Abstract
Tetracycline (TC) is widely used in medicine and livestock farming. TC is difficult to degrade and tends to persist and accumulate in aquatic environments, and it has gradually become an emerging pollutant. Biochar (BC) has strong potential for removing TC from water. This potential arises from its excellent surface properties, low-cost raw materials, and renewable nature. However, raw biomass materials are highly diverse, and their preparation conditions vary significantly. Modification methods differ in specificity and the application scenarios are complex. These factors collectively cause unstable TC removal efficiency by biochar. The chemical activation process using KOH/H3PO4 significantly enhanced porosity and surface functionality, transforming raw biochar into an activated carbon material with targeted adsorption capacity. Adjusting the application dosage and environmental factors (particularly pH) further enhanced the removal performance. Solution pH critically governs the adsorption efficiency: optimal conditions (pH 5–7) increased removal by 35–40% through strengthened electrostatic attraction, whereas acidic/alkaline extremes disrupted ionizable functional groups. The dominant adsorption mechanisms of biochar involved π–π interactions, pore filling, hydrophobic interactions, hydrogen bonding, electrostatic interactions, and surface complexation. In addition, the main challenges currently hindering the large-scale application of biochar for the removal of TC from water are highlighted: (i) secondary pollution risks of biochar application from heavy metals, persistent free radicals, and toxic organic leaching; (ii) economic–environmental conflicts due to high preparation/modification costs; and (iii) performance gaps between laboratory studies and real water applications.
1. Introduction
Tetracycline (TC) is a low-cost antibiotic with extensive global use. Its residues create serious ecological risks in aquatic environments. Medical wastewater serves as a primary TC contamination source. TC concentrations exhibit substantial regional variations. For example, Beijing hospital wastewater contains 0.02081–0.07633 μg/L TC [1] while Portuguese wastewater contains 23.2–54.7 μg/L TC [2]. Lower levels have been recorded in Vietnam (0.1 ± 0.0 μg/L) [3] and Iran (0.068–0.144 μg/L) [4]. In addition, the excessive use of antibiotics in the livestock industry is also a significant contributor to TC contamination in the aquatic environment. TC consumption in Asia is projected to surge from 25,260 tons/year (2010) to 51,851 tons/year by 2030 [5]. The EU and USA currently use 2575 and 3230 tons/year, respectively [6]. Poor TC absorption leads to environmental release through animal manure. Chinese farm manure contains 290.5 μg/kg TC [7], and pig operations produce 6500–9600 μg/kg TC [8]. Aquaculture contributes significantly to TC pollution. Global aquaculture used 4242 tons of total TC in 2018 [9]. The detection rates reached 64% in Vietnamese aquaculture systems and 10.8% in Korean fish samples [10,11]. Thai aquaculture wastewater contains 180 ng/L TC, which exceeds China’s Pearl River Basin level (80 ng/L) [12]. Approximately 23% of residual antibiotics (1563 tons) enter freshwater systems annually, with 18% (1247 tons) reaching oceans. China’s Liao River Basin has a TC concentration of 160 ng/L, which is 1.6 times higher than that of Nigerian rivers [13]. Zimbabwe’s surface water contains extreme concentrations of 150 μg/L [14].
The global TC consumption has a strong geographical concentration. Russia, China, and the United States are the top three consumers. Russia uses 13,579 tons annually [15], followed by China at 6950 tons [16], and the U.S. at 3230 tons [17]. Europe represents a major regional consumer, with an annual TC use of 2575 tons [5]. However, significant disparities exist among European countries: France uses 185 tons/year [18], the UK 308 tons, and Germany 542 tons [19]. In contrast, South Korea’s annual TC consumption has reached 1278 tons [20], exceeding that of most European nations. Table 1 summarizes the global TC concentrations in major rivers.

Table 1.
Global TC concentrations in major urban rivers.
TC demonstrates chemical stability and environmental persistence and remains in water environments for long periods. This pollutant accumulates in food chains through biomagnification. The accumulation creates a cross-media transport pathway of “water–soil–organisms”. Toxicological studies have shown that TC inhibits aquatic organism development. Exposure to 20 μg/L TC significantly inhibited zebrafish embryo development, which led to delayed hatching, reduced body length, impaired yolk sac absorption, and the underdevelopment of swim bladder cavities [36]. TC enters agricultural systems through irrigation. Gudda et al. reported TC accumulation in vegetables irrigated with contaminated water. The TC concentrations in leafy vegetables were 4.2 times greater than those in the soil. The enrichment factor reached 6.8 for these vegetables [37]. Recent studies revealed hepatocyte membrane damage at TC concentrations above 50 μg/L. TC disrupts hepatocyte membrane lipid bilayers. This disruption increases membrane permeability. As a result, mitochondria and endoplasmic reticulum dysfunction occur [38].
In recent years, major advances in TC removal from water have been reported. Primary technologies include biodegradation [39], photocatalytic degradation [40], adsorption [41,42], membrane filtration, and advanced oxidation processes [43]. Adsorption is a key method for removing TC and offers low operational costs. This process generates minimal secondary pollution. The adsorbents demonstrated strong resistance to degradation. Biochar is widely regarded as the most promising green adsorbent. It uses renewable biomass feedstocks. Biochar production supports carbon sequestration. Its surface properties allow for chemical modifications [44]. TC adsorption efficiency depends on two physicochemical characteristics: (1) multiple pore structures provide mass-transfer channels [45], and (2) oxygen-containing functional groups (carboxyl and hydroxyl groups) and aromatic carbon skeletons enable molecular trapping. Key mechanisms include π–π electron donor–acceptor interactions, hydrogen bonding, and electrostatic interactions [46]. Current biochar research focuses on three areas: the optimization of production parameters (pyrolysis temperature, duration, and atmosphere); surface modification techniques (magnetic particle incorporation, heteroatom doping, microbial loading); and adsorption performance evaluation under various conditions (pH, temperature, coexisting ions). Practical applications face unresolved challenges. Environmental interference reduces field effectiveness while secondary pollution risks create implementation barriers. Therefore, a significant knowledge gap persists between laboratory studies and real-world deployment.
This manuscript provides a comprehensive review of biochar modification schemes as well as the effectiveness and removal mechanisms of biochar for TC in water. The scope, aims, and objectives of the manuscript’s exploration include (i) biochar surface characteristics; (ii) methods for modifying biochar to remove TC; (iii) analysis of the effects of biochar on the removal of TC from water; (iv) mechanisms of modified BC adsorption to TC; and (v) present knowledge gaps. These results emphasize the great potential of biochar for the deep purification of TC-contaminated water. The manuscript fills the key gaps in the field of TC removal by biochar, from basic mechanisms to engineering translation. In addition, this work highlights current challenges in scaling up biochar applications. These challenges include secondary pollution risks (e.g., heavy metal leaching, toxic persistent free radicals), conflicts between economic and environmental benefits (high energy costs for pyrolysis), and performance gaps between laboratory tests and real-world water treatment. We propose targeted solutions to address these limitations. These recommendations aim to advance biochar technology development and practical implementation.
2. Biochar Surface Characteristics
The surface of biochar serves as the critical zone for adsorption and catalytic reactions. Surface properties directly determine the pollutant removal efficiency. The main characteristics are specific surface area, pore arrangement, types of functional groups, and how charges are distributed. The active site distribution depends on the specific surface area and pore count. A larger surface area and more developed mesopores expose more catalytic sites. These properties increase organic pollutant adsorption by enhancing π–π electron donor–acceptor (EDA) interactions [47,48]. Multilayered pore structures (micropore combinations) provide additional adsorption sites and diffusion pathways [49]. For example, the surface area of ZnCl2-treated wheat straw activated carbon increased from 515 to 2944 m2/g. The pore volume of biochar expands to 1.33 cm3/g, creating a synergistic micromesoporous structure that increases its adsorption capacity [50]. The degree of the graphitization of biochar (as measured by Raman ID/IG) regulates π–π EDA interactions. Pyrolysis above 500 °C strengthens the effects of EDA. High temperatures promote aromatic ring condensation and graphite microcrystal growth. This process lowers the ID/IG and enhances π-electron delocalization [51]. Chen et al. reported that pyrolysis at 800 °C reduced the number of oxygen-containing functional groups in woodchip biochar by 40%. The dominant adsorption mechanism shifted from hydrogen bonding to π–π interactions [52]. The surface charge influences pollutant binding via electrostatic forces. Positively charged surfaces attract negatively charged contaminants when the solution pH falls below the zero-charge point (pHpzc) of biochar [53]. Deprotonation enhances the electron transfer efficiency. The generation of PFRs also improves catalytic oxidation processes [54].
The surface properties of biochar depend on the type of raw material and the conditions of preparation. The composition of the feedstocks determines the initial pore structure and chemical characteristics of biochar. Feedstocks with high lignin contents (e.g., wood) produce biochar with highly aromatic structures and low ID/IG values, indicating a high degree of graphitization, which enhances electron donor–acceptor (EDA) interactions [55]. The hydrophobicity of biochar is correlated with the O/C ratio and the (N+O)/C ratio of the material. A lower ratio corresponds to greater hydrophobicity, which favors the adsorption of nonpolar pollutants [56]. The specific surface area of biochar varies with the type of feedstock. Woody biomass-derived biochar achieves specific surface areas of 2.35 to 1081 m2/g under optimized pyrolysis conditions. Feedstocks rich in cellulose and lignin (e.g., macadamia nut shells, pine sawdust) yield biochar with specific surface areas exceeding 300 m2/g under suitable preparation protocols [57]. The functional group composition of biochar reflects the nature of the raw materials. Cellulose-rich feedstocks favor the formation of oxygen-containing functional groups, whereas lignin-rich feedstocks retain aromatic functional groups [58]. The pyrolysis temperature controls the surface properties of biochar. Temperatures below 500 °C retain oxygen-containing functional groups but result in low specific surface areas. Temperatures above 500 °C promote graphitization at the expense of functional group loss [59]. For example, the activation of poplar biochar-derived carbon with KOH at 300 °C increases the content of oxygenated functional groups (SOFGs). The adsorption capacity of tetracycline increased from 4.30 to 21.17 mg/g. At 700 °C, the specific surface area increases from 106.80 to 337.81 m2/g, but the adsorption performance decreases [60]. Extended residence times during pyrolysis increase the development of micropores and increase specific surface areas [61]. The preparation of biochar involves environmental risks. These include the leaching of heavy metals, the generation of persistent free radicals (PFRs), and the formation of toxic compounds such as polycyclic aromatic hydrocarbons (PAHs) [62].
3. Methods for Modifying Biochar to Remove TC
3.1. Physical Modification
3.1.1. Heat-Treated Micropores
Heat treatment is a key method used to modify biochar surface properties. This process improves critical physicochemical properties including the specific surface area and pore volume. It also adjusts the distribution of surface functional groups. Studies indicate that biochar prepared in a CO2 atmosphere has better pore connectivity than biochar produced in a N2 atmosphere. CO2-treated biochar also has increased hydrophilicity and surface acidic functional group density [63]. For example, Zhu et al. treated biochar with a N2/air mixed atmosphere at 600–800 °C. This increased its mesopore specific surface area to 316 m2/g (3.8 times greater than that of untreated biochar). The mesopore volume also increased to 0.284 cm3/g. These changes increased the TC adsorption capacity by 5.5–9.2 times [64]. In another study, softwood sawdust-derived biochar was pyrolyzed at 700 °C and its adsorption capacity reached 96.1 mg/g. This high performance was attributed to two factors: a well-developed pore structure and abundant oxygen-containing functional groups. The pore structure and functional groups work together to enhance the adsorption mechanism. These interactions include hydrogen bonding and π–π electron donor–acceptor interactions [65].
3.1.2. Ball Milling
The ball milling technique reduces the biochar particle size to the nanometer scale. This process uses mechanical energy to break the chemical bonds, optimizing the pore structure and surface properties. It enhances the specific surface area, pore size distribution, and density of active sites on the particle surfaces and edges. For example, Zhang et al. studied crayfish shell-derived biochar. When the pyrolysis temperature increased to 800 °C, the specific surface area increased from 127.9 m2/g to 289.7 m2/g (a 2.26-fold increase). The modification increased the TC adsorption capacity by approximately 50% (39.1 mg/g to 60.7 mg/g) [66]. Yu et al. demonstrated that ball milling increased the number of oxygen-containing functional groups. The hydroxyl and carboxyl group contents were 3–5 times greater than those of the untreated biochar. This led to an increase in the TC adsorption capacity from 20.1 mg/g to 103.7 mg/g (a 4.2-fold increase) [67]. Jiang et al. further confirmed these findings. Ball milling improved the sawdust biochar adsorption capacity by approximately four times. This enhancement was correlated with an optimized surface charge distribution and improved particle dispersion stability [68].
3.1.3. Steam Activation
Steam activation improves biochar adsorption performance by modifying the surface properties. This method focuses on enhancing the SSA and pore structure [69]. For example, bamboo-based biochar treated with steam activation at 500 °C presented an increase in SSA from 156.2 m2/g to 312.5 m2/g. Its TC adsorption capacity reached 98.66 mg/g [70]. The improved adsorption arose from synergistic pore structure optimization. Micropores trap pollutants via size-sieving effects, whereas mesopores increase the mass transfer efficiency [71,72]. However, steam activation faces challenges. High energy consumption and pore clogging in humid environments limit its efficiency. Future studies could balance the adsorption performance and energy costs by combining chemical activation (e.g., KOH-assisted methods) or adjusting the steam partial pressure. Additionally, integrating steam activation with other techniques, such as metal loading or heteroatom doping, may lead to the development of biochar adsorbents with greater practical potential.
3.2. Chemical Modification
3.2.1. Acid–Base Modification
Acid–base modification enhances carbon material properties through ion exchange and chemical etching. This method improves the SSA, pore volume, and surface chemistry. Acid treatments (e.g., H3PO4 and H2SO4) selectively remove ash and introduce oxygen-rich functional groups. For example, H3PO4-activated oily tea husk carbon (pyrolyzed at 600 °C) presented an SSA increase from 384.2 m2/g to 1392.4 m2/g. Its pore volume increased 3.2-fold, and the TC adsorption capacity increased 6.8-fold to 451.5 mg/g [73]. Similarly, H2SO4-activated rice husk carbon presented a 36% increase in SSA and a 137% increase in adsorption capacity due to enhanced microporosity [74]. Acid modification also alters the surface chemical properties. The hydroxyl group density increases by 3–5 times, and the carboxyl group content increases by 2.8–4.6 times. These changes increase the surface positivity and pollutant affinity [75]. For example, KOH/MnCl2 activation of banana peels resulted in 339-fold SSA expansion (from <1 m2/g to 338.6 m2/g) and a TC adsorption capacity of 295 mg/g [76]. KOH-activated rice husk carbon presented a 3.5-fold increase in adsorption (Qmax = 58.82 mg/g). The increased number of oxygen-rich surface groups and improved mesopore proportions drove this increase. The surface carbon content of activated biochar increased from 71.6% to 83.2% [74]. These improvements result from synergistic tuning of the physical structure (SSA, pore size) and chemical traits (functional groups, surface charge) of activated carbons, enabling precise property optimization for adsorption applications.
3.2.2. Heteroatom Codoping Modification
Heteroatom codoping modifies biochar by introducing two or more heteroatoms (e.g., N, S, B, P, or Fe). This method enhances the surface functional groups and active site density. The added heteroatoms increase biochar alkalinity and reduce electron density on its carbon surface, improving adsorption performance [77]. For example, N/S-codoped biochar presented a TC adsorption capacity of 156 mg/g, which was 5.4-fold greater than that of pristine biochar (29 mg/g). This improvement resulted from enhanced surface alkalinity and reduced electron density [73]. Similarly, the Fe/S-codoped biochar achieved an equilibrium adsorption capacity of 174.06 mg/g because the Fe–S and Fe–O bonds formed uniform metal oxide clusters. This capacity was 5.3 times greater than that of unmodified biochar [78].
3.2.3. Magnetic Modification
Magnetic biochar adsorbents enhance the biochar properties by loading iron-based nanoparticles (e.g., Fe3O4). These modifications improve the SSA, pore volume, and surface characteristics. For example, FeCl-modified pine sawdust biochar resulted in a 51-fold increase in SSA, increasing from 13.08 m2/g to 666.22 m2/g [79]. Similarly, magnetically modified wood chip biochar increased the pore volume from 0.003 cm3/g to 1.301 cm3/g, with an adsorption capacity of 423.7 mg/g [52]. Iron-based nanoparticles also increase the accessibility of adsorption sites. Fe–O bonding modulates the surface charge, improving pollutant interactions [80]. However, excessive iron oxide loading can clog pores and reduce performance. For example, FeCl3-modified biochar experienced an SSA reduction due to Fe3+-induced pore structure damage [81]. Overloading may also lower the adsorption capacity [82,83]. Therefore, it is necessary to balance the quality of the iron source with the retention of the pore structure during the modification process to achieve synergistic optimization of the adsorption performance and separation efficiency.
The adsorption performance of various biochars for tetracycline removal is systematically compared in Table 2, which summarizes key parameters including feedstock type, modification method, pyrolysis temperature, specific surface area, maximum adsorption capacity, initial TC concentration, and removal efficiency.

Table 2.
The removal effects of different modification methods on TC.
4. Analysis of the Effects of Biochar on the Removal of TC from Water
4.1. Influence of Different Biomass Feedstocks, Preparation Conditions, and Modification Methods on the TC Removal Effect
The TC adsorption performance of biochar depends on the synergistic effects of the type of biomass feedstock, the pyrolysis temperature, and the modification methods. An analysis of 76 studies (Figure 1) revealed a prevalence of chemical modification methods (58% of studies), followed by physical modification (32%) and unmodified biochar (10%), highlighting chemical modification as the primary strategy for optimizing TC adsorption. Agricultural waste (AFW) accounts for 80% of feedstocks in these studies, whereas organic waste (OW) represents 20%. Organic waste (OW) dominated 60% of the studies under high initial TC concentrations (≥100 mg/L). This reflects the high ash content of OW, which is rich in mineral elements such as P, K, and Ca (Figure 1), demonstrating its suitability for high-pollution scenarios.
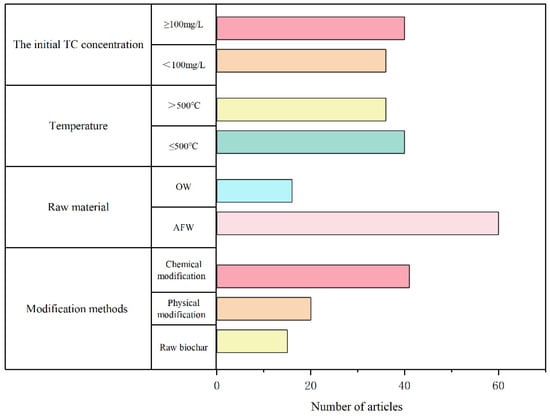
Figure 1.
Analysis of the published literature on the effectiveness of biochar and modified biochar applied to adsorb TC in water (data from 76 publications, OW: Organic Waste, AFW: Agricultural and Forestry Waste).
The chemical composition of raw materials determines the surface properties of biochar. Agricultural waste-derived biochar (e.g., rice husk and straw) has a high carbon content (>60% C) and porous structures with an SSA exceeding 300 m2/g. In contrast, sludge- or manure-based biochar contains abundant metal oxides (e.g., CaO, Fe2O3, and Al2O3), enhancing TC fixation through complexation and coprecipitation [122]. The pyrolysis temperature influences adsorption by regulating carbonization and functional group abundance [123]. At temperatures > 500 °C, biochar achieved higher values of Qmax and SSA under low TC concentrations (C0 < 100 mg/L) than the ≤500 °C groups (Figure 2a). This indicates enhanced pore development and active site exposure via high-temperature chemical modification. High-temperature biochar (>500 °C) relies primarily on micropore filling and van der Waals forces but also stabilizes TC through mineral phases (e.g., CaCO3 and Fe3O4) via chemical bonding [124]. The initial TC concentration significantly affects adsorption (Figure 2a–d). At low concentrations (C0 < 100 mg/L), chemically modified biochar at >500 °C presented a 42% increase in Qmax (p < 0.01) versus unmodified biochar, driven by pore optimization and surface charge modulation. At C0 ≥ 100 mg/L, the modified biochar maintained superior Qmax and SSA values (p < 0.01), confirming its stable performance in high-concentration environments. For example, H2SO4 modification introduces -COOH and sulfur groups, increasing the negative surface charge and enhancing TC adsorption by 2.2-fold at pH 5 via electrostatic interactions [125].
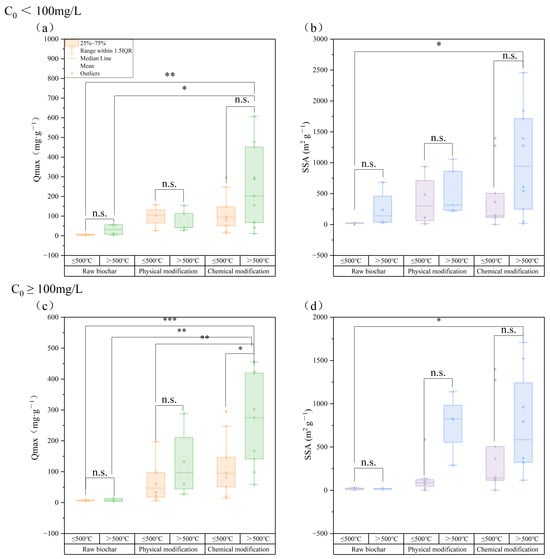
Figure 2.
Effect of initial concentration and pyrolysis temperature on Qmax and SSA in biochar (data from 76 publications, (a) Qmax at C0 < 100 mg/L; (b) SSA at C0 < 100 mg/L; (c) Qmax at C0 ≥ 100 mg/L; (d) SSA at C0 ≥ 100 mg/L, * indicates a significant difference (p < 0.05), ** indicates a highly significant difference (p < 0.01, *** indicates an extremely significant difference (p < 0.001), n.s. indicates no significant difference).
4.2. Influence of the Environmental Factors of the Application Scenarios on the TC Removal Effect
The removal efficiency of TC by biochar is influenced by environmental factors. The solution pH has the most critical effect. The solution pH controls the surface charge of biochar. The solution pH determines the ionization state of TC. The solution pH regulates the surface functional group activity. These groups include carboxyl and hydroxyl groups. This control governs the adsorption equilibrium. Coexisting cations and anions reduce the TC removal capacity, compete for adsorption sites, and change the ionic strength. Coexisting soluble organic matter (DOM) forms complexes with TC. DOM blocks biochar pores, which decreases the adsorption efficiency. These factors are frequently ignored in real water systems. Ionic interference and DOM interact synergistically. This interaction diverges from laboratory predictions under ideal conditions.
4.2.1. pH
Solution pH significantly affects the biochar TC adsorption performance by regulating the biochar surface charge and TC speciation. The mechanism involves three stages. (1) Under acidic conditions (pH < 4), biochar becomes protonated with positive charges. TCs exist mainly as TCH3+ cations (>85%). Electrostatic repulsion between them reduces the adsorption efficiency. (2) At neutral pH values (5–7), biochar becomes deprotonated and has negative charges. TCs primarily exist in neutral (TCH20) or monoanionic (TCH−) forms. Electrostatic attraction and hydrogen bonding jointly increase the adsorption capacity to peak levels. (3) Under alkaline conditions (pH > 8), TC fully deprotonates to TC2−. Strong Coulomb repulsion occurs between negatively charged biochar and TC2−. This significantly increases the adsorption energy barrier [126]. Experimental evidence confirms this pH dependence. Wang et al. reported that the adsorption capacity increased from 52 mg/g (pH = 2) to 128 mg/g (pH = 5) and then decreased to 85 mg/g (pH > 7) due to electrostatic repulsion [70]. Meng et al. reported the maximum adsorption (243.76 mg/g) at pH = 4.41 for BM-S/ZVI@BC, which was maintained at >200 mg/g at pH = 7.08. Under alkaline conditions (pH 10.77), adsorption decreased to 105.71 mg/g because Fe0 oxidation formed Fe(OH)3 passivation layers [106]. Zhang et al. reported that the TC removal efficiency improved from 55.3% (pH = 2.0) to 69.3% (pH = 6.0) and then decreased to 65.6% (pH = 10.0) as the TC2− proportion increased. These findings demonstrate that pH optimization is crucial for maximizing the biochar adsorption efficiency [66].
4.2.2. Coexisting Cations/Anions
Coexisting cations (e.g., Ca2+ and Mg2+) and anions (e.g., Cl− and SO42−) in water reduce the biochar TC removal efficiency through multiple mechanisms [127]. Cations primarily compete with TC for adsorption sites on biochar. This competition weakens electrostatic attraction and cation–π interactions. Chen et al. demonstrated this effect. Increasing the concentrations of Na+, K+, Mg2+, and Ca2+ from 0 to 0.1 M reduced the TC adsorption by 12.5%, 13%, 25%, and 37.5%, respectively. The inhibition strength was correlated with the cation properties [128]. A larger hydrated ion radius (Ca2+ > Mg2+ > K+ > Na+) and higher charge density (Ca2+ = Mg2+ > K+ = Na+) enhance interference. Anions affect adsorption through complexation and conductivity changes. Li et al. reported greater TC removal (99.72%) in mixed SO42−/Cl− systems (0.1–0.5 M) than in single-anion solutions. This improvement stems from the optimized biochar surface charge distribution through anion synergy [98]. However, HCO3− has distinct inhibitory effects. Meng et al. reported a 59% decrease in TC adsorption by BM-S/ZVI@BC as the HCO3− concentration increased from 0 to 0.1 M (128–52 mg/g). The mechanism involves HCO3− forming passivation layers on iron surfaces. These layers block electron transfer, suppressing Fe2+/Fe3+-TC complexation [106].
4.2.3. Dissolved Organic Matter
Dissolved organic matter (DOM) widely exists in environments and interferes with biochar TC adsorption through multiple pathways. DOM components (e.g., humic acid, fulvic acid, and polysaccharides) compete with TC for biochar active sites. DOM also reduces the adsorption efficiency via pore blockage and surface charge neutralization [129,130]. Xiong and Bi demonstrated the structural impact of DOM. Corn stover biochar pyrolyzed at 300 °C released biodegradable DOM (BDOM) during adsorption. This caused a 54% decrease in surface area (4.02 to 1.83 m2/g). The H/C ratio increased from 0.80 to 0.91, indicating reduced aromaticity and weakened π–π interactions. DOM effects vary by concentration [131]. Wang et al. reported that low humic acid concentrations (0–10 mg/L) enhanced TC adsorption on Zn-LBC. The adsorption capacity increased by 14.6% (157.36–180.37 mg/g) due to DOM-TC complex bridging. Conversely, BDOM removal significantly improved performance [132]. Yin et al. reported that residual biochar (RMBC) after BDOM removal achieved a Qmax = 328.030 mg/g, which was 25.01% greater than that of the KMnO4-modified biochar (262.392 mg/g). This enhancement stems from the exposed Mn4+ sites on RMBC, which form complexes with the phenolic hydroxyl groups of TC [133].
5. Mechanisms of Modified BC Adsorption to TC
The adsorption mechanisms of TC onto biochar include pore filling, π–π interactions, hydrophobic interactions, hydrogen bonding, electrostatic interactions, and surface complexation (Figure 3). Among these interactions, π–π interactions dominate due to strong electron coupling between the aromatic carbon structures of biochar and the benzene rings of TC. This mechanism contributes most significantly under neutral to weakly alkaline conditions. Pore filling plays a key role in high-surface-area biochar but depends on pore size matching with TC molecules. The adsorption efficiency decreases sharply when the biochar pores are smaller than the TC molecules [134]. Electrostatic interactions and hydrogen bonding have limited effects and rely heavily on the solution pH and TC ionization state. At pH > 8, TC deprotonation increases the electrostatic repulsion, reducing adsorption by 20–35%. Hydrophobic interactions and surface complexation act as secondary mechanisms. Hydrophobic interactions contribute up to 15% of the total adsorption in high-ionic-strength environments. Iron-modified biochar enhances adsorption via Fe-O-TC complexation, resulting in a 1.8-fold greater capacity than that of unmodified biochar [120].
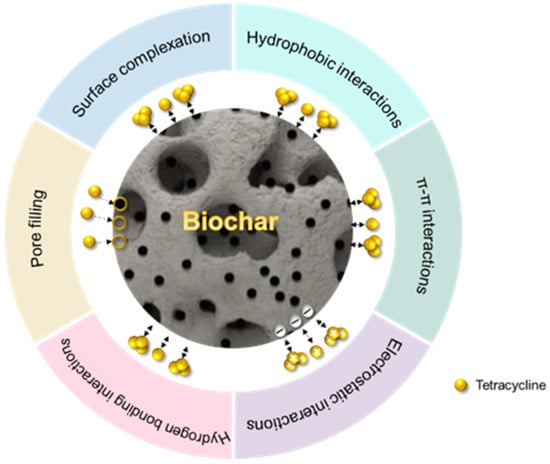
Figure 3.
The six mechanisms of TC adsorption in water by modified biochar are π–π interactions (the main mechanism), electrostatic interactions, hydrogen bonding, pore filling, surface complexation, and hydrophobic interactions.
5.1. π–π Interactions
π–π interactions serve as a primary driver in TC adsorption mechanisms. The surface functional groups (carboxyl, nitro, and ketone groups) of biochar act as electron acceptors. These groups form π–π electron donor–acceptor (EDA) interactions with the aromatic rings of TC [135]. Experimental evidence confirms this dominance. Jiang et al. analyzed Fe@MBC via Raman spectroscopy. After TC adsorption, the D-band (1307 cm−1) and G-band (1587 cm−1) intensities decreased significantly. The ID/IG ratio increased by 8.14, confirming the major role of π–π interactions. Iron-modified biochar also showed spectral changes, indicating cation–π interactions between the biochar’s π-bonds and Fe2+/Fe3+ [81]. Wang et al. further validated this mechanism. The C=C characteristic peak (1588 cm−1) of the alkali-activated carbon was reduced by 68% after TC adsorption. This strongly supports π–π EDA interactions as the key pathway [56].
5.2. Electrostatic Interactions
The surface charge of biochar governs its TC adsorption mechanism. Biochar surfaces typically carry negative charges due to the deprotonation of oxygen-containing groups (e.g., carboxyl and hydroxyl groups), enabling the electrostatic adsorption of positively charged pollutants [136]. The zero-charge pH (pHpzc) directly controls the adsorption performance. At pH < pHpzc, biochar surfaces become positively charged, preferentially adsorbing deprotonated TC−/TC2−. At pH > pHpzc, negatively charged biochar surfaces favor protonated TC+ adsorption [137]. Experimental studies have confirmed these principles. Oladipo and Ifebajo reported reduced adsorption under acidic conditions (pH < pHpzc) due to electrostatic repulsion between the positively charged MCB and cationic TC. At pH 8.0, MCB achieved 93.2% TC removal through electrostatic attraction with anionic TCH−. Adsorption decreased at pH > pHpzc, as negatively charged MCB repelled TC2− [119]. Yu et al. identified pH 5–9 as the optimal range for electrostatic-driven TC adsorption. Direct evidence comes from surface analysis [113]. Liu et al. detected significant changes in the C=O and C-OH/O-C=O peaks via XPS O 1 s spectra after TC adsorption, confirming electrostatic interactions between TC and these functional groups on IKBCH surfaces [134].
5.3. Hydrogen Bonding
Hydrogen bonding significantly contributes to TC adsorption on biochar. The oxygen-containing functional groups (-OH, -COOH) introduced during biochar modification enable the formation of hydrogen bonds. These bonds provide stable and persistent adsorption due to their high bond energy [138]. Iron-modified biochar (Fe-BC) demonstrates this mechanism. Its hydroxyl and carboxyl groups act as hydrogen bond donors/acceptors through oxygen atoms, enhancing the TC adsorption capacity [115]. Nitrogen-doped biochar also utilizes hydrogen bonding. Mei et al. confirmed that nitrogen functional groups (e.g., -NH2) participate in TC adsorption via hydrogen bonds [73]. Meng et al. revealed hydrogen-bonding interactions at multiple sites. The hydroxyl (-OH), methyl (-CH3), and amino (-NH2) groups of TC function as hydrogen bond acceptors. Interactions with the surface oxygen groups and iron oxides of BM-S/ZVI@BC highlight the essential role of hydrogen bonding in adsorption [106].
5.4. Pore Filling
Pore filling is a critical mechanism in TC adsorption by biochar, with the efficiency governed by the SSA and hierarchical pore structure. The diffusion of TC molecules within modified biochar pores significantly influences the adsorption kinetics [139]. Zhang et al. demonstrated this via diffusion models: TC exhibited a 3.2-fold higher diffusion coefficient in mesoporous biochar (MPBC) than in conventional biochar, which was attributed to rapid mass transfer through mesoporous channels. Pore structure optimization further enhances adsorption [114]. Zhu et al. adjusted the pyrolysis atmosphere (air/N2 mixture replacing pure N2) to develop mesoporous carbons (A050, A070), with the TC adsorption capacities exceeding 90–5.5–9.2 times greater than those of microporous biochars [64]. Three mesoporous advantages explain this: (1) the pore size (2–50 nm) matches the TC dimensions (1.2 × 0.6 nm) for efficient capture; (2) open channels minimize the diffusion resistance; and (3) a high SSA ensures dense active sites for stable adsorption. Microporous structures (<2 nm) exhibit limited TC adsorption due to steric hindrance, underscoring the importance of hierarchical porous designs [140,141]. These findings highlight pore engineering as a strategic approach to maximize the biochar TC removal performance.
5.5. Surface Complexation
Surface complexation significantly contributes to TC adsorption on biochar and is strongly related to the density and distribution of oxygen-containing functional groups [142]. Zhang et al. provided direct evidence through C1s XPS analysis. Post-adsorption analysis revealed reductions in C-O-C groups (16.61% to 11.77%) and O-C=O groups (12.6% to 5.65%), with a 0.18 decrease in O/C. These changes confirm the dominance of surface coordination mechanisms [114]. Meng et al. further quantified this mechanism via the use of sulfur-doped ZVI biochar (S-ZVI@BC). The C=O and -OH group contents decreased by 23.4% (37.6–28.5%) and 12.8% (42.1–36.7%), respectively. These reductions were strongly negatively correlated (R2 = 0.9949–0.9167) with the maximum TC adsorption capacity (Qmax = 505.68 mg/g), highlighting hydroxyl complexation as a key pathway [106]. Wei et al. identified Fe-O-TC bonds via Fe 2p XPS analysis. A characteristic peak at 531.2 eV confirmed stable coordination between TC and iron oxides (FeO/FeOOH) on the biochar, with a coordination number of 4.2 [143].
5.6. Hydrophobic Interactions
Hydrophobic interactions enhance TC adsorption on modified biochar through multiscale mechanisms. These interactions originate from van der Waals forces between hydrophobic groups (e.g., methyl groups, aromatic carbon clusters) on biochar and nonpolar TC moieties (e.g., benzene rings, methyl groups). Carbon skeleton topology optimization further strengthens the performance. High-temperature pyrolysis promotes the graphitization and aromatization of carbon structures. This process significantly increases material hydrophobicity, increasing the TC adsorption capacity by 3–5 times. Zou et al. demonstrated this enhancement. Compared with raw biochar, biochar pyrolyzed at 700 °C presented a 12.23-fold greater TC adsorption capacity (49.92 to 610.72 mg/g). This improvement correlated with reduced structural defects (ID/IG = 1.06) and increased graphitic carbon content. This behavior can be explained by hydrophobicity-driven phase partitioning [144]. TC migrates from polar aqueous phases to nonpolar biochar surfaces. The partition coefficient (Kd) was strongly negatively correlated with the biochar O/C ratio. Ma et al. validated this relationship. Vinyl chloride-modified biochar (CDBC) reduced the O/C ratio from 0.704 to 0.532. This decreased surface polarity and enhanced hydrophobicity significantly improved the TC removal efficiency. The synergy between hydrophobic and π-π electron donor–acceptor (EDA) effects further optimizes adsorption [145]. Tang et al. achieved 95% TC removal within 9 h via NaOH-modified biochar (NBC). Its maximum adsorption capacity reached 101.01 mg/g, demonstrating that hydrophobic–π interactions were the dominant mechanism [146].
6. Present Knowledge Gaps
6.1. Risk of Secondary Contamination of Raw Charcoal
Despite the potential of biochar for TC removal, its large-scale use faces risks of secondary pollution. Biochar from sludge or industrial waste may retain heavy metals (e.g., Cd and Pb) if the feedstocks are incompletely decomposed during pyrolysis, causing dual contamination during TC adsorption [147]. Wang et al. reported that the Cd content in textile dye sludge biochar increased from 0.793 mg·kg−1 (300 °C) to 0.928 mg·kg−1 (500 °C) and then decreased to 0.139 mg·kg−1 at 600 °C [148]. The presence of persistent free radicals (PFRs) in biochar poses environmental hazards. Light or heat activates PFRs to produce reactive oxygen species (ROS). These ROS directly damage DNA [149] and convert pollutants into toxic by-products (e.g., nitro-PAHs) [150]. Zhang et al. reported plant membrane damage from biochar-borne PFRs: catalase activity increased by 38.1% at 20 g/kg biochar but decreased by 25.2% at 80 g/kg [151]. Lieke et al. demonstrated neurotoxicity in Caenorhabditis elegans exposed to straw-derived biochar (500 °C, 6.37 × 105 C/O-centered radicals), resulting in impaired excretion and chemotaxis [152]. Physical hazards include inhalable dust from biochar grinding and crystalline silica in rice husk biochar pyrolyzed at >550 °C [153]. These risks underscore the need for safety assessments before large-scale biochar deployment.
6.2. Economic–Environmental Efficiency Conflicts
Biochar has potential for pollutant adsorption but faces economic and practical limitations. Compared with activated carbon, its adsorption processes often require longer contact times. This necessitates either increased biochar dosages (increasing costs) or extended treatment durations (reducing efficiency). High production costs stem from energy-intensive pyrolysis (≥500 °C) and chemical activation requirements. For example, U.S.-produced wood-derived biochar costs up to USD 17.80 per kg, typically exceeding the activated carbon prices [154]. Snyder quantified cost disparities: rapid pyrolysis at 500 °C yielded triple the net present value of slow pyrolysis at 400 °C, confirming performance–cost trade-offs. Low-cost agricultural wastes (e.g., straw and nut shells) are affordable but suffer from inconsistent adsorption capacities [155]. Katiyar et al. reported 15–40% performance variations in real wastewater treatments due to fluctuating pollutant concentrations. Additional barriers include limited regeneration efficiency (≤65% capacity retention after 3 cycles) and pH-dependent stability, which results in 15–40% performance variations in real wastewater treatments due to fluctuating pollutant concentrations [156]. Addressing these challenges requires multistrategy optimization: (1) developing low-temperature activation methods (<400 °C), (2) standardizing waste-derived feedstocks through pretreatment protocols, and (3) engineering hybrid biochar composites for enhanced regeneration. These improvements could reduce the production costs by 30–50% while maintaining a ≥80% adsorption efficiency.
6.3. Laboratory–Actual Water Efficiency Deviation
Compared with laboratory conditions, practical water matrices significantly alter the contaminant removal efficiency of modified biochar. For example, Wang et al. reported 84.5% TC and 84.0% Cu2+ simultaneous removal by SBC in Yangtze River water. However, the TC removal efficiency at low Cu2+ concentrations (0.2 mmol/L) was lower than that at ultrapure water concentrations because of ion competition. At high Cu2+ concentrations (2.0 mmol/L), the TC removal efficiency surpassed the laboratory results through complex bridging effects between Cu2+ and adsorbents [70]. Similarly, Meng et al. reported reduced TC adsorption capacities for BM-S/ZVI@BC in artificial lake water (266.09 mg/g) and river water (246.29 mg/g) compared with those of deionized water (387.22 mg/g). This reduction stems from three factors: competitive ions, microbial interference, and pore blockage by suspended particles [106]. Wang et al. reported a 50% decrease in TC adsorption (159.64 → 85.64 mg/g) for Zn-LBC in hospital wastewater versus controlled settings. The performance gap likely arises from complex pollutant competition for active sites in real water samples [132].
7. Conclusions and Prospects
This study investigated the surface properties of biochar. These properties affect TC adsorption in water. This study investigated the adsorption performance, mechanisms, and environmental factors. Pristine biochar has a limited TC adsorption capacity. This limitation comes from constraints. For example, pristine biochar has a small particle size and also causes electrostatic repulsion. Physical and chemical modifications significantly improve biochar performance. Compared with the other methods, modified biochar better removed TC. Both physical and chemical modifications enhanced performance. Treatment with H3PO4 at high temperatures (>500 °C) was particularly effective and increased the adsorption capacity by 13.5 times, reaching 451.5 mg/g. This showed superior TC removal efficiency. The adsorption mechanisms involved pore filling, π–π interactions (dominant), electrostatic interactions, hydrogen bonding, hydrophobic effects, and surface complexation. π–π interactions primarily originated from strong electronic coupling between the aromatic carbon structures of biochar and the benzene rings of TC. The solution pH critically affects the adsorption efficiency. pH controls the surface charge of biochar as well as the ionization state of the TC. It also controls functional group activity. Coexisting cations such as Ca2+ and Mg2+ reduce TC removal. Coexisting anions such as Cl− and SO42− also reduce removal and cause competitive adsorption. These compounds alter the ionic strength. The DOM indirectly lowers the efficiency. DOM causes complexation and blocks pores. For practical use, medium- to high-temperature (>500 °C) biochar works best. Chemical modifiers such as KOH or H3PO4 improve this effect, resulting in optimal TC removal. However, high-temperature pyrolysis uses more energy, increasing consumption by approximately 30% compared with that at low temperatures. Field trials have shown only a 15–20% capacity increase in real wastewater, which is caused by ion interference. This highlights a gap between the laboratory results and real water performance. Scale-up also faces challenges, and secondary contamination risks exist. Examples include heavy metal leaching and toxic radicals. Economic and environmental trade-offs are difficult. High-cost pyrolysis results in only marginal gains. Therefore, there is a need for matrix-specific modification protocols. These aspects need urgent attention and will help bridge the lab-to-industry gap. These aspects need to be considered in the near future to address the challenges being faced.
- (i).
- Secondary pollution risks of biochar
The secondary pollution risk of biochar requires control across the production and application stages. Agricultural residues such as corncobs and rice husks have lower heavy metal contents than sludge or industrial waste, making them safer feedstocks. Staged pyrolysis can reduce risks: low-temperature treatment (300–400 °C) removes volatile pollutants, followed by brief high-temperature pulses (e.g., 600 °C) to break down free radicals. Surface coatings with humic acid or zeolite seal heavy metals and reduce radical activity through mineral adsorption.
- (ii).
- Economic–environmental trade-offs
High production costs and high energy consumption limit the large-scale use of biochar. Solutions include developing low-cost, low-energy modification methods. Multifunctional applications, such as combining water treatment with soil remediation or carbon sequestration, improve the economic viability.
- (iii).
- Lab-to-field performance gaps
The real-world performance of biochar often falls below the laboratory results because of ion competition, microbial interference, and pore blockage. Addressing this requires testing under realistic water conditions and optimizing biochar for complex environments. Smart monitoring technologies could adjust biochar dosing in real-time to maintain stable efficiency.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w17131960/s1, Text S1: Calculation of biochar removal rate of TC in water [42].
Funding
This project was financially supported by the Guizhou University Student Innovation and Entrepreneurship Training Program (gzusc2024137).
Data Availability Statement
The original contributions presented in this study are included in the article and Supplementary Material. Further inquiries can be directed to the corresponding author(s).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Liu, X.; Zhang, G.; Liu, Y.; Lu, S.; Qin, P.; Guo, X.; Bi, B.; Wang, L.; Xi, B.; Wu, F.; et al. Occurrence and fate of antibiotics and antibiotic resistance genes in typical urban water of Beijing, China. Environ. Pollut. 2019, 246, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Pena, A.; Paulo, M.; Silva, L.J.G.; Seifrtová, M.; Lino, C.M.; Solich, P. Tetracycline antibiotics in hospital and municipal wastewaters: A pilot study in Portugal. Anal. Bioanal. Chem. 2010, 396, 2929–2936. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, X.; Feng, C.; Songtao, Z. Technologies for the Removal of Antibiotics in the Environment: A Review. Int. J. Electrochem. Sci. 2022, 17, 220768. [Google Scholar] [CrossRef]
- Kafaei, R.; Papari, F.; Seyedabadi, M.; Sahebi, S.; Tahmasebi, R.; Ahmadi, M.; Sorial, G.A.; Asgari, G.; Ramavandi, B. Occurrence, distribution, and potential sources of antibiotics pollution in the water-sediment of the northern coastline of the Persian Gulf, Iran. Sci. Total Environ. 2018, 627, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef]
- Leichtweis, J.; Vieira, Y.; Welter, N.; Silvestri, S.; Dotto, G.L.; Carissimi, E. A review of the occurrence, disposal, determination, toxicity and remediation technologies of the tetracycline antibiotic. Process Saf. Environ. 2022, 160, 25–40. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, J.; Lu, C.; Liao, Q.; Gudda, F.O.; Ling, W. Antibiotics in animal manure and manure-based fertilizers: Occurrence and ecological risk assessment. Chemosphere 2020, 255, 127006. [Google Scholar] [CrossRef]
- Li, S.; Hofstra, N.; van de Schans, M.G.M.; Yang, J.; Li, Y.; Zhang, Q.; Ma, L.; Strokal, M.; Kroeze, C.; Chen, X.; et al. Riverine Antibiotics from Animal Production and Wastewater. Environ. Sci. Technol. Lett. 2023, 10, 1059–1067. [Google Scholar] [CrossRef]
- Li, S.; Zhu, Y.; Zhong, G.; Huang, Y.; Jones, K.C. Comprehensive Assessment of Environmental Emissions, Fate, and Risks of Veterinary Antibiotics in China: An Environmental Fate Modeling Approach. Environ. Sci. Technol. 2024, 58, 5534–5547. [Google Scholar] [CrossRef]
- Pham, D.K.; Chu, J.; Do, N.T.; Brose, F.; Degand, G.; Delahaut, P.; De Pauw, E.; Douny, C.; Nguyen, K.V.; Vu, T.D.; et al. Monitoring Antibiotic Use and Residue in Freshwater Aquaculture for Domestic Use in Vietnam. Ecohealth 2015, 12, 480–489. [Google Scholar] [CrossRef]
- Kang, H.; Lee, S.; Shin, D.; Jeong, J.; Hong, J.; Rhee, G. Occurrence of veterinary drug residues in farmed fishery products in South Korea. Food Control 2018, 85, 57–65. [Google Scholar] [CrossRef]
- Shimizu, A.; Takada, H.; Koike, T.; Takeshita, A.; Saha, M.; Rinawati; Nakada, N.; Murata, A.; Suzuki, T.; Suzuki, S.; et al. Ubiquitous occurrence of sulfonamides in tropical Asian waters. Sci. Total Environ. 2013, 452, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Chen, B.; Nie, X.; Shi, Z.; Huang, X.; Li, X. The distribution and partitioning of common antibiotics in water and sediment of the Pearl River Estuary, South China. Chemosphere 2013, 92, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Faleye, A.C.; Adegoke, A.A.; Ramluckan, K.; Bux, F.; Stenström, T.A. Antibiotic Residue in the Aquatic Environment: Status in Africa. Open Chem. 2018, 16, 890–903. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P. Tetracycline antibiotics in the environment: A review. Environ. Chem. Lett. 2013, 11, 209–227. [Google Scholar] [CrossRef]
- Amangelsin, Y.; Semenova, Y.; Dadar, M.; Aljofan, M.; Bjørklund, G. The Impact of Tetracycline Pollution on the Aquatic Environment and Removal Strategies. Antibiotics 2023, 12, 440. [Google Scholar] [CrossRef]
- Yan, C.; Yang, Y.; Zhou, J.; Liu, M.; Nie, M.; Shi, H.; Gu, L. Antibiotics in the surface water of the Yangtze Estuary: Occurrence, distribution and risk assessment. Environ. Pollut. 2013, 175, 22–29. [Google Scholar] [CrossRef]
- Charuaud, L.; Jarde, E.; Jaffrezic, A.; Thomas, M.; Le Bot, B. Veterinary pharmaceutical residues from natural water to tap water: Sales, occurrence and fate. J. Hazard. Mater. 2019, 361, 169–186. [Google Scholar] [CrossRef]
- Torres-Acosta, M.A.; Pereira, J.F.B.; Freire, M.G.; Aguilar-Yáñez, J.M.; Coutinho, J.A.P.; Titchener-Hooker, N.J.; Rito-Palomares, M. Economic evaluation of the primary recovery of tetracycline with traditional and novel aqueous two-phase systems. Sep. Purif. Technol. 2018, 203, 178–184. [Google Scholar] [CrossRef]
- Kim, K.; Owens, G.; Kwon, S.; So, K.; Lee, D.; Ok, Y.S. Erratum to: Occurrence and Environmental Fate of Veterinary Antibiotics in the Terrestrial Environment. Water Air Soil Pollut. 2012, 223, 6213–6214. [Google Scholar] [CrossRef]
- Wang, D.; Li, J.; Xu, Z.; Zhu, Y.; Chen, G.; Cui, Z. Synthesis of g-C3N4/NiO p–n heterojunction materials with ball-flower morphology and enhanced photocatalytic performance for the removal of tetracycline and Cr6+. J. Mater. Sci. 2019, 54, 11417–11434. [Google Scholar] [CrossRef]
- Xu, Y.; Guo, C.; Luo, Y.; Lv, J.; Zhang, Y.; Lin, H.; Wang, L.; Xu, J. Occurrence and distribution of antibiotics, antibiotic resistance genes in the urban rivers in Beijing, China. Environ. Pollut. 2016, 213, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Wu, Y.; Zhang, W.; Zhong, J.; Lou, Q.; Yang, P.; Fang, Y. Occurrence, distribution, and risk assessment of antibiotics in the surface water of Poyang Lake, the largest freshwater lake in China. Chemosphere 2017, 184, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Suthar, S. Occurrence, seasonal variations, and ecological risk of pharmaceuticals and personal care products in River Ganges at two holy cities of India. Chemosphere 2021, 268, 129331. [Google Scholar] [CrossRef]
- Burke, V.; Richter, D.; Greskowiak, J.; Mehrtens, A.; Schulz, L.; Massmann, G. Occurrence of Antibiotics in Surface and Groundwater of a Drinking Water Catchment Area in Germany. Water Environ. Res. 2016, 88, 652–659. [Google Scholar] [CrossRef]
- Topal, M.; Topal, E.I.A. Investigation of tetracycline and degradation products in Euphrates river receiving outflows of trout farms. Aquac. Res. 2016, 47, 3837–3844. [Google Scholar] [CrossRef]
- Harnisz, M.; Korzeniewska, E.; Golas, I. The impact of a freshwater fish farm on the community of tetracycline-resistant bacteria and the structure of tetracycline resistance genes in river water. Chemosphere 2015, 128, 134–141. [Google Scholar] [CrossRef]
- Nantaba, F.; Wasswa, J.; Kylin, H.; Palm, W.; Bouwman, H.; Kümmerer, K. Occurrence, distribution, and ecotoxicological risk assessment of selected pharmaceutical compounds in water from Lake Victoria, Uganda. Chemosphere 2020, 239, 124642. [Google Scholar] [CrossRef]
- Chan, R.; Wandee, S.; Wang, M.; Chiemchaisri, W.; Chiemchaisri, C.; Yoshimura, C. Fate, transport and ecological risk of antibiotics from pig farms along the bang pakong River, Thailand. Agric. Ecosyst. Environ. 2020, 304, 107123. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, K.; Choi, K. Effect of runoff discharge on the environmental levels of 13 veterinary antibiotics: A case study of Han River and Kyungahn Stream, South Korea. Mar. Pollut. Bull. 2016, 107, 347–354. [Google Scholar] [CrossRef]
- Al-Haideri, H.H.; Hassan, F.M.; Sattar, S.; El-Sheekh, M.M. Anthropocentric perspective on climate variability: The destination of antibiotics in the Tigris river is not restricted. J. Water Health 2024, 22, 1306–1327. [Google Scholar] [CrossRef]
- Wilkinson, J.L.; Boxall, A.B.A.; Kolpin, D.W.; Leung, K.M.Y.; Lai, R.W.S.; Galbán-Malagón, C.; Adell, A.D.; Mondon, J.; Metian, M.; Marchant, R.A.; et al. Pharmaceutical pollution of the world’s rivers. Proc. Natl. Acad. Sci. USA 2022, 119, e2113947119. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Li, N.; Ying, G. Antibiotic distribution, risk assessment, and microbial diversity in river water and sediment in Hong Kong. Environ. Geochem. Health 2018, 40, 2191–2203. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.H.; Hoang, L.; Nghiem, L.D.; Nguyen, N.; Ngo, H.H.; Guo, W.S.; Trinh, Q.T.; Mai, N.; Chen, H.T.; Nguyen, D.D.; et al. Occurrence and risk assessment of multiple classes of antibiotics in urban canals and lakes in Hanoi, Vietnam. Sci. Total Environ. 2019, 692, 157–174. [Google Scholar] [CrossRef]
- Liu, X.; Lu, S.; Guo, W.; Xi, B.; Wang, W. Antibiotics in the aquatic environments: A review of lakes, China. Sci. Total Environ. 2018, 627, 1195–1208. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, J.; Xin, Q. Effects of tetracycline on developmental toxicity and molecular responses in zebrafish (Danio rerio) embryos. Ecotoxicology 2015, 24, 707–719. [Google Scholar] [CrossRef]
- Gudda, F.; Odinga, E.S.; Tang, L.; Waigi, M.G.; Wang, J.; Abdalmegeed, D.; Gao, Y. Tetracyclines uptake from irrigation water by vegetables: Accumulation and antimicrobial resistance risks. Environ. Pollut. 2023, 338, 122696. [Google Scholar] [CrossRef]
- Yao, X.; Cheng, Z.; Agathokleous, E.; Wei, Y.; Feng, X.; Li, H.; Zhang, T.; Li, S.; Dhawan, G.; Luo, X. Tetracycline and sulfadiazine toxicity in human liver cells Huh-7. Environ. Pollut. 2024, 345, 123454. [Google Scholar] [CrossRef]
- Ferreira, L.; Rosales, E.; Danko, A.S.; Sanromán, M.A.; Pazos, M.M. Bacillus thuringiensis a promising bacterium for degrading emerging pollutants. Process Saf. Environ. Prot. 2016, 101, 19–26. [Google Scholar] [CrossRef]
- Ajala, O.A.; Akinnawo, S.O.; Bamisaye, A.; Adedipe, D.T.; Adesina, M.O.; Okon-Akan, O.A.; Adebusuyi, T.A.; Ojedokun, A.T.; Adegoke, K.A.; Bello, O.S. Adsorptive removal of antibiotic pollutants from wastewater using biomass/biochar-based adsorbents. RSC Adv. 2023, 13, 4678–4712. [Google Scholar] [CrossRef]
- Munuhe, L.N.; Madivoli, E.S.; Nzilu, D.M.; Lemeitaron, P.N.; Kimani, P.K. Advances in Adsorbent Materials for Pharmaceutical Pollutant Removal: A Review of Occurrence, Fate, and State-of-the-Art Remediation. J. Chem. 2025, 2025, 4477822. [Google Scholar] [CrossRef]
- Chen, Y.; Mao, W.; Yang, W.; Niazi, N.K.; Wang, B.; Wu, P. A novel phosphate rock-magnetic biochar for Pb2+ and Cd2+ removal in wastewater: Characterization, performance and mechanisms. Environ. Technol. Innov. 2023, 32, 103268. [Google Scholar] [CrossRef]
- Teodosiu, C.; Gilca, A.; Barjoveanu, G.; Fiore, S. Emerging pollutants removal through advanced drinking water treatment: A review on processes and environmental performances assessment. J. Clean. Prod. 2018, 197, 1210–1221. [Google Scholar] [CrossRef]
- Cui, T.; Xie, Y.; Zhang, M.; Raise, A. Tetracycline removal from aqueous media and hospital wastewater using a magnetic composite of mango lignocellulosic kernel biochar/MnFe2O4/Cu@Zn-BDC MOF. Int. J. Biol. Macromol. 2025, 297, 139774. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, H.; Yu, H. Development of Biochar-Based Functional Materials: Toward a Sustainable Platform Carbon Material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef]
- Peiris, C.; Gunatilake, S.R.; Mlsna, T.E.; Mohan, D.; Vithanage, M. Biochar based removal of antibiotic sulfonamides and tetracyclines in aquatic environments: A critical review. Bioresour. Technol. 2017, 246, 150–159. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, W.; Wang, J.; Chen, G.; Li, N.; Song, Y.; Cheng, Z.; Yan, B.; Hou, L.; Wang, S. Sulfamethoxazole degradation by regulating active sites on distilled spirits lees-derived biochar in a continuous flow fixed bed peroxymonosulfate reactor. Appl. Catal. B-Environ. 2022, 310, 121342. [Google Scholar] [CrossRef]
- Yu, J.; Tang, L.; Pang, Y.; Zeng, G.; Feng, H.; Zou, J.; Wang, J.; Feng, C.; Zhu, X.; Ouyang, X.; et al. Hierarchical porous biochar from shrimp shell for persulfate activation: A two-electron transfer path and key impact factors. Appl. Catal. B-Environ. 2020, 260, 118160. [Google Scholar] [CrossRef]
- Kumar, A.; Bhattacharya, T. Biochar: A sustainable solution. Environ. Dev. Sustain. 2021, 23, 6642–6680. [Google Scholar] [CrossRef]
- Shi, W.; Wang, H.; Yan, J.; Shan, L.; Quan, G.; Pan, X.; Cui, L. Wheat straw derived biochar with hierarchically porous structure for bisphenol A removal: Preparation, characterization, and adsorption properties. Sep. Purif. Technol. 2022, 289, 120796. [Google Scholar] [CrossRef]
- Oginni, O.; Singh, K. Influence of high carbonization temperatures on microstructural and physicochemical characteristics of herbaceous biomass derived biochars. J. Environ. Chem. Eng. 2020, 8, 104169. [Google Scholar] [CrossRef]
- Chen, S.; Chen, Y.; Jiang, H. Slow Pyrolysis Magnetization of Hydrochar for Effective and Highly Stable Removal of Tetracycline from Aqueous Solution. Ind. Eng. Chem. Res. 2017, 56, 3059–3066. [Google Scholar] [CrossRef]
- Li, X.; Jia, Y.; Zhang, J.; Qin, Y.; Wu, Y.; Zhou, M.; Sun, J. Efficient removal of tetracycline by H2O2 activated with iron-doped biochar: Performance, mechanism, and degradation pathways. Chin. Chem. Lett. 2022, 33, 2105–2110. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, R.; Zimmerman, A.R.; Wang, H.; Gao, B. Applications, impacts, and management of biochar persistent free radicals: A review. Environ. Pollut. 2023, 327, 121543. [Google Scholar] [CrossRef]
- Gholizadeh, M.; Hu, X.; Liu, Q. A mini review of the specialties of the bio-oils produced from pyrolysis of 20 different biomasses. Renew. Sustain. Energy Rev. 2019, 114, 109313. [Google Scholar] [CrossRef]
- Wang, K.; Yao, R.; Zhang, D.; Peng, N.; Zhao, P.; Zhong, Y.; Zhou, H.; Huang, J.; Liu, C. Tetracycline Adsorption Performance and Mechanism Using Calcium Hydroxide-Modified Biochars. Toxics 2023, 11, 841. [Google Scholar] [CrossRef]
- Mei, Y.; Zhuang, S.; Wang, J. Biochar: A potential and green adsorbent for antibiotics removal from aqueous solution. Rev. Environ. Sci. Biol. Technol. 2024, 23, 1065–1103. [Google Scholar] [CrossRef]
- Janu, R.; Mrlik, V.; Ribitsch, D.; Hofman, J.; Sedláček, P.; Bielská, L.; Soja, G. Biochar surface functional groups as affected by biomass feedstock, biochar composition and pyrolysis temperature. Carbon Resour. Convers. 2021, 4, 36–46. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, X.; Geng, M.; Chen, D.; Lin, H.; Zhang, H. Catalytic oxidation of clofibric acid by peroxydisulfate activated with wood-based biochar: Effect of biochar pyrolysis temperature, performance and mechanism. Chem. Eng. J. 2019, 374, 1253–1263. [Google Scholar] [CrossRef]
- Huang, H.; Tang, J.; Gao, K.; He, R.; Zhao, H.; Werner, D. Characterization of KOH modified biochars from different pyrolysis temperatures and enhanced adsorption of antibiotics. RSC Adv. 2017, 7, 14640–14648. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, W.; Zou, Y.; Wu, Y.; Mao, W.; Zhang, J.; Zia-ur-Rehman, M.; Wang, B.; Wu, P. Quantification of the effect of biochar application on heavy metals in paddy systems: Impact, mechanisms and future prospects. Sci. Total Environ. 2024, 912, 168874. [Google Scholar] [CrossRef] [PubMed]
- Gasim, M.F.; Lim, J.; Low, S.; Lin, K.A.; Oh, W. Can biochar and hydrochar be used as sustainable catalyst for persulfate activation. Chemosphere 2022, 287, 132458. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Song, X.; Miao, J.; Ma, Y.; Zhao, T.; Yin, M. Removal of tetracycline from water by adsorption with biochar: A review. J. Water Process Eng. 2024, 60, 105215. [Google Scholar] [CrossRef]
- Zhu, X.; Li, C.; Li, J.; Xie, B.; Lu, J.; Li, Y. Thermal treatment of biochar in the air/nitrogen atmosphere for developed mesoporosity and enhanced adsorption to tetracycline. Bioresour. Technol. 2018, 263, 475–482. [Google Scholar] [CrossRef]
- Huang, H.; Niu, Z.; Shi, R.; Tang, J.; Lv, L.; Wang, J.; Fan, Y. Thermal oxidation activation of hydrochar for tetracycline adsorption: The role of oxygen concentration and temperature. Bioresour. Technol. 2020, 306, 123096. [Google Scholar] [CrossRef]
- Zhang, W.; Yan, L.; Wang, Q.; Li, X.; Guo, Y.; Song, W.; Li, Y. Ball milling boosted the activation of peroxymonosulfate by biochar for tetracycline removal. J. Environ. Chem. Eng. 2021, 9, 106870. [Google Scholar] [CrossRef]
- Yu, Z.; Ji, L.; Zuo, Y.; Zhang, F.; Wei, C.; Jiang, F.; Fu, X.; Wu, W.; Du, J.; Chen, C.; et al. Removal of Tetracycline Hydrochloride by Ball-Milled Mulberry Biochar. Water Air Soil Pollut. 2023, 234, 14. [Google Scholar] [CrossRef]
- Jiang, W.; Cai, Y.; Liu, D.; Shi, Q.; Wang, Q. Adsorption properties and mechanism of suaeda biochar and modified materials for tetracycline. Environ. Res. 2023, 235, 116549. [Google Scholar] [CrossRef]
- Shim, T.; Yoo, J.; Ryu, C.; Park, Y.; Jung, J. Effect of steam activation of biochar produced from a giant Miscanthus on copper sorption and toxicity. Bioresour. Technol. 2015, 197, 85–90. [Google Scholar] [CrossRef]
- Wang, R.; Huang, D.; Liu, Y.; Zhang, C.; Lai, C.; Wang, X.; Zeng, G.; Zhang, Q.; Gong, X.; Xu, P. Synergistic removal of copper and tetracycline from aqueous solution by steam-activated bamboo-derived biochar. J. Hazard. Mater. 2020, 384, 121470. [Google Scholar] [CrossRef]
- Azargohar, R.; Dalai, A.K. Steam and KOH activation of biochar: Experimental and modeling studies. Micropor. Mesopor. Mater. 2008, 110, 413–421. [Google Scholar] [CrossRef]
- Sewu, D.D.; Jung, H.; Kim, S.S.; Lee, D.S.; Woo, S.H. Decolorization of cationic and anionic dye-laden wastewater by steam-activated biochar produced at an industrial-scale from spent mushroom substrate. Bioresour. Technol. 2019, 277, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Xu, J.; Zhang, Y.; Li, B.; Fan, S.; Xu, H. Effect of Fe-N modification on the properties of biochars and their adsorption behavior on tetracycline removal from aqueous solution. Bioresour. Technol. 2021, 325, 124732. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Liu, W.; Jiang, H.; Chen, J.; Li, W.; Yu, H. Modification of bio-char derived from fast pyrolysis of biomass and its application in removal of tetracycline from aqueous solution. Bioresour. Technol. 2012, 121, 235–240. [Google Scholar] [CrossRef]
- Joshi, M.; Bhatt, D.; Srivastava, A. Enhanced Adsorption Efficiency through Biochar Modification: A Comprehensive Review. Ind. Eng. Chem. Res. 2023, 62, 13748–13761. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Chen, K.; Shen, S.; Hu, H.; Chang, M.; Chen, D.; Wu, Y.; Yuan, H.; Wang, Y. Engineering banana-peel-derived biochar for the rapid adsorption of tetracycline based on double chemical activation. Resour. Conserv. Recycl. 2023, 190, 106821. [Google Scholar] [CrossRef]
- Thines, K.R.; Abdullah, E.C.; Mubarak, N.M.; Ruthiraan, M. Synthesis of magnetic biochar from agricultural waste biomass to enhancing route for waste water and polymer application: A review. Renew. Sustain. Energy Rev. 2017, 67, 257–276. [Google Scholar] [CrossRef]
- Ma, J.; Zhou, B.; Zhang, H.; Zhang, W. Fe/S modified sludge-based biochar for tetracycline removal from water. Powder Technol. 2020, 364, 889–900. [Google Scholar] [CrossRef]
- Liang, J.; Fang, Y.; Luo, Y.; Zeng, G.; Deng, J.; Tan, X.; Tang, N.; Li, X.; He, X.; Feng, C.; et al. Magnetic nanoferromanganese oxides modified biochar derived from pine sawdust for adsorption of tetracycline hydrochloride. Environ. Sci. Pollut. Res. 2019, 26, 5892–5903. [Google Scholar] [CrossRef]
- Shin, J.; Lee, Y.; Kwak, J.; Kim, S.; Lee, S.; Park, Y.; Lee, S.; Chon, K. Adsorption of radioactive strontium by pristine and magnetic biochars derived from spent coffee grounds. J. Environ. Chem. Eng. 2021, 9, 105119. [Google Scholar] [CrossRef]
- Jiang, F.; Wei, C.; Yu, Z.; Ji, L.; Liu, M.; Cao, Q.; Wu, L.; Li, F. Fabrication of Iron-Containing Biochar by One-Step Ball Milling for Cr(VI) and Tetracycline Removal from Wastewater. Langmuir 2023, 39, 18958–18970. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Zhang, S.; Song, J.; Zhao, Y.; Yang, F. Activation of porous magnetized biochar by artificial humic acid for effective removal of lead ions. J. Hazard. Mater. 2020, 389, 122115. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J.; Liu, X.; Liu, H.; Wang, L.; Cheng, D.; Wang, Y.; Guo, W.; Ngo, H.H. Efficient antibiotics removal by pig manure-based magnetic biochar-driven catalytic degradation. J. Water Process Eng. 2025, 70, 107013. [Google Scholar] [CrossRef]
- Naghipour, D.; Hoseinzadeh, L.; Taghavi, K.; Jaafari, J.; Amouei, A. Effective removal of tetracycline from aqueous solution using biochar prepared from pine bark: Isotherms, kinetics and thermodynamic analyses. Int. J. Environ. Anal. Chem. 2023, 103, 5706–5719. [Google Scholar] [CrossRef]
- Wang, H.; Fang, C.; Wang, Q.; Chu, Y.; Song, Y.; Chen, Y.; Xue, X. Sorption of tetracycline on biochar derived from rice straw and swine manure. RSC Adv. 2018, 8, 16260–16268. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Cao, Y.; Han, L. Characteristics of tetracycline adsorption by cow manure biochar prepared at different pyrolysis temperatures. Bioresour. Technol. 2019, 285, 121348. [Google Scholar] [CrossRef]
- Liu, D.; Cai, Y.; Yu, X.; Wang, Q. Removal of tetracycline with grape leaves–based biochar: Adsorption properties and mechanism. Biomass Convers. Biorefin. 2025, 14. [Google Scholar] [CrossRef]
- Dai, Y.; Li, J.; Shan, D. Adsorption of tetracycline in aqueous solution by biochar derived from waste Auricularia auricula dregs. Chemosphere 2020, 238, 124432. [Google Scholar] [CrossRef]
- Hoslett, J.; Ghazal, H.; Katsou, E.; Jouhara, H. The removal of tetracycline from water using biochar produced from agricultural discarded material. Sci. Total Environ. 2021, 751, 141755. [Google Scholar] [CrossRef]
- Zhang, D.; He, Q.; Hu, X.; Zhang, K.; Chen, C.; Xue, Y. Enhanced adsorption for the removal of tetracycline hydrochloride (TC) using ball-milled biochar derived from crayfish shell. Colloids Surf. A Physicochem. Eng. Asp. 2021, 615, 126254. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Ren, X.; Dong, W.; Chen, H.; Cai, T.; Zeng, W.; Li, W.; Tang, L. Soybean residue based biochar prepared by ball milling assisted alkali activation to activate peroxydisulfate for the degradation of tetracycline. J. Colloid. Interf. Sci. 2021, 599, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, H.; Cai, S.; Hu, Z.; Guo, D.; Sha, L. Efficient removal of tetracycline by boosting persulfate activation with ball milling biochar derived from iron-rich paper sludge. J. Water Process Eng. 2025, 69, 106858. [Google Scholar] [CrossRef]
- Zhu, J.; Yan, L.; Li, X.; Song, W.; Yan, T.; Li, Y. Ball-milled MoS2/biochar as peroxymonosulfate activator efficiently removes tetracycline: Multiple active sites-triggered radical/non-radical pathways. Ind. Crop Prod. 2023, 205, 117450. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Y.; Zhou, C.; Luo, G.; Zhang, S.; Chen, J. A novel porous carbon derived from hydrothermal carbon for efficient adsorption of tetracycline. Carbon 2014, 77, 627–636. [Google Scholar] [CrossRef]
- Torres-Perez, J.; Gerente, C.; Andres, Y. Sustainable Activated Carbons from Agricultural Residues Dedicated to Antibiotic Removal by Adsorption. Chin. J. Chem. Eng. 2012, 20, 524–529. [Google Scholar] [CrossRef]
- Chen, T.; Luo, L.; Deng, S.; Shi, G.; Zhang, S.; Zhang, Y.; Deng, O.; Wang, L.; Zhang, J.; Wei, L. Sorption of tetracycline on H3PO4 modified biochar derived from rice straw and swine manure. Bioresour. Technol. 2018, 267, 431–437. [Google Scholar] [CrossRef]
- Liu, Q.; Li, D.; Cheng, H.; Cheng, J.; Du, K.; Hu, Y.; Chen, Y. High mesoporosity phosphorus-containing biochar fabricated from Camellia oleifera shells: Impressive tetracycline adsorption performance and promotion of pyrophosphate-like surface functional groups (C-O-P bond). Bioresour. Technol. 2021, 329, 124922. [Google Scholar] [CrossRef]
- Li, H.; Hu, J.; Meng, Y.; Su, J.; Wang, X. An investigation into the rapid removal of tetracycline using multilayered graphene-phase biochar derived from waste chicken feather. Sci. Total Environ. 2017, 603–604, 39–48. [Google Scholar] [CrossRef]
- Cheng, D.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Zhang, X.; Varjani, S.; Liu, Y. Feasibility study on a new pomelo peel derived biochar for tetracycline antibiotics removal in swine wastewater. Sci. Total Environ. 2020, 720, 137662. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, W.; Zhang, H.; Bai, H.; Liu, K.; Zhang, J.; Li, Z.; Zhu, W. Porous biochar derived from walnut shell as an efficient adsorbent for tetracycline removal. Bioresour. Technol. 2023, 383, 129213. [Google Scholar] [CrossRef]
- Jang, H.M.; Kan, E. Engineered biochar from agricultural waste for removal of tetracycline in water. Bioresour. Technol. 2019, 284, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.M.; Yoo, S.; Choi, Y.; Park, S.; Kan, E. Adsorption isotherm, kinetic modeling and mechanism of tetracycline on Pinus taeda-derived activated biochar. Bioresour. Technol. 2018, 259, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.C.; Pezoti, O.; Cazetta, A.L.; Bedin, K.C.; Yamazaki, D.A.S.; Bandoch, G.F.G.; Asefa, T.; Visentainer, J.V.; Almeida, V.C. Removal of tetracycline by NaOH-activated carbon produced from macadamia nut shells: Kinetic and equilibrium studies. Chem. Eng. J. 2015, 260, 291–299. [Google Scholar] [CrossRef]
- Nguyen, V.; Nguyen, T.; Huang, C.P.; Chen, C.; Bui, X.; Dong, C. Alkaline modified biochar derived from spent coffee ground for removal of tetracycline from aqueous solutions. J. Water Process Eng. 2021, 40, 101908. [Google Scholar] [CrossRef]
- Zhao, J.; Dai, Y. Tetracycline adsorption mechanisms by NaOH-modified biochar derived from waste Auricularia auricula dregs. Environ. Sci. Pollut. Res. 2022, 29, 9142–9152. [Google Scholar] [CrossRef]
- Meng, Y.; Chen, X.; Ai, D.; Wei, T.; Fan, Z.; Wang, B. Sulfur-doped zero-valent iron supported on biochar for tetracycline adsorption and removal. J. Clean. Prod. 2022, 379, 134769. [Google Scholar] [CrossRef]
- Wu, J.F.; Hou, B.W.; Wang, X.Q.; Liu, Z.W.; Wang, Z.D.; Liu, B.A.; Li, S.Y.; Gao, H.B.; Zhu, X.F.; Mao, Y.L. Preparation of N,S-codoped magnetic bagasse biochar and adsorption characteristics for tetracycline. RSC Adv. 2022, 12, 11786–11795. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, B.; Zhang, H.; Ma, J.; Mu, B.; Zhang, W. A novel Biochar modified by Chitosan-Fe/S for tetracycline adsorption and studies on site energy distribution. Bioresour. Technol. 2019, 294, 122152. [Google Scholar] [CrossRef]
- Gao, W.; Lin, Z.; Yan, S.; Gao, Y.; Zhang, H.; Hu, X.; Sun, H.; Zhang, S. Preparation of N-, O-, and S-Tri-Doped Biochar through One-Pot Pyrolysis of Poplar and Urea Formaldehyde and Its Enhanced Removal of Tetracycline from Wastewater. Energies 2022, 15, 8081. [Google Scholar] [CrossRef]
- Zeng, Z.; Ye, S.; Wu, H.; Xiao, R.; Zeng, G.; Liang, J.; Zhang, C.; Yu, J.; Fang, Y.; Song, B. Research on the sustainable efficacy of g -MoS2 decorated biochar nanocomposites for removing tetracycline hydrochloride from antibiotic-polluted aqueous solution. Sci. Total Environ. 2019, 648, 206–217. [Google Scholar] [CrossRef]
- Huang, K.; Yang, S.; Liu, X.; Zhu, C.; Qi, F.; Wang, K.; Wang, J.; Wang, Q.; Wang, T.; Ma, P. Adsorption of antibiotics from wastewater by cabbage-based N, P co-doped mesoporous carbon materials. J. Clean. Prod. 2023, 391, 136174. [Google Scholar] [CrossRef]
- Peng, H.; Wang, H.; Wang, L.; Huang, C.; Zheng, X.; Wen, J. Efficient adsorption-photocatalytic removal of tetracycline hydrochloride over La2S3-modified biochar with S,N-codoping. J. Water Process Eng. 2022, 49, 103038. [Google Scholar] [CrossRef]
- Yu, D.; Zeng, S.; Wu, Y.; Niu, J.; Tian, H.; Yao, Z.; Wang, X. Removal of tetracycline in the water by a kind of S/N co-doped tea residue biochar. J. Environ. Manag. 2024, 365, 121601. [Google Scholar] [CrossRef]
- Zhang, X.; Zhen, D.; Liu, F.; Chen, R.; Peng, Q.; Wang, Z. An achieved strategy for magnetic biochar for removal of tetracyclines and fluoroquinolones: Adsorption and mechanism studies. Bioresour. Technol. 2023, 369, 128440. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, S.; Wang, Q.; Hu, K.; Zhang, H.; Chang, J.; Liu, N.; Kokyo, O.H.; Cheng, H. Tetracycline removal from aqueous solution by magnetic biochar modified with different iron valences: A comparative study. Sep. Purif. Technol. 2024, 339, 126614. [Google Scholar] [CrossRef]
- Peng, L.; Ren, Y.; Gu, J.; Qin, P.; Zeng, Q.; Shao, J.; Lei, M.; Chai, L. Iron improving bio-char derived from microalgae on removal of tetracycline from aqueous system. Environ. Sci. Pollut. Res. 2014, 21, 7631–7640. [Google Scholar] [CrossRef]
- Varadharajan, V.; Senthilkumar, D.S.; Senthilkumar, K.; Sundramurthy, V.P.; Manikandan, R.; Senthilarasan, H.; Ganesan, H.; Kesavamoorthy, I.; Ramasamy, A. Process modeling and toxicological evaluation of adsorption of tetracycline onto the magnetized cotton dust biochar. J. Water Process Eng. 2022, 49, 103046. [Google Scholar] [CrossRef]
- Qu, J.; Wang, S.; Jin, L.; Liu, Y.; Yin, R.; Jiang, Z.; Tao, Y.; Huang, J.; Zhang, Y. Magnetic porous biochar with high specific surface area derived from microwave-assisted hydrothermal and pyrolysis treatments of water hyacinth for Cr(VI) and tetracycline adsorption from water. Bioresour. Technol. 2021, 340, 125692. [Google Scholar] [CrossRef]
- Oladipo, A.A.; Ifebajo, A.O. Highly efficient magnetic chicken bone biochar for removal of tetracycline and fluorescent dye from wastewater: Two-stage adsorber analysis. J. Environ. Manag. 2018, 209, 9–16. [Google Scholar] [CrossRef]
- Zhao, T.; Chen, R.; Wang, J. A Mild Method for Preparation of Highly Selective Magnetic Biochar Microspheres. Int. J. Mol. Sci. 2020, 21, 3752. [Google Scholar] [CrossRef]
- Shan, D.; Deng, S.; Zhao, T.; Wang, B.; Wang, Y.; Huang, J.; Yu, G.; Winglee, J.; Wiesner, M.R. Preparation of ultrafine magnetic biochar and activated carbon for pharmaceutical adsorption and subsequent degradation by ball milling. J. Hazard. Mater. 2016, 305, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, A.; Sokolowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biol. Technol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Cao, X.; Harris, W. Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresour. Technol. 2010, 101, 5222–5228. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Li, Y.; Zhang, J.; Wu, S.; Cao, Y.; Liang, P.; Zhang, J.; Wong, M.H.; Wang, M.; Shan, S.; et al. Influence of pyrolysis temperature on properties and environmental safety of heavy metals in biochars derived from municipal sewage sludge. J. Hazard. Mater. 2016, 320, 417–426. [Google Scholar] [CrossRef]
- Liu, Y.; Li, F.; Deng, J.; Wu, Z.; Lei, T.; Tan, M.; Wu, Z.; Qin, X.; Li, H. Mechanism of sulfamic acid modified biochar for highly efficient removal of tetracycline. J. Anal. Appl. Pyrolysis 2021, 158, 105247. [Google Scholar] [CrossRef]
- Xiang, W.; Wan, Y.; Zhang, X.; Tan, Z.; Xia, T.; Zheng, Y.; Gao, B. Adsorption of tetracycline hydrochloride onto ball-milled biochar: Governing factors and mechanisms. Chemosphere 2020, 255, 127057. [Google Scholar] [CrossRef]
- Hong, G.; Yu, Z.; Kong, D.; Huhe, T.; Shan, R.; Yuan, H.; Chen, Y. Removal of tetracycline from aqueous solution by magnetic biochar modified with different iron valence and K2C2O4: A comparative study and mechanism. J. Anal. Appl. Pyrolysis 2025, 187, 107005. [Google Scholar] [CrossRef]
- Chen, J.; Li, H.; Li, J.; Chen, F.; Lan, J.; Hou, H. Efficient removal of tetracycline from water by tannic acid-modified rice straw-derived biochar: Kinetics and mechanisms. J. Mol. Liq. 2021, 340, 117237. [Google Scholar] [CrossRef]
- Lian, F.; Sun, B.; Chen, X.; Zhu, L.; Liu, Z.; Xing, B. Effect of humic acid (HA) on sulfonamide sorption by biochars. Environ. Pollut. 2015, 204, 306–312. [Google Scholar] [CrossRef]
- Zhu, Y.; Yan, J.; Sui, F.; Wang, H.; Quan, G.; Cui, L. Interaction mechanism of biochar dissolved organic matter (BDOM) and tetracycline for environmental remediation. Environ. Res. 2025, 275, 121405. [Google Scholar] [CrossRef]
- Xiong, Y.; Bi, E. Effect of endogenetic dissolved organic matter on tetracycline adsorption by biochar. Environ. Sci. Pollut. Res. 2023, 30, 77022–77031. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Gao, M.; Cao, M.; Dan, J.; Yang, H. Self-propagating synthesis of Zn-loaded biochar for tetracycline elimination. Sci. Total Environ. 2021, 759, 143542. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Wang, J.; Tian, X.; Yu, N.; Zhang, X.; Zhao, Y.; Liu, Y.; Sui, S.; Wang, C.; Lian, F.; et al. Effect of biochar-derived dissolved organic matter on tetracycline sorption by KMnO4-modified biochar. Chem. Eng. J. 2023, 474, 145872. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, W.; Yin, S.; Liu, R.; Li, Z. Efficient removal of tetracycline from aqueous solution by K2CO3 activated penicillin fermentation residue biochar. Front. Chem. 2022, 10, 1078877. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Johir, M.A.H.; Sun, L.; Asadullah, M.; Belhaj, D. Sorption of hydrophobic organic contaminants on functionalized biochar: Protagonist role of π-π electron-donor-acceptor interactions and hydrogen bonds. J. Hazard. Mater. 2018, 360, 270–278. [Google Scholar] [CrossRef]
- Rosales, E.; Meijide, J.; Pazos, M.; Sanromán, M.A. Challenges and recent advances in biochar as low-cost biosorbent: From batch assays to continuous-flow systems. Bioresour. Technol. 2017, 246, 176–192. [Google Scholar] [CrossRef]
- Jha, S.; Gaur, R.; Shahabuddin, S.; Tyagi, I. Biochar as Sustainable Alternative and Green Adsorbent for the Remediation of Noxious Pollutants: A Comprehensive Review. Toxics 2023, 11, 117. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, B.; Wu, P.; Lee, X.; Xing, Y.; Chen, M.; Gao, B. Adsorption of emerging contaminants from water and wastewater by modified biochar: A review. Environ. Pollut. 2021, 273, 116448. [Google Scholar] [CrossRef]
- Qin, Y.; Chai, B.; Wang, C.; Yan, J.; Fan, G.; Song, G. Removal of tetracycline onto KOH-activated biochar derived from rape straw: Affecting factors, mechanisms and reusability inspection. Colloid. Surface A 2022, 640, 128466. [Google Scholar] [CrossRef]
- Li, C.; Zhu, X.; He, H.; Fang, Y.; Dong, H.; Lu, J.; Li, J.; Li, Y. Adsorption of two antibiotics on biochar prepared in air-containing atmosphere: Influence of biochar porosity and molecular size of antibiotics. J. Mol. Liq. 2019, 274, 353–361. [Google Scholar] [CrossRef]
- Yang, J.; Dai, J.; Wang, L.; Ge, W.; Xie, A.; He, J.; Yan, Y. Ultrahigh adsorption of tetracycline on willow branche-derived porous carbons with tunable pore structure: Isotherm, kinetics, thermodynamic and new mechanism study. J. Taiwan. Inst. Chem. Eng. 2019, 96, 473–482. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, W.; Chen, Y.; Zou, Y.; Wang, S.; Zhang, J.; Yang, L.; Niazi, N.K.; Wang, B.; Zhou, H.; et al. Acid mine drainage sludge-modified biochar for the efficient removal of As(III) in wastewater: Adsorption performance and mechanism. J. Water Process Eng. 2025, 71, 107279. [Google Scholar] [CrossRef]
- Wei, J.; Liu, Y.; Li, J.; Zhu, Y.; Yu, H.; Peng, Y. Adsorption and co-adsorption of tetracycline and doxycycline by one-step synthesized iron loaded sludge biochar. Chemosphere 2019, 236, 124254. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Yang, W.; Chen, Y.; Zhang, J.; Wang, B.; Niazi, N.K.; Yang, L.; Wang, S.; Zhou, H.; Wu, P. Efficient removal of tetracycline from wastewater via KHCO3-activated biochar: Characterization, performance, and mechanism. J. Water Process Eng. 2024, 68, 106450. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, B.; Wang, S.; Liu, C.; Zhu, L.; Zhao, Z.; Li, W.; Shao, Z.; Liu, X.; Dai, Y. Enhanced removal of tetracycline by vitamin C-modified cow manure biochar in water. Sci. Rep. 2024, 14, 16. [Google Scholar] [CrossRef]
- Tang, Y.; Li, Y.; Zhan, L.; Wu, D.; Zhang, S.; Pang, R.; Xie, B. Removal of emerging contaminants (bisphenol A and antibiotics) from kitchen wastewater by alkali-modified biochar. Sci. Total Environ. 2022, 805, 150158. [Google Scholar] [CrossRef]
- Sen, U.; Esteves, B.; Aguiar, T.; Pereira, H. Removal of Antibiotics by Biochars: A Critical Review. Appl. Sci. 2023, 13, 11963. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Li, Z.; Yu, G.; Wang, Y. Effect of pyrolysis temperature on characteristics, chemical speciation and risk evaluation of heavy metals in biochar derived from textile dyeing sludge. Ecotoxicol. Environ. Saf. 2019, 168, 45–52. [Google Scholar] [CrossRef]
- Jia, H.; Zhao, S.; Nulaji, G.; Tao, K.; Wang, F.; Sharma, V.K.; Wang, C. Environmentally Persistent Free Radicals in Soils of Past Coking Sites: Distribution and Stabilization. Environ. Sci. Technol. 2017, 51, 6000–6008. [Google Scholar] [CrossRef]
- Godlewska, P.; Ok, Y.S.; Oleszczuk, P. THE DARK SIDE OF BLACK GOLD: Ecotoxicological aspects of biochar and biochar-amended soils. J. Hazard. Mater. 2021, 403, 123833. [Google Scholar] [CrossRef]
- Zhang, R.; Zimmerman, A.R.; Zhang, R.; Li, P.; Zheng, Y.; Gao, B. Persistent free radicals generated from a range of biochars and their physiological effects on wheat seedlings. Sci. Total Environ. 2024, 908, 168260. [Google Scholar] [CrossRef] [PubMed]
- Lieke, T.; Zhang, X.; Steinberg, C.E.W.; Pan, B. Overlooked Risks of Biochars: Persistent Free Radicals trigger Neurotoxicity in Caenorhabditis elegans. Environ. Sci. Technol. 2018, 52, 7981–7987. [Google Scholar] [CrossRef]
- Ndirangu, S.M.; Liu, Y.; Xu, K.; Song, S. Risk Evaluation of Pyrolyzed Biochar from Multiple Wastes. J. Chem. 2019, 2019, 4506314. [Google Scholar] [CrossRef]
- Alhashimi, H.A.; Aktas, C.B. Life cycle environmental and economic performance of biochar compared with activated carbon: A meta-analysis. Resour. Conserv. Recycl. 2017, 118, 13–26. [Google Scholar] [CrossRef]
- Snyder, B.F. Costs of biomass pyrolysis as a negative emission technology: A case study. Int. J. Energy Res. 2019, 43, 1232–1244. [Google Scholar] [CrossRef]
- Katiyar, R.; Chen, C.; Singhania, R.R.; Tsai, M.; Saratale, G.D.; Pandey, A.; Dong, C.; Patel, A.K. Efficient remediation of antibiotic pollutants from the environment by innovative biochar: Current updates and prospects. Bioengineered 2022, 13, 14730–14748. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).