Maifanstone Powder-Modified PE Filler for Enhanced MBBR Start-Up in Treating Marine RAS Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Reagents and Materials
2.2. Liquid-Phase Oxidation Modification Process
2.3. Liquid-Phase Oxidation—Surface Coating Modification by MaifanStone Powder
2.4. MBBRs: Systems and Start-Up
2.5. MBBR Performance Tests on HRT, Shock Load, and Temperature
2.6. Analysis and Testing Methods
2.6.1. Testing Method for Water Quality
2.6.2. Bacterial Analysis
2.7. Characterization of Filler Surface
2.7.1. Testing Procedure for Contact Angle
2.7.2. Scanning Electron Microscopy (SEM) Test
2.7.3. X-Ray Energy Dispersive Spectroscopy (EDS) Test
2.7.4. Fourier Transform Infrared Spectroscopy (FTIR) Test
3. Results and Discussion
3.1. Characterization of Modified MBBR Filler
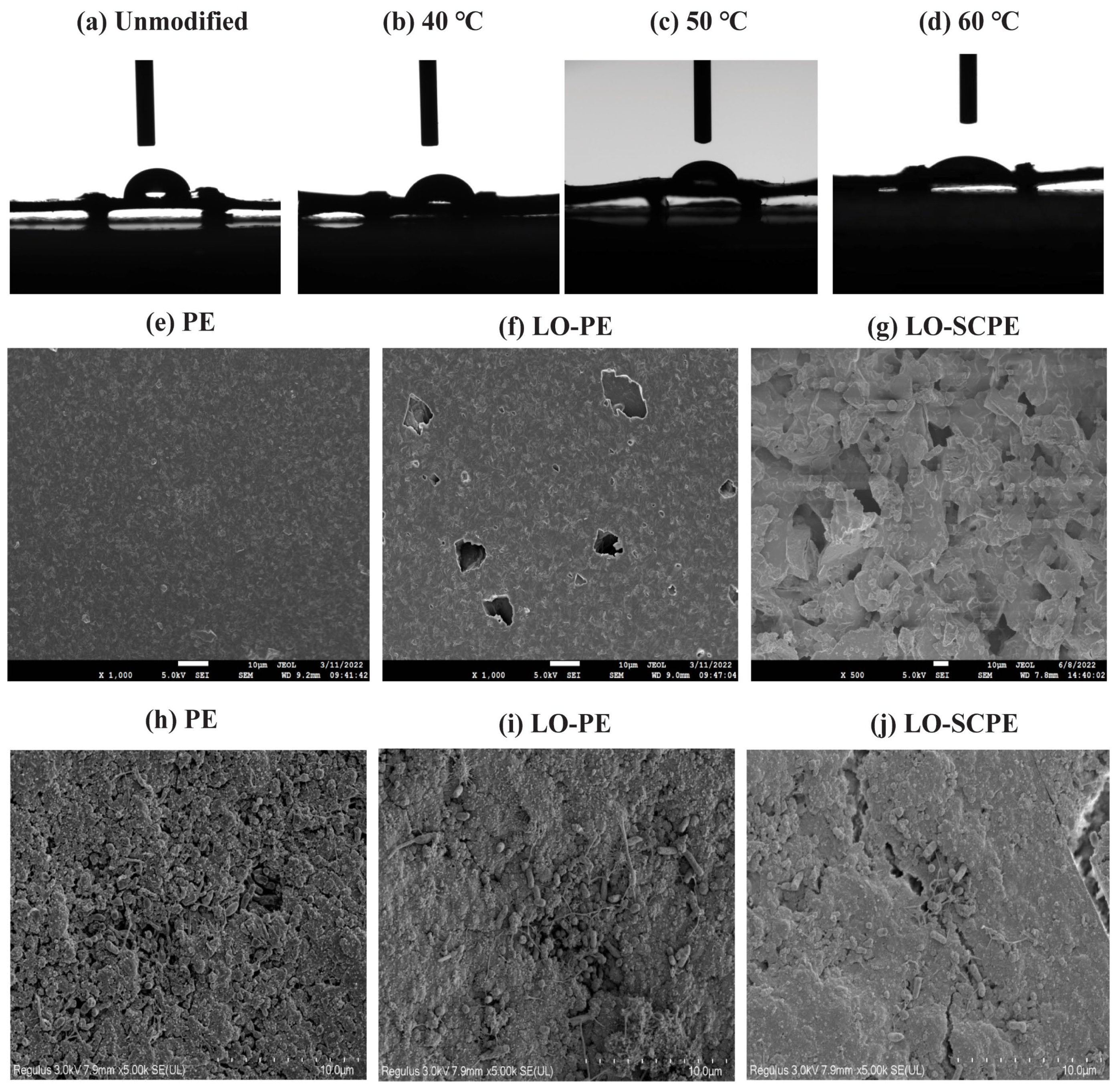
3.1.1. Effect of Liquid-Phase Oxidation Modification on Static Contact Angle
3.1.2. Effect of Modification on the Filler Surface Morphology
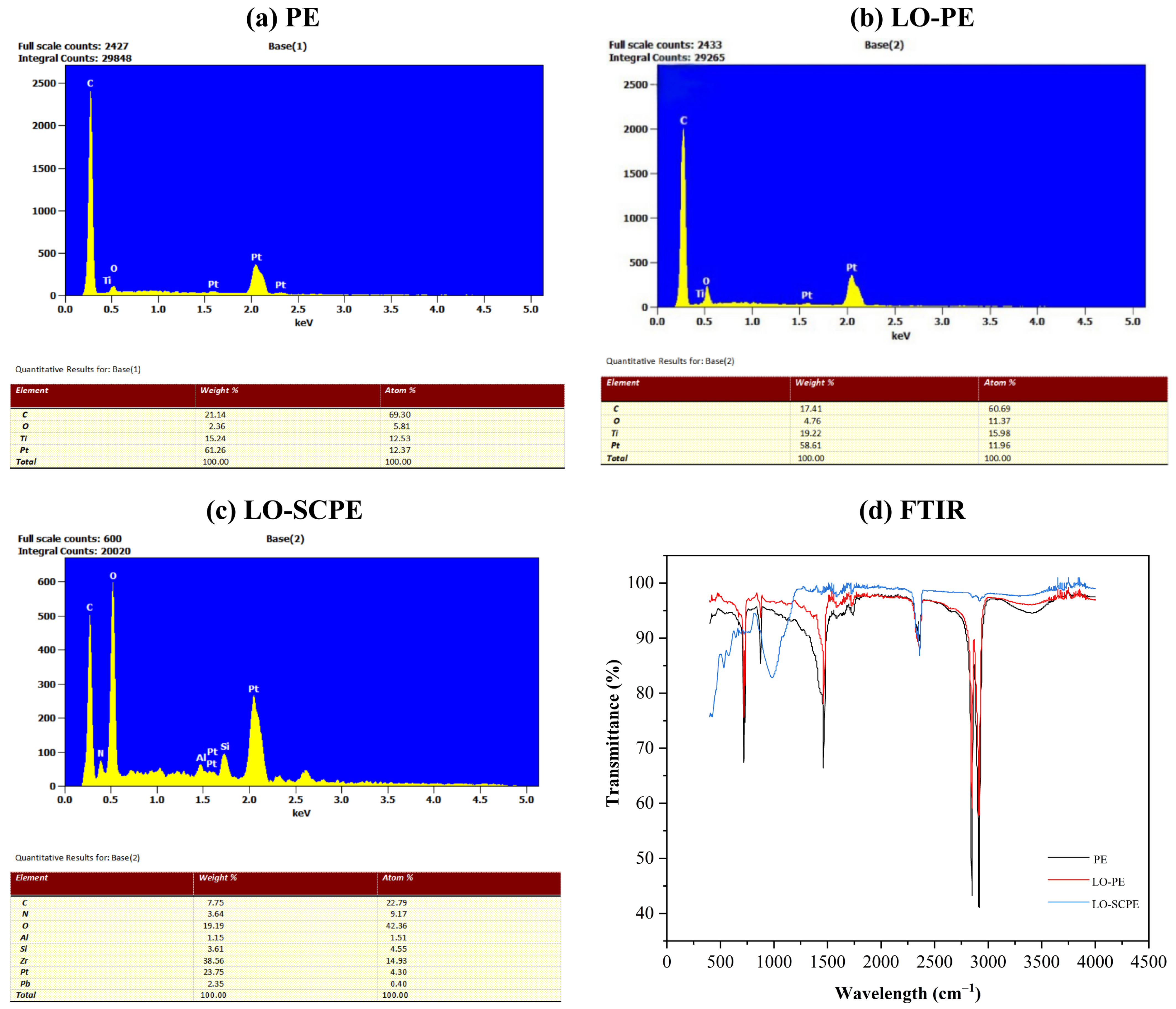
3.1.3. Bacterial Morphology Analysis, Elemental Composition, and Chemical Structure of Filler Surfaces
3.2. MBBR Start-Up Comparison of Marine RAS Wastewater Treatment
3.3. Bacterial Community Analysis
3.3.1. Phylum-Level Bacterial Community Composition Analysis
3.3.2. Genus-Level Bacterial Community Composition Analysis
3.3.3. Quantitative Analysis of AOBs and NOBs
3.4. Performance Comparison During MBBR Operation
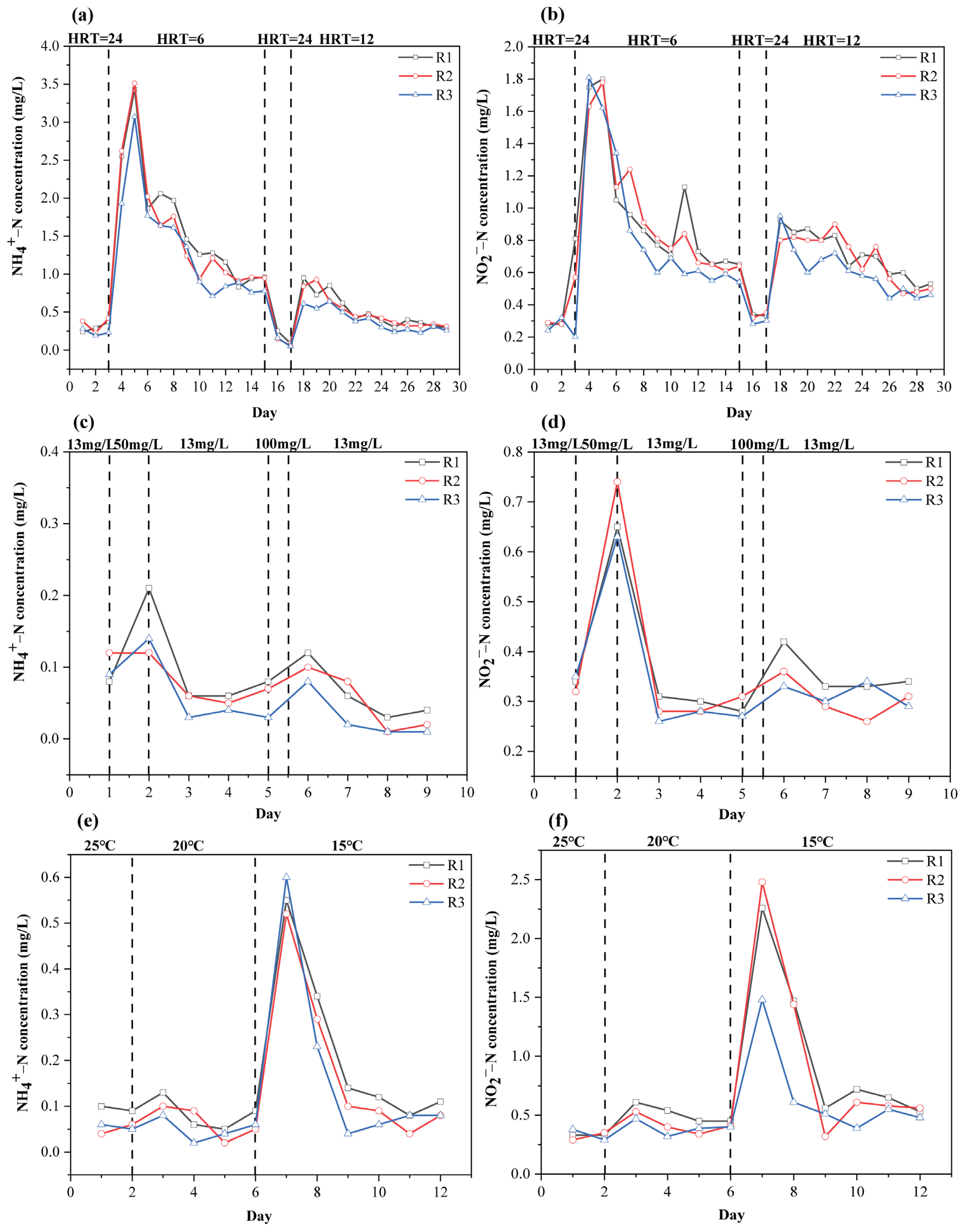
3.4.1. Influence of HRT on MBBR Performance
3.4.2. Impact of Ammonium Shock Load on MBBRs’ Performance
3.4.3. Influence of Temperature on MBBRs’ Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, Q.; Bhattarai, N.; Pahlow, M.; Xu, Z. Environmental sustainability and footprints of global aquaculture. J. Resour. Conserv. Recycl. 2022, 180, 106183. [Google Scholar] [CrossRef]
- Ahmed, N.; Turchini, G.M. Recirculating aquaculture systems (RAS): Environmental solution and climate change adaptation. J. Clean. Prod. 2021, 297, 126604. [Google Scholar] [CrossRef]
- Díaz, V.; Ibáñez, R.; Gómez, P.; Urtiaga, A.M.; Ortiz, I. Kinetics of nitrogen compounds in a commercial marine Recirculating Aquaculture System. Aquac. Eng. 2012, 50, 20–27. [Google Scholar] [CrossRef]
- Liu, M.J.; Guo, H.Y.; Zhu, K.C.; Liu, B.S.; Liu, B.; Guo, L.; Zhang, N.; Yang, J.W.; Jiang, S.G.; Zhang, D.C. Effects of acute ammonia exposure and recovery on the antioxidant response and expression of genes in the Nrf2-Keap1 signaling pathway in the juvenile golden pompano (Trachinotus ovatus). Aquat. Toxicol. 2021, 240, 105969. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Chen, S. An experimental study on nitrification biofilm performances using a series reactor system. Aquac. Eng. 1999, 20, 245–259. [Google Scholar] [CrossRef]
- Dutra, F.M.; Ronnau, M.; Sponchiado, D.; Forneck, S.C.; Freire, C.A.; Ballester, E.L.C. Histological alterations in gills of Macrobrachium amazonicum juveniles exposed to ammonia and nitrite. Aquat. Toxicol. 2017, 187, 115–123. [Google Scholar] [CrossRef]
- He, H.; Chen, Y.; Li, X.; Cheng, Y.; Yang, C.; Zeng, G. Influence of salinity on microorganisms in activated sludge processes: A review. Int. Biodeterior. Biodegrad. 2017, 119, 520–527. [Google Scholar] [CrossRef]
- Lusinier, N.; Seyssiecq, I.; Sambusiti, C.; Jacob, M.; Lesage, N.; Roche, N. A comparative study of conventional activated sludge and fixed bed hybrid biological reactor for oilfield produced water treatment: Influence of hydraulic retention time. Chem. Eng. J. 2021, 420, 127611. [Google Scholar] [CrossRef]
- Madan, S.; Madan, R.; Hussain, A. Advancement in biological wastewater treatment using hybrid moving bed biofilm reactor (MBBR): A review. Appl. Water Sci. 2022, 12, 141. [Google Scholar] [CrossRef]
- Almeida, J.C.R.; Bega, J.M.M.; Leite, L.D.S.; Oliveira, J.N.D.; Albertin, L.L.; Matsumoto, T. Membrane aerated biofilm reactor in recirculating aquaculture system for effluent treatment. Environ. Technol. 2023, 44, 4071–4083. [Google Scholar] [CrossRef]
- Arabgol, R.; Vanrolleghem, P.A.; Delatolla, R. Influence of MBBR carrier geometrical properties and biofilm thickness restraint on biofilm properties, effluent particle size distribution, settling velocity distribution, and settling behaviour. J. Environ. Sci. 2022, 122, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wu, T.; Wang, H.; Dong, W.; Zhao, Y.; Chu, Z.; Yan, G.; Chang, Y. Comparative study of denitrifying-MBBRs with different polyethylene carriers for advanced nitrogen removal of real reverse osmosis concentrate. Int. J. Environ. Res. Public Health 2020, 17, 2667. [Google Scholar] [CrossRef]
- Sen, D.; Copithorn, R.R.; Randall, C.W. Successful evaluation of Ten IFAS and MMBR facilities by applying the unified model to quantify biofilm surface area requirements for nitrification, determine its accuracy in predicting effluent characteristics, and understand the contribution of media towards organics removal and nitrification. In Proceedings of the Water Environment Federation, Dallas, TX, USA, 30 September–4 October 2006; pp. 185–199. [Google Scholar]
- Fayzullin, I.; Gorbachev, A.; Volfson, S.; Serikbayev, Y.; Nakyp, A.; Akylbekov, N. Composite material based on polypropylene and modified natural fillers. J. Polym. 2024, 16, 1703. [Google Scholar] [CrossRef]
- Mao, Y.; Quan, X.; Zhao, H.; Zhang, Y.; Chen, S.; Liu, T.; Quan, W. Accelerated startup of moving bed biofilm process with novel electrophilic suspended biofilm carriers. Chem. Eng. J. 2017, 315, 364–372. [Google Scholar] [CrossRef]
- Hu, J.; Li, F.; Wang, B.; Zhang, H.; Ji, C.; Wang, S.; Zhou, Z. A two-step combination strategy for significantly enhancing the interfacial adhesion of CF/PPS composites: The liquid-phase oxidation followed by grafting of silane coupling agent. Compos. Part B Eng. 2020, 191, 107966. [Google Scholar] [CrossRef]
- Duan, L.; Jiang, W.; Song, Y.; Xia, S.; Hermanowicz, S.W. The characteristics of extracellular polymeric substances and soluble microbial products in moving bed biofilm reactor-membrane bioreactor. Bioresour. Technol. 2013, 148, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Pervissian, A.; Parker, W.J.; Legge, R.L. Combined MBBR-MF for industrial wastewater treatment. Environ. Prog. Sustain. Energy 2012, 31, 288–295. [Google Scholar] [CrossRef]
- Tadda, M.A.; Altaf, R.; Gouda, M.; Rout, P.R.; Shitu, A.; Ye, Z.; Zhu, S.; Liu, D. Impact of Saddle-Chips biocarrier on treating mariculture wastewater by moving bed biofilm reactor (MBBR): Mechanism and kinetic study. J. Environ. Chem. Eng. 2021, 9, 106710. [Google Scholar] [CrossRef]
- Chunmei, L.; Haiyan, W.; Youle, W.; Yu, H.Q.; Zirui, C.; Kai, L. Influence of the liquid-phase chemical method modified MBBR carriers on advanced nitrogen removal of urban wastewater treatment plant effluent. J. Environ. Eng. Technol. 2016, 6, 336–342. [Google Scholar]
- Rejeb, M.; Koubaa, A.; Elleuch, F.; Godard, F.; Migneault, S.; Bouslimi, B.; Khlif, M.; Mrad, H. Effects of chromic treatment on the surface properties of polypropylene (PP) wood composites. Coatings 2021, 11, 851. [Google Scholar] [CrossRef]
- Kowalczuk, D.; Pitucha, M. Application of FTIR method for the assessment of immobilization of active substances in the matrix of biomedical materials. Materials 2019, 12, 2972. [Google Scholar] [CrossRef]
- Zhang, Q.; Liang, S.; Tan, S.; Chen, W.; Liu, H.; Yang, C.; Qin, S.; Long, K. MBBR start-up with HN-AD bacteria inoculation: Comparative analysis in simulated and real wastewater for performance, microbial characteristics, and nitrogen removal mechanism. J. Water Process Eng. 2024, 58, 104786. [Google Scholar] [CrossRef]
- Lim, J.W.; Seng, C.E.; Lim, P.E.; Ng, S.L.; Sujari, A.N.A. Nitrogen removal in moving bed sequencing batch reactor using polyurethane foam cubes of various sizes as carrier materials. Bioresour. Technol. 2011, 102, 9876–9883. [Google Scholar] [CrossRef]
- Bassin, J.P.; Kleerebezem, R.; Rosado, A.S.; Loosdrecht, M.C.M.V.; Dezotti, M. Effect of different operational conditions on biofilm development, nitrification, and nitrifying microbial population in moving-bed biofilm reactors. Environ. Sci. Technol. 2012, 46, 1546–1555. [Google Scholar] [CrossRef] [PubMed]
- Pishgar, R.; Dominic, J.A.; Tay, J.H.; Chu, A. Pilot-scale investigation on nutrient removal characteristics of mineral-rich aerobic granular sludge: Identification of uncommon mechanisms. Water Res. 2020, 168, 115151. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhu, Y.; Gan, D.; Cai, X.; Li, X.; Liu, X.; Xia, S. Enhancing biofilm formation with powder carriers for efficient nitrogen and phosphorus removal. Sci. Total Environ. 2024, 951, 175812. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, X.; Ni, H.; Rong, X.; Zhang, Q.; Xiao, X.; Huan, H.; Liu, J.F.; Wu, Z. Surface modification of basalt fiber with organic/Inorganic composites for biofilm carrier used in wastewater treatment. ACS Sustain. Chem. Eng. 2018, 6, 2596–2602. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X.; Li, J.; Lee, C.T.; Ong, P.Y.; Zhang, Z.; Li, C. Effect of aquaculture salinity on nitrification and microbial community in moving bed bioreactors with immobilized microbial granules. Bioresour. Technol. 2020, 297, 122427. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Z.; Yang, Y.; Mei, X.; Wu, Z. Correlating microbial community structure and composition with aeration intensity in submerged membrane bioreactors by 454 high-throughput pyrosequencing. Water Res. 2013, 47, 859–869. [Google Scholar] [CrossRef]
- Atabek, A.; Camesano, T.A. Atomic force microscopy study of the effect of lipopolysaccharides and extracellular polymers on adhesion of pseudomonas aeruginosa. J. Bacteriol. 2007, 189, 8503–8509. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, J.; Zhao, H.; Yang, H. Microbial community and N removal of aerobic granular sludge at high COD and N loading rates. Bioresour. Technol. 2013, 143, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, H.; Jie, M.; Zhang, K.; Qian, Y.; Ma, J. Performance and microbial communities of different biofilm membrane bioreactors with pre-anoxic tanks treating mariculture wastewater. Bioresour. Technol. 2020, 295, 122302. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.D.; Kim, T.S.; Yu, G.H.; Jung, J.H.; Park, H.D. Bacterial community composition and diversity of a full-scale integrated fixed-film activated sludge system as investigated by pyrosequencing. J. Microbiol. Biotechnol. 2010, 20, 1717–1723. [Google Scholar] [PubMed]
- He, T.; Zhang, M.; Ding, C.; Wu, Q.; Chen, M.; Mou, S.; Cheng, D.; Duan, S.; Wang, Y. New insight into the nitrogen removal capacity and mechanism of Streptomyces mediolani EM-B2. Bioresour. Technol. 2022, 348, 126819. [Google Scholar] [CrossRef]
- Sorokin, D.Y.; Lücker, S.; Vejmelkova, D.; Kostrikina, N.A.; Kleerebezem, R.; Rijpstra, W.I.C.; Damste, J.S.S.; Paslier, D.L.; Muyzer, G.; Wagner, M.; et al. Nitrification expanded: Discovery, physiology and genomics of a nitrite-oxidizing bacterium from the phylum Chloroflexi. ISME J. 2012, 6, 2245–2256. [Google Scholar] [CrossRef]
- Li, C.; Liang, J.; Lin, X.; Xu, H.; Tadda, M.A.; Lan, L.; Liu, D. Fast start-up strategies of MBBR for mariculture wastewater treatment. J. Environ. Manag. 2019, 248, 109267. [Google Scholar] [CrossRef]
- Rud, I.; Kolarevic, J.; Holan, A.B.; Berget, I.; Calabrese, S.; Terjesen, B.F. Deep-sequencing of the bacterial microbiota in commercial-scale recirculating and semi-closed aquaculture systems for Atlantic salmon post-smolt production. Aquac. Eng. 2017, 78, 50–62. [Google Scholar] [CrossRef]
- Angeles, V.D.R.; Pichel, A.; Fernandez-Gonzalez, N.; Pedrouso, A.; Fra-Vazquez, A.; Morales, N.; Mendez, R.; Campos, J.L.; Mosquera-Corral, A. Performance and microbial features of the partial nitritation-anammox process treating fish canning wastewater with variable salt concentrations. J. Environ. Manag. 2018, 208, 112–121. [Google Scholar]
- Shitu, A.; Zhu, S.; Qi, W.; Tadda, M.A.; Liu, D.; Ye, Z. Performance of novel sponge biocarrier in MBBR treating recirculating aquaculture systems wastewater: Microbial community and kinetic study. J. Environ. Manag. 2020, 275, 111264. [Google Scholar] [CrossRef]
- Sonwani, R.K.; Swain, G.; Giri, B.S.; Singh, R.S.; Rai, B.N. A novel comparative study of modified carriers in moving bed biofilm reactor for the treatment of wastewater: Process optimization and kinetic study. Bioresour. Technol. 2019, 281, 335–342. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Z.; Guo, W.; Lu, Y.; Qi, L.; Wen, H.; Ngo, H.H. Behavior of nitrogen removal in an aerobic sponge based moving bed biofilm reactor. Bioresour. Technol. 2017, 245, 1282–1285. [Google Scholar] [CrossRef] [PubMed]
- Navada, S.; Vadstein, O.; Gaumet, F.; Tveten, A.K.; Spanu, C.; Mikkelsen, O.; Kolarevic, J. Biofilms remember: Osmotic stress priming as a microbial management strategy for improving salinity acclimation in nitrifying biofilms. Water Res. 2020, 176, 115732. [Google Scholar] [CrossRef]
- Shore, J.L.; M’Coy, W.S.; Gunsch, C.K.; Deshusses, M.A. Application of a moving bed biofilm reactor for tertiary ammonia treatment in high temperature industrial wastewater. Bioresour. Technol. 2012, 112, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Cabrol, L.; Poly, F.; Malhautier, L.; Pommier, T.; Lerondelle, C.; Verstraete, W.; Lepeuple, A.S.; Fanlo, J.L.; Roux, X.L. Management of microbial communities through transient disturbances enhances the functional resilience of nitrifying gas-biofilters to future disturbances. Environ. Sci. Technol. 2016, 50, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, D.; Liang, Y.; Zeng, H.; He, Y.; Zhang, Y.; Zhang, J. Performance and microbial community of completely autotrophic nitrogen removal over nitrite (CANON) process in two membrane bioreactors (MBR) fed with different substrate levels. Bioresour. Technol. 2014, 152, 185–191. [Google Scholar] [CrossRef]
- Rotthauwe, J.H.; Witzel, K.P.; Liesack, W. The ammonia monooxygenase structural gene amoA as a functional marker: Molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl. Environ. Microbiol. 1997, 63, 4704–4712. [Google Scholar] [CrossRef]


| Time Period (Days) | Stages | Action/Factors | Designed Values | Operational Conditions |
|---|---|---|---|---|
| 0–160 | Reactor start-up | R1 (PE), R2 (LO-PE), and R3 (LO-SCPE) | HRT = 24 h | |
| 161–180 | Performance comparison during stable operation | R1, R2, and R3 | ||
| 181–210 | MBBRs’ stability performance under HRT | Variation of effluent NH4+-N and NO2−-N during HRT | HRT (h) = 6, 12, 24 | Temp (°C) = 25, DO (6.5 ± 1 mg/L) and pH (7.5 ± 0.5) |
| 211–220 | MBBRs’ performance on ammonia nitrogen shock loading | Varied influent NH4+-N concentrations | Concentration (mg/L) = 13, 50, 100 | |
| 221–232 | MBBRs’ performance at temperature level | Effluent concentration of NH4+-N and NO2−-N varied by temperature | Temp (°C) = 15, 20, 25 | DO (6.5 ± 1 mg/L) and pH (7.5 ± 0.5) |
| Sample | Chao1 | Faith_pd | Observed_Species | Pielou_e | Shannon | Simpson |
|---|---|---|---|---|---|---|
| PE | 350.26 | 43.609 | 338.7 | 0.61473 | 4.9562 | 0.92563 |
| LO-PE | 406.38 | 24.75 | 404.7 | 0.55633 | 4.9383 | 0.90270 |
| LO-SCPE | 498.29 | 30.085 | 480.6 | 0.57020 | 5.16611 | 0.89864 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altaf, R.; Xiao, T.; Wang, K.; Guo, J.; Li, Q.; Zou, J.; Jaafarzadeh, N.; Wu, D.; Liu, D. Maifanstone Powder-Modified PE Filler for Enhanced MBBR Start-Up in Treating Marine RAS Wastewater. Water 2025, 17, 1888. https://doi.org/10.3390/w17131888
Altaf R, Xiao T, Wang K, Guo J, Li Q, Zou J, Jaafarzadeh N, Wu D, Liu D. Maifanstone Powder-Modified PE Filler for Enhanced MBBR Start-Up in Treating Marine RAS Wastewater. Water. 2025; 17(13):1888. https://doi.org/10.3390/w17131888
Chicago/Turabian StyleAltaf, Rubina, Tianyu Xiao, Kai Wang, Jianlin Guo, Qian Li, Jing Zou, Neemat Jaafarzadeh, Daoji Wu, and Dezhao Liu. 2025. "Maifanstone Powder-Modified PE Filler for Enhanced MBBR Start-Up in Treating Marine RAS Wastewater" Water 17, no. 13: 1888. https://doi.org/10.3390/w17131888
APA StyleAltaf, R., Xiao, T., Wang, K., Guo, J., Li, Q., Zou, J., Jaafarzadeh, N., Wu, D., & Liu, D. (2025). Maifanstone Powder-Modified PE Filler for Enhanced MBBR Start-Up in Treating Marine RAS Wastewater. Water, 17(13), 1888. https://doi.org/10.3390/w17131888









