 1408 cm−1 are due to the COO− asymmetrical and symmetric stretching vibration of carboxylic acid (COOH groups), and these bands are related to polygalacturonic acid; other bands at 1234 and 1141 cm−1 corresponded to the C-O-C stretching vibration of other groups and glycoside bonds, with the latter possibly being related to long molecules. The pentopyranoses are characterized by the presence of a band that appears at around 1076 cm−1, which can be assigned to CH-O-H, C-H and C-C-; the main characteristic of polysaccharides rich in polygalacturonic acid is the presence of a band at around 1018 cm−1, as observed in the pectin sample. The bands between 1200 and 800 cm−1 correspond to the fingerprint region specific to each polysaccharide. Meanwhile, the pectin spectra were compared before and after the removal of heavy metals for the central and peripheral samples, thus showing the disappearance of bands at 2951, 2893, 1739, 1141, 921 and 829 cm−1 and/or the shifting of 3371 to 3251, 1616 to 1635, 1076 to 1087, and 1018 to 1002 cm−1 after the removal process with pectin, demonstrating that polysaccharides are involved in removal by bridging between carbonyl, carboxyl, and hydroxyl groups of pectin and heavy metals in water samples. The results are consistent with Castañeda-Figueredo et al. [42], who studied the effect of particle size and different bio-adsorbent materials (organic peels) on the removal of lead and chromium from solutions. They reported similar bands and changes in the FTIR spectra of orange peel (pectin) after the removal process, as shown in our study, that were attributed to the adsorption of the cations.
The results obtained in this study demonstrate that pectin can be used as an adsorbent material in the treatment of water contaminated by heavy metals. Therefore, after fulfilling its function, this adsorbent could be treated with an acidic or alkaline solution that breaks the bonds generated with the metal ions and makes it possible to use the pectin in several adsorption/desorption cycles [52]. Furthermore, the HM-free pectin polymer, as a biodegradable material, could biodegrade in the soil after completing its useful life, increasing the organic matter content in soils and providing a carbon source for the microbial community [53]. This would positively contribute to the profitability of the adsorption process and to the environment [54,55].
4. Conclusions
The physicochemical parameters (temperature, pH, conductivity, and turbidity) and total and dissolved concentration of heavy metals (Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb, Zn, As and Hg) in the waters of Tequesquitengo Lake, as well as the removal of heavy metals with pectin studied, contribute to the generation of a first-hand database on the pollution of the lake in relation to heavy metals and the use of pectin as a bio-adsorbent material in heavy metal removal, respectively. Overall, the pH of the water in the lake varied little, in association with the geochemical composition of the region. The concentrations of heavy metals also differed between the peripheral and central areas, with the higher values of Pb, Cr, and Mn in the peripheral area and Fe in the central area being associated with aquatics and hotel activities during day-bridge holidays, sunken objects in the lake and local geological formations. Pb and Cr exceeded the maximum permissible limits for human consumption established by national and international standards.
The removal of heavy metals from water samples obtained from Tequesquitengo Lake at a pectin dose of 1 mg/mL had a 100%rHM of Cr and there were variations in the %rHM of Mn, Cd, Ni and Pb in water samples from the peripheral and central area; this was due to bridging between the functional groups and heavy metals, where pectin showed the ability to reduce the concentration of Cr to values within the permissible ranges for human use and consumption.
Author Contributions
Conceptualization, S.V.V.-S., J.A.S.-C. and F.R.-G.; methodology, S.V.V.-S., Y.Y.L.-D., F.R.-G. and A.O.-R.; software, S.V.V.-S., F.R.-G. and S.S.M.-G.; validation, S.V.V.-S., Y.Y.L.-D., F.R.-G. and S.S.M.-G.; formal analysis, S.V.V.-S., Y.Y.L.-D., F.R.-G. and A.O.-R.; investigation, S.V.V.-S., Y.Y.L.-D., J.A.S.-C., R.M.-V., M.L.C.R. and F.R.-G.; resources, F.R.-G.; data curation, S.V.V.-S., F.R.-G. and A.O.-R.; writing—original draft preparation, S.V.V.-S., J.A.S.-C. and F.R.-G.; writing—review and editing, S.V.V.-S., A.O.-R. and F.R.-G.; visualization, S.V.V.-S., Y.Y.L.-D. and F.R.-G.; supervision, S.V.V.-S. and F.R.-G.; project administration, F.R.-G.; funding acquisition, F.R.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Secretaria de Investigación y Posgrado del Instituto Politécnico Nacional (SIP-IPN). Projects Nos. 20230817, 20240661 and 20250066.
Data Availability Statement
The raw/processed data required to reproduce these findings are available upon request.
Acknowledgments
This research was supported by Secretaria de Investigación y Posgrado del Instituto Politécnico Nacional (SIP-IPN) (Projects Nos. 20230817, 20240661 and 20250066). Y.Y.L.-D. wishes to thank CONAHCyT (Mexico) for the master fellowship. A.O.-R. wishes to thank SECIHTI (Mexico) for the postdoctoral Fellowship.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| HMs | Heavy metals |
| HEI | The metal evaluation index |
| AAS | Atomic Absorption Spectrophotometer |
| FTIR | Fourier Transform Infrared Spectrophotometry |
References
- Viana, J.L.M.; Souza, A.F.; Hernández, A.H.; Elias, L.P.; Eismann, C.E.; Rezende-Filho, A.T.; Barbiero, L.; Menegario, A.A.; Fostier, A.H. In situ arsenic speciation at the soil/water interface of saline-alkaline lakes of the Pantanal, Brazil: A DGT-based approach. Sci. Total Environ. 2022, 804, 150113. [Google Scholar] [CrossRef] [PubMed]
- Hoaghia, M.; Cadar, O.; Moisa, C.; Roman, C.; Kovacs, E. Heavy metals and health risk assessment in vegetables grown in the vicinity of a former non-metallic facility located in Romania. Environ. Sci. Pollut. Res. 2022, 29, 40079–40093. [Google Scholar] [CrossRef] [PubMed]
- Muneer, J.; AlObaid, A.; Ullah, R.; Rehman, K.U.; Erinle, K.O. Appraisal of toxic metals in water, bottom sediments and fish of fresh water lake. J. King Saud. Univ. Sci. 2022, 34, 101685. [Google Scholar] [CrossRef]
- Wurtsbaugh, W.A.; Leavitt, P.R.; Moser, K.A. Effects of a century of mining and industrial production on metal contamination of a model saline ecosystem, Great Salt Lake, Utah. Environ. Pollut. 2020, 266, 115072. [Google Scholar] [CrossRef]
- Barhoumi, B.; Le Menach, K.; Clérandeau, C.; Ameur, W.B.; Budzinski, H.; Driss, M.R.; Cachot, J. Assessment of pollution in the Bizerte lagoon (Tunisia) by the combined use of chemical and biochemical markers in mussels, Mytilus galloprovincialis. Mar. Pollut. Bull. 2014, 84, 379–390. [Google Scholar] [CrossRef]
- Ghribi, F.; Richir, J.; Bejaoui, S.; Boussouf, A.D.; Marengo, M.; El Cafsi, M.; Gobert, S. Trace elements and oxidative stress in the Ark shell Arca noae from a Mediterranean coastal lagoon (Bizerte lagoon, Tunisia): Are there health risks associated with their consumption? Environ. Sci. Pollut. Res. 2020, 27, 15607–145623. [Google Scholar] [CrossRef]
- Helal, U.A.B.; Khalid, R.S.; Khan, U.A.; Abba, S.A. A review Heavy metals contamination in traditional medicinal products. J. Appl. Pharm. 2013, 4, 748–756. [Google Scholar]
- Zhang, C.; Richard, A.; Hao, W.; Liu, C.; Tang, Z. Trace metals in saline waters and brines from China: Implications for tectonic and climatic controls on basin-related mineralization. J. Asian Earth Sci. 2022, 233, 105263. [Google Scholar] [CrossRef]
- Edet, A.E.; Offiong, O.E. Evaluation of water quality pollution indices for heavy metal contamination for monitoring. A case study from Akpabuyo-Odukpani area, Lower cross River Basin (Southeastern Nigeria). GeoJournal 2002, 57, 295–304. [Google Scholar] [CrossRef]
- Li, D.; Yu, R.; Chen, J.; Leng, X.; Zhao, D.; Jia, H.; An, S. Ecological risk of heavy metals in lake sediments of China: A national-scale integrated analysis. J. Clean. Prod. 2022, 334, 130206. [Google Scholar] [CrossRef]
- González-Avilez, E.; Rodríguez-González, F.; Vargas-Solano, S.V.; Osorio-Ruiz, A.; Jonathan, M.P.; Campos-Villegas, L.E. Effect of the concentration of uronic acids in Opuntia mucilage on the removal of heavy metals and water quality of the Yautepec River, Mexico. Arab. J. Chem. 2024, 17, 105636. [Google Scholar] [CrossRef]
- Kuczajowska-Zadrożnaa, M.; Filipkowska, U.; Jóźwiak, T. Adsorption of Cu (II) and Cd (II) from aqueous solutions by chitosan immobilized in alginate beads. J. Environ. Chem. Eng. 2020, 8, 103878. [Google Scholar] [CrossRef]
- Vargas-Solano, S.V.; Rodríguez-González, F.; Martínez-Velarde, R.; Morales-García, S.S.; Jonathan, M.P. Removal of heavy metals present in water from the Yautepec River Morelos México, using Opuntia ficus-indica mucilage. Environ. Adv. 2022, 7, 100160. [Google Scholar] [CrossRef]
- Vecino, X.; Devesa-Rey, R.; Lima, S.D.M.; Moldes, A.B.; Cruz, J.M.; Alcantar, N.A. Evaluation of cactus mucilage biocomposite to remove total arsenic from water. Environ. Technol. Innov. 2016, 6, 69–79. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Dai, T.; He, X.; Chen, M.; Liu, C.; Liang, R.; Chen, J. Preparation of pectin/poly (m-phenylenediamine) microsphere and its application for Pb2+ removal. Carbohydr. Polym. 2021, 260, 117811. [Google Scholar] [CrossRef]
- García-González, C.A.; Alnaief, M.; Smirnova, I. Polysaccharide-based aerogels—Promising biodegradable carriers for drug delivery systems. Carbohydr. Polym. 2011, 86, 1425–1438. [Google Scholar] [CrossRef]
- Kyomugasho, C.; Christiaens, S.; Shpigelman, A.; Van Loey, A.M.; Hendrickx, M.E. FT-IR spectroscopy, a reliable method for routine analysis of the degree of methylesterification of pectin in different fruit- and vegetable-based matrices. Food Chem. 2015, 176, 82–90. [Google Scholar] [CrossRef]
- Gandhi, N.; Sirisha, D.; Sekhar, K.B.C. Biodepollution of paint manufacturing industry waste water containing chromium by using coagulation process. Int. Ref. Res. J. 2013, 4, 110–118. [Google Scholar]
- Shao, Z.; Lu, J.; Ding, J.; Fan, F.; Sun, X.; Li, P.; Fang, Y.; Hu, Q. Novel green chitosan-pectin gel beads for the removal of Cu (II), Cd (II), Hg (II) and Pb (II) from aqueous solution. Int. J. Biol. Macromol. 2021, 176, 217–225. [Google Scholar] [CrossRef]
- Messager, M.; Lehner, B.; Grill, G.; Nedeva, I.; Schmitt, O. Estimating the volume and age of water stored in global lakes using a geo–statistical approach. Nat. Commun. 2016, 7, 13603. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, J.B.; Gutiérrez-Ojeda, C. Why are there level variations in Lake Tequesquitengo? 1. Calibration of a model of the hydrological system. Tecnol. Cienc. Agua 2015, 19, 33–46. [Google Scholar]
- Castellanos-Trujillo, L.; Palacios-Mayorga, S. Contribución al estudio de las aguas del lago de Tequesquitengo, estado de Morelos. Rev. Mex. Cienc. Geol. 1977, 1, 218–224. (In Spanish) [Google Scholar]
- INEGI (Instituto Nacional de Estadística y Geografía). Manantiales en el Estado de Morelos: Inventario y Caracterización Físico-Química; Instituto Nacional de Estadística y Geografía: Aguascalientes, Mexico, 2020. (In Spanish) [Google Scholar]
- CONAGUA (Comisión Nacional del Agua). Estadística del Agua en México. SEMARNAT, 2021. México. Available online: https://www.gob.mx/conagua (accessed on 7 May 2025). (In Spanish).
- SSA (Secretaría de Salud). Norma Oficial Mexicana NOM-230-SSA1-2002, Salud Ambiental, Agua Para Uso y Consumo Humano, Requisitos Sanitarios Que se Deben Cumplir en Los Sistemas de Abastecimiento Públicos y Privados Durante el Manejo del Agua Procedimientos Sanitarios Para el Muestreo. Diario Oficial de la Federación: México. 2005. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=2081772&fecha=12/07/2005#gsc.tab=0 (accessed on 7 May 2025). (In Spanish).
- ASTM D2035-08; (American Society for Testing and Materials) Standard Practice for Coagulation-Flocculation Jar Test of Water ASTM Annual Book of Standard Edition. ASTM International: West Conshohocken, PA, USA, 2008. (In Spanish)
- CONAGUA (Comisión Nacional del Agua). El Reporte del Clima en México Reporte Anual 2019. Coordinación General del Servicio Meteorológico Nacional de la Comisión Nacional del Agua, México. Available online: https://smn.conagua.gob.mx/tools/DATA/Climatolog%C3%ADa/Diagn%C3%B3stico%20Atmosf%C3%A9rico/Reporte%20del%20Clima%20en%20M%C3%A9xico/Anual2019.pdf (accessed on 7 May 2025). (In Spanish).
- Hernández, D.U.; Tapia Peña, M.I. Ecología del fitoplancton primaveral de superficie en el lago de Tequesquitengo, Morelos, México. Rev. Biol. Trop. 2016, 35, 31–39. (In Spanish) [Google Scholar]
- Chandrasekhar, S.; Sharma, H.; Mohanty, K.K. Dependence of wettability on brine composition in high temperature carbonate rocks. Fuel 2018, 225, 573–587. [Google Scholar] [CrossRef]
- Roldán Pérez, G.; Ramírez Restrepo, J.J. Fundamentos Limnología Neotropical, 2nd ed.; Universidad de Antioquia: Medellín, Colombia, 2008; pp. 49–71. [Google Scholar]
- Retama, I.; Jonathan, M.P.; Roy, P.D.; Rodríguez-Espinosa, P.F.; Nagarajan, R.; Sarkar, S.K.; Morales-García, S.S.; Muñoz-Sevilla, N.P. Metal concentration in sediments from tourist beaches of Huatulco, Oxaca, Mexico: An evaluation of post-Easter week vacation. Environ. Earth Sci. 2016, 75, 375. [Google Scholar] [CrossRef]
- SSA (Secretaría de Salud). Norma Oficial Mexicana NOM 127-SSA1-2021, Agua Para uso y Consumo Humano-Límites Permisibles de la Calidad del Agua; Diario Oficial de la Federación: Mexico City, Mexico, 2022. (In Spanish) [Google Scholar]
- WHO (World Health Organization). Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First and Second Addenda; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- El-Hak, H.N.G.; El-Din, M.I.S.; Elrayess, R.A. Bioaccumulation of heavy metals and histopathological impact on Mugil cephalus from the North Eastern Region of Manzala Lake, Egypt. Reg. Stud. Mar. Sci. 2021, 45, 101841. [Google Scholar] [CrossRef]
- Galindo Domínguez, R.E.; Bandy, W.L.; Mortera Gutiérrez, C.A.; Ortega Ramírez, J. Geophysical-Archaeological Survey in Lake Tequesquitengo, Morelos, Mexico. Geofís Int. 2013, 52, 261–275. [Google Scholar] [CrossRef]
- Morrison, J.M.; Goldhaber, M.B.; Mills, C.T.; Breit, G.N.; Hooper, R.L.; Holloway, J.M.; Diehl, S.F.; Ranville, J.F. Weathering and transport of chromium and nickel from serpentinites in the Coast Range ophiolite to the Sacramento valley, California, USA. Appl. Geochem. 2015, 61, 72–86. [Google Scholar] [CrossRef]
- Cheng, H.; Zhou, T.; Li, Q.; Lu, L.; Lin, C. Anthropogenic chromium emissions in China from 1990 to 2009. PLoS ONE 2014, 9, e87753. [Google Scholar] [CrossRef]
- Saha, R.; Nandi, R.; Saha, B. Sources and toxicity of hexavalent chromium. J. Coord. Chem. 2011, 64, 1782–1806. [Google Scholar] [CrossRef]
- Nharingo, T.; Zivurawa, M.T.; Guyo, U. Exploring the use of cactus Opuntia ficus indica in the biocoagulation-flocculation of Pb(II) ions from wastewaters. Int. J. Environ. Sci. Technol. 2015, 12, 3791–3802. [Google Scholar] [CrossRef]
- Onditi, M.; Adelodun, A.A.; Changamu, E.O.; Ngila, J.C. Removal of Pb2+ and Cd2+ from drinking water using polysaccharide extract isolated from cactus pads (Opuntia ficus indica). J. Appl. Polym. Sci. 2016, 133, 43913. [Google Scholar] [CrossRef]
- Buttice, A.L.; Stroot, J.M.; Lim, D.M.; Stroot, P.G.; Alcantar, N.A. Removal of sediment and Bacteria from Water Using Green Chemistry. Environ. Sci. Technol. 2010, 44, 3514–3519. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Figueredo, J.S.; Torralba-Dotor, A.I.; Pérez-Rodríguez, C.C.; Moreno-Bedoya, A.M.; Mosquera-Vivas, C.S. Removal of lead and chromium from solution by organic peels: Effect of particle size and bio-adsorbent. Heliyon 2022, 8, e10275. [Google Scholar] [CrossRef]
- Ehsanpour, S.; Riahi, S.M.; Toghraie, D. Removal of chromium (VI) from aqueous solution using Eggshell/poly pyrrole composite. Alex. Eng. J. 2023, 64, 581–589. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, J.; Jiang, Z.; Shan, D.; Lu, Y. Biosorption of Fe(II) and Mn(II) ions from aqueous solution by rice husk ash. BioMed Res. Int. 2014, 2014, 973095. [Google Scholar] [CrossRef]
- Singh, T.L.; Parmar, H.; Kumar, V.A.; Kumar, C.A.; Mondal, P. Removal of manganese from synthetic wastewater by Vetiveria zizanioides. Mat. Today Proc. 2022, 72, 2687–2690. [Google Scholar] [CrossRef]
- Ho, T.L. Hard and Soft Acid and Bases Principle in Organic Chemistry; Academic Press, Inc. (London) LTD.: London, UK, 1977. [Google Scholar]
- Ibarra-Rodriguez, D.; Lizardi-Mendoza, J.; López-Maldonado, E.A.; Oropeza-Guzmán, M.T. Capacity of ’nopal’ pectin as a dual coagulant-flocculant agent for heavy metals removal. Chem. Eng. J. 2017, 323, 19–28. [Google Scholar] [CrossRef]
- Wang, R.; Liang, R.; Dai, T.; Chen, J.; Shuai, X.; Liu, C. Pectin-based adsorbents for heavy metal ions: A review. Trends Food Sci. Technol. 2019, 91, 319–329. [Google Scholar] [CrossRef]
- Feng, N.; Guo, X. Characterization of adsorptive capacity and mechanisms on adsorption of copper, lead and zinc by modified orange peel. Trans. Nonferrous Met. Soc. China 2012, 22, 1224–1231. [Google Scholar] [CrossRef]
- Martínez-Sabando, J.; Coin, F.; Melillo, J.H.; Goyanes, S.; Cerveny, S. A Review of Pectin-Based Material for Applications in Water Treatment. Materials 2023, 16, 2207. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, C.; Hansen, H.K.; Hernández, P.; Pinilla, C. Biosorption of cadmium with brown macroalgae. Chemosphere 2015, 138, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Fouda-Mbanga, B.G.; Velempini, T.; Pillay, K.; Tywaby-Ngeva, Z. Heavy metals removal from wastewater and reuse of the metal loaded adsorbents in various applications: A review. Hybrid Adv. 2024, 6, 100193. [Google Scholar] [CrossRef]
- Soliman, E.; Mansour, M.M. Enhancing Soil Organic Carbon Content and Water Retention Using Polyvinyl Alcohol Cross-linked with Chitosan and Pectin. J. Soil Sci. Plant Nutr. 2024, 24, 791–803. [Google Scholar] [CrossRef]
- Suresh Kumar, P.; Ejerssa, W.W.; Wegener, C.C.; Korving, L.; Dugulan, A.I.; Temmink, H.; van Loosdrecht, M.C.M.; Witkamp, G.J. Understanding and improving the reusability of phosphate adsorbents for wastewater effluent polishing. Water Res. 2018, 145, 365–374. [Google Scholar] [CrossRef]
- de Farias, A.B.V.; da Costa, T.B.; da Silva, M.G.C.; Vieira, M.G.A. Cerium recovery from aqueous solutions by bio/adsorption: A review in a circular economy context. J. Clean. Prod. 2021, 326, 129395. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
