Bacterial Community in Foam-Sand Filter Media in Domestic Sewage Treatment: A Case Study of Elevated Ammonium Nitrogen Content
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Methodology
2.2. Analytical Methods
2.2.1. Illumina Sequencing of 16S rRNA Gene
2.2.2. 16S rRNA Gene Sequence Analysis
2.2.3. The Adsorbed Biomass Analysis
2.2.4. SEM Observations
2.2.5. The Nitrogen Removal Process
2.3. Statistical Analysis
3. Results and Discussion
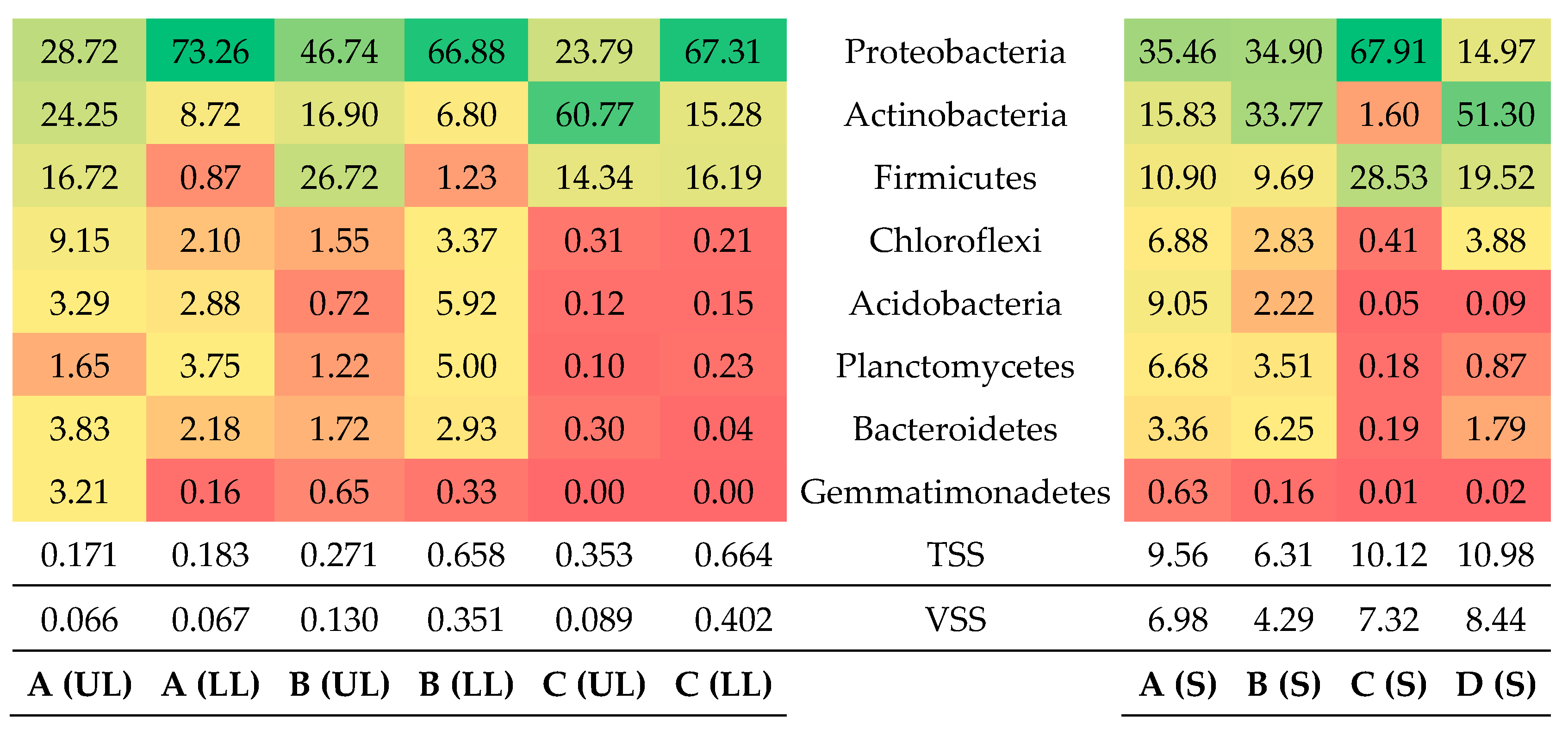
3.1. Microbial Community Analysis
3.1.1. Main Bacteria Phyla
3.1.2. Main Bacteria Classes
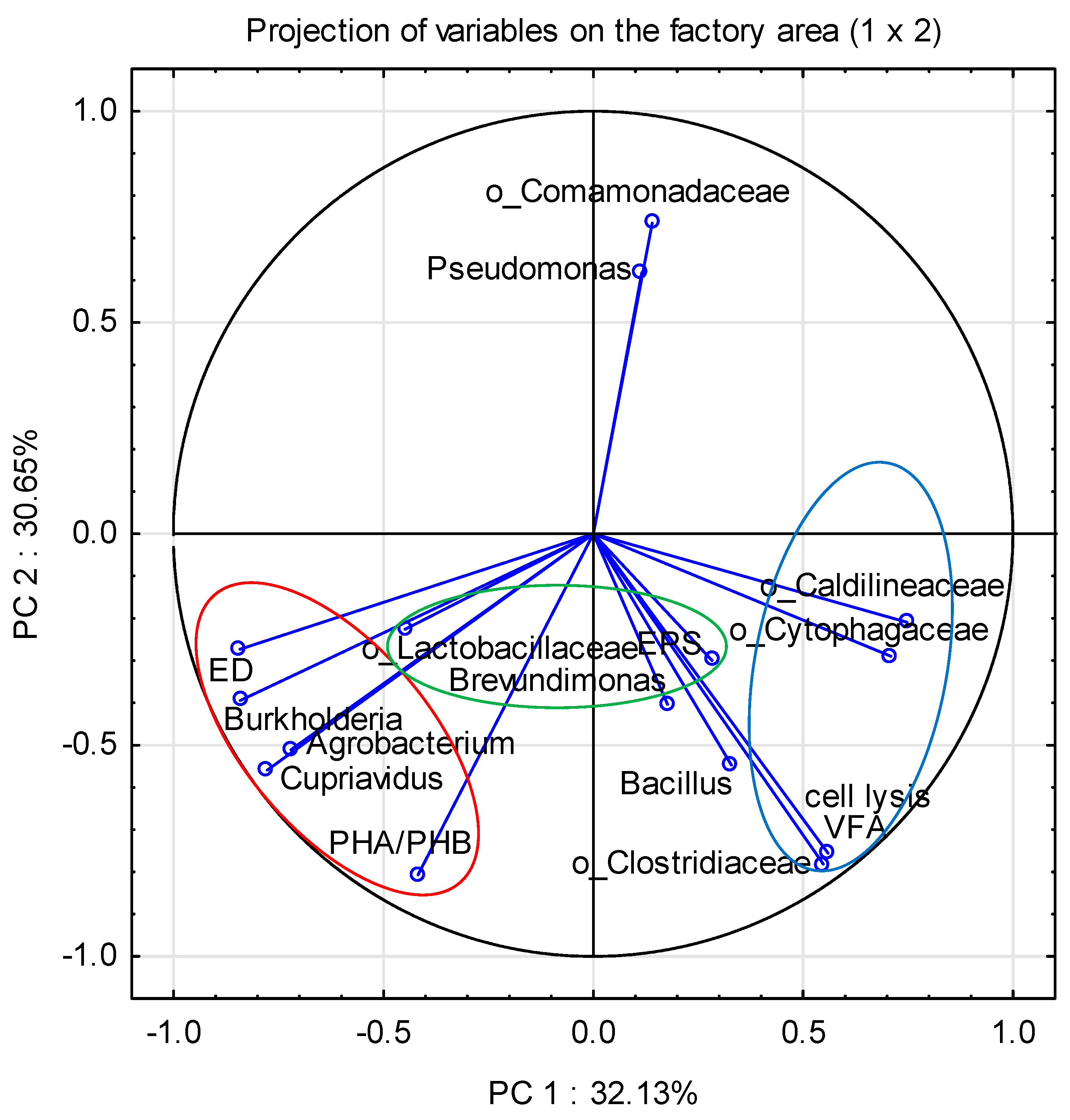
3.1.3. Claster Analysis of All Bacterial Community
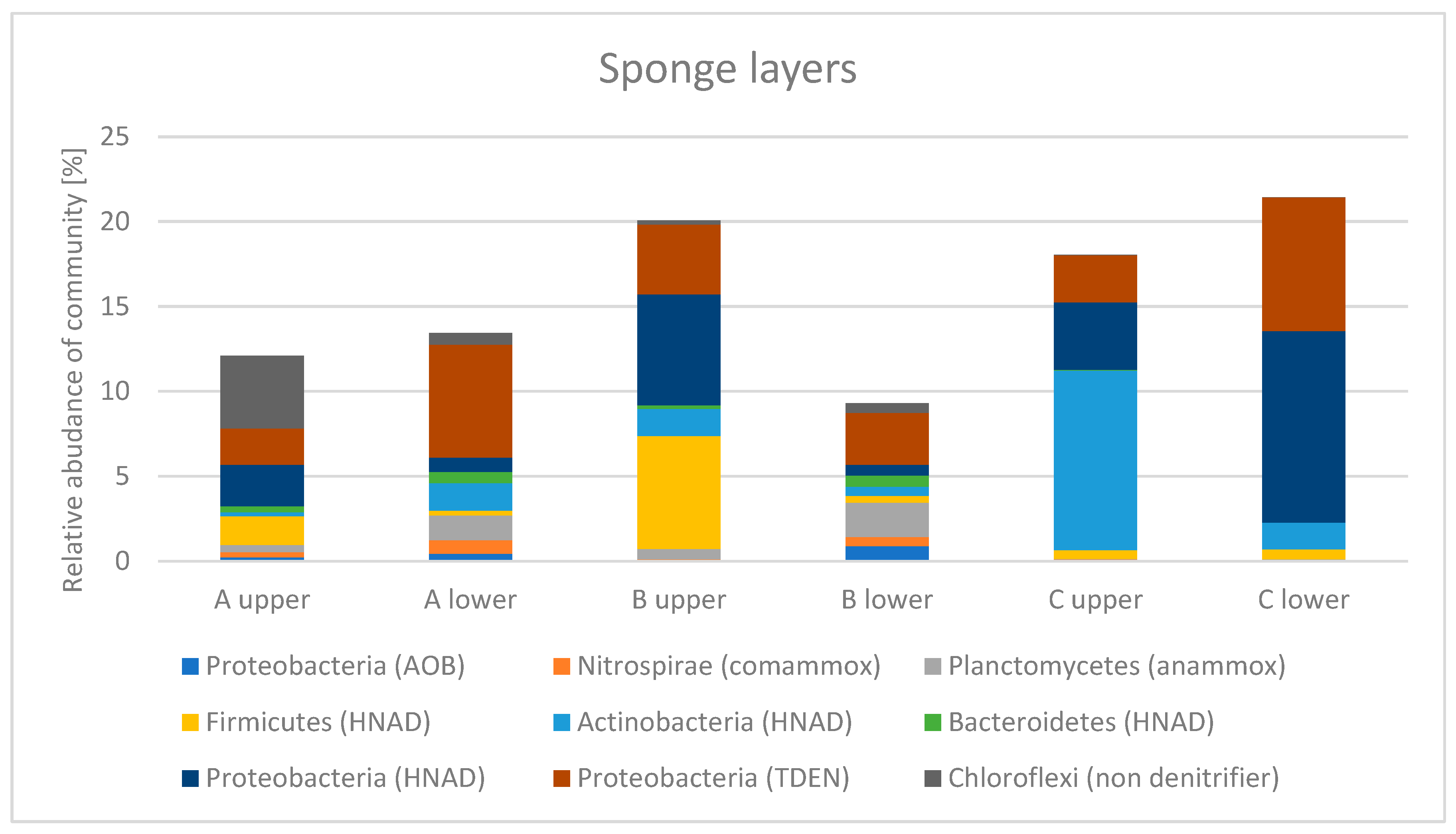
3.2. Microbial Community of Sponges
3.3. Microbial Community of Schmutzdecke
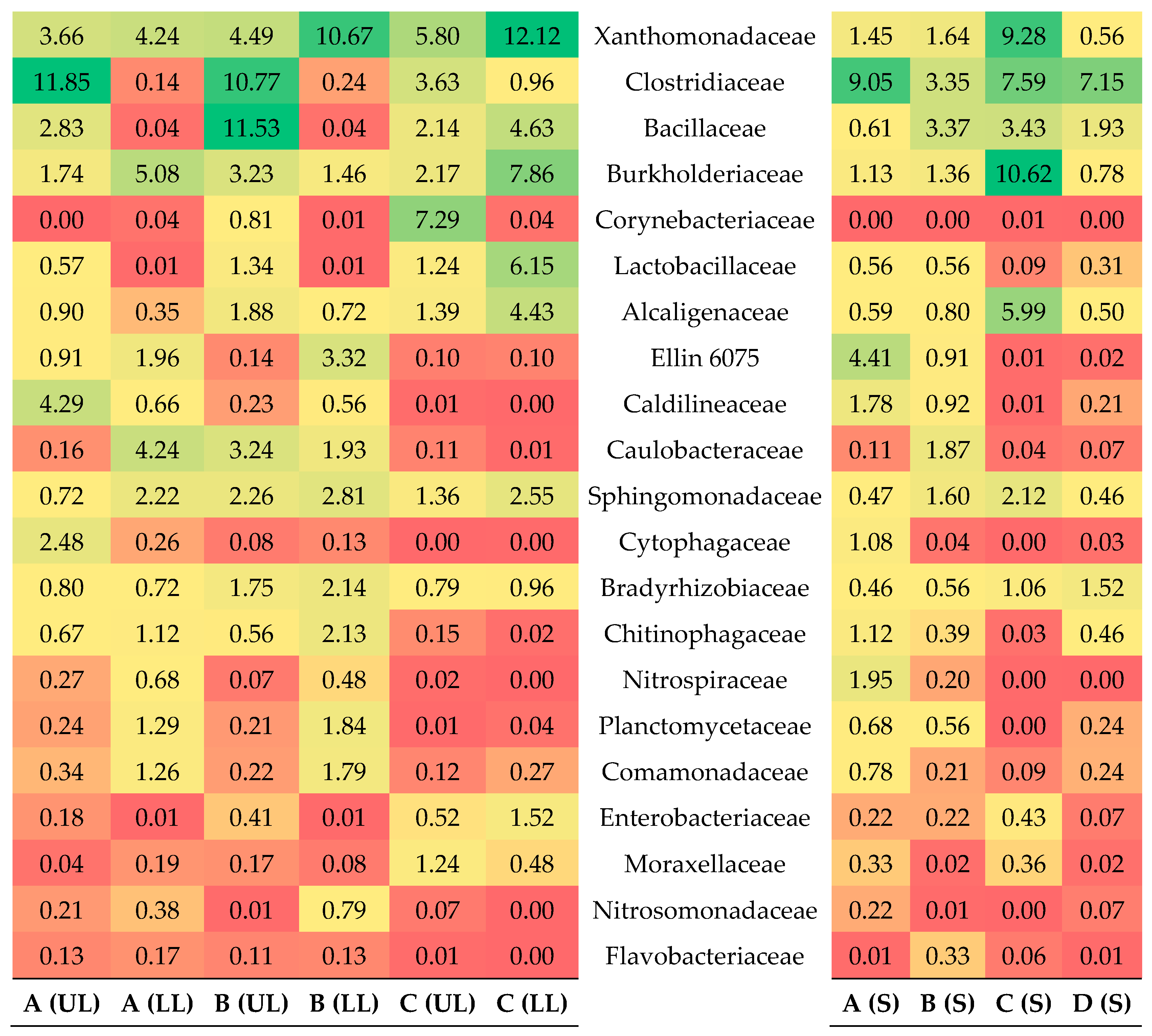
3.4. Main Bacteria Families
3.4.1. Microbial Community of Sponge
3.4.2. Microbial Community of Schmutzdecke
3.5. Bacteria Involved in the Removal of Organic Carbon
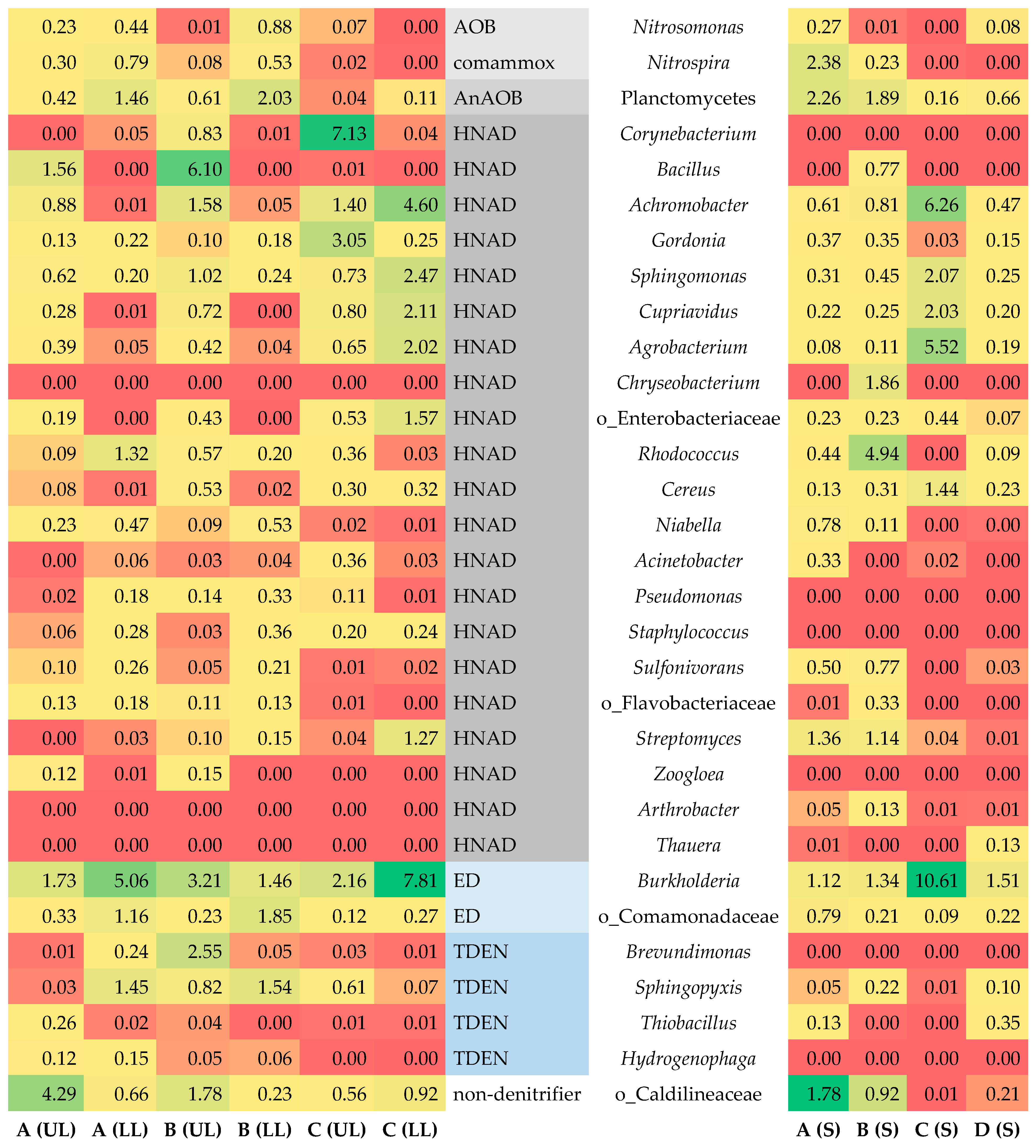
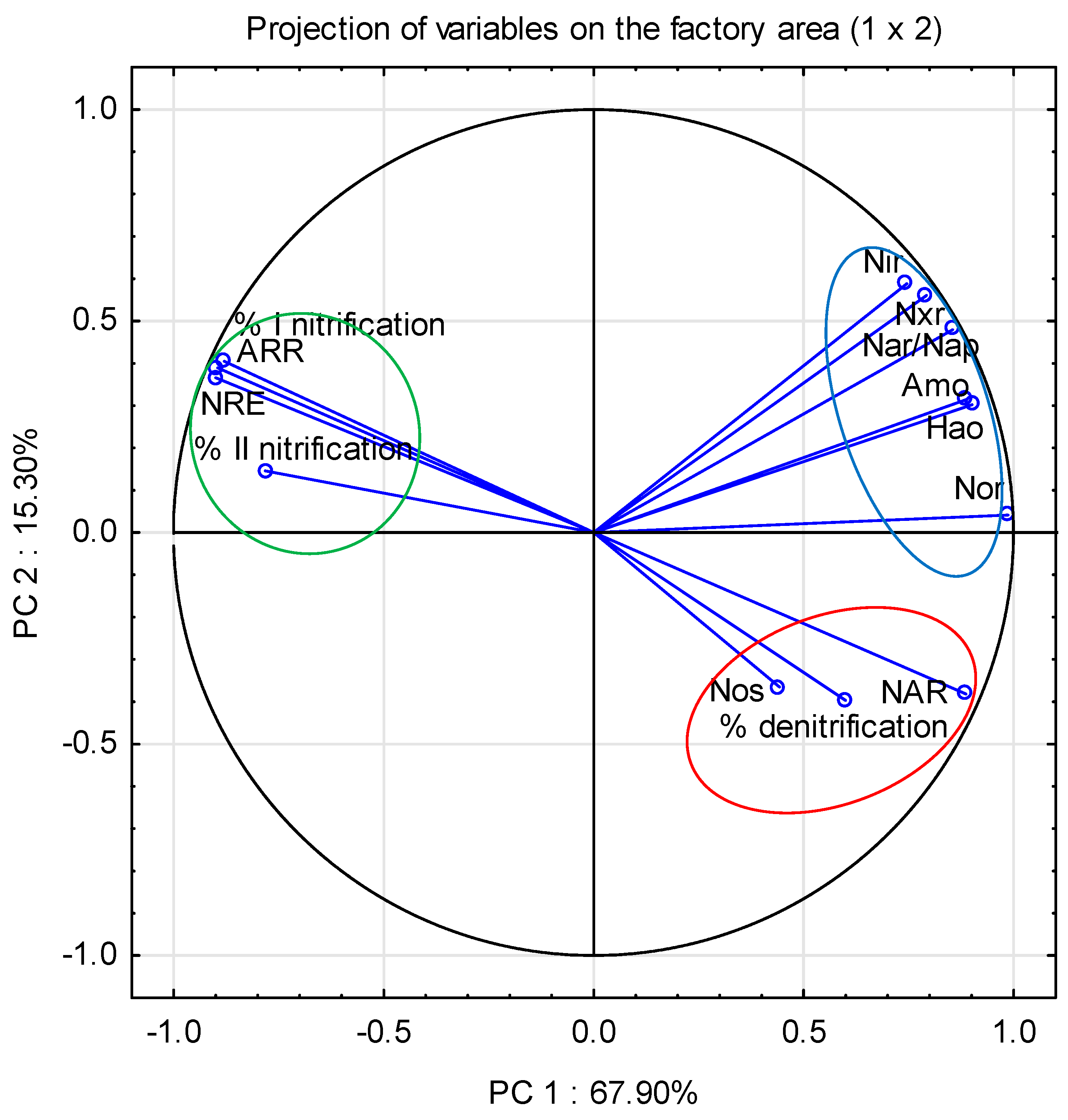
3.6. Bacteria Involved in Nitrogen Removal
3.6.1. Microbial Community of Sponge
3.6.2. Microbial Community of Schmutzdecke
3.6.3. Nitrogen Removal Efficiency
3.7. Bacteria Involved in Phosphate Removal
4. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AOB | Ammonia Oxidizing Bacteria |
| comammox | complete ammonia oxidation |
| HNAD | Heterotrophic Nitrification-Aerobic Denitrification |
| NOB | Nitrite Oxidizing Bacteria |
| Amo | Ammonia monooxygenase |
| Hao | Hydroxylamine oxidoreductase |
| Nxr | Nitrite oxidoreductase |
| Nar | Nitrate reductase |
| Nap | Periplasmic nitrate reductases |
| Nir | Nitrite reductase |
| Nor | Nitric oxide reductase |
| Nos | Nitrous oxide reductase |
| DO | dissolved oxygen |
| C/N | organic carbon to nitrogen ratio |
| WWTP | Wastewater Treatment Plant |
| SND | Simultaneous Nitrification-Denitrification |
| PN/A | Partial Nitritation /Anammox |
| anammox | anaerobic ammonia oxidation |
| ED | Endogenous Denitrification |
| PHA | Poly-β-hydroxyalkanoate |
| PHB | Poly-β-hydroxybutyrate |
| VFA | Volatile Fatty Acids |
| EPS | Extracellular Polymeric Substances |
| TDEN | Total Denitrifying |
| NAR | Nitrite Accumulation Rate |
| ARR | Ammonium Nitrogen removal Rate |
| NRR | inorganic Nitrogen Removal Rate |
| SEM | Scanning Electron Microscope |
| OTU | operational taxonomic unit |
| PCA | Principal Component Analysis |
| PC | Principal Component |
| DHS | Downflow Hanging Sponge |
| SpSF | Slow Sponge Sand Filter |
| UL | Upper Layer |
| LL | Lower Layer |
| S | Schmutzdecke |
References
- Soler-Jofra, A.; Pérez, J.; Van Loosdrecht, M.C. Hydroxylamine and the nitrogen cycle: A review. Water Res. 2021, 190, 116723. [Google Scholar] [CrossRef]
- Simon-Várhelyi, M.; Cristea, V.M.; Luca, A.V. Reducing energy costs of the wastewater treatment plant by improved scheduling of the periodic influent load. J. Environ. Manag. 2020, 262, 110294. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Vo, T.S.; Tran-Nguyen, P.L.; Nguyen, M.N.; Pham, V.H.; Matsuhashi, R.; Kim, K.; Vo, T.T.B.C. A comprehensive review of aeration and wastewater treatment. Aquaculture 2024, 591, 741113. [Google Scholar] [CrossRef]
- Yan, Y.M.; Lu, H.W.; Zhang, J.; Zhu, S.G.; Wang, Y.Q.; Lei, Y.; Zhang, R.; Song, L.Y. Simultaneous heterotrophic nitrification and aerobic denitrification (SND) for nitrogen removal: A review and future perspectives. Environ. Adv. 2022, 9, 100254. [Google Scholar] [CrossRef]
- Song, T.; Zhang, X.; Li, J.; Wu, X.; Feng, H.; Dong, W. A review of research progress of heterotrophic nitrification and aerobic denitrification microorganisms (HNADMs). Sci. Total Environ. 2021, 801, 149319. [Google Scholar] [CrossRef]
- Rout, P.R.; Bhunia, P.; Dash, R.R. Simultaneous removal of nitrogen and phosphorous from domestic wastewater using Bacillus cereus GS-5 strain exhibiting heterotrophic nitrification, aerobic denitrification and denitrifying phosphorous removal. Bioresour. Technol. 2017, 244, 484–495. [Google Scholar] [CrossRef]
- Jin, P.; Chen, Y.; Xu, T.; Cui, Z.; Zheng, Z. Efficient nitrogen removal by simultaneous heterotrophic nitrifying-aerobic denitrifying bacterium in a purification tank bioreactor amended with two-stage dissolved oxygen control. Bioresour. Technol. 2019, 281, 392–400. [Google Scholar] [CrossRef]
- Sriwiriyarat, T.; Jangkorn, S.; Charoenpanich, J.; Chinwetkitvanich, S.; Fongsatitkul, P. Occurrence of aerobic denitrifying bacteria in integrated fixed film activated sludge system. Chemosphere 2021, 285, 131504. [Google Scholar] [CrossRef]
- Hu, B.; Quan, J.; Huang, K.; Zhao, J.; Xing, G.; Wu, P.; Chen, Y.; Ding, X.; Hu, Y. Effects of C/N ratio and dissolved oxygen on aerobic denitrification process: A mathematical modeling study. Chemosphere 2021, 272, 129521. [Google Scholar] [CrossRef]
- Gu, X.; Leng, J.; Zhu, J.; Zhang, K.; Zhao, J.; Wu, P.; Xing, Q.; Tang, K.; Hu, B. Influence mechanism of C/N ratio on heterotrophic nitrification-aerobic denitrification process. Bioresour. Technol. 2022, 343, 126116. [Google Scholar] [CrossRef]
- Xi, H.; Zhou, X.; Arslan, M.; Luo, Z.; Wei, J.; Wu, Z.; El-Din, M.G. Heterotrophic nitrification and aerobic denitrification process: Promising but a long way to go in the wastewater treatment. Sci. Total Environ. 2022, 805, 150212. [Google Scholar] [CrossRef]
- Fu, W.; Song, G.; Wang, Y.; Wang, Q.; Duan, P.; Liu, C.; Zhang, X.; Rao, Z. Advances in research into and applications of heterotrophic nitrifying and aerobic denitrifying microorganisms. Front. Environ. Sci. 2022, 10, 887093. [Google Scholar] [CrossRef]
- Ji, B.; Yang, K.; Zhu, L.; Jiang, Y.; Wang, H.; Zhou, J.; Zhang, H. Aerobic denitrification: A review of important advances of the last 30 years. Biotechnol. Bioprocess Eng. 2015, 20, 643–651. [Google Scholar] [CrossRef]
- Prasetyo, R.A.; Pertiwiningrum, A.; Erwanto, Y.; Yusiati, L.M.; Fitriyanto, N.A. Characterization of Pseudomonas sp. LS3K as Nitrate Removal Agent at Different C/N Ratios Under Aerobic Condition. In Proceeding of the 2nd International Conference on Tropical Agriculture; Springer: Cham, Switzerland, 2018; pp. 185–194. [Google Scholar]
- Anjali, G.; Sabumon, P.C. The Heterotrophic Nitrification and Aerobic Denitrification (HN–AD) Process. In Ammonia Oxidizing Bacteria: Applications in Industrial Wastewater Treatment; Shah, M.P., Ed.; Royal Society of Chemistry: London, UK, 2023; pp. 103–134. [Google Scholar]
- Sun, H.; Wang, T.; Yang, Z.; Yu, C.; Wu, W. Simultaneous removal of nitrogen and pharmaceutical and personal care products from the effluent of waste water treatment plants using aerated solid-phase denitrification system. Bioresour. Technol. 2019, 287, 121389. [Google Scholar] [CrossRef]
- Li, W.; Zhuang, J.; Zhou, Y.; Meng, F.; Kang, D.; Zheng, P.; Shapleigh, J.P.; Liu, Y. Metagenomics reveals microbial community differences lead to differential nitrate production in anammox reactors with differing nitrogen loading rates. Water Res. 2020, 169, 115279. [Google Scholar] [CrossRef]
- Sparacino-Watkins, C.; Stolz, J.F.; Basu, P. Nitrate and periplasmic nitrate reductases. Chem. Society Rev. 2014, 43, 676–706. [Google Scholar] [CrossRef]
- Gupta, R.K.; Poddar, B.J.; Nakhate, S.P.; Chavan, A.R.; Singh, A.K.; Purohit, H.J.; Khardenavis, A.A. Role of heterotrophic nitrifiers and aerobic denitrifiers in simultaneous nitrification and denitrification process: A nonconventional nitrogen removal pathway in wastewater treatment. Lett. Appl. Microbiol. 2022, 74, 159–184. [Google Scholar] [CrossRef]
- Zhu, Z.; Yang, Y.; Fang, A.; Lou, Y.; Xie, G.; Ren, N.; Xing, D. Quorum sensing systems regulate heterotrophic nitrification-aerobic denitrification by changing the activity of nitrogen-cycling enzymes. Environ. Sci. Ecotechnol. 2020, 2, 100026. [Google Scholar] [CrossRef]
- Ouyang, L.; Zhang, W.; Chen, X.; Huang, Q.; Wang, H.; Li, S. Insights into the Nitrogen Removal Mechanism of Heterotrophic Nitrification and Aerobic Denitrification Bacterium Delfitia sp. B7. Water 2024, 16, 3042. [Google Scholar] [CrossRef]
- Yang, R.C.; Cui, Y.W.; Li, Z.Y.; Li, M.T.; Jiang, L.X.; Mi, Y.N.; Sui, Y.; Liang, H.K. Molecular identification of heterotrophic nitrification and aerobic denitrification bacteria: From methods development to application demonstration. Water Res. 2025, 280, 123542. [Google Scholar] [CrossRef]
- Chen, J.; Gu, S.; Hao, H.; Chen, J. Characteristics and metabolic pathway of Alcaligenes sp. TB for simultaneous heterotrophic nitrification-aerobic denitrification. Appl. Microbiol. Biotechnol. 2016, 100, 9787–9794. [Google Scholar] [CrossRef]
- Zhang, S.; Sha, C.; Jiang, W.; Li, W.; Zhang, D.; Li, J.; Meng, L.; Piao, Y. Ammonium removal at low temperature by a newly isolated heterotrophic nitrifying and aerobic denitrifying bacterium Pseudomonas fluorescens wsw-1001. Environ. Technol. 2015, 36, 2488–2494. [Google Scholar] [CrossRef]
- Ma, T.; Chen, Q.; Gui, M.; Li, C.; Ni, J. Simultaneous denitrification and phosphorus removal by Agrobacterium sp. LAD9 under varying oxygen concentration. Appl. Microbiol. Biotechnol. 2016, 100, 3337–3346. [Google Scholar] [CrossRef]
- Huang, M.Q.; Cui, Y.W.; Yang, H.J.; Xu, M.J.; Cui, Y.B.; Chen, Z.B. A halophilic aerobic-heterotrophic strain Halomonas venusta SND-01: Nitrogen removal by ammonium assimilation and heterotrophic nitrification-aerobic denitrification. Bioresour. Technol. 2023, 374, 128758. [Google Scholar] [CrossRef]
- He, T.; Xie, D.; Ni, J.; Li, Z.; Li, Z. Nitrous oxide produced directly from ammonium, nitrate and nitrite during nitrification and denitrification. J. Hazard. Mater. 2020, 388, 122114. [Google Scholar] [CrossRef]
- KEGG. Pathway Maps for Organism Groups—Nitrogen Metabolism. Available online: https://www.kegg.jp/kegg-bin/organism_stat?mapno=00910 (accessed on 4 April 2025).
- Wang, Z.; Zhang, X.X.; Lu, X.; Liu, B.; Li, Y.; Long, C.; Li, A. Abundance and diversity of bacterial nitrifiers and denitrifiers and their functional genes in tannery wastewater treatment plants revealed by high-throughput sequencing. PLoS ONE 2014, 9, e113603. [Google Scholar] [CrossRef]
- Strous, M.; Fuerst, J.A.; Kramer, E.H.M.; Logemann, S.; Muyzer, G.; van de Pas-Schoonen, K.T.; Webb, R.; Kuenen, J.G.; Jetten, M.S.M. Missing lithotroph identified as new Planctomycete. Nature 1999, 400, 446–449. [Google Scholar] [CrossRef]
- Ji, J.; Peng, Y.; Wang, B.; Wang, S. Achievement of high nitrite accumulation via endogenous partial denitrification (EPD). Bioresour. Technol. 2017, 224, 140–146. [Google Scholar] [CrossRef]
- Pham, V.H.T.; Ahn, J.; Kim, J.; Lee, S.; Lee, I.; Kim, S.; Chang, S.; Chung, W. Volatile fatty acid production from food waste leachate using enriched bacterial culture and soil bacteria as co-digester. Sustainability 2021, 13, 9606. [Google Scholar] [CrossRef]
- Grzelak, J.; Ślęzak, R.; Krzystek, L.; Ledakowicz, S. Effect of pH on the production of volatile fatty acids in dark fermentation process of organic waste. Ecol. Chem. Eng. 2018, 25, 295. [Google Scholar] [CrossRef]
- Sathiyanarayanan, G.; Bhatia, S.K.; Song, H.S.; Jeon, J.M.; Kim, J.; Lee, Y.K.; Kim, Y.G.; Yang, Y.H. Production and characterization of medium-chain-length polyhydroxyalkanoate copolymer from Arctic psychrotrophic bacterium Pseudomonas sp. PAMC 28620. Int. J. Biol. Macromol. 2017, 97, 710–720. [Google Scholar] [CrossRef]
- Chang, Y.C.; Reddy, M.V.; Mawatari, Y.; Sarkar, O. Enhanced polyhydroxyalkanoate biosynthesis by Cupriavidus sp. CY-1 utilizing CO2 under controlled non-explosive conditions. Chemosphere 2025, 373, 144181. [Google Scholar] [CrossRef]
- Bolla, M.; Pettinato, M.; Ferrari, P.F.; Fabiano, B.; Perego, P. Polyhydroxyalkanoates production from laboratory to industrial scale: A review. Int. J. Biol. Macromol. 2025, 310, 143255. [Google Scholar] [CrossRef]
- Khan, S.T.; Horiba, Y.; Yamamoto, M.; Hiraishi, A. Members of the family Comamonadaceae as primary poly (3-hydroxybutyrate-co-3-hydroxyvalerate)-degrading denitrifiers in activated sludge as revealed by a polyphasic approach. Appl. Environ. Microbiol. 2002, 68, 3206–3214. [Google Scholar] [CrossRef]
- Palleroni, N.J. Burkholderia. In Bergey’s Manual of Systematics of Archaea and Bacteria; Whitman, W.B., Rainey, F., Kämpfer, P., Trujillo, M., Chun, J., DeVos, P., Hedlund, B., Dedysh, S., Eds.; Wiley: Hoboken, NJ, USA, 2015; Volume 410. [Google Scholar]
- Kemmou, L.; Amanatidou, E. Factors affecting nitrous oxide emissions from activated sludge wastewater treatment plants—A review. Resources 2023, 12, 114. [Google Scholar] [CrossRef]
- Sugira Murekezi, J.; Chen, W.; Zhao, B.; Manzi, H.P.; Nizeyimana, J.C.; Habimana Simbi, C.; Getu, A.A.; Hazzan, O.O.; Xiao, Y. The Impact of Bacteria on Nitrous Oxide Emission from Wastewater Treatment Plants: Bibliometric Analysis. Sustainability 2025, 17, 1592. [Google Scholar] [CrossRef]
- Zhou, X.; Manna, B.; Lyu, B.; Singhal, N. Enhancing NosZ Activity to Reduce N2O Emissions from Biological Wastewater Treatment Systems. Available online: https://www.biorxiv.org/content/10.1101/2024.09.22.614384v2.full (accessed on 12 March 2025).
- Chen, X.; Ni, B.J.; Sin, G. Nitrous oxide production in autotrophic nitrogen removal granular sludge: A modeling study. Biotechnol. Bioeng. 2019, 116, 1280–1291. [Google Scholar] [CrossRef]
- Wigginton, S.K.; Brannon, E.Q.; Kearns, P.J.; Lancellotti, B.V.; Cox, A.; Moseman-Valtierra, S.; Loomis, G.W.; Amador, J.A. Nitrifying and denitrifying microbial communities in centralized and decentralized biological nitrogen removing wastewater treatment systems. Water 2020, 12, 1688. [Google Scholar] [CrossRef]
- Xiang, H.; Hong, Y.; Wu, J.; Wang, Y.; Ye, F.; Hu, Z.; Qu, Z.; Long, A. NosZ–II–type N2O-reducing bacteria play dominant roles in determining the release potential of N2O from sediments in the Pearl River Estuary, China. Environ. Pol. 2023, 329, 121732. [Google Scholar] [CrossRef]
- Mozumder, M.S.I.; Picioreanu, C.; Van Loosdrecht, M.C.; Volcke, E.I. Effect of heterotrophic growth on autotrophic nitrogen removal in a granular sludge reactor. Environ. Technol. 2014, 35, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, Q.; Chen, X.; Zhu, Y.; Yuan, C.; Zhang, C.; Zhao, T. Efficiency and microbial diversity of aeration solid-phase denitrification process bioaugmented with HN-AD bacteria for the treatment of low C/N wastewater. Environ. Res. 2021, 202, 111786. [Google Scholar] [CrossRef] [PubMed]
- Dacewicz, E.; Lenart-Boroń, A. Waste polyurethane foams as biomass carriers in the treatment process of domestic sewage with increased ammonium nitrogen content. Materials 2023, 16, 619. [Google Scholar] [CrossRef] [PubMed]
- Dacewicz, E.; Grzybowska-Pietras, J. Polyurethane foams for domestic sewage treatment. Materials 2021, 14, 933. [Google Scholar] [CrossRef] [PubMed]
- Dacewicz, E.; Jurik, Ľ. Application of a double layer sand filter with a PUR foams layer in the treatment of domestic sewage with an increased content of ammonia nitrogen. Acta Sci. Pol. Form. Circumiectus 2019, 2, 67–81. [Google Scholar] [CrossRef]
- Dacewicz, E. Impact of the sponge structure of a multilayer sand filter on the treatment of domestic sewage with an increased content of ammonia nitrogen. Acta Sci. Polonorum. Form. Circumiectus 2020, 19, 53–75. [Google Scholar] [CrossRef]
- KEGG Pathway Maps for Organism Groups—Exopolysaccharide Biosynthesis. Available online: https://www.kegg.jp/kegg-bin/organism_stat?mapno=00543 (accessed on 15 March 2025).
- Braeken, J.; Van Assen, M.A. An empirical Kaiser criterion. Psychol. Methods 2017, 22, 450. [Google Scholar] [CrossRef]
- Nomoto, N.; Hatamoto, M.; Hirakata, Y.; Ali, M.; Jayaswal, K.; Iguchi, A.; Harada, H. Defining microbial community composition and seasonal variation in a sewage treatment plant in India using a down-flow hanging sponge reactor. Appl. Microbiol. Biotechnol. 2018, 102, 4381–4392. [Google Scholar] [CrossRef]
- Marín, I. Proteobacteria. In Encyclopedia of Astrobiology; Gargaud, W.M., Irvine, R., Amils, H.J., Cleaves, D., Pinti, J., Cernicharo, D., Rouan, T., Spohn, S., Tirard, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 2036–2037. [Google Scholar]
- Xie, N.; Zhong, L.; Ouyang, L.; Xu, W.; Zeng, Q.; Wang, K.; Zaynab, M.; Chen, H.; Xu, F.; Li, S. Community composition and function of bacteria in activated sludge of municipal wastewater treatment plants. Water 2021, 13, 852. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, X.; Zhu, L. Activated sludge bacterial communities of typical wastewater treatment plants: Distinct genera identification and metabolic potential differential analysis. AMB Express 2018, 8, 184. [Google Scholar] [CrossRef]
- Hatamoto, M.; Okubo, T.; Kubota, K.; Yamaguchi, T. Characterization of downflow hanging sponge reactors with regard to structure, process function, and microbial community compositions. Appl. Microbiol. Biotechnol. 2018, 102, 10345–10352. [Google Scholar] [CrossRef]
- Watari, T.; Cuong Mai, T.; Tanikawa, D.; Hirakata, Y.; Hatamoto, M.; Syutsubo, K.; Fukuda, M.; Nguyen, N.B.; Yamaguchi, T. Development of downflow hanging sponge (DHS) reactor as post treatment of existing combined anaerobic tank treating natural rubber processing wastewater. Water Sci. Technol. 2017, 75, 57–68. [Google Scholar] [CrossRef]
- Watari, T.; Mai, T.C.; Tanikawa, D.; Hirakata, Y.; Hatamoto, M.; Syutsubo, K.; Fukuda, M.; Nguyen, N.B.; Yamaguchi, T. Performance of the pilot scale upflow anaerobic sludge blanket—Downflow hanging sponge system for natural rubber processing wastewater treatment in South Vietnam. Bioresour. Technol. 2017, 237, 204–212. [Google Scholar] [CrossRef]
- Maharjan, N.; Kuroda, K.; Dehama, K.; Hatamoto, M.; Yamaguchi, T. Development of slow sponge sand filter (SpSF) as a post-treatment of UASB-DHS reactor effluent treating municipal wastewater. Water Sci. Technol. 2016, 74, 65–72. [Google Scholar] [CrossRef]
- Wei, J.; Huang, X.; Wang, H.; Wang, F.; Liu, X.; Yan, Y.; Qu, Y. Insight into biofilm formation of wastewater treatment processes: Nitrogen removal performance and biological mechanisms. Sci. Total Environ. 2023, 903, 166550. [Google Scholar] [CrossRef]
- Seong, C.N.; Kang, J.W.; Lee, J.H.; Seo, S.Y.; Woo, J.J.; Park, C.; Bae, K.S.; Kim, M.S. Taxonomic hierarchy of the phylum Firmicutes and novel Firmicutes species originated from various environments in Korea. J. Microbiol. 2018, 56, 1–10. [Google Scholar] [CrossRef]
- André, S.; Vallaeys, T.; Planchon, S. Spore-forming bacteria responsible for food spoilage. Res. Microbiol. 2017, 168, 379–387. [Google Scholar] [CrossRef]
- Zhang, L.; Shen, Z.; Fang, W.; Gao, G. Composition of bacterial communities in municipal wastewater treatment plant. Sci. Total Environ. 2019, 689, 1181–1191. [Google Scholar] [CrossRef]
- Bovio-Winkler, P.; Guerrero, L.D.; Erijman, L.; Oyarzúa, P.; Suárez-Ojeda, M.E.; Cabezas, A.; Etchebehere, C. Genome-centric metagenomic insights into the role of Chloroflexi in anammox, activated sludge and methanogenic reactors. BMC Microbiol. 2023, 23, 45. [Google Scholar] [CrossRef]
- Speirs, L.B.; Rice, D.T.; Petrovski, S.; Seviour, R.J. The phylogeny, biodiversity, and ecology of the Chloroflexi in activated sludge. Front. Microbiol. 2019, 10, 2015. [Google Scholar] [CrossRef]
- Nierychlo, M.; Miłobędzka, A.; Petriglieri, F.; McIlroy, B.; Nielsen, P.H.; McIlroy, S.J. The morphology and metabolic potential of the Chloroflexi in full-scale activated sludge wastewater treatment plants. FEMS Microbiol. Ecol. 2019, 95, fiy228. [Google Scholar] [CrossRef]
- Botchkova, E.; Vishnyakova, A.; Popova, N.; Sukhacheva, M.; Kolganova, T.; Litti, Y.; Safonov, A. Characterization of enrichment cultures of anammox, nitrifying and denitrifying bacteria obtained from a cold, heavily nitrogen-polluted aquifer. Biology 2023, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Du, W.L.; Miao, L.L.; Liu, Y.; Liu, Z.P. Microbial community dynamics in an ANAMMOX reactor for piggery wastewater treatment with startup, raising nitrogen load, and stable performance. AMB Express 2018, 8, 156. [Google Scholar] [CrossRef]
- Yue, X.; Xiao, X.; Liang, J.; Lin, Y.; Xiao, K.; Che, K. Firmicutes and Bacteroidetes as the dominant microorganisms for ammonium nitrogen wastewater treatment with a low C/N ratio in BCOR. J. Water Process Eng. 2024, 65, 105851. [Google Scholar] [CrossRef]
- Huang, S.; Kong, Y.; Chen, Y.; Huang, X.; Ma, P.; Liu, X. Microbial denitrification characteristics of typical decentralized wastewater treatment processes based on 16S rRNA sequencing. Front. Microbiol. 2023, 14, 1242506. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, X.; Zhu, L. Structure and function of the microbial consortia of activated sludge in typical municipal wastewater treatment plants in winter. Sci. Rep. 2017, 7, 17930. [Google Scholar] [CrossRef]
- Mujakić, I.; Piwosz, K.; Koblížek, M. Phylum Gemmatimonadota and its role in the environment. Microorganisms 2022, 10, 151. [Google Scholar] [CrossRef]
- Samad, M.S.; Biswas, A.; Bakken, L.R.; Clough, T.J.; de Klein, C.A.; Richards, K.G.; Lanigan, G.J.; Morales, S.E. Phylogenetic and functional potential links pH and N2O emissions in pasture soils. Sci. Rep. 2016, 6, 35990. [Google Scholar]
- Park, D.; Kim, H.; Yoon, S. Nitrous oxide reduction by an obligate aerobic bacterium, Gemmatimonas aurantiaca strain T-27. Appl. Environ. Microbiol. 2017, 83, e00502-17. [Google Scholar] [CrossRef]
- Zeng, Y.; Baumbach, J.; Barbosa, E.G.V.; Azevedo, V.; Zhang, C.; Koblížek, M. Metagenomic evidence for the presence of phototrophic Gemmatimonadetes bacteria in diverse environments. Environ. Microbiol. Rep. 2016, 8, 139–149. [Google Scholar] [CrossRef]
- Hunter, P.J.; Calvo-Bado, L.A.; Parsons, N.R.; Pettitt, T.R.; Petch, G.M.; Shaw, E.; Morgan, J.A.W.; Whipps, J.M. Variation in microbial communities colonizing horticultural slow sand filter beds: Implications for filter function. Irrigation Sci. 2013, 31, 631–642. [Google Scholar] [CrossRef]
- Maharjan, A.K.; Kamei, T.; Amatya, I.M.; Mori, K.; Kazama, F.; Toyama, T. Ammonium-nitrogen (NH4+-N) removal from groundwater by a dropping nitrification reactor: Characterization of NH4+-N transformation and bacterial community in the reactor. Water 2020, 12, 599. [Google Scholar] [CrossRef]
- Kubota, K.; Hayashi, M.; Matsunaga, K.; Iguchi, A.; Ohashi, A.; Li, Y.Y.; Yamaguchi, T.; Harada, H. Microbial community composition of a down-flow hanging sponge (DHS) reactor combined with an up-flow anaerobic sludge blanket (UASB) reactor for the treatment of municipal sewage. Bioresour. Technol. 2014, 151, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Dinkla, I.J.; Muyzer, G. Microbial ecology of biofiltration used for producing safe drinking water. Appl. Microbiol. Biotechnol. 2022, 106, 4813–4829. [Google Scholar] [CrossRef]
- Wang, L.; Pang, Q.; Peng, F.; Zhang, A.; Zhou, Y.; Lian, J.; Zhang, Y.; Yang, F.; Zhu, Y.; Ding, C. Response Characteristics of Nitrifying Bacteria and Archaea Community Involved in Nitrogen Removal and Bioelectricity Generation in Integrated Tidal Flow Constructed Wetland-Microbial Fuel Cell. Front. Microbiol. 2020, 11, 1385. [Google Scholar] [CrossRef]
- Hasan, H.A.; Rahim, N.F.M.; Alias, J.; Ahmad, J.; Said, N.S.M.; Ramli, N.N.; Buhari, J.; Abdullah, S.R.S.; Othman, A.R.; Jusoh, H.H.W.; et al. A Review on the Roles of Extracellular Polymeric Substances (EPSs) in Wastewater Treatment: Source, Mechanism Study, Bioproducts, Limitations, and Future Challenges. Water 2024, 16, 2812. [Google Scholar] [CrossRef]
- Mandic-Mulec, I.; Stefanic, P.; van Elsas, J.D. Ecology of Bacillaceae. In The Bacterial Spore: From Molecules to Systems; Wiley: Hoboken, NJ, USA, 2016; pp. 59–85. [Google Scholar]
- Zhang, L.; Long, B.; Wu, J.; Cheng, Y.; Zhang, B.; Zeng, Y.; Huang, S.; Zeng, M. Evolution of microbial community during dry storage and recovery of aerobic granular sludge. Heliyon 2019, 5, e03023. [Google Scholar] [CrossRef]
- Sotres, A.; Cerrillo, M.; Viñas, M.; Bonmatí, A. Nitrogen removal in a two-chambered microbial fuel cell: Establishment of a nitrifying–denitrifying microbial community on an intermittent aerated cathode. Chem. Eng. J. 2016, 284, 905–916. [Google Scholar] [CrossRef]
- Reichenbach, H. The order Cytophagales. In The Prokaryotes; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; pp. 549–590. [Google Scholar]
- Louime, C.; Abazinge, M.; Johnson, E. Location, formation and biosynthetic regulation of cellulases in the gliding bacteria Cytophaga hutchinsonii. Int. J. Mol. Sci. 2006, 7, 1–11. [Google Scholar] [CrossRef]
- Świątczak, P.; Cydzik-Kwiatkowska, A.; Rusanowska, P. Microbiota of anaerobic digesters in a full-scale wastewater treatment plant. Arch. Environ. Prot. 2017, 43, 53–60. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, S.; Ji, B.; Liu, Y. Towards mainstream deammonification of municipal wastewater: Partial nitrification-anammox versus partial denitrification-anammox. Sci. Total Environ. 2019, 692, 393–401. [Google Scholar] [CrossRef]
- Tanikawa, D.; Yamashita, S.; Kataoka, T.; Sonaka, H.; Hirakata, Y.; Hatamoto, M.; Yamaguchi, T. Non-aerated single-stage nitrogen removal using a down-flow hanging sponge reactor as post-treatment for nitrogen-rich wastewater treatment. Chemosphere 2019, 233, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E. The family Chitinophagaceae. In The Prokaryotes: Other Major Lineages of Bacteria and the Archaea; Rosenberg, E., Long, E.F.D., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 493–495. [Google Scholar]
- Cania, B.; Vestergaard, G.; Krauss, M.; Fliessbach, A.; Schloter, M.; Schulz, S. A long-term field experiment demonstrates the influence of tillage on the bacterial potential to produce soil structure-stabilizing agents such as exopolysaccharides and lipopolysaccharides. Environ. Microbiome 2019, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Paulo, E.M.; Vasconcelos, M.P.; Oliveira, I.S.; Affe, H.M.D.J.; Nascimento, R.; Melo, I.S.D.; de Abreu Roque, M.R.; de Assis, S.A.D. An alternative method for screening lactic acid bacteria for the production of exopolysaccharides with rapid confirmation. Food Sci. Technol. 2012, 32, 710–714. [Google Scholar] [CrossRef]
- Garibay, M.V.; del Castillo, A.F.; Torres, O.D.; de Anda, J.; Yebra-Montes, C.; Senés-Guerrero, C.; Gradilla-Hernández, M.S. Characterization of the spatial variation of microbial communities in a decentralized subtropical wastewater treatment plant using passive methods. Water 2021, 13, 1157. [Google Scholar] [CrossRef]
- Maheepala, S.S.; Hatamoto, M.; Mitsuishi, Y.; Watari, T.; Yamaguchi, T. A syphon-downflow hanging sponge (DHS) reactor for improving the denitrification efficiency of sewage water treatment. Environ. Technol. Innov. 2023, 31, 103205. [Google Scholar] [CrossRef]
- Loi, J.X.; Syutsubo, K.; Rabuni, M.F.; Takemura, Y.; Aoki, M.; Chua, A.S.M. Downflow sponge biofilm reactors for polluted raw water treatment: Performance optimisation, kinetics, and microbial community. Chemosphere 2024, 358, 142156. [Google Scholar] [CrossRef]
- Petrilli, R.; Fabbretti, A.; Cerretani, A.; Pucci, K.; Pagliaretta, G.; Picciolini, M.; Falconi, M. Selection, identification and functional performance of ammonia-degrading microbial communities from an activated sludge for landfill leachate treatment. Microorganisms 2023, 11, 311. [Google Scholar] [CrossRef]
- Xu, C.; Ruan, H.; Cai, W.; Staehelin, C.; Dai, W. Identification of an exopolysaccharide biosynthesis gene in Bradyrhizobium diazoefficiens USDA110. Microorganisms 2021, 9, 2490. [Google Scholar] [CrossRef]
- Chen, S.; Dougherty, M.; Chen, Z.; Zuo, X.; He, J. Managing biofilm growth and clogging to promote sustainability in an intermittent sand filter (ISF). Sci. Total Environ. 2021, 755, 142477. [Google Scholar] [CrossRef]
- Morvan, C.; Folgosa, F.; Kint, N.; Teixeira, M.; Martin-Verstraete, I. Responses of Clostridia to oxygen: From detoxification to adaptive strategies. Environ. Microbiol. 2021, 23, 4112–4125. [Google Scholar] [CrossRef] [PubMed]
- Kampmann, K.; Ratering, S.; Kramer, I.; Schmidt, M.; Zerr, W.; Schnell, S. Unexpected stability of Bacteroidetes and Firmicutes communities in laboratory biogas reactors fed with different defined substrates. Appl. Environ. Microbiol. 2012, 78, 2106–2119. [Google Scholar] [CrossRef]
- Wiegel, J.; Tanner, R.; Rainey, F.A. An Introduction to the Family Clostridiaceae. In The Prokaryotes; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; pp. 654–678. [Google Scholar]
- Jiang, X.T.; Guo, F.; Zhang, T. Population dynamics of bulking and foaming bacteria in a full-scale wastewater treatment plant over five years. Sci. Rep. 2016, 6, 24180. [Google Scholar] [CrossRef]
- Oren, A. The Family Xanthobacteraceae. In The Prokaryotes; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 709–726. [Google Scholar]
- Kragelund, C.; Thomsen, T.R.; Mielczarek, A.T.; Nielsen, P.H. Eikelboom’s morphotype 0803 in activated sludge belongs to the genus Caldilinea in the phylum Chloroflexi. FEMS Microbiol. Ecol. 2011, 76, 451–462. [Google Scholar] [CrossRef]
- Zeng, T.; Wang, L.; Zhang, X.; Song, X.; Li, J.; Yang, J.; Chen, S.; Zhang, J. Characterization of microbial communities in wastewater treatment plants containing heavy metals located in chemical industrial zones. Int. J. Environ. Res. Public Health 2022, 19, 6529. [Google Scholar] [CrossRef]
- Naz, I.; Hodgson, D.; Smith, A.; Marchesi, J.; Sehar, S.; Ahmed, S.; Lynch, J.; Avignone-Rossa, C.; Saroj, D.P. Investigation of the active biofilm communities on polypropylene filter media in a fixed biofilm reactor for wastewater treatment. J. Chem. Technol. Biotechnol. 2018, 93, 3264–3275. [Google Scholar] [CrossRef]
- Dossounon, Y.D.D.; Moutia, M.; Lee, K.; Habti, N.; Blaghen, M. Bioactive Exopolysaccharides (EPS) Synthesized From Exiguobacterium aurantiacum and Brevundimonas diminuta with Myeloid Cancer Cells Inhibiting and Flocculating Activity. Sch. Acad. J. Biosci. 2017, 5, 699–707. [Google Scholar]
- Wei, W.; Isobe, K.; Nishizawa, T.; Zhu, L.; Shiratori, Y.; Ohte, N.; Koba, K.; Otsuka, S.; Senoo, K. Higher diversity and abundance of denitrifying microorganisms in environments than considered previously. ISME J. 2015, 9, 1954–1965. [Google Scholar] [CrossRef]
- Bovio-Winkler, P.; Cabezas, A.; Etchebehere, C. Unveiling the hidden diversity and functional role of Chloroflexota in full-scale wastewater treatment plants through genome-centric analyses. ISME Commun. 2024, 4, ycae050. [Google Scholar] [CrossRef]
- Petriglieri, F.; Kondrotaite, Z.; Singleton, C.; Nierychlo, M.; Dueholm, M.K.; Nielsen, P.H. A comprehensive overview of the Chloroflexota community in wastewater treatment plants worldwide. Msystems 2023, 8, e0066723. [Google Scholar] [CrossRef]
- Anthonisen, A.C.; Loehr, R.C.; Prakasam, T.B.S.; Srinath, E.G. Inhibition of nitrification by ammonia and nitrous acid. J. Water Pollut. Control Fed. 1976, 48, 835–852. [Google Scholar]
- Watari, T.; Vazquez, C.L.; Hatamoto, M.; Yamaguchi, T.; van Lier, J.B. Development of a single-stage mainstream anammox process using a sponge-bed trickling filter. Environ. Technol. 2021, 42, 3036–3047. [Google Scholar] [CrossRef]
- Onodera, T.; Okubo, T.; Uemura, S.; Yamaguchi, T.; Ohashi, A.; Harada, H. Long-term performance evaluation of down-flow hanging sponge reactor regarding nitrification in a full-scale experiment in India. Bioresour. Technol. 2016, 204, 177–184. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.; Poursat, B.A.; de Ridder, D.; Smidt, H.; van der Wal, A.; Sutton, N.B. Unravelling the contribution of nitrifying and methanotrophic bacteria to micropollutant co-metabolism in rapid sand filters. J. Hazard. Mater. 2022, 424, 127760. [Google Scholar] [CrossRef]
- Riahi, H.S.; Heidarieh, P.; Fatahi-Bafghi, M. Genus Pseudonocardia: What we know about its biological properties, abilities and current application in biotechnology. J. Appl. Microbiol. 2022, 132, 890–906. [Google Scholar] [CrossRef]
- Feng, M.; Xie, Y.; Mao, W.; Lu, Y.; Wang, Y.; Li, H.; Zhang, C. Efficient biodegradation of tris-(2-chloroisopropyl) phosphate by a novel strain Amycolatopsis sp. FT-1: Process optimization, mechanism studies and toxicity changes. J. Hazard. Mater. 2023, 443, 130149. [Google Scholar] [CrossRef]
- Zuo, N.; He, J.; Ma, X.; Peng, Y.; Li, X. Phosphorus removal performance and population structure of phosphorus-accumulating organisms in HA-A/A-MCO sludge reduction process. Bioengineered 2016, 7, 327–333. [Google Scholar] [CrossRef][Green Version]
| A | B | C | D | |
|---|---|---|---|---|
| Total sequence reads | 132,398 | 156,902 | 264,629 | 59,462 |
| OTUs | 915 | 966 | 766 | 670 |
| Shannon diversity index | 4.64 | 4.83 | 3.30 | 3.24 |
| Simpson index | 0.05 | 0.02 | 0.10 | 0.21 |
| Dominant phyla (% of total sequence reads) | ||||
| Proteobacteria alphaproteobacteria betaproteobacteria gammaproteobacteria | 45.96 | 51.44 | 52.97 | 14.97 |
| 29.96 | 31.98 | 27.82 | 8.98 | |
| 7.73 | 6.77 | 13.46 | 4.26 | |
| 7.62 | 12.18 | 10.88 | 1.14 | |
| Actinobacteria | 16.81 | 17.63 | 24.82 | 51.30 |
| Firmicutes | 9.78 | 11.72 | 20.72 | 19.52 |
| Chloroflexi | 6.14 | 2.64 | 0.33 | 3.88 |
| Acidobacteria | 4.50 | 3.22 | 0.10 | 0.09 |
| Planctomycetes | 3.57 | 3.37 | 0.17 | 0.87 |
| Bacteroidetes | 3.14 | 3.48 | 0.19 | 1.79 |
| Gemmatimonadetes | 4.00 | 1.13 | 0.01 | 0.02 |
| Principal Component | PC 1 | PC 2 | PC 3 | PC 4 |
|---|---|---|---|---|
| Eigenvalue | 5.14 | 4.90 | 2.30 | 1.80 |
| % cumulative variance | 32.13 | 62.79 | 77.20 | 88.45 |
| Principal Component | PC 1 | PC 2 | PC 3 |
|---|---|---|---|
| Eigenvalue | 8.83 | 1.99 | 1.38 |
| % cumulative variance | 67.90 | 83.20 | 93.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dacewicz, E. Bacterial Community in Foam-Sand Filter Media in Domestic Sewage Treatment: A Case Study of Elevated Ammonium Nitrogen Content. Water 2025, 17, 1957. https://doi.org/10.3390/w17131957
Dacewicz E. Bacterial Community in Foam-Sand Filter Media in Domestic Sewage Treatment: A Case Study of Elevated Ammonium Nitrogen Content. Water. 2025; 17(13):1957. https://doi.org/10.3390/w17131957
Chicago/Turabian StyleDacewicz, Ewa. 2025. "Bacterial Community in Foam-Sand Filter Media in Domestic Sewage Treatment: A Case Study of Elevated Ammonium Nitrogen Content" Water 17, no. 13: 1957. https://doi.org/10.3390/w17131957
APA StyleDacewicz, E. (2025). Bacterial Community in Foam-Sand Filter Media in Domestic Sewage Treatment: A Case Study of Elevated Ammonium Nitrogen Content. Water, 17(13), 1957. https://doi.org/10.3390/w17131957






