Investigation of TiO2 Nanoparticles Added to Extended Filamentous Aerobic Granular Sludge System: Performance and Mechanism
Abstract
1. Introduction
2. Materials and Methods
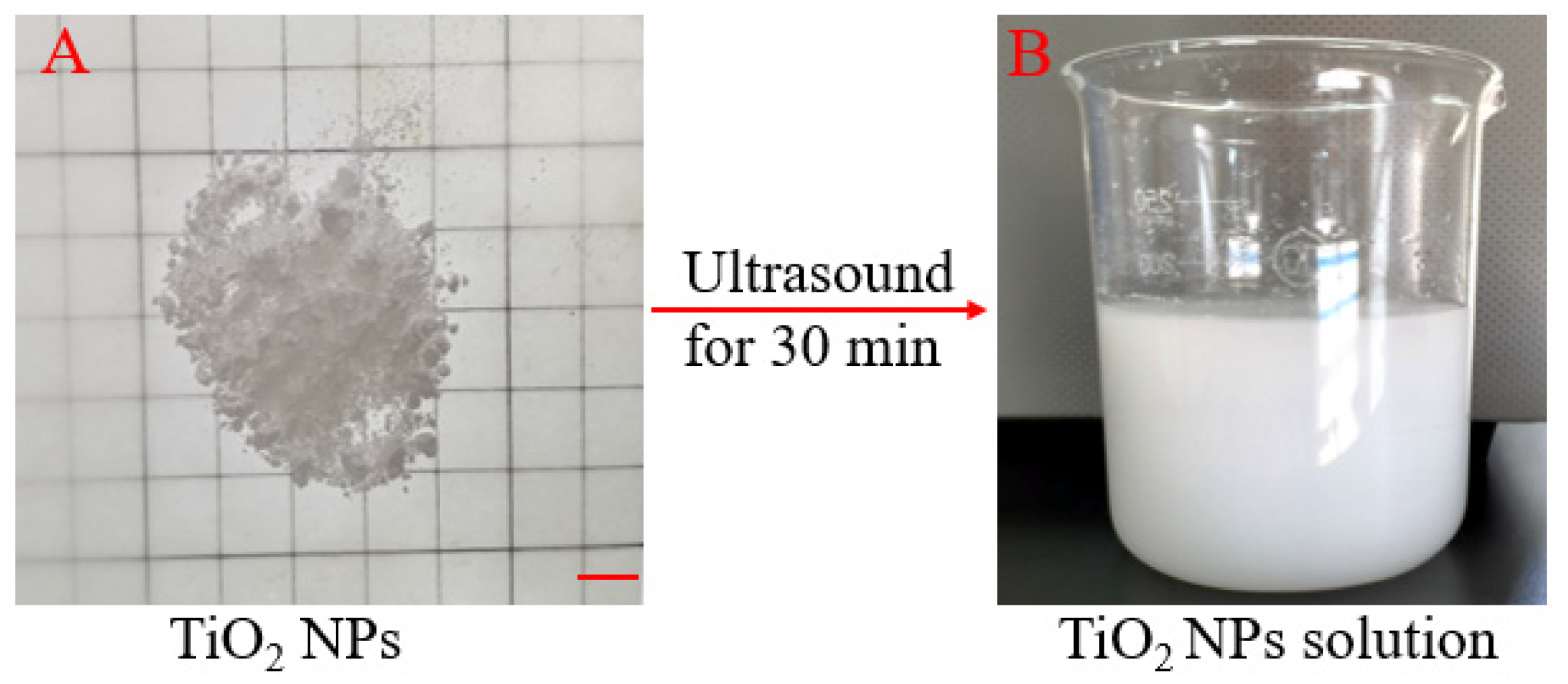
2.1. Preparation of TiO2 NPs
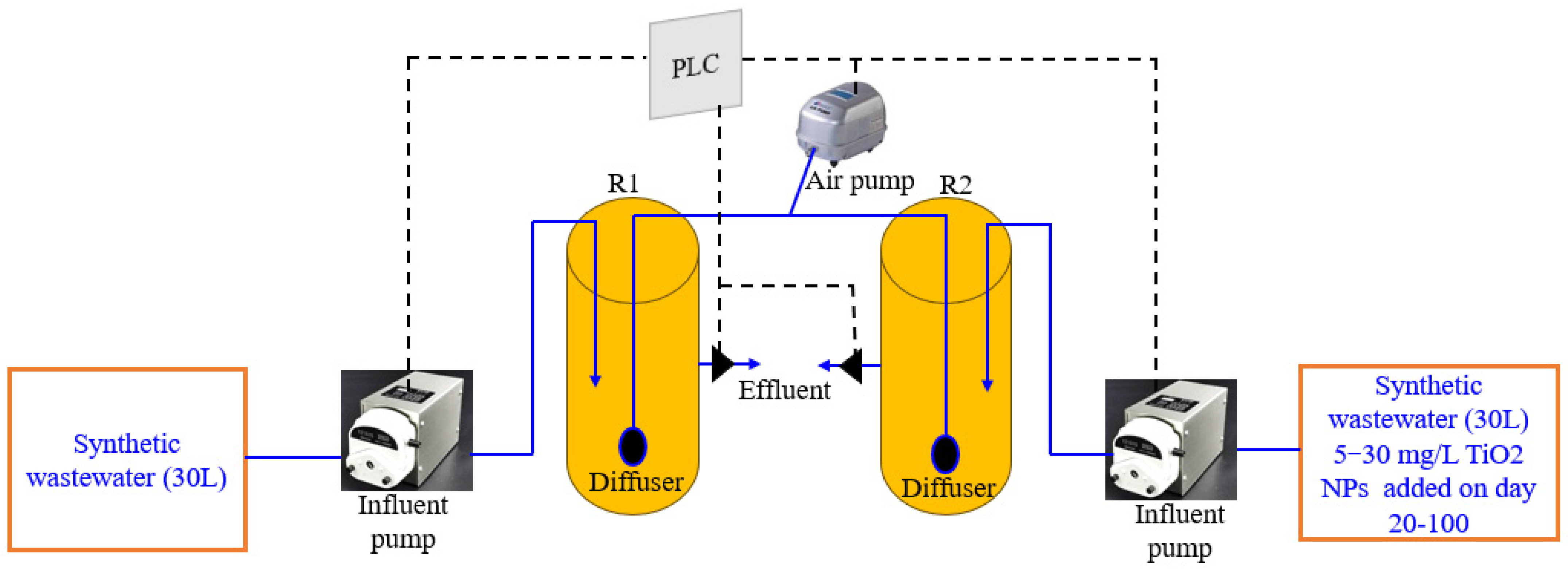
2.2. Operation of the SBRs
2.3. Wastewater and Seeding Sludge
2.4. Analytical Methods
2.4.1. Analysis of Water Quality and Sludge Properties
2.4.2. Analysis of Microbial Community
3. Results and Discussion
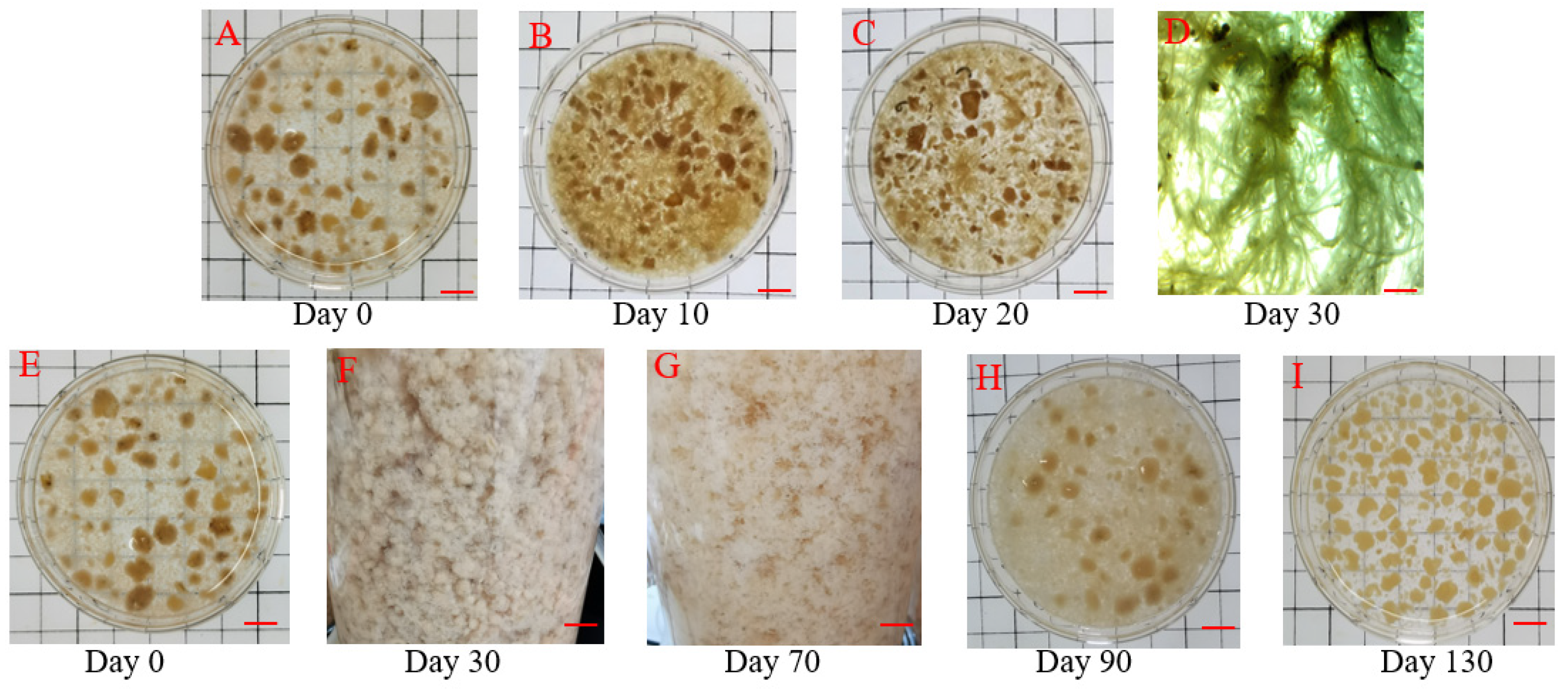
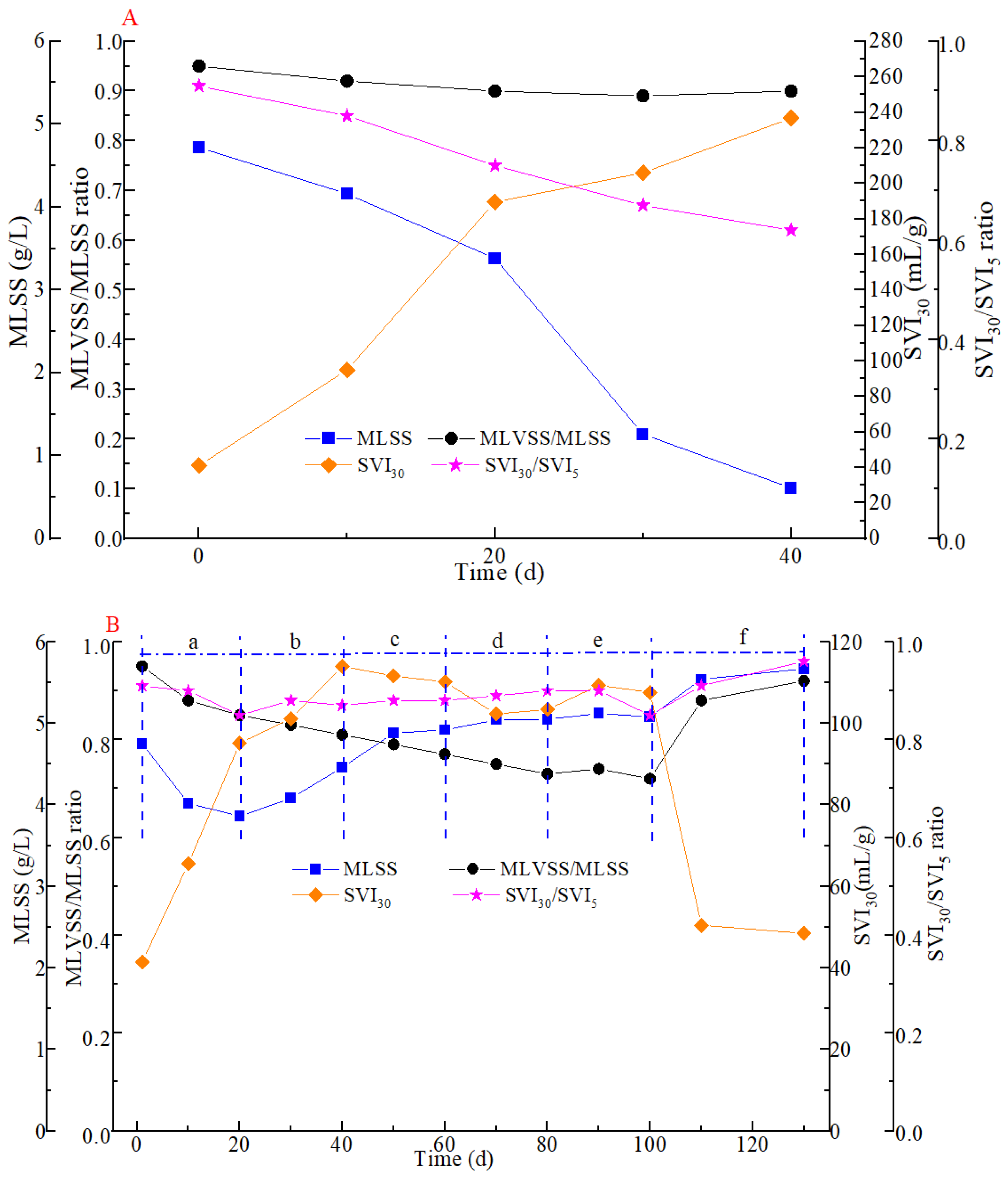
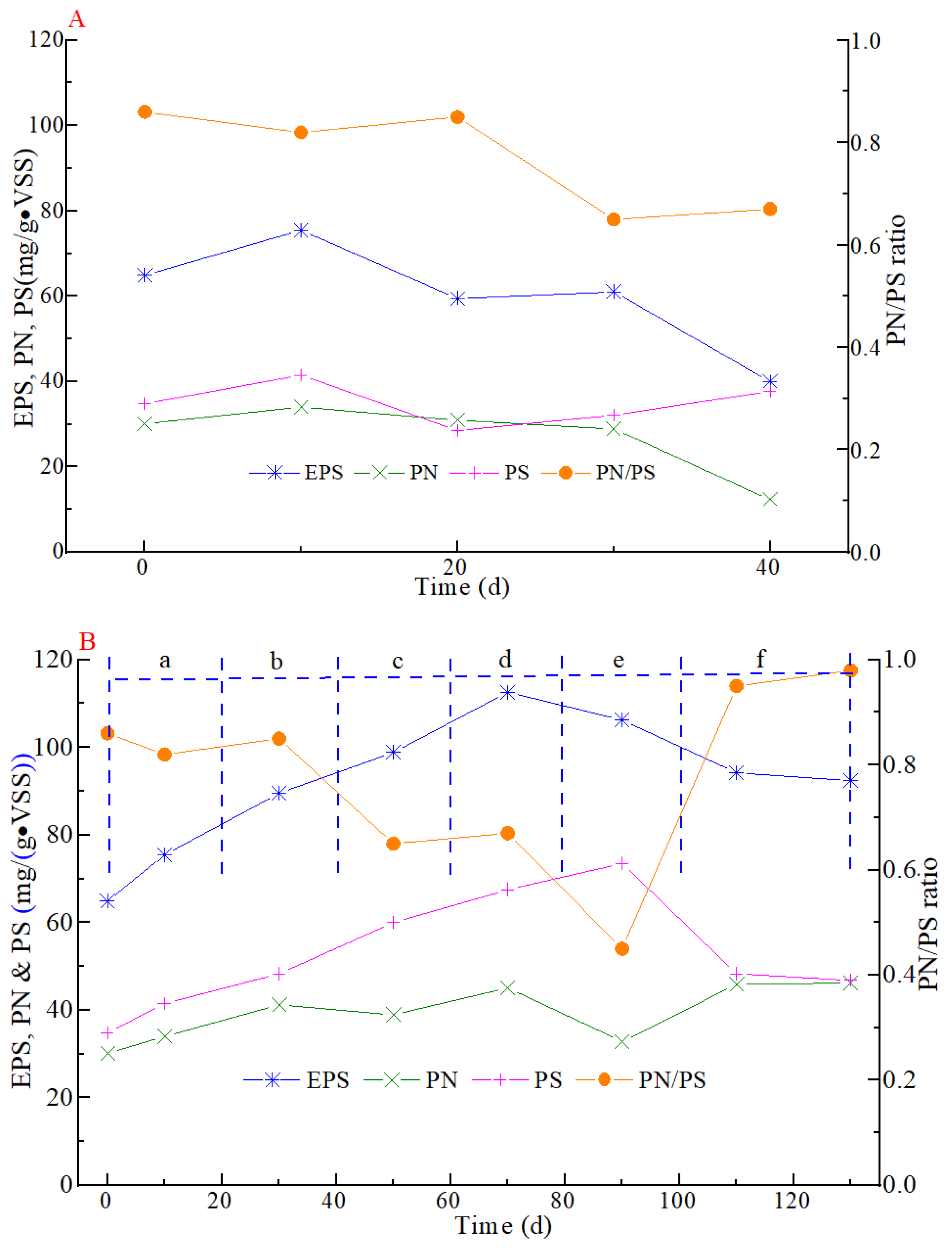
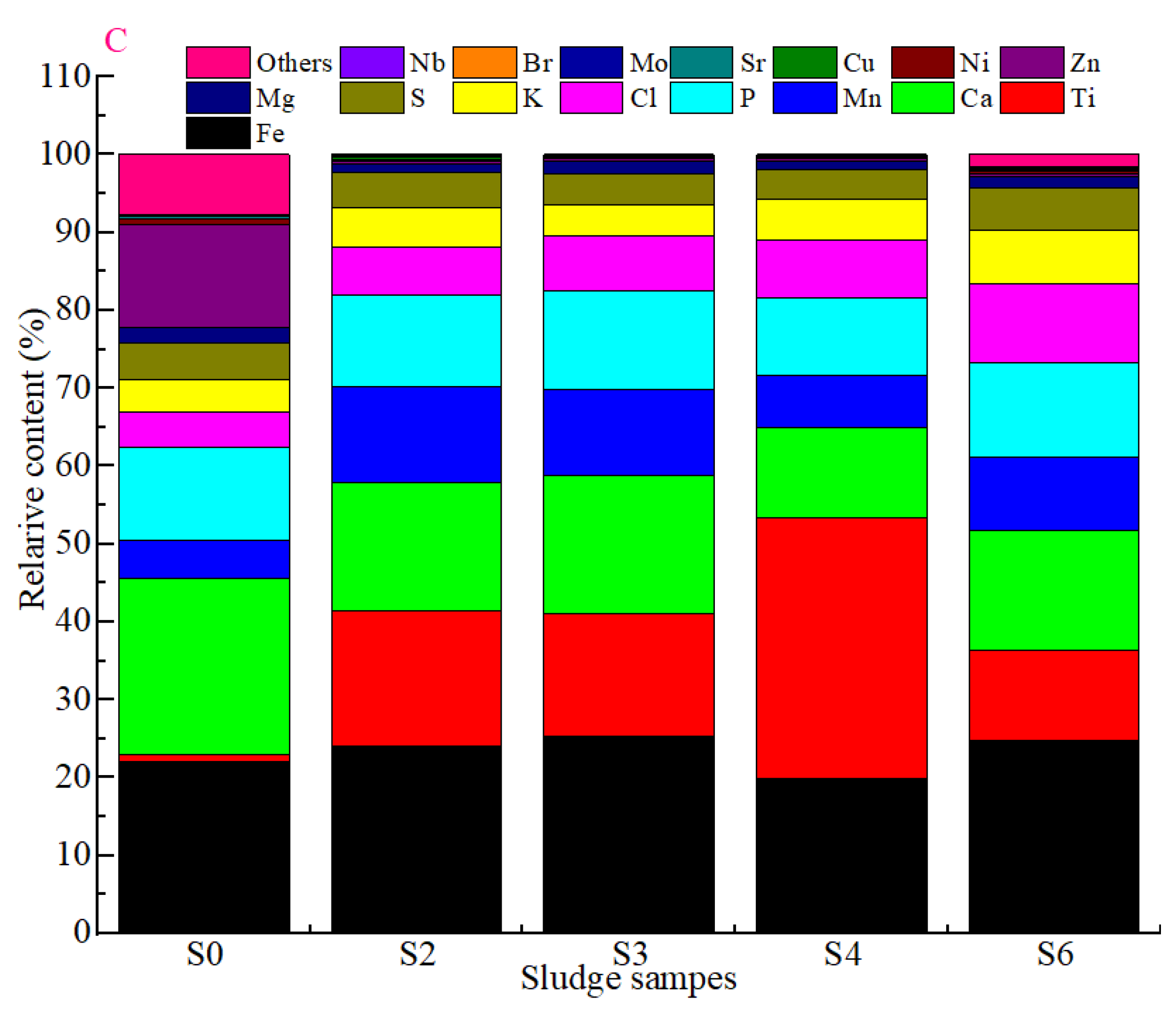
3.1. Variations in Sludge Characteristics
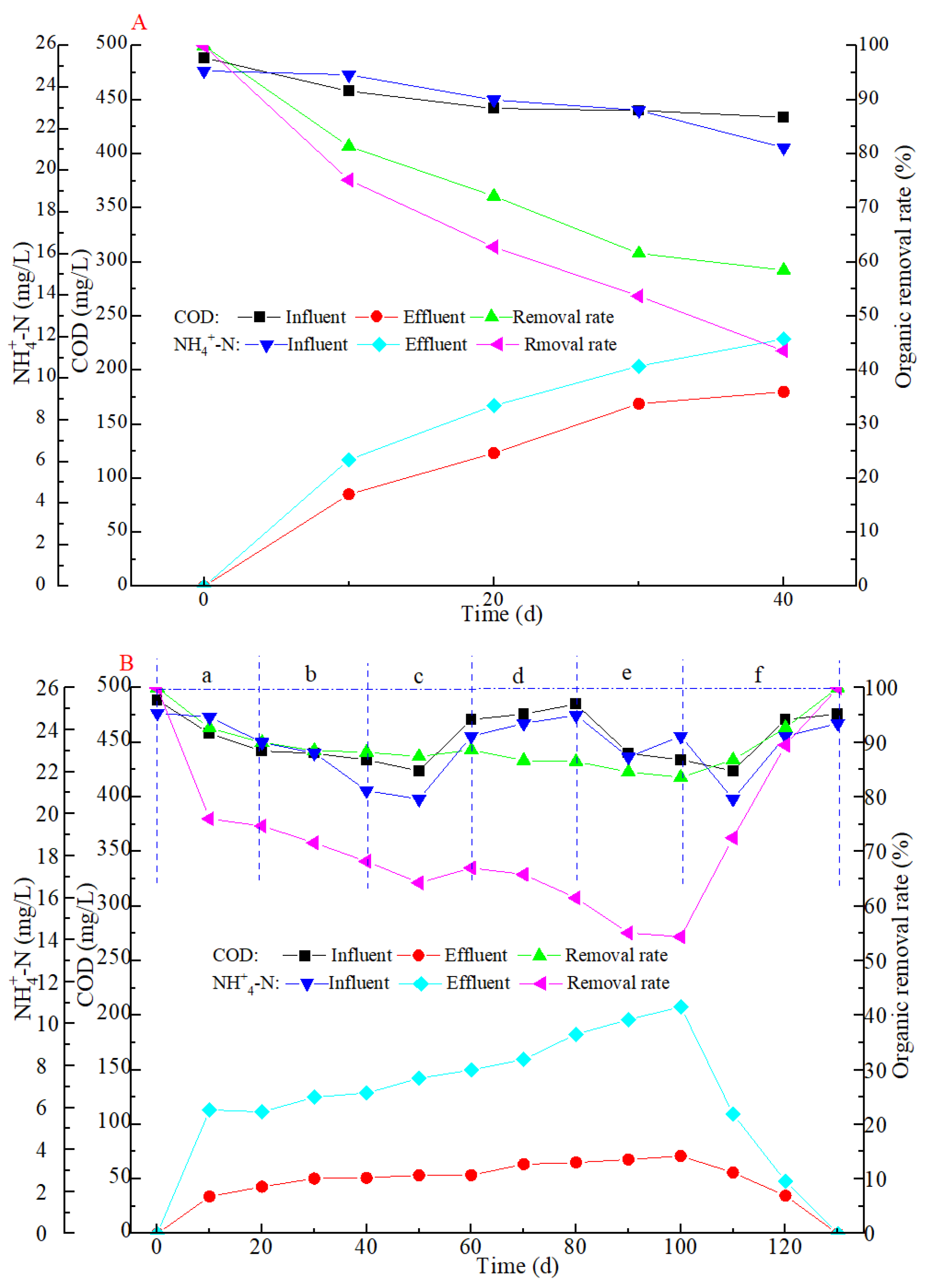
3.2. Performance of Organic Degradation in Both SBRs
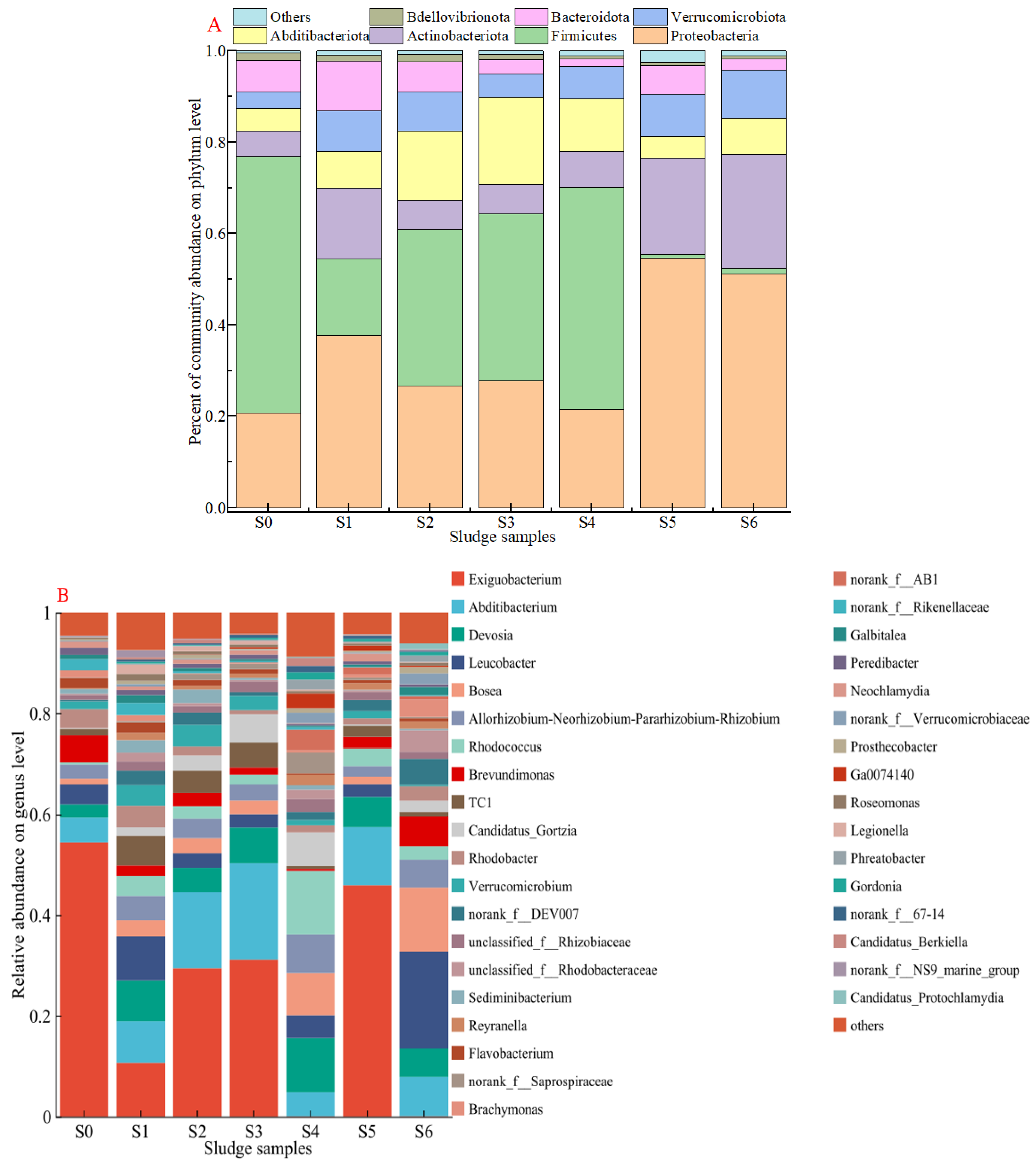
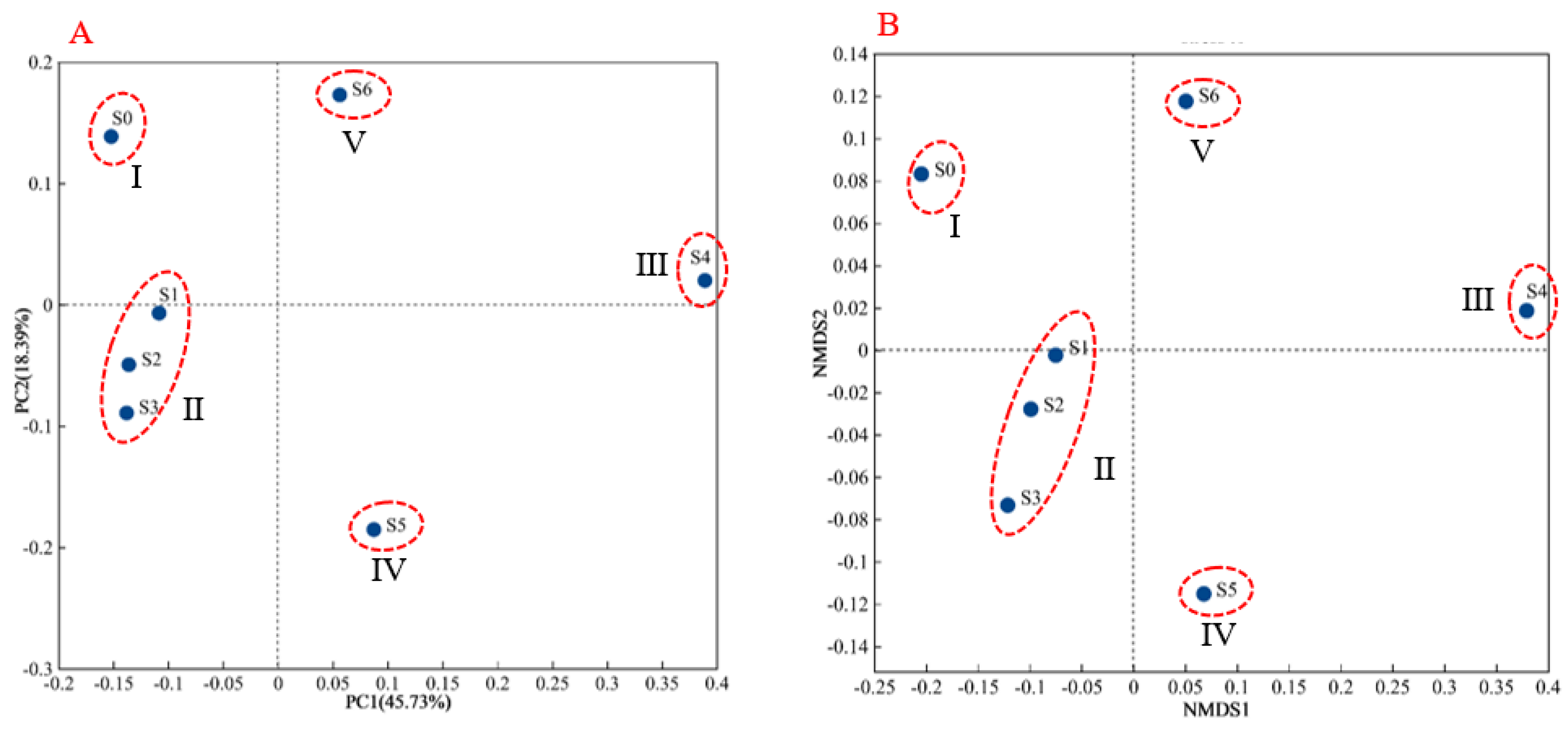
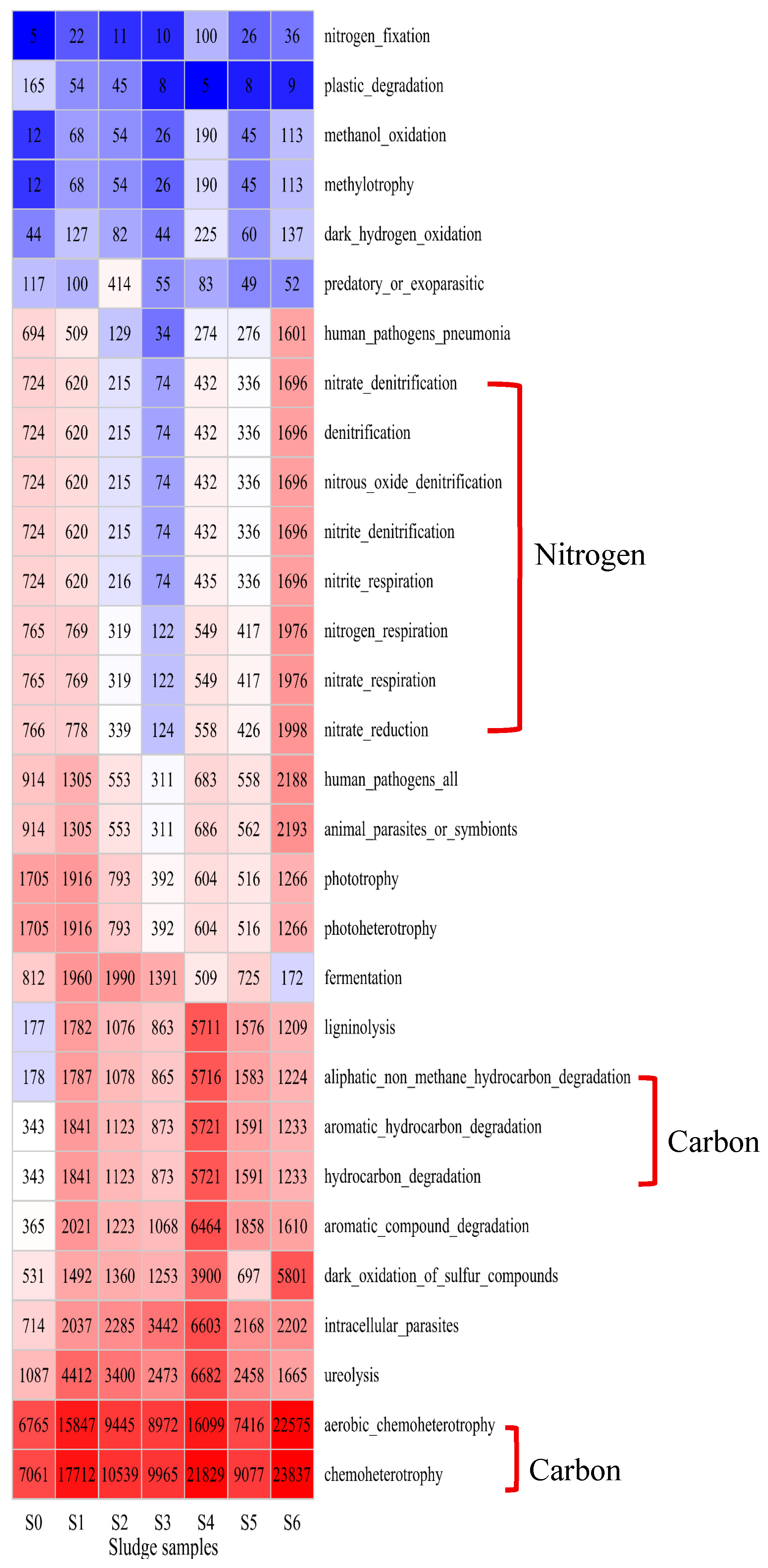
3.3. Microbial Composition and Functional Groups
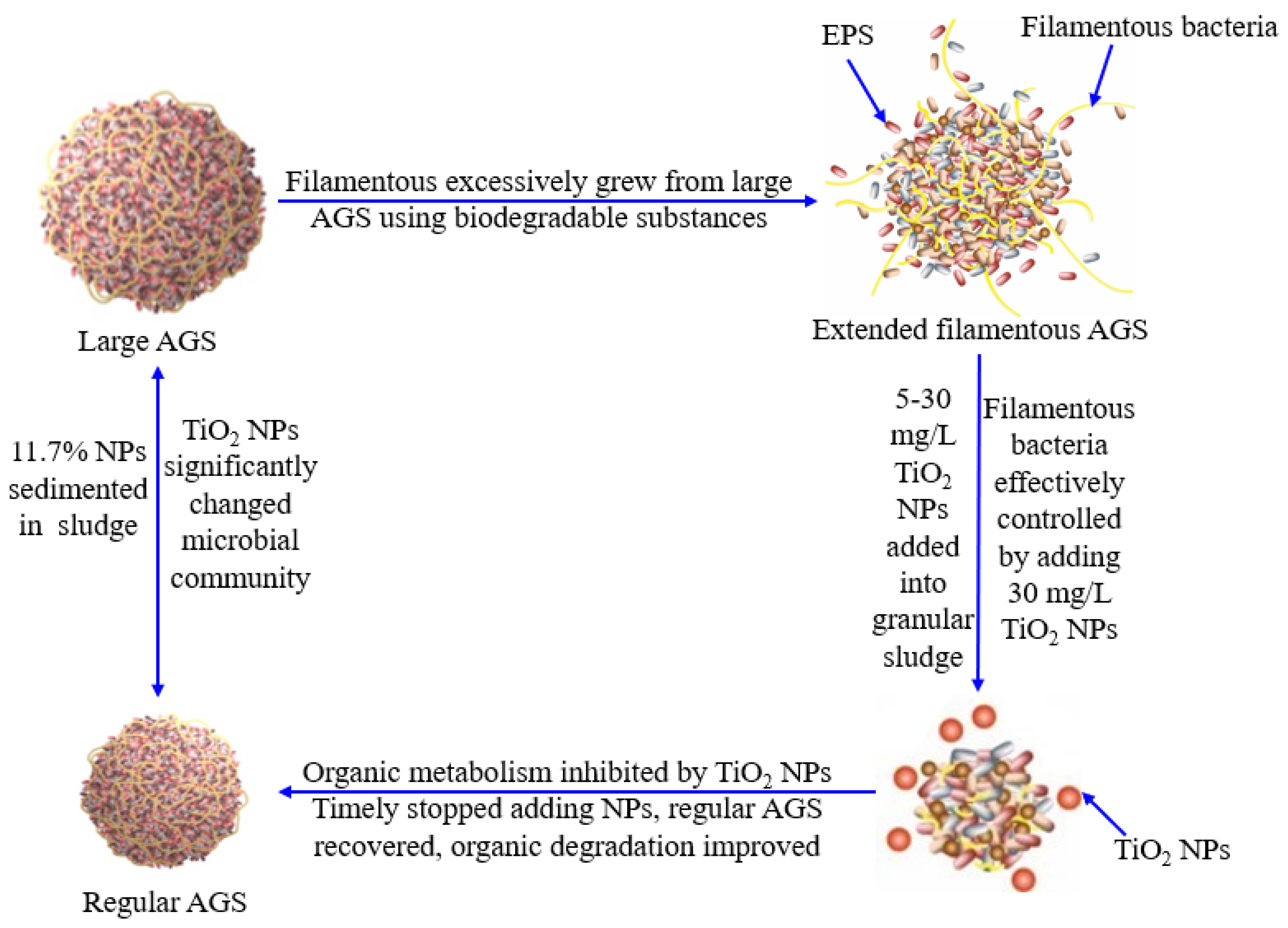
3.4. Proposed Model of TiO2 NPs for Extended Filamentous AGS
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Thakura, N.; Kumar, A.; Thakurc, V.K.; Kaliad, S.; Aryae, V.; Kumare, A.; Kumarf, S.; Kyzas, G.Z. A critical review on the recent trends of photocatalytic, antibacterial, antioxidant and nanohybrid applications of anatase and rutile TiO2 nanoparticles. Sci. Total Environ. 2024, 914, 169815. [Google Scholar]
- Navidpour, A.H.; Xu, B.T.; Ahmed, M.B.; Zhou, J.L. Immobilization of TiO2 and ZnO by facile surface engineering methods to improve semiconductor performance in photocatalytic wastewater treatment: A review. Mat. Sci. Semicon. Proc. 2024, 179, 108518. [Google Scholar]
- Jiang, Y.; Shang, Y.; Zhang, W.; Zhang, X.L.; Li, J.Y.; Shao, S.L. Assessing the effect of SiO2 and TiO2 nanoparticles on granule stability and microbial community shift in aerobic granular sludge process. Chemosphere 2022, 307, 135677. [Google Scholar]
- Wang, F.; Zhou, L.; Mu, D.H.; Zhang, H.; Zhang, G.; Huang, X.M.; Xiong, P.Z. Current research on ecotoxicity of metal-based nanoparticles: From exposure pathways, ecotoxicological effects to toxicity mechanisms. Front. Public Health 2024, 12, 1390099. [Google Scholar]
- Yu, C.; Kim, S.E.; Jang, M.; Park, C.M.; Yoon, Y.M. Occurrence and removal of engineered nanoparticles in drinking water treatment and wastewater treatment processes: A review. Environ. Eng. Res. 2022, 27, 210339. [Google Scholar]
- Li, B.; Huang, W.L.; Zhang, C.; Feng, S.S.; Zhang, Z.Y.; Lei, Z.F.; Sugiura, N. Effect of TiO2 nanoparticles on aerobic granulation of algal-bacterial symbiosis system and nutrients removal from synthetic wastewater. Bioresour. Technol. 2015, 187, 214–220. [Google Scholar]
- He, W.Y.; Wang, Q.B.; Zhu, Y.; Wang, K.J.; Mao, J.H.; Xue, X.F.; Shi, Y.W. Innovative technology of municipal wastewater treatment for rapid sludge sedimentation and enhancing pollutants removal with nano-material. Bioresour. Technol. 2012, 324, 124675. [Google Scholar]
- Chen, Y.G.; Wang, D.B.; Zhu, X.Y.; Zheng, X.; Feng, L.Y. Long-term effects of copper nanoparticles on wastewater biological nutrient removal and N2O generation in the activated sludge process. Environ. Sci. Technol. 2012, 46, 12452–12458. [Google Scholar]
- Ni, B.J.; Xie, W.M.; Liu, S.G.; Yu, H.Q.; Wang, Y.Z.; Wang, G.; Dai, L. Granulation of activated sludge in a pilot-scale sequencing batch reactor for the treatment of low-strength municipal wastewater. Water Res. 2009, 43, 751–761. [Google Scholar]
- Li, J.; Ding, L.B.; Cai, A.; Huang, G.X.; Horn, H. Aerobic sludge granulation in a full-scale sequencing batch reactor. BioMed Res. Int. 2014, 268789. [Google Scholar]
- Pronk, M.; Kreuk, M.K.D.; Bruin, B.D.; Kamminga, P.; Kleerebezem, R.; Van Loosdrecht, M.C.M. Full scale performance of the aerobic granular sludge process for sewage treatment. Water Res. 2015, 84, 207–217. [Google Scholar] [PubMed]
- Jenkins, D.; Richard, M.G.; Daigger, G.T. Manual on the Causes and Control of Activated Sludge Bulking and Forming; Lodos Ativados; CRC-Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Martins, A.M.P.; Pagilla, K.; Heijnen, J.J.; Van Loosdrecht, M.C.M. Filamentous bulking sludge a critical review. Water Res. 2004, 34, 793–817. [Google Scholar]
- Liu, Y.; Liu, Q.S. Causes and control of filamentous bacteria growth in aerobic granular sludge sequencing batch reactors. Biotechnol. Adv. 2006, 24, 115–117. [Google Scholar]
- Zhou, J.H.; Zhang, Z.L.; Zhao, H.; Yu, H.T.; Alvarez, P.J.J.; Xu, X.Y.; Zhu, L. Optimizing granules size distribution for aerobic granular sludge stability: Effect of a novel funnel-shaped internals on hydraulic shear stress. Bioresour. Technol. 2016, 216, 562–570. [Google Scholar]
- He, Q.L.; Zhang, J.; Gao, S.X.; Chen, L.; Lyu, W.L.; Zhang, W.; Song, J.Y.; Hu, X.L.; Chen, R.F.; Wang, H.Y.; et al. Comprehensive comparison between non-bulking and bulking aerobic granular sludge in microbial communities. Bioresour. Technol. 2019, 294, 122151. [Google Scholar]
- Guo, J.H.; Peng, Y.Z.; Wang, Z.W.; Yuan, Z.G.; Yang, X.; Wang, S.Y. Control filamentous bulking by chlorine-resistant Type 021N bacteria through adding a biocide CATB. Water Res. 2012, 46, 6531–6542. [Google Scholar]
- Wang, B.B.; Zhang, L.; Peng, D.C.; Hou, Y.P.; Pei, L.Y.; Yu, L.F. Extended filaments of bulking sludge sink in the floc layer with particulate substrate. Chemosphere 2013, 93, 2715–2731. [Google Scholar]
- Liu, J.; Xu, D.; He, W.Q.; He, Q.L.; Chu, W.H.; Li, S.B.; Li, J. Results of adding sludge micropowder for microbial structure and partial nitrification and denitrification in a filamentous AGS-SBR using high-ammonia wastewater. Water 2023, 15, 508. [Google Scholar]
- He, Q.L.; Zhe, Y.; Zhang, J.; Zhang, S.L.; Zhang, W.; Zou, Z.C.; Wang, H.Y. Insight into the impact of ZnO nanoparticles on aerobic granular sludge under shock loading. Chemosphere 2017, 173, 411–416. [Google Scholar]
- Cheng, Y.F.; Zhang, Z.Z.; Li, G.F.; Zhu, B.Q.; Zhang, Q.; Liu, Y.Y.; Zhu, W.Q.; Fan, N.S.; Jin, R.C. Effects of ZnO nanoparticles on high-rate denitrifying granular sludge and the role of phosphate in toxicity attenuation. Environ. Pollut. 2019, 251, 166–174. [Google Scholar]
- Zheng, X.Y.; Lu, D.; Chen, W.; Gao, Y.J.; Zhou, G.; Zhang, Y.; Zhou, X.; Jin, M.Q. Response of aerobic granular sludge to the long-term presence of CuO NPs in A/O/A SBRs: Nitrogen and phosphorus removal, enzymatic activity, and the microbial community. Environ. Sci. Technol. 2017, 51, 10503–10510. [Google Scholar]
- Li, Y.Q.; Zhao, B.H.; Yang, H.S.; Zhang, Y.Q.; Zhang, X.Y. Effects of polyvinylchloride microplastics on the toxicity of nanoparticles and antibiotics to aerobic granular sludge: Nitrogen removal, microbial community and resistance genes. Environ. Res. 2023, 238, 117151. [Google Scholar] [PubMed]
- Li, Y.Q.; Zhao, B.H.; Zhang, Y.Q.; Yang, H.S.; Zhang, B.L. Insight into response mechanism of aerobic granular sludge to combined nanoparticle stress: Nitrogen removal, microbial community and heavy metal resistance genes. Chem. Eng. J. 2024, 496, 154327. [Google Scholar]
- Pan, K.L.; Wei, Y.X.; Qiu, C.; Li, H.Y.; Wang, L.; Cheng, L.H.; Bi, X.J. Comprehensive analysis of effects of magnetic nanoparticles on aerobic granulation and microbial community composition: From the perspective of acyl-homoserine lactones mediated communication. Bioresour. Technol. 2024, 393, 13017. [Google Scholar]
- Liang, X.Y.; Gao, B.Y.; Ni, S.Q. Effects of magnetic nanoparticles on aerobic granulation process. Bioresour. Technol. 2017, 227, 44–49. [Google Scholar] [PubMed]
- Daraei, H.; Rafiee, M.; Yazdanbakhsh, A.R.; Amoozegard, M.A.; Qiu, G.L. A comparative study on the toxicity of nano zero valent iron (nZVI) on aerobic granular sludge and flocculent activated sludge: Reactor performance, microbial behavior, and mechanism of toxicity. Process Saf. Environ. 2019, 129, 238–248. [Google Scholar]
- Zhang, B.; Mao, X.; Ma, T.F.; Zhang, B.; Li, X.H.; Liu, B.; Iorhemen, O.T.; Shi, W.X. Enhanced performance of aerobic granular sludge-membrane system through the utilization of different iron-based nano-metal oxides for mitigating membrane fouling. Desalination 2014, 591, 118020. [Google Scholar]
- Sudharsan, G.; Sarvajith, M.; Nancharaiah, Y.V. Selenite reduction and biogenesis of selenium-nanoparticles by different size groups of aerobic granular sludge under aerobic conditions. J. Environ. Manag. 2013, 334, 117482. [Google Scholar]
- Nancharaiah, Y.V.; Sarvajith, M. Aerobic granular sludge for efficient biotransformation of chalcogen SeIV and TeIV oxyanions: Biological nutrient removal and biogenesis of Se0 and Te0 nanostructures. J. Hazard Mater. 2022, 422, 126833. [Google Scholar]
- Zhao, X.; Hou, N.; Wan, C.L.; Zhang, L.; Liu, X. Gold nanoparticles synthesis mediated by fungus isolated from aerobic granular sludge: Process and mechanisms. Heliyon 2024, 10, e28281. [Google Scholar]
- Li, Y.Q.; Zhao, B.H.; Chen, X.T.; Zhang, Y.Q.; Yang, H.S. Co-existence effect of copper oxide nanoparticles and ciprofloxacin on simultaneous nitrification, endogenous denitrification, and phosphorus removal by aerobic granular sludge. Chemosphere 2023, 312, 137254. [Google Scholar]
- Liu, J.; Li, J.; Sarah, P.C. The combination of external conditioning and Ca2+ addition prior to the reintroduction of effluent sludge into SBR sharply accelerates the formation of aerobic granules. J. Water Process Eng. 2020, 36, 101269. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association/American Water Work Association/Water Environmental Federation: Washington, DC, USA, 2012. [Google Scholar]
- Liu, H.; Fang, H.H.P. Extraction of extracellular polymeric substance (EPS) of sludge. J. Biotechnol. 2002, 95, 249–256. [Google Scholar] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar]
- Liu, Y.Q.; Liu, Y.; Tay, J.H. The effects of extracellular polymeric substances on the formation and stability of biogranules. Appl. Microbiol. Biotechnol. 2004, 65, 143–148. [Google Scholar]
- Xu, N.; Zhang, X.; Guo, P.C.; Xie, D.H.; Sheng, G.P. Biological self-protection inspired engineering of nanomaterials to construct a robust bio-nano system for environmental applications. Sci. Adv. 2024, 10, eadp2179. [Google Scholar] [PubMed]
- Bahgat, N.T.; Wilfert, P.; Eustance, S.J.; Korving, L.; Van Loosdrecht, M.C.M. Phosphorous speciation in EPS extracted from aerobic granular sludge. Water Res. 2024, 262, 122077. [Google Scholar]
- Kedves, A.; Konya, Z. Effects of nanoparticles on anaerobic, anammox, aerobic, and algal-bacterial granular sludge: A comprehensive review. Biofilm 2024, 8, 100234. [Google Scholar]
- Zheng, X.; Chen, Y.G.; Wu, R. Long-term effects of Titanium dioxide nanoparticles on nitrogen and phosphorus removal from wastewater and bacterial community shift in activated sludge. Environ. Sci. Technol. 2011, 45, 7284–7290. [Google Scholar]
- Liu, J.; Han, X.S.; Zhu, X.W.; Li, J.; Zhong, D.; Wei, L.L.; Liang, H. A systemic evaluation of aerobic granular sludge among granulation, operation, storage, and reactivation processes in an SBR. Environ. Res. 2023, 235, 116594. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Li, S.; Yin, S.; Chang, Z.; Ma, X.; Xing, B. Investigation of TiO2 Nanoparticles Added to Extended Filamentous Aerobic Granular Sludge System: Performance and Mechanism. Water 2025, 17, 2052. https://doi.org/10.3390/w17142052
Liu J, Li S, Yin S, Chang Z, Ma X, Xing B. Investigation of TiO2 Nanoparticles Added to Extended Filamentous Aerobic Granular Sludge System: Performance and Mechanism. Water. 2025; 17(14):2052. https://doi.org/10.3390/w17142052
Chicago/Turabian StyleLiu, Jun, Songbo Li, Shunchang Yin, Zhongquan Chang, Xiao Ma, and Baoshan Xing. 2025. "Investigation of TiO2 Nanoparticles Added to Extended Filamentous Aerobic Granular Sludge System: Performance and Mechanism" Water 17, no. 14: 2052. https://doi.org/10.3390/w17142052
APA StyleLiu, J., Li, S., Yin, S., Chang, Z., Ma, X., & Xing, B. (2025). Investigation of TiO2 Nanoparticles Added to Extended Filamentous Aerobic Granular Sludge System: Performance and Mechanism. Water, 17(14), 2052. https://doi.org/10.3390/w17142052





