Abstract
Sewage sludge, as a by-product of wastewater treatment, poses severe environmental challenges due to its high moisture, ash, and heavy metal content. Thermochemical conversion technologies, including pyrolysis and gasification, offer promising pathways for transforming sludge into valuable products such as bio-oil, biochar, and syngas. This paper systematically reviews recent advancements in pyrolysis and gasification, focusing on process optimization and catalyst development to enhance product quality and energy recovery. In pyrolysis, factors such as temperature, residence time, and heating rate significantly influence product yields and properties, while catalytic and co-pyrolysis approaches further improve product structure and reduce environmental risks. In gasification, parameters like the equivalence ratio, steam-to-sludge ratio, and catalyst application are key to enhancing syngas yield and quality, with biomass co-gasification offering additional benefits. Despite substantial progress, commercialization remains challenged by high operational costs, catalyst durability, and environmental impacts. Future research should emphasize improving sludge pretreatment, optimizing thermochemical processes, developing efficient and cost-effective catalysts, and addressing critical issues such as bio-oil quality, tar management, and syngas purification to promote the industrial application of these technologies.
1. Introduction
Sewage sludge is the solid or semi-solid residue remaining after wastewater treatment. It is an inevitable and significant by-product of wastewater treatment, and its specific composition is a complex heterogeneous microbial mixture [1,2]. At the same time, due to the rapid development of industrialization and the continuous increase in population, the production of sewage sludge has increased sharply [3,4,5]. Sewage sludge is rich in organic and inorganic matter content and is used as an important biomass fuel for energy recovery [6].
Thermochemical conversion technologies for wastewater sludge, including pyrolysis, gasification, and hydrothermal liquefaction, each have certain advantages and development potential [1,4,7]. Thermochemical processes can effectively destroy pathogens and potentially increase the value of energy-rich substances [3]. Moreover, through appropriate technical strategies, valuable nutrients and metals can also be recovered [8,9]. The advantages of thermochemical conversion technology are its excellent economic benefits, high processing efficiency for sludge in the thermochemical process, and good volume reduction effect for sludge. However, its disadvantage is that it is easily affected by complex thermochemical conversion equipment and processes [10,11].
Against the backdrop of continuously rising global carbon emissions, countries have set carbon reduction targets, especially within the framework of the Paris Agreement, aiming to achieve carbon neutrality [12]. Sewage sludge generated during the wastewater treatment process is a significant potential source of carbon emissions. Traditional treatment methods such as landfilling and composting often result in the release of greenhouse gases (such as CO2 and CH4), which have negative impacts on the environment. With the increasing demand for carbon reduction and resource utilization, exploring low-carbon sewage sludge treatment technologies has become a critical issue to be addressed [13]. In this context, the thermochemical conversion of sewage sludge, as an efficient resource recovery method, not only reduces carbon emissions but also converts it into biofuels, thereby achieving the dual goals of resource utilization and carbon reduction in sewage sludge [14,15]. Biomass raw materials such as sewage sludge have complex compositions and high impurity contents, which pose challenges to the thermochemical conversion process, such as low-quality biofuels. Various strategies such as drying, gasification, pyrolysis, and hydrothermal treatment need to be adopted to address issues like high water content, high ash content, and heavy metals in sludge. Although technologies like pyrolysis and gasification show promising prospects for energy recovery, they have relatively high capital and operational costs. Future research should further explore how to effectively remove water, ash, and pollutants from sludge; optimize various thermochemical conversion processes; and achieve sustainable development in the resource utilization of sludge [16,17].
The thermochemical conversion of sewage sludge is influenced by the specific parameters of technology. The products generated by thermochemical processes are often affected by various parameters within the reactor. The type of reactor and reaction parameters directly influence the reaction mechanism and product characteristics [6]. The main challenges of thermochemical conversion technologies for sewage sludge are the combination of large-scale application, economic competitiveness, and environmental friendliness [18]. Better parameter adjustment can help achieve the goal.
This article is structured according to the technological roadmap (Figure 1) and reviews the progress of thermochemical treatment technologies for sewage sludge, focusing on the potential of thermochemical conversion in realizing sludge resource utilization and reducing environmental burdens. It also discusses the understanding of sludge value-added through different thermochemical processes, with a focus on pyrolysis and gasification as the two main thermochemical conversion technologies due to their higher technological maturity, scalability, and practical relevance in current industrial applications. In contrast, although hydrothermal technologies show greater potential for treating wet biomass, they typically require high-pressure reaction systems, involve complex reactor designs, and pose higher operational risks, which have limited their commercial deployment.
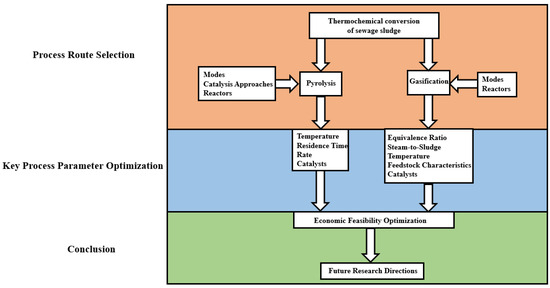
Figure 1.
Technology roadmap.
Although sewage sludge is well known for its high moisture content, this review specifically focuses on pyrolysis and gasification rather than hydrothermal technologies such as hydrothermal liquefaction or carbonization. The rationale lies in the comparative technological maturity, scalability, and relevance of pyrolysis and gasification in current industrial applications. While hydrothermal processes are indeed promising for wet biomass treatment, they typically require high-pressure systems and are associated with complex reactor designs, higher operational risks, and limited commercial deployment. In contrast, pyrolysis and gasification have seen broader research efforts and practical implementations for sludge valorization, offering more established pathways for producing biochar, bio-oil, and syngas. These technologies also align better with the current emphasis on energy recovery, carbon reduction, and the development of circular economy strategies. Therefore, this review prioritizes the comprehensive analysis of pyrolysis and gasification technologies to provide actionable insights into process optimization, catalyst development, and potential for industrial-scale applications in sludge management.
2. Materials and Methods
2.1. Literature Selection Criteria
This review primarily focuses on English-language literature related to the thermochemical conversion of sewage sludge published between 2010 and 2024. The literature was collected from major academic databases, including Web of Science, Scopus, ScienceDirect, and Google Scholar. Keywords used in the search included “sewage sludge”, “pyrolysis”, “gasification”, “thermochemical conversion”, “biochar”, “bio-oil”, and “syngas”. To ensure the comprehensiveness and global relevance of the review, the selected literature is not restricted to any specific country or region and aims to cover research findings from major countries in Asia, Europe, and the Americas. Inclusion criteria for the literature were as follows: (1) the study must focus explicitly on the thermochemical treatment of sewage sludge; (2) it must include experimental data or clearly defined process parameters; and (3) it must be published in peer-reviewed international journals.
2.2. Component Analysis of Sewage Sludge
According to Table 1, the various components of sewage sludge have been classified in detail. “The characteristics of sewage sludge have been analyzed from four aspects”: ultimate analysis, industrial analysis, proximate analysis, and other properties. These classifications not only help to understand the physical and chemical properties of sludge but also provide an important basis for sludge treatment and resource utilization.

Table 1.
Components of sewage sludge.
The data in Table 1 are primarily derived from studies on municipal and domestic sludge, with some also including industrial or mixed sludge (e.g., from tanneries). Parameter measurements were conducted using standard analytical methods, such as elemental and proximate analyses, typically in accordance with ASTM D3172 and D3176 standards [11,19,20,21] to ensure data consistency across different studies. The data on grease and fat content were mainly obtained from Reference [20], in which the total lipid content in sludge samples was determined using Soxhlet extraction and gravimetric methods.
In addition, the harmful components contained in sewage sludge cannot be ignored; therefore, it is urgent to find suitable sustainable technologies to treat sewage sludge [26,27]. Another issue that cannot be ignored is that the water content of sewage sludge has a significant impact on subsequent treatment. Its water content is extremely high, almost reaching 98%. In the treatment of sludge, methods such as natural drying, mechanical drying, thermal drying, and biological drying are often used to reduce the water content of sewage sludge to a suitable level for further processing. According to the research of Wu [11,28,29], physical dehydration has a relatively low energy consumption, generally ranging from 30 to 50 kJ/kg dry matter. The energy consumption of technologies such as acoustic wave treatment is between 600 and 800 kJ/kg dry matter. The energy consumption for removing each kilogram of water by thermal treatment methods is around 1100 kJ, while the freeze–thaw method consumes 10,000 to 11,000 kJ per kilogram of water removed. The energy consumption for removing each kilogram of water by electric field dehydration is between 180 and 360 kJ. Also, according to the research of Rao et al. (2022), it is found that during the sludge drying process, the application of conduction drying, convection and conduction integration technology, as well as the addition of drying accelerators and other methods, can effectively reduce energy consumption [11,28,29].
Sewage sludge contains a large amount of organic matter. During the sewage treatment process, various organic pollutants (OC) remain on the sewage sludge, causing them to be contaminated by various compounds [30]. The concentration of organic pollutants in sewage sludge is influenced by the concentration of organic pollutants in the influent wastewater, the physicochemical properties of organic compounds in the sewage, as well as the characteristics of the sludge and the operational parameters of the sewage treatment plant, which also have a certain impact on the treatment effect. At the same time, organic pollutants are persistent and can accumulate through the food chain, causing various health problems, including risks such as endocrine disruption, carcinogenicity, and the spread of antibiotic resistance genes [3,31,32].
Sewage sludge also contains various heavy metals, which pose potential ecological risks due to their bioavailability, accumulation, and non-degradability in sludge [2,33,34]. Both organic and heavy metal pollutants have bioaccumulation properties. Direct application as compost to land may lead to the enrichment of pollutants along the food chain, ultimately causing harm to the ecological environment and organisms [35]. Heavy metals accumulate through the food chain, and pollutants may enter the human body, increasing the risks of cancer, cardiovascular diseases, and neurological disorders [36].
Relevant studies have shown that sewage sludge contains various bacteria, viruses, fungi and parasites [3,32,35]. These pathogenic microorganisms may enter the environment through land fertilization or sludge treatment, posing a threat to human and animal health.
2.3. The Relationship Between Sludge Types and Thermochemical Processes
The type of sludge has a significant influence on the behavior and product characteristics of pyrolysis and gasification processes. Primary sludge (PS) is mainly composed of organic suspended solids formed through sedimentation and contains higher levels of organic matter and lipids. In contrast, secondary sludge (SS) is primarily derived from residual active biomass in biological treatment systems and is richer in proteins and polysaccharides and has a higher ash content [37,38].
Analyze based on Table 2 during pyrolysis, PS exhibits a higher oil yield and greater heating value, reflecting superior fuel quality, which can be attributed to its higher carbon and hydrogen content and lower oxygen content [38]. In comparison, SS tends to produce higher-quality biochar, characterized by high fixed carbon content, low ash content, high energy yield, and better combustion stability, making it more similar to coal-based fuels and suitable for development as solid fuel or environmental remediation materials [37].

Table 2.
Comparative composition of primary and secondary sludge.
According to Table 2 sludge type strongly affects gasification outcomes. Primary sludge (PS) has more volatile matter and less ash, leading to higher yields of energy-rich gases like H2 and CH4, but may cause more tar due to its lipid content. Secondary sludge (SS) contains more ash and nitrogen, producing more CO2 and NOx and needing higher temperatures [26,39]. PS is better for energy-rich gas production, while SS is useful for catalytic or nitrogen-related applications.
2.4. The Necessity of Resource Utilization and Environmental Relief
According to relevant data, among the European Union (EU) countries, Germany generates the driest sludge, with an annual output of 1.85 million tons, followed by the United Kingdom with 1.14 million tons and Spain with 1.03 million tons. China’s sewage sludge production is steadily increasing. According to the 2022 Annual Report on Ecological and Environmental Statistics, 79.34 billion tons of domestic sewage were treated in 2022, accounting for 88.7% of the total sewage treated [40].
The huge amount of sludge produced has stimulated research on the energy utilization of sludge. With the increasing demand for carbon reduction and resource utilization, exploring low-carbon wastewater sludge treatment technologies has become a key issue that needs to be urgently addressed [13]. The products of the thermochemical conversion of sewage sludge can replace fossil fuels and reduce direct carbon emissions. For instance, gasification or pyrolysis can convert the carbon in sludge into biomass fuels. Against this backdrop, the thermochemical conversion of wastewater sludge, as an efficient resource recovery method, not only reduces carbon emissions but also generates biofuels, thereby achieving the dual goals of resource utilization and carbon reduction in wastewater sludge [21,41].
2.5. Traditional Treatment Methods for Sewage Sludge
With the research on sewage sludge, traditional sludge disposal methods have many deficiencies. The main treatment methods include composting, direct landfilling, and anaerobic digestion (AD) [42]. Sewage sludge is treated as fertilizer to recover nutrients and carbon, but it is often affected by heavy metals and pathogens in it [43]. The US Environmental Protection Agency (EPA) defines biosolids as treated sewage sludge that can be used as fertilizer only when it reaches an appropriate level of pollutants or pathogens. Direct landfilling, as another major method for sewage sludge disposal, is more widely applied. However, for countries with small land areas, landfilling is not reasonable [27,36]. Anaerobic digestion is a relatively slow biological process and is highly sensitive to the working environment. The limitations of traditional treatment methods have prompted people to seek more efficient alternative technologies. Thermochemical conversion technology has attracted much attention due to its high efficiency and the generation of multiple products.
2.5.1. Landfilling Method
The landfilling method involves the simultaneous disposal of sewage sludge and other solid wastes. Its simplicity and cost-effectiveness have made it a primary option for handling large volumes of sludge [44]. From the 1970s to the mid-1990s, research on the landfilling of sewage sludge decreased significantly. With the advancement of sludge treatment technologies, the proportion of studies focusing on landfilling sewage sludge has declined [45]. As landfilling progresses, it can lead to construction difficulties and instability of the landfill slope structure. Additionally, long-term landfilling of sludge may cause blockages in the systems for collecting leachate and gases produced by the landfill. The heavy metals and organic substances contained within the sludge may cause secondary pollution to water and soil through leachate, and the accumulated methane gas may cause secondary pollution to the atmosphere. Moreover, as society places greater emphasis on land resources, the landfilling method is gradually being rejected [27,46,47].
2.5.2. Composting Treatment
Composting treatment relies on natural microorganisms to decompose organic matter in sewage sludge through aerobic decomposition, converting it into fertilizer suitable for soil improvement and plant growth. This process also enables the recycling of nitrogen and phosphorus nutrients, enhances the destruction of pathogens and organic pollutants, and, to a certain extent, passivates the heavy metals contained, significantly reducing the environmental impact of antibiotics [48,49,50]. The overall process is time-consuming and dependent on microbial activity. If the composting time is not reduced, it may cause the composting design to be overloaded. If the time is reduced, it will affect the stability of the final compost product [49,51]. During the composting of sewage sludge, malodorous gases are produced, mainly due to the decomposition of numerous organic compounds and sulfur- and nitrogen-containing compounds in the sewage sludge, causing air pollution [52,53,54].
2.5.3. Anaerobic Digestion
Anaerobic digestion is a technology that uses microorganisms to anaerobically decompose macromolecular organic substances in sewage sludge, resulting in the final product of methane (CH4). The main commercial value of this process lies in its ability to produce renewable bioenergy–methane, which can be used for the generation of electricity and heat [55,56,57]. However, anaerobic digestion for treating sewage sludge also has its drawbacks. It relies on biological reactions, which have a relatively low reaction rate. Residual high-molecular-weight compounds and complex organic substances in sewage sludge affect the hydrolysis process of anaerobic digestion, and its economic benefits are significantly influenced by the volume of the reactor and the retention time [55,57,58]. The process is also relatively fragile. Untreated sewage sludge can lead to a high load, and antibiotics in the sewage sludge, toxic intermediate products generated, and overall pH changes can have a certain negative impact on the microbial community structure during anaerobic digestion, affecting the overall anaerobic digestion results and the production and utilization of biogas [59,60,61,62].
2.6. Potential Application Value of Thermochemical Conversion Technology
Thermochemical conversion is also a major resource recovery method in sludge treatment, as shown in Figure 2. Different from the traditional transformation technologies for sewage sludge, it includes gasification, pyrolysis and hydrothermal liquefaction. Compared with traditional transformation technologies, its advantage lies in the ability to convert the entire organic part of the raw material. It is more efficient and timesaving than biological transformation and can produce a wide range of products that can be separated and used for various purposes [6]. In addition to increasing energy recovery capacity and efficiency, thermochemical conversion processes also have the advantages of significantly reducing the volume of sludge after treatment, effectively killing pathogens, and converting the carbon in sewage sludge into fuel gas, bio-oil and biochar. The thermochemical process increases the energy content of sewage sludge and reduces the emission of unstructured greenhouse gases [40,63]. At the same time, through appropriate technical means, valuable nutrients and metals can also be recovered [20]. The thermochemical conversion of sewage sludge can reduce the environmental burden, such as reducing the formation of dioxins, furans, SOX and NOX [1], and meet the increasingly strict environmental standards.
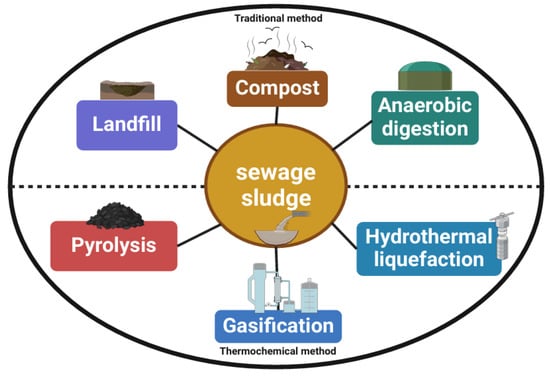
Figure 2.
Methods for treating sewage sludge.
3. Pyrolysis Process of Sewage Sludge and Influencing Factors
3.1. Main Products of Sewage Sludge Pyrolysis
Sewage sludge pyrolysis produces three main products: bio-oil, biochar, and pyrolysis gas. Bio-oil primarily contains hydrocarbons, organic acids, phenols, and nitrogen compounds, with a typical HHV of around 30 MJ/kg. Biochar’s HHV ranges from 11 to 15 MJ/kg, while its production may release small amounts of furan and malodorous gases [11,64]. The main gas components produced by sludge pyrolysis are CO2, CO, H2 and CH4, with a total content of nearly 90% [65]. Figure 3 shows the main products of pyrolysis of sewage sludge.
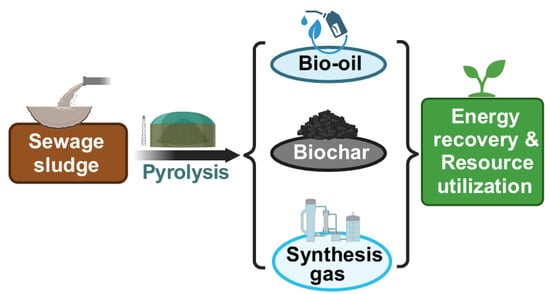
Figure 3.
Main products of pyrolysis of sewage sludge.
The Relationship Between Bio-Oil, Bio-Char and Pyrolysis Gas in Pyrolysis
Based on the different properties of the three products, different raw materials, different processing procedures and adjustments to key parameters, the proportions of the three products vary. According to this, a better method with higher economic benefits can be selected. The moisture content, high oxygen content and high ash content of raw materials may reduce the calorific value of the produced bio-oil [11]. The calorific value of biochar produced by pyrolysis mainly depends on the raw materials. Its basic properties, such as pH value, ash content, specific surface area of biochar and heavy metal content, are sensitive to the temperature and heating rate of the pyrolysis process. During the pyrolysis process, as the temperature rises, the gas output will increase, but at the same time, nitrogen oxides, sulfur oxides, tar, polycyclic aromatic hydrocarbons and particulate matter (PM) may increase [11,66].
3.2. Parameter Influence
3.2.1. Temperature
The gas yield increases with the extension of residence time and the rise in temperature [17], especially significantly when a certain temperature is reached. The main reason is that at high temperatures, the extended residence time enhances the secondary cracking reactions (SCRs) of volatile substances, promotes the decomposition of pyrolysis oil, generates more non-condensable gases, and increases gas production.
The yield of biochar decreases with the increase in temperature [67]. The content of fixed carbon is positively affected by temperature, but the H/C ratio shows a negative correlation with temperature, and aromaticity increases. This is mainly due to dehydrogenation, condensation (aromatization), and secondary cracking at high temperatures. The decrease in biochar yield with the reduction in temperature is attributed to the degradation of organic matter, the participation of thermochemical cracking reactions, and the extremely high carbonization temperature that accelerates the volatilization reaction, thereby reducing the biochar yield at high temperatures [68,69].
The yield of bio-oil shows a peak characteristic with respect to temperature. After reaching a certain temperature, the bio-oil yield reaches its peak and then begins to decline with further temperature increase [70]. The main reason for the increase in the rising stage is that more energy is available to break organic bonds and promote the volatilization of organic matter as the temperature rises. The decline after the peak is due to SCRs [19,71]. For the bio-oil produced from the pyrolysis of sewage sludge, its calorific value is influenced by oxygen, carbon, and hydrogen elements. The final oxygen content of the product decreases with the increase in temperature, while the percentages of carbon and hydrogen increase with the rise in temperature [72]. Temperature is the most significant influencing factor in the pyrolysis process for generating bio-oil, and it is crucial to identify the maximum temperature at which the yield reaches its peak.
3.2.2. Residence Time
According to Shen’s research, the yield of biochar decreases as the residence time increases but stabilizes after a certain period [73]. Meanwhile, Trinh et al. pointed out that the shorter the residence time of bio-oil, the higher the liquid yield, as a shorter residence time can prevent steam from participating in secondary cracking reactions, thereby reducing the conversion of liquid to gas [70]. Secondary cracking reactions decrease the yields of biochar and bio-oil, causing the yield of pyrolysis gas to gradually increase with the extension of residence time. Especially at high temperatures (525–625 °C), prolonging the residence time promotes the generation of gas. Short residence times are favorable for the formation of liquids, while long residence times are beneficial for increasing the gas yield but reduce the yields of liquids and biochar. The residence time can be utilized to regulate the final products.
3.2.3. Heating Rate
Heating rates are also an important influencing parameter for the pyrolysis of sewage sludge and have a significant impact on the final products. Zhai et al. compared the results of pyrolysis from 20 °C to 900 °C at heating rates of 5, 20, and 35 °C/min. They found that when the heating rate was lower, the heat conduction in the sludge particles was more thorough, and the mass loss was greater [74]. As the heating rate increased, the temperature corresponding to the maximum weight loss rate (TDTGmax) rose, indicating that the higher the heating rate, the temperature at which the main stage of the pyrolysis reaction occurred. According to Shahraki [75] under a pyrolysis temperature of 600 °C and a nitrogen flow rate of 100 cm3/min, heating rates of 20, 40, or 60 °C/s affected the yield of pyrolysis products. Higher heating rates promoted dehydration reactions, increasing the bio-oil yield, which then decreased after reaching a peak. Additionally, according to Kan et al., this was because high temperatures (above 600 °C) led to secondary cracking reactions during pyrolysis, resulting in less oil and more gas. Heating rates of 10, 40, 70, and 100 °C/min were studied. The results show that the heating rate is positively correlated with the temperatures of endothermic and exothermic reactions [76]. Higher heating rates promoted primary pyrolysis reactions and inhibited secondary reactions.
Overall, heating rate, along with residence time and temperature, often jointly determine the final pyrolysis products. For instance, increasing the heating rate and shortening the residence time can reduce the formation of NOx precursors throughout the pyrolysis process. Conversely, decreasing the heating rate and extending the residence time can reduce the formation of SOx [20].
3.3. Non-Catalytic Pyrolysis of Sewage Sludge
Pyrolysis of sewage sludge is a process of thermal degradation of chemical molecules in sewage sludge under high temperature and inert atmosphere, with the reaction temperature ranging from 300 °C to 1000 °C. This process converts high-molecular-weight carbon-containing substances in sewage sludge into small molecules, and the main products are bio-oil, low-molecular-weight gases, and solid fractions [77,78]. Due to its ability to generate renewable energy and concentrate heavy metals in the final products, pyrolysis technology is regarded as an environmentally friendly technology.
Pyrolysis technology mainly consists of two stages. The first stage is the primary reaction, where heat is transferred to the surface of sewage sludge particles and accumulates on the surface of sewage sludge particles, causing the temperature to rise and pyrolysis volatiles to start to be released. When the temperature exceeds 500 °C, some of the products from the first stage enter the second stage and undergo various complex chemical reactions [27,79].
The pyrolysis of sewage sludge can be further classified into slow pyrolysis, fast pyrolysis, and flash pyrolysis based on the temperature, heating rate of the reactor, and residence time [80,81]. The products of sewage sludge pyrolysis are influenced by multiple factors. The most significant influencing factors are the operating conditions, the composition of the raw material, temperature, pressure, reaction residence time, and reactor type [77,82].
3.3.1. Fast Pyrolysis
Fast pyrolysis of sewage sludge is a process of heating the sludge in an oxygen-free environment at high temperatures, typically with a heating rate of about 500 °C to 700 °C, a gas residence time of a few seconds, and under conditions specifically designed for feedstocks with particle sizes less than 3 mm. The main product of this process is bio-oil [27,81,83].
According to Table 3 the common reactor types include fluidized bed reactors, circulating fluidized bed reactors, vacuum pyrolysis reactors, ablative pyrolysis reactors, and Rotating Cone Reactors. The majority of research focuses on fast pyrolysis carried out in fixed fluidized bed and rotary kiln reactors [77,84]. The water content in the sewage sludge feedstock can cause phase separation in the bio-oil, resulting in two or more liquid phases, which may affect the uniformity of the bio-oil and increase the difficulty of subsequent processing and application. This can lead to a decrease in combustion performance and combustion reaction rate and significantly affect combustion efficiency and calorific value [10].

Table 3.
Non-catalytic pyrolysis modes of sewage sludge.
3.3.2. Slow Pyrolysis
Slow pyrolysis is suitable for raw materials with particle sizes less than 50 mm. The heating temperature is relatively low, generally ranging from 400 °C to 500 °C. During slow pyrolysis, the steam stays in the conversion zone for a longer time, resulting in more biochar formation and a reduction in the generation of gases and bio-oil. At the same time, the quality of the small amount of bio-oil produced in the end is relatively low [27,77,81]. In slow pyrolysis, the formation of biochar is divided into three stages. In the first stage, below 150 °C, the internal moisture of the raw material evaporates. In the second stage, the reaction heat temperature is between 150 °C and 300 °C, during which the internal chemical bonds of the biomass break and rearrange. The third stage is the carbonization stage. Maintaining a relatively slow heating rate and a temperature between 300 °C and 400 °C can produce high-quality biochar [85]. The biochar produced by slow pyrolysis can be used as a catalyst to continue to act on the pyrolysis process [88,89].
According to Table 4 the main reactors for slow pyrolysis are the screw reactor and the fixed-bed reactor. In the screw reactor, the screw is used for feeding and mixing the particles, as well as controlling the reaction residence time. The wall around the heating screw is heated to generate heat for the reaction. The advantages of the screw reactor are that it is suitable for larger particles and the temperature and residence time can be stably controlled. The disadvantages are that there is a risk of blockage and possible wear at high temperatures. The fixed-bed reactor consists of a cylindrical reactor, with nitrogen introduced to create an inert environment, and bio-oil collected through a tubular condenser. Its advantages include a simple structure and convenient operation, allowing for effective control of parameters such as reaction temperature and residence time. The main disadvantages are uneven temperature distribution, resulting in low heat transfer efficiency, and limited reactor capacity, making large-scale production difficult [84,90,91,92].

Table 4.
Comparison of sewage sludge pyrolysis reactors.
3.3.3. Flash Pyrolysis
Flash pyrolysis is also a high-temperature and high-heating-rate pyrolysis process, with a bio-oil yield that can reach 75%. This method involves rapid de-volatilization under an inert atmosphere at around 1000 °C with a heating rate as high as 2500 °C/s, and the vapor residence time is less than 1 s, requiring very small substrate particle sizes. The higher heating rate and shorter residence time of flash pyrolysis result in a higher bio-oil yield compared to fast pyrolysis. However, this process has the disadvantages of insufficient thermal stability and the bio-oil becoming more viscous due to the catalytic effect of the carbon present in the products [27,84].
3.3.4. Co-Pyrolysis
The co-pyrolysis technology of sewage sludge is a technique that involves adding sewage sludge to other feedstocks for pyrolysis. The main purpose of this technology is to add raw materials that have a synergistic effect with the pyrolysis of sewage sludge. Under inert conditions, the thermal decomposition generates biochar, thereby achieving high-quality and high-yield output substances. Usually, raw materials of different properties are combined to leverage their synergistic effects, improve the quality of pyrolysis products, and enhance resource utilization efficiency; at the same time, it can not only fix heavy metals and reduce environmental risks but also efficiently treat sludge [86,93]. Relevant studies have shown that coconut fibers [87], manure [94], kitchen waste [95], walnut shells [96], cotton stalks [24,97], straw and minerals [98], and rice husks and bamboo sawdust [99] all have certain synergistic effects with sewage sludge. These raw materials have optimized the co-pyrolysis products to some extent, such as improving the surface structure of biochar and enhancing the oxygenated compounds in bio-oil [11]. As shown in Table 5, co-pyrolysis has demonstrated a higher biochar yield and improved the performance of the produced biochar.

Table 5.
Co-pyrolysis of sewage sludge with different materials.
3.4. Catalytic Pyrolysis
Co-pyrolysis mainly relies on the synergy among multiple raw materials to improve the characteristics of the products and the reaction process. Different from co-pyrolysis, catalytic pyrolysis uses catalysts to provide active sites, reduce reaction activation energy, and adjust reaction pathways, effectively improving the properties of pyrolysis products and reducing the potential environmental hazards of heavy metals and pollutants [100]. The current development trend of sewage sludge pyrolysis is to improve the properties of pyrolysis products by adding catalysts to enhance their commercial value and usability. At present, the commonly used catalysts for sewage sludge catalytic pyrolysis are biochar catalysts, metal oxide catalysts, and zeolite catalysts.
3.4.1. Biochar Catalysts
Biochar, as a catalyst, is characterized by low cost and effectiveness. Biochar is often produced during the pyrolysis process, so using biochar as a catalyst can reduce potential waste treatment costs and increase its added value. Relevant studies have shown that when biochar is used as a catalyst in catalytic pyrolysis, the type and content of metals jointly determine the reaction pathway orientation, enabling biochar-based catalysts to promote the formation of hydrocarbons while suppressing the generation of oxygen-containing compounds (such as acids and phenols) and nitrogen-containing compounds (such as amides/amines and non-heterocyclic compounds). This results in a higher heating value and increased pH of the bio-oil, thereby improving its overall quality [101].
The metal content significantly influences the performance of biochar-based catalysts. In the studies by Shrestha and Liu [102,103], it was found that the appropriate incorporation of metal elements (such as Fe, Ni, Co, Mn) can markedly enhance the overall catalytic activity of the catalysts. This enhancement is primarily reflected in two aspects: firstly, the increase in surface active sites, and secondly, the acceleration of tar cracking and conversion efficiency. Furthermore, research has shown that the catalytic activity of metals is positively correlated with their loading amount. However, when the metal loading exceeds a certain threshold, the catalytic performance declines. This is mainly attributed to the agglomeration of active sites and blockage of pore structures. Low to moderate metal loadings—generally below 10 wt%—can typically maintain or even improve the specific surface area and pore volume of the catalysts, thereby facilitating sufficient contact between reactants and active sites. In contrast, high metal loadings—above 10 wt%—tend to cause metal particle agglomeration and micropore blockage, which in turn suppresses the diffusion capacity and catalytic activity of the material [102,103].
3.4.2. Metal Oxide Catalysts
Metal oxides are also frequently used as catalysts for the pyrolysis of sewage sludge. Common metal oxide catalysts include Al2O3, CaO, Fe2O3, ZnO, and TiO2. According to relevant studies, Al2O3 mainly functions as a catalyst for coal pyrolysis at high temperatures but has little effect on the high-temperature pyrolysis of sludge. CaO can increase the conversion rate when added within a certain temperature range, because it promotes the decomposition of hemicellulose and lignin while inhibiting the decomposition of cellulose in the sample. Fe2O3 promotes the conversion of sludge at temperatures below 510 K but inhibits it above 510 K. ZnO also shows a shift from positive to negative effects on the conversion of organic matter in sludge as the temperature changes. TiO2 consistently promotes the conversion of organic matter and shows a stable promoting effect within a certain temperature range [104].
Al2O3 and TiO2 can promote the degradation of organic matter in sludge, reduce solid residues, and accelerate the pyrolysis process, ultimately shortening the pyrolysis time. CaO promotes the initial decomposition of hemicellulose and the further decomposition of lignin but may inhibit the decomposition of cellulose, which could lead to an extended pyrolysis time. Fe2O3 and ZnO inhibit the decomposition of organic matter within certain temperature ranges, resulting in increased solid residues and potentially prolonged pyrolysis time.
3.4.3. Zeolite Catalytic Pyrolysis
Zeolites have been widely applied as catalysts in sludge pyrolysis due to their unique structural and physicochemical properties. High specific surface area and strong acidic sites facilitate the cracking reactions, allowing large biomass molecules to enter the pore channels and undergo catalytic conversion. Moreover, zeolites possess distinctive shape-selective catalytic capabilities, enabling precise control over the types of target products—particularly favoring the formation of aromatic compounds, thereby enhancing the heating value and stability of the resulting bio-oil. In addition, zeolites exhibit excellent adaptability for modification; their pore structure and acidity can be further optimized through methods such as mesopore formation and metal doping to suit different reaction pathways and desired products [105]. In comparison, the effectiveness of natural catalysts like kaolin and diatomite depends on their Si/Al ratio and structural characteristics. Kaolin activates its structure at 570 °C, significantly promoting the generation of H2 and CH4, and showing a more pronounced deoxygenation reaction in cellulose cracking. Diatomite, with its higher Si/Al ratio, mainly promotes the generation of CO, but its overall catalytic effect is weaker than that of synthetic zeolites [106].
According to the research by Csutoras and Miskolczi, ZSM-5 zeolite enhances the cracking reaction at high temperatures, promoting the generation of gaseous products such as hydrogen and carbon monoxide [106]. Meanwhile, Y-type zeolite shows higher catalytic activity at 600 °C, with its larger specific surface area and moderate pore size facilitating the production and increase in yield of gases like CO2 and H2. Additionally, Y-type zeolite significantly contributes to the cracking of small-molecule hydrocarbons, resulting in the generation of more low-carbon-number gas products. Furthermore, according to the study by Valizadeh et al., HZSM-5 and β-type zeolites demonstrate remarkable catalytic performance due to their acidity and pore structure. HZSM-5 at 600 °C effectively promotes the formation of benzene, toluene, ethylbenzene, and xylene by enhancing deoxygenation and aromatization reactions [107], while at 400 °C, its main role is to crack small-molecule organics and inhibit coke formation. In contrast, β-type zeolite exhibits high cracking efficiency at 500–600 °C, with its larger pore size and high acidity contributing to an increase in the yield of light gases such as alkanes and alkenes, while also having a certain inhibitory effect on coke formation.
In summary, zeolite catalysts not only optimize the product distribution but also significantly enhance the cracking reaction in sludge pyrolysis, especially at high temperatures. Moreover, zeolite catalytic pyrolysis can reduce the content of acidic products and improve the fuel quality of the products by regulating acidity, pore size, and oxygen vacancy distribution, making it have significant application potential in sludge pyrolysis technology. In conclusion, using zeolites as catalysts in the pyrolysis of sewage sludge is expected to achieve efficient generation of high-value chemicals and clean fuels during the pyrolysis process.
3.5. Comprehensive Evaluation and Challenges of Pyrolysis Technology
Pyrolysis technology decomposes the organic components in sewage sludge at high temperatures, achieving energy and resource utilization. It has significant advantages in reducing sludge volume, concentrating heavy metals, and reducing greenhouse gas emissions. Different pyrolysis processes and the application of catalysts significantly affect the characteristics and distribution of the products, which can be used as alternative energy sources. At the same time, the target products and raw material properties can be changed according to different processes and catalysts used.
At present, the commercialization of pyrolysis technology still faces problems such as high equipment investment and high operating costs. In addition, the cost of some catalysts is high, and there are difficulties in their recovery and reuse, which limits the feasibility of large-scale application. Developing low-cost, efficient and stable catalysts is one of the important research directions in the future. However, it is inevitable that by-products during the pyrolysis process (such as malodorous gases and tar) may still pose potential threats to the environment, which need to be controlled through the optimization of process conditions and supporting treatment measures.
4. Gasification of Sewage Sludge
The gasification process of sewage sludge is a thermochemical conversion method that uses sewage sludge as raw material to convert the organic components in the sludge into high-calorific synthetic gas in a high-temperature and reducing atmosphere; the by-products include tar and ash. The advantage of this technology is that it offers the dual benefits of volume reduction and the production of clean energy [18]. Any carbon-containing material can be used as gasification feedstock, and the carbon content in the raw material directly affects the energy content in the final synthetic gas, while the presence of oxygen and volatiles influences the thermal stability of the final product. However, the gasification of sludge still faces challenges such as high tar content and the formation of NOx from subsequent combustion due to by-products like NH3 and HCN. Additionally, high moisture and ash content may reduce energy conversion efficiency and affect equipment performance [108,109,110].
4.1. Gasification Process of Sewage Sludge
Gasification is a purely chemical reduction process, mainly divided into four stages: drying (70–200 °C, to remove moisture), pyrolysis (350–500 °C, generating coke, volatiles, and gases), oxidation (providing necessary heat), and reduction (converting pyrolysis products into permanent gases) [110,111]. The chemical reactions occurring in the oxidation zone and reduction zone are, respectively, shown in Figure 4 [111].
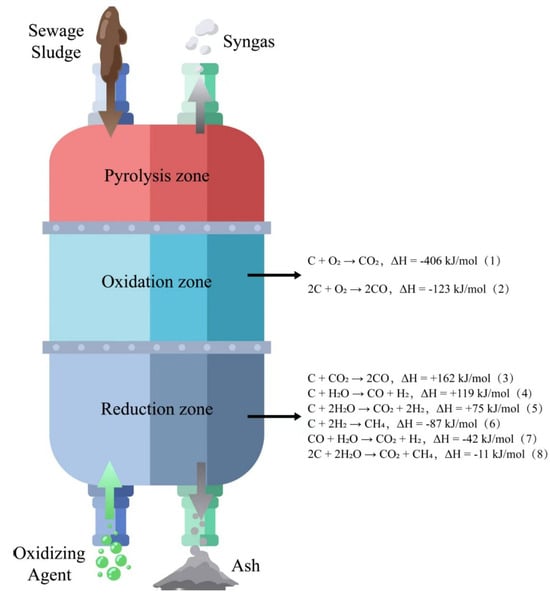
Figure 4.
Oxidation-reduction reaction of the gasifier.
4.2. Types of Gasification Reactors for Sewage Sludge
According to Table 6 the common types of sludge gasification reactors include fixed-bed reactors, which can be further classified into downdraft gasifiers, updraft gasifiers, and crossflow gasifiers [18]. The downdraft gasifier operates on the principle of co-current flow of gasifying agent and fuel, resulting in low tar content and high gas purity in the final product. However, it is only suitable for small-scale applications. The updraft gasifier, on the other hand, uses counter-current gas–solid contact, offering high thermal efficiency and the ability to handle high-moisture fuels. Its main drawback is the relatively high tar content in the produced gas. Both types of fixed-bed reactors have the disadvantage of low feed rates and are limited to specific particle sizes of the feedstock [112].

Table 6.
Comparison of sewage sludge gasification reactors.
Fluidized bed reactors include bubbling fluidized bed (BFB), circulating fluidized bed (CFB), and dual fluidized bed pyrolysis furnace. The overall operating temperature of a fluidized bed reactor is usually lower than that of a fixed bed reactor, and fluidized bed reactors typically use raw materials with a particle size of approximately 0.6 mm. Smaller particle size facilitates rapid heat transfer. A bubbling fluidized bed operates at a lower temperature and is suitable for processing smaller particle-size fuels. Good gas–solid contact and high heat and mass transfer efficiency are achieved in the reactor. Circulating fluidized beds have a high gas velocity, with solid particles being carried and recycled back into the reactor, resulting in a fast reaction rate and large processing capacity, making them suitable for large-scale wastewater sludge gasification applications [80,112]. In the dual fluidized bed, the gasification zone and combustion zone are located in separate fluidized beds, featuring high hydrogen production, flexible raw material mixing, and energy efficiency improvement through recycling. Entrained bed gasifiers require high-temperature and pressure operating conditions and ensure fine particles are fed into the reactor, using oxygen or steam as the gasification agent. Their main advantages include high-quality syngas, almost complete carbon conversion, and minimal heat loss. Disadvantages include the need to crush raw materials and the high cost of operating the facility [111,112].
4.3. Operating Parameters of Sewage Sludge Gasification Process
Operating parameters are of great significance for the gasification of sewage sludge. The final products of sewage sludge are often influenced by the operating parameters during the gasification process. Table 7 lists the operating parameters that affect the gasification results and analyzes their influence on the process of sewage sludge.

Table 7.
Key parameters of sewage sludge gasification.
4.3.1. Equivalence Ratio
The equivalence ratio (ER) is defined as the ratio of the actual air mass entering the gasifier to the air mass required for the complete combustion of wastewater sludge. Changes in the equivalence ratio directly affect the composition of syngas, tar content, and the conversion rate of final products [27,114]. An increase in the equivalence ratio leads to a greater introduction of oxygen or air into the reactor, shifting the overall gasification process towards oxidation, which reduces the production of CO and H2 while increasing the production of CO2 and H2O. The tar yield is also directly influenced by the equivalence ratio; a certain increase in ER raises the temperature inside the reactor, enhancing the tar cracking reaction and ultimately reducing the tar content produced. However, an excessively high ER reduces the overall syngas yield. An excessively low ER (<0.2) results in incomplete gasification, leading to the formation of high tar and char [10]. Therefore, relevant studies indicate that an ER value between 0.2 and 0.35 is effective [11]. Thus, the equivalence ratio plays a crucial role in the output results of syngas during the gasification process.
4.3.2. Ratio of Steam to Sewage Sludge (S/SS)
S/SS represents the amount of steam relative to the quantity of sewage sludge introduced into the gasifier. It is an important parameter in sewage sludge gasification. The introduction of steam in the gasification process has been optimized in terms of gasification efficiency, syngas composition, and overall process performance, especially when the S/SS is between 0 and 2 [114]. Compared to the introduction of air, the results show an increase in the production of H2 and CO, an increase in the calorific value of syngas, and a reduction in the production of tar and char. Relevant studies have shown that the introduction of steam in biomass gasification can increase the carbon conversion rate, mainly due to the higher reactivity of carbon in sewage sludge with steam [10]. As an important parameter in the sewage sludge gasification process, it should be regulated to achieve the best product and process performance.
4.3.3. Temperature
Temperature, as a crucial parameter, directly affects the gas production rate, calorific value, cold gas efficiency, and the yield of coke and tar, as well as the composition of the gas and the carbon conversion rate during the gasification process. As the temperature rises during gasification, endothermic reactions such as the water–gas reaction and the Boudouard reaction are promoted, resulting in an increase in the production of H2 and CO, and an increase in the calorific value of the syngas. At the same time, at high temperatures, the content of tar decreases while the number of gaseous products increases. This is because tar undergoes secondary cracking and reforming reactions at high temperatures. The amount of solid residue generated decreases with the increase in temperature [114,115]. Overall, the gasification temperature plays a key role in influencing the reaction rate and system efficiency. Appropriate temperature control is essential for optimizing the gasification process.
4.3.4. Parameters of Sewage Sludge as Raw Material
The characteristics of sewage sludge as raw material significantly affect the efficiency of the gasification process and the quality of the product. The high moisture content of sludge increases the energy consumption of the drying process and reduces the overall efficiency of the system. The selection of appropriate pretreatment technology directly affects the final quality and output of syngas from sewage sludge, which is crucial [11,110]. At the same time, the particle size of sewage sludge as raw material is also very important. High conversion rates can be achieved at very small particle sizes because grinding solid raw materials into fine particles improves the fluidization performance in the dense fluidized system [107,113]. Smaller particle sizes can be more evenly distributed in the reactor, improving heat transfer efficiency. An efficient heat transfer rate can enhance the gasification reaction, thereby increasing the H2 and CO values and reducing tar content. Optimizing raw material parameters can significantly improve gasification performance and economic benefits.
4.3.5. Catalyst Addition
Catalysts play a crucial role in the gasification of sewage sludge, as they can lower the reaction temperature, enhance the reaction rate, and increase the gas yield. They are mainly classified into homogeneous and heterogeneous catalysts [117]. Homogeneous catalysts are in the same phase as the reaction substrates. They are mainly alkali metals, whose main function is to promote the water–gas shift reaction, which can promote tar cracking and increase hydrogen production but may cause equipment corrosion. Heterogeneous catalysts are in a different phase from the reactants, including activated carbon catalysts or metal catalysts as well as metal oxide catalysts. Activated carbon catalysts mainly reduce tar content by adsorbing tar. Metal catalysts mainly increase hydrogen production by promoting tar cracking and gas product reforming [117,118,119]. Selecting the appropriate catalyst can significantly improve the gasification efficiency of sewage sludge and the calorific value of syngas. Relevant studies have shown that natural minerals such as low-cost dolomite, olivine, and limonite have good catalytic effects on various gasification conditions. Their low cost is accompanied by efficient catalytic capabilities, making them a catalytic method with relatively strong economic competitiveness [120].
4.4. Co-Gasification
Co-gasification is a thermochemical conversion technology that gasifies sludge together with other carbon-containing raw materials to produce syngas mainly composed of hydrogen, carbon monoxide, and methane. This technology effectively optimizes gasification efficiency and the quality of gas products [108]. Compared with single gasification, co-gasification technology enhances energy conversion efficiency by taking advantage of synergistic effects.
The performance improvement in co-gasification is mainly attributed to the synergistic effect of raw materials and catalytic effects. The high volatile matter content of biomass enhances the pyrolysis and initial reaction rate of sludge, but an excessively high sludge ratio may limit the reaction activity due to ash accumulation [121,122]. According to the research by Yan et al. [123], co-gasification of cotton stalks and sludge significantly increased hydrogen production and reduced tar formation and solid residues, demonstrating excellent synergistic effects. Experiments on co-gasification of waste tire carbon and sludge showed that the alkali and alkaline earth metal components in sludge can act as natural catalysts, reducing energy activation and increasing carbon conversion rate. Additionally, the co-gasification of straw and sludge in a CO2 atmosphere indicated that the high volatile matter content of straw can significantly enhance the reactivity and gas yield of sludge [124]. By adjusting the mixing ratio and gasification temperature, the proportion of H2 and CO in the syngas can be effectively optimized, reducing methane and tar formation. Appropriate mixing ratios and gasification temperatures can significantly increase carbon conversion rate and reduce tar production [121,123].
4.5. Comprehensive Evaluation and Challenges of Gasification Technology
Sludge gasification technology, as an important thermochemical conversion method, has demonstrated significant potential in reducing sludge volume, optimizing resource utilization, and producing high-calorific synthetic gas [11]. By rationally selecting reactor types, optimizing operating parameters, and introducing catalysts and co-gasification strategies, the efficiency of the gasification process and the quality of the product gas have been significantly enhanced. However, this technology still faces numerous challenges in practical applications. In practical applications, sludge gasification technology faces several major challenges. One of the most critical issues is the inherently high moisture and ash content of sewage sludge, which significantly increases the system’s energy consumption and operational maintenance costs while also posing risks to the long-term stability of gasification equipment. In addition, the gasification process often leads to the formation of undesirable by-products such as tar, NH3, HCN, and slag. These by-products not only reduce the purity of the resulting syngas but may also cause secondary environmental pollution. While the use of catalysts can improve product quality, it still suffers from high costs, limited durability, and susceptibility to poisoning or deactivation during operation.
To address these challenges, current research efforts generally follow two technological approaches. The first involves the optimization of operational parameters—such as temperature, equivalence ratio, and residence time—as well as the development of more efficient and robust catalysts in order to adjust reaction pathways and suppress the generation of unwanted by-products at the source. The second approach focuses on the post-treatment of product gases, where existing tars or contaminants are removed through gas cleaning technologies such as wet gas cleaning (WGC) and dry gas cleaning (DGC), thereby improving the usability and environmental compatibility of the final products [125].
5. Economic Feasibility Considerations
Although thermochemical conversion technologies demonstrate great potential for sustainable sludge management, economic feasibility remains a significant constraint to their widespread adoption. High capital and operational costs, complex reactor designs, expensive catalysts, and the need for pretreatment processes collectively elevate the overall investment threshold [41]. Among various technologies, gasification generally requires a higher initial investment but offers better energy recovery efficiency, while pyrolysis presents more flexible applications with relatively lower operational costs. Co-processing sludge with biomass and developing recyclable, low-cost catalysts are effective strategies to improve cost-effectiveness [17,126]. Additionally, integrating energy recovery systems and optimizing process parameters—such as temperature, residence time, and equivalence ratio—can reduce production costs and improve energy efficiency.
According to Table 8, the main economic challenges in thermochemical sludge treatment technologies are concentrated in four key areas: high capital investment, substantial energy consumption, complex pretreatment requirements, and the environmental impacts of byproduct formation. Nonetheless, recent studies and practical engineering efforts have proposed a range of feasible optimization strategies. These include lowering initial costs through catalyst innovation and modular equipment design, improving systemic efficiency by combining sludge with biomass and incorporating heat recovery, and increasing the value proposition by implementing advanced pollutant control and byproduct valorization technologies. Together, these measures establish a systematic approach to improving economic feasibility.

Table 8.
Economic feasibility factors and optimization strategies in thermochemical sludge treatment.
Furthermore, integrating energy recovery systems and optimizing process parameters can substantially reduce production costs and enhance economic returns. Future advancements must focus not only on technical optimization but also on comprehensive techno-economic assessments to facilitate the large-scale commercialization of thermochemical sludge treatment technologies [78]. Going forward, these technological and process innovations should be incorporated into robust techno-economic assessment frameworks and verified through pilot-scale and demonstration projects. Such efforts are essential to bridging the gap between laboratory research and industrial-scale deployment, thereby accelerating the commercialization of thermochemical sludge treatment technologies.
6. Conclusions and Prospects
6.1. Conclusions
Efficient disposal of sewage sludge remains a major challenge in wastewater treatment processes. Thermochemical technologies, including pyrolysis, gasification, and others, offer an effective means of recycling sludge resources; however, product characteristics are influenced by factors such as sludge composition, temperature, residence time, reactor type, co-pyrolysis, and catalysts. Research indicates that increasing temperature and residence time during pyrolysis boosts gas yield while reducing biochar output, yet enhances the carbon content and aromaticity of the biochar. An optimal temperature range exists for bio-oil production; however, excessively high temperatures result in a reduced yield. By selecting appropriate reactors, reaction conditions can be effectively controlled to optimize product quality. Furthermore, co-pyrolysis significantly enhances the quality of biochar and bio-oil, whereas catalytic pyrolysis further reduces bio-oil viscosity and heavy metal content. Key parameters of the gasification process include the equivalence ratio, steam ratio, temperature, and sludge particle size. The optimal equivalence ratio ranges from 0.2 to 0.35. An appropriate steam ratio improves syngas quality and reduces the formation of tar and carbon deposits. Higher temperatures promote syngas formation and reduce residue amounts. Moreover, reducing sludge moisture content and particle size further enhances gasification efficiency. In conclusion, optimizing pyrolysis and gasification parameters and selecting appropriate reactors, additives, and catalysts can significantly enhance the efficiency of sludge resource utilization, thereby providing an effective technical approach for sludge treatment and energy recovery.
6.2. Prospects
Future research must focus on sludge pretreatment, process optimization, catalyst innovation, and commercialization strategies to effectively advance the industrial application of thermochemical conversion technologies for sewage sludge. Future research and engineering applications should focus on the following key areas:
- Enhancement of sludge pretreatment technologies: Effective pretreatment methods and co-pyrolysis with biomass reduce moisture, ash, and heavy metal content, enhancing sludge safety and product quality.
- Optimization of process parameters and development of cost-effective catalysts: During pyrolysis and gasification processes, continuous adjustment of key parameters—such as temperature, residence time, and equivalence ratio—is essential to identify optimal operating conditions. Additionally, designing recyclable and low-cost catalysts can significantly improve product quality, energy output, and overall cost-effectiveness.
- Improvement of product quality and resolution of key technical bottlenecks: Enhancing bio-oil and biochar quality and tackling gasification challenges like tar management and syngas purity are key to achieving commercial viability.
Author Contributions
Y.H., investigation, data curation, visualization, writing—original draft; Z.C., writing—review and editing, supervision, project administration, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
The financial support of the “Fundamental Research Funds for the Central Universities” (2024JBMC044) is acknowledged.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
The following abbreviations are used in this manuscript:
| HHV | Higher Heating Value |
| PS | Primary sludge |
| SS | Secondary sludge |
| OC | Organic Pollutants |
| EU | European Union |
| EPA | Environmental Protection Agency |
| AD | Aerobic Digestion |
| PM | Particulate Matter |
| SCRs | Secondary Cracking Reactions |
| TDTGmax | Maximum Weight Loss Rate |
| BFB | Bubbling Fluidized Bed |
| CFB | Circulating Fluidized Bed |
| ER | Equivalent Ratio |
| S/SS | Ratio of Steam to Sewage Sludge |
References
- Capodaglio, A.G.; Callegari, A. Energy and resources recovery from excess sewage sludge: A holistic analysis of opportunities and strategies. Resour. Conserv. Recycl. Adv. 2023, 19, 200184. [Google Scholar] [CrossRef]
- Vali, N.; Zabihi, S.; Shamim, S.; Mohsenzadeh, A.; Pettersson, A. Slow-pyrolysis of municipal sewage sludge: Biochar characteristics and advanced thermodynamics. Biomass Convers. Biorefin. 2025, in press. [Google Scholar] [CrossRef]
- Wang, B.; Liu, Y.; Guan, Y.; Feng, Y. Characteristic of the production of hydrogen-rich combustible gas by pyrolysis of high-ash sewage sludge. J. Clean. Prod. 2022, 334, 130224. [Google Scholar] [CrossRef]
- Wang, Y.C.; Zhang, T.; Westerhoff, P.; Jiang, R.F.; Ren, H.Q.; Yang, Y.; Li, Z. Microwave-assisted digestion and NaOH treatment of waste-activated sludge to recover phosphorus by crystallizing struvite. Environ. Technol. 2017, 38, 1211–1222. [Google Scholar] [CrossRef]
- Zhu, Y.; Wen, X.; Guo, Z. Research progress on high-value utilization technology of sludge solid waste in China. J. Mater. Cycles Waste Manag. 2025, 27, 654–665. [Google Scholar] [CrossRef]
- Hu, M.; Ye, Z.; Zhang, H.; Chen, B.; Pan, Z.; Wang, J. Thermochemical conversion of sewage sludge for energy and resource recovery: Technical challenges and prospects. Environ. Pollut. Bioavailab. 2021, 33, 145–163. [Google Scholar] [CrossRef]
- Veli Sezgin, İ.; Merdun, H. Estimation of fast pyrolysis product yields of different biomasses by artificial neural networks. Chem. Eng. Res. Des. 2025, 215, 32–42. [Google Scholar] [CrossRef]
- He, X.Y.; Zhang, T.; Xue, Q.; Zhou, Y.L.; Wang, H.L.; Bolan, N.S.; Jiang, R.F.; Tsang, D.C.W. Enhanced adsorption of Cu(II) and Zn(II) from aqueous solution by polyethyleneimine modified straw hydrochar. Sci. Total Environ. 2021, 778, 146116. [Google Scholar] [CrossRef]
- Xie, S.Y.; He, X.Y.; Ali Alshehri, M.; Abou-Elwafa, S.F.; Zhang, T. Elevated effect of hydrothermal treatment on phosphorus transition between solid-liquid phase in swine manure. Results Eng. 2024, 24, 102887. [Google Scholar] [CrossRef]
- Syed-Hassan, S.S.A.; Wang, Y.; Hu, S.; Su, S.; Xiang, J. Thermochemical processing of sewage sludge to energy and fuel: Fundamentals, challenges and considerations. Renew. Sustain. Energy Rev. 2017, 80, 888–913. [Google Scholar] [CrossRef]
- Gao, N.; Kamran, K.; Quan, C.; Williams, P.T. Thermochemical conversion of sewage sludge: A critical review. Prog. Energy Combust. Sci. 2020, 79, 100843. [Google Scholar] [CrossRef]
- Nabernegg, S.; Bednar-Friedl, B.; Muñoz, P.; Titz, M.; Vogel, J. National policies for global emission reductions: Effectiveness of carbon emission reductions in international supply chains. Ecol. Econ. 2019, 158, 146–157. [Google Scholar] [CrossRef]
- Gu, G.; Wang, Z.; Wu, L. Carbon emission reductions under global low-carbon technology transfer and its policy mix with R&D improvement. Energy 2021, 216, 119300. [Google Scholar]
- Lim, H.Y.; Rashidi, N.A.; Othman, M.F.H.; Ismail, I.S.; Saadon, S.Z.A.H.; Chin, B.L.F.; Yusup, S.; Rahman, M.N. Recent advancement in thermochemical conversion of biomass to biofuel. Biofuels 2024, 15, 587–604. [Google Scholar] [CrossRef]
- Deng, Y.X.; Zhang, T.; Clark, J.; Aminabhavi, T.; Kruse, A.; Tsang, D.C.W.; Sharma, B.K.; Zhang, F.S.; Ren, H.Q. Mechanisms and modelling of phosphorus solid–liquid transformation during the hydrothermal processing of swine manure. Green Chem. 2020, 22, 5628–5638. [Google Scholar] [CrossRef]
- Poornima, S.; Manikandan, S.; Prakash, R.; Deena, S.R.; Subbaiya, R.; Karmegam, N.; Kim, W.; Govarthanan, M. Biofuel and biochemical production through biomass transformation using advanced thermochemical and biochemical processes—A review. Fuel 2024, 372, 132204. [Google Scholar] [CrossRef]
- Rijo, B.; Nobre, C.; Brito, P.; Ferreira, P. An overview of the thermochemical valorization of sewage sludge: Principles and current challenges. Energies 2024, 17, 2417. [Google Scholar] [CrossRef]
- Manara, P.; Zabaniotou, A. Towards sewage sludge-based biofuels via thermochemical conversion—A review. Renew. Sustain. Energy Rev. 2012, 16, 2566–2582. [Google Scholar] [CrossRef]
- Fonts, I.; Gea, G.; Azuara, M.; Ábrego, J.; Arauzo, J. Sewage sludge pyrolysis for liquid production: A review. Renew. Sustain. Energy Rev. 2012, 16, 2781–2805. [Google Scholar] [CrossRef]
- Djandja Oraléou, S.; Wang, Z.C.; Wang, F.; Xu, Y.P.; Duan, P.G. Pyrolysis of municipal sewage sludge for biofuel production: A review. Ind. Eng. Chem. Res. 2020, 59, 16939–16956. [Google Scholar] [CrossRef]
- Chan, Y.H.; Lock, S.S.M.; Chin, B.L.F.; Wong, M.K.; Loy, A.C.M.; Foong, S.Y.; Yiin, C.L.; Lam, S.S. Progress in thermochemical co-processing of biomass and sludge for sustainable energy, value-added products and circular economy. Bioresour. Technol. 2023, 380, 129061. [Google Scholar] [CrossRef]
- Chen, M.; Oshita, K.; Mahzoun, Y.; Takaoka, M.; Fukutani, S.; Shiota, K. Survey of elemental composition in dewatered sludge in Japan. Sci. Total Environ. 2021, 752, 141857. [Google Scholar] [CrossRef] [PubMed]
- Wiśniowska, E.; Kowalczyk, M. Recovery of cellulose, extracellular polymeric substances and microplastics from sewage sludge: A review. Energies 2022, 15, 7744. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, L.; Liu, K.; Wang, J.; Zhu, H.; Song, Q.; Shu, X. Co-pyrolysis of sewage sludge and cotton stalks. Waste Manag. 2019, 89, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Burke, I.T.; Chen, X.; Stewart, D.I. Assessing metal contamination and speciation in sewage sludge: Implications for soil application and environmental risk. Rev. Environ. Sci. Biotechnol. 2023, 22, 1037–1058. [Google Scholar] [CrossRef]
- Naqvi, S.R.; Tariq, R.; Shahbaz, M.; Naqvi, M.; Aslam, M.; Khan, Z.; Mackey, H.; McKay, G.; Al-Ansari, T. Recent developments on sewage sludge pyrolysis and its kinetics: Resources recovery, thermogravimetric platforms, and innovative prospects. Comput. Chem. Eng. 2021, 150, 107325. [Google Scholar] [CrossRef]
- Gururani, P.; Bhatnagar, P.; Bisht, B.; Jaiswal, K.K.; Kumar, V.; Kumar, S.; Vlaskin, M.S.; Grigorenko, A.V.; Rindin, K.G. Recent advances and viability in sustainable thermochemical conversion of sludge to bio-fuel production. Fuel 2022, 316, 123351. [Google Scholar] [CrossRef]
- Wu, B.; Dai, X.; Chai, X. Critical review on dewatering of sewage sludge: Influential mechanism, conditioning technologies and implications to sludge re-utilizations. Water Res. 2020, 180, 115912. [Google Scholar] [CrossRef]
- Rao, B.; Wang, G.; Xu, P. Recent advances in sludge dewatering and drying technology. Dry. Technol. 2022, 40, 3049–3063. [Google Scholar] [CrossRef]
- Patureau, D.; Mailler, R.; Delgenes, N.; Danel, A.; Vulliet, E.; Deshayes, S.; Moilleron, R.; Rocher, V.; Gasperi, J. Fate of emerging and priority micropollutants during the sewage sludge treatment—Part 2: Mass balances of organic contaminants on sludge treatments are challenging. Waste Manag. 2021, 125, 122–131. [Google Scholar] [CrossRef]
- Yakamercan, E.; Aygün, A. Anaerobic/aerobic cycle effect on di(2-ethylhexyl) phthalate and pentachlorophenol removal from real textile wastewater in sequencing batch biofilm reactor. J. Clean. Prod. 2020, 273, 122975. [Google Scholar] [CrossRef]
- Wasserman, M.; Moretti, A.; Goi, D.; Mainardis, M. Integrating renewable energy in sewage sludge treatment through greenhouse solar drying: A review. Sci. Total Environ. 2025, 965, 178634. [Google Scholar] [CrossRef]
- Tang, J.; Tang, H.; Sima, W.; Wang, H.; Zou, D.; Qiu, B.; Qu, J.; Liang, R.; Dong, J.; Liao, Y.; et al. Heavy metal pollution level and potential ecological risk assessment of sludge landfill. Environ. Prog. Sustain. Energy 2022, 41, e13761. [Google Scholar]
- Qi, C.; Cao, D.; Gao, X.; Jia, S.; Yin, R.; Nghiem, L.D.; Li, G.; Luo, W. Optimising organic composition of feedstock to improve microbial dynamics and symbiosis to advance solid-state anaerobic co-digestion of sewage sludge and organic waste. Appl. Energy 2023, 351, 121857. [Google Scholar] [CrossRef]
- Galey, B.; Gautier, M.; Kim, B.; Blanc, D.; Chatain, V.; Ducom, G.; Dumont, N.; Gourdon, R. Trace metal elements vaporization and phosphorus recovery during sewage sludge thermochemical treatment—A review. J. Hazard. Mater. 2022, 424, 127360. [Google Scholar] [CrossRef] [PubMed]
- Buss, W. Pyrolysis solves the issue of organic contaminants in sewage sludge while retaining carbon: Making the case for sewage sludge treatment via pyrolysis. ACS Sustain. Chem. Eng. 2021, 9, 10048–10053. [Google Scholar] [CrossRef]
- Menezes, L.N.B.; Silveira, E.A.; Mazzoni, J.V.S.; Evaristo, R.B.W.; Rodrigues, J.S.; Lamas, G.C.; Suarez, P.A.Z.; Ghesti, G.F. Alternative valuation pathways for primary, secondary, and tertiary sewage sludge: Biochar and bio-oil production for sustainable energy. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Kulikova, Y.; Babich, O.; Tsybina, A.; Sukhikh, S.; Mokrushin, I.; Noskova, S.; Orlov, N. Feasibility of Thermal Utilization of Primary and Secondary Sludge from a Biological Wastewater Treatment Plant in Kaliningrad City. Energies 2022, 15, 5639. [Google Scholar] [CrossRef]
- Purkayastha, R.; Choudhury, B.J.; Mahanta, P.; Suami, A.; Itaya, Y.; Moholkar, V.S.; Kobayashi, N. Investigations in pyrolysis kinetics of sludges of different origins: Chemical sludge, biological sludge and oily sludge. J. Mater. Cycles Waste Manag. 2024, 26, 2754–2769. [Google Scholar] [CrossRef]
- Hu, M.; Hu, H.; Ye, Z.; Tan, S.; Yin, K.; Chen, Z.; Guo, D.; Rong, H.; Wang, J.; Pan, Z.; et al. A review on turning sewage sludge to value-added energy and materials via thermochemical conversion towards carbon neutrality. J. Clean. Prod. 2022, 379, 134657. [Google Scholar] [CrossRef]
- Chang, H.; Yuan, J.; Zhao, Y.; Bisinella, V.; Damgaard, A.; Christensen, T.H. Carbon footprints of incineration, pyrolysis, and gasification for sewage sludge treatment. Resour. Conserv. Recycl. 2025, 212, 107939. [Google Scholar] [CrossRef]
- An, Q.; Chen, D.; Han, M.; Feng, Y. High-quality hydrochar production through co-hydrothermal treatment of sewage sludge and wheat straw: Novel properties. Biomass Convers. Biorefin. 2025, 15, 141–153. [Google Scholar] [CrossRef]
- Jiang, G.; Xu, D.; Hao, B.; Liu, L.; Wang, S.; Wu, Z. Thermochemical methods for the treatment of municipal sludge. J. Clean. Prod. 2021, 311, 127811. [Google Scholar] [CrossRef]
- O’Kelly, B.C. Sewage sludge to landfill: Some pertinent engineering properties. J. Air Waste Manag. Assoc. 2005, 55, 765–771. [Google Scholar] [CrossRef]
- Bagheri, M.; Bauer, T.; Burgman, L.E.; Wetterlund, E. Fifty years of sewage sludge management research: Mapping researchers’ motivations and concerns. J. Environ. Manag. 2023, 325, 116412. [Google Scholar] [CrossRef]
- Liu, S.; Liu, H.; Wei, G.; Zhu, Y.; Zhao, H.; Shi, H.; Lian, Y. Comparative life cycle assessment of landfill sludge treatment technologies in China. Environ. Sci. Pollut. Res. Int. 2024, 31, 41208–41220. [Google Scholar] [CrossRef]
- Yang, Y.; Luan, J.; Nie, J.; Zhang, X.; Du, J.; Zhao, G.; Dong, L.; Fan, Y.; Cui, H.; Li, Y. Reprocessing and resource utilization of landfill sludge—A case study in a Chinese megacity. Water 2024, 16, 468. [Google Scholar] [CrossRef]
- Yuan, J.; Chadwick, D.; Zhang, D.; Li, G.; Chen, S.; Luo, W.; Du, L.; He, S.; Peng, S. Effects of aeration rate on maturity and gaseous emissions during sewage sludge composting. Waste Manag. 2016, 56, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Chen, S.; Tan, X.; Li, X.; Pan, H.; Ma, P.; Wu, Z.; Xie, Q. Microbial community succession in response to sludge composting efficiency and heavy metal detoxification during municipal sludge composting. Front. Microbiol. 2022, 13, 1015949. [Google Scholar] [CrossRef]
- Manea, E.E.; Bumbac, C. Sludge composting—Is this a viable solution for wastewater sludge management? Water 2024, 16, 2241. [Google Scholar] [CrossRef]
- Stürmer, B.; Waltner, M. Best available technology for P-recycling from sewage sludge—An overview of sewage sludge composting in Austria. Recycling 2021, 6, 82. [Google Scholar] [CrossRef]
- González, D.; Colón, J.; Gabriel, D.; Sánchez, A. The effect of the composting time on the gaseous emissions and the compost stability in a full-scale sewage sludge composting plant. Sci. Total Environ. 2019, 654, 311–323. [Google Scholar] [CrossRef]
- González, D.; Colón, J.; Sánchez, A.; Gabriel, D. A systematic study on the VOCs characterization and odour emissions in a full-scale sewage sludge composting plant. J. Hazard. Mater. 2019, 373, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Nie, E.; Zheng, G.; Gao, D.; Chen, T.; Yang, J.; Wang, Y.; Wang, X. Emission characteristics of VOCs and potential ozone formation from a full-scale sewage sludge composting plant. Sci. Total Environ. 2019, 659, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Neumann, P.; Pesante, S.; Venegas, M.; Vidal, G. Developments in pre-treatment methods to improve anaerobic digestion of sewage sludge. Rev. Environ. Sci. Biotechnol. 2016, 15, 173–211. [Google Scholar] [CrossRef]
- Lafratta, M.; Thorpe, R.B.; Ouki, S.K.; Shana, A.; Germain, E.; Willcocks, M.; Lee, J. Dynamic biogas production from anaerobic digestion of sewage sludge for on-demand electricity generation. Bioresour. Technol. 2020, 310, 123415. [Google Scholar] [CrossRef]
- Khanh Nguyen, V.; Kumar Chaudhary, D.; Hari Dahal, R.; Hoang Trinh, N.; Kim, J.; Chang, S.W.; Hong, Y.; Duc La, D.; Nguyen, X.C.; Hao Ngo, H.; et al. Review on pretreatment techniques to improve anaerobic digestion of sewage sludge. Fuel 2021, 285, 119105. [Google Scholar] [CrossRef]
- Khawer, M.U.B.; Naqvi, S.R.; Ali, I.; Arshad, M.; Juchelková, D.; Anjum, M.W.; Naqvi, M. Anaerobic digestion of sewage sludge for biogas and biohydrogen production: State-of-the-art trends and prospects. Fuel 2022, 329, 125416. [Google Scholar] [CrossRef]
- Di Capua, F.; Spasiano, D.; Giordano, A.; Adani, F.; Fratino, U.; Pirozzi, F.; Esposito, G. High-solid anaerobic digestion of sewage sludge: Challenges and opportunities. Appl. Energy 2020, 278, 115608. [Google Scholar] [CrossRef]
- Wu, Q.; Zou, D.; Zheng, X.; Liu, F.; Li, L.; Xiao, Z. Effects of antibiotics on anaerobic digestion of sewage sludge: Performance of anaerobic digestion and structure of the microbial community. Sci. Total Environ. 2022, 845, 157384. [Google Scholar] [CrossRef]
- Feng, L.; Hu, T.; Ma, H.; Gao, Z.; Liu, Y.; He, S.; Ding, J.; Jiang, J.; Zhao, Q.; Wei, L. Impacts of biochar derived from oil sludge on anaerobic digestion of sewage sludge: Performance and associated mechanisms. J. Clean. Prod. 2023, 425, 138838. [Google Scholar] [CrossRef]
- Zielińska, M.; Cydzik-Kwiatkowska, A. Effect of emerging micropollutants on the anaerobic digestion of sewage sludge. Energies 2024, 17, 1033. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Liu, F.; Zhang, X.; Song, M.; Li, R. Pyrolysis of sewage sludge to biochar: Transformation mechanism of phosphorus. J. Anal. Appl. Pyrolysis 2023, 173, 106065. [Google Scholar] [CrossRef]
- Yuan, Z.; Luo, J.; Ndudi, E.A.; Ma, W.; Zhu, N.; Lou, Z. Systematic understanding of char-volatile evolution and interaction mechanism during sewage sludge pyrolysis through in-situ tracking solid-state reaction and products fate. J. Hazard. Mater. 2022, 432, 128669. [Google Scholar] [CrossRef]
- Moško, J.; Pohořelý, M.; Skoblia, S.; Beňo, Z.; Jeremiáš, M. Detailed analysis of sewage sludge pyrolysis gas: Effect of pyrolysis temperature. Energies 2020, 13, 4087. [Google Scholar] [CrossRef]
- Racek, J.; Sevcik, J.; Chorazy, T.; Kucerik, J.; Hlavinek, P. Biochar—Recovery material from pyrolysis of sewage sludge: A review. Waste Biomass Valorization 2020, 11, 3677–3709. [Google Scholar] [CrossRef]
- Li, X.; Cen, K.; Wang, L.; Jia, D.; Zhu, X.; Chen, D. Co-pyrolysis of cellulose and lignin: Effects of pyrolysis temperature, residence time, and lignin percentage on the properties of biochar using response surface methodology. Ind. Crops Prod. 2024, 219, 119071. [Google Scholar] [CrossRef]
- Das, S.K.; Ghosh, G.K.; Avasthe, R.K.; Sinha, K. Compositional heterogeneity of different biochar: Effect of pyrolysis temperature and feedstocks. J. Environ. Manag. 2021, 278, 111501. [Google Scholar] [CrossRef]
- Guo, S.; Xiong, X.; Che, D.; Liu, H.; Sun, B. Effects of sludge pyrolysis temperature and atmosphere on characteristics of biochar and gaseous products. Korean J. Chem. Eng. 2021, 38, 55–63. [Google Scholar] [CrossRef]
- Trinh, T.N.; Jensen, P.A.; Dam-Johansen, K.; Knudsen, N.O.; Sørensen, H.R. Influence of the pyrolysis temperature on sewage sludge product distribution, bio-oil, and char properties. Energy Fuels 2013, 27, 1419–1427. [Google Scholar] [CrossRef]
- Huang, X.; Cao, J.P.; Shi, P.; Zhao, X.Y.; Feng, X.B.; Zhao, Y.P.; Fan, X.; Wei, X.Y.; Takarada, T. Influences of pyrolysis conditions in the production and chemical composition of the bio-oils from fast pyrolysis of sewage sludge. J. Anal. Appl. Pyrolysis 2014, 110, 353–362. [Google Scholar] [CrossRef]
- Guedes, R.E.; Luna, A.S.; Torres, A.R. Operating parameters for bio-oil production in biomass pyrolysis: A review. J. Anal. Appl. Pyrolysis 2018, 129, 134–149. [Google Scholar] [CrossRef]
- Shen, Q.; Wu, H. Rapid pyrolysis of biochar prepared from slow and fast pyrolysis: The effects of particle residence time on char properties. Proc. Combust. Inst. 2023, 39, 3371–3378. [Google Scholar] [CrossRef]
- Zhai, Y.; Peng, W.; Zeng, G.; Fu, Z.; Lan, Y.; Chen, H.; Wang, C.; Fan, X. Pyrolysis characteristics and kinetics of sewage sludge for different sizes and heating rates. J. Therm. Anal. Calorim. 2012, 107, 1015–1022. [Google Scholar] [CrossRef]
- Shahraki, S.; Miri, M.; Motahari-Nezhad, M. Experimental analysis of pyrolysis of sewage sludge. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 2037–2043. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T. Effect of the heating rate on the thermochemical behavior and biofuel properties of sewage sludge pyrolysis. Energy Fuels 2016, 30, 1564–1570. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhai, Y.; Li, S.; Liu, X.; Wang, B.; Liu, X.; Fan, Y.; Shi, H.; Li, C.; Zhu, Y. Thermal treatment of sewage sludge: A comparative review of the conversion principle, recovery methods and bioavailability-predicting of phosphorus. Chemosphere 2022, 291, 133053. [Google Scholar] [CrossRef]
- Sajid, M.; Raheem, A.; Ullah, N.; Asim, M.; Saif Ur Rehman, M.; Ali, N. Gasification of municipal solid waste: Progress, challenges, and prospects. Renew. Sustain. Energy Rev. 2022, 168, 112815. [Google Scholar] [CrossRef]
- Ofori-Boateng, C.; Service, S.L.O. Sustainability of Thermochemical Waste Conversion Technologies; Springer International Publisher: Cham, Switzerland, 2024. [Google Scholar]
- Siwal, S.S.; Sheoran, K.; Saini, A.K.; Vo, D.V.N.; Wang, Q.; Thakur, V.K. Advanced thermochemical conversion technologies used for energy generation: Advancement and prospects. Fuel 2022, 321, 124107. [Google Scholar] [CrossRef]
- Kasiński, S.; Dębowski, M. Municipal solid waste as a renewable energy source: Advances in thermochemical conversion technologies and environmental impacts. Energies 2024, 17, 4704. [Google Scholar] [CrossRef]
- Basar, I.A.; Liu, H.; Eskicioglu, C. Effects of municipal sludge composition on hydrothermal liquefaction products: Aqueous phase characterization and biodegradability assessment. Bioresour. Technol. 2024, 400, 130671. [Google Scholar] [CrossRef]
- Nandhini, R.; Berslin, D.; Sivaprakash, B.; Rajamohan, N.; Vo, D.V.N. Thermochemical conversion of municipal solid waste into energy and hydrogen: A review. Environ. Chem. Lett. 2022, 20, 1645–1669. [Google Scholar] [CrossRef] [PubMed]
- Pahnila, M.; Koskela, A.; Sulasalmi, P.; Fabritius, T. A review of pyrolysis technologies and the effect of process parameters on biocarbon properties. Energies 2023, 16, 6936. [Google Scholar] [CrossRef]
- Li, J.; Xu, K.; Yao, X.; Liu, J. Investigation of biomass slow pyrolysis mechanisms based on the generation trends in pyrolysis products. Process Saf. Environ. Prot. 2024, 183, 327–338. [Google Scholar] [CrossRef]
- Mohamed, B.A.; Li, L.Y. Biofuel production by co-pyrolysis of sewage sludge and other materials: A review. Environ. Chem. Lett. 2023, 21, 153–182. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, X.; Zhang, J.; Ma, X.; Sun, P.; Zhao, L. Sewage sludge–coconut fiber co-pyrolysis biochar: Mechanisms underlying synergistic heavy metal stabilization and ciprofloxacin adsorption. J. Clean. Prod. 2022, 375, 134149. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Ganeshan, P.; Gohil, N.; Kumar, V.; Singh, V.; Rajendran, K.; Harirchi, S.; Solanki, M.K.; Sindhu, R.; Binod, P.; et al. Advanced approaches for resource recovery from wastewater and activated sludge: A review. Bioresour. Technol. 2023, 384, 129250. [Google Scholar] [CrossRef]
- Khan, R.; Shukla, S.; Kumar, M.; Zuorro, A.; Pandey, A. Sewage sludge-derived biochar and its potential for sustainable environment in circular economy: Advantages and challenges. Chem. Eng. J. 2023, 471, 144495. [Google Scholar] [CrossRef]
- del Pozo, C.; Rego, F.; Puy, N.; Bartrolí, J.; Fàbregas, E.; Yang, Y.; Bridgwater, A.V. The effect of reactor scale on biochars and pyrolysis liquids from slow pyrolysis of coffee silverskin, grape pomace and olive mill waste, in auger reactors. Waste Manag. 2022, 148, 106–116. [Google Scholar] [CrossRef]
- Vieira, F.R.; Romero Luna, C.M.; Arce, G.L.A.F.; Ávila, I. Optimization of slow pyrolysis process parameters using a fixed bed reactor for biochar yield from rice husk. Biomass Bioenergy 2020, 132, 105412. [Google Scholar] [CrossRef]
- Tabakaev, R.; Astafev, A.; Shanenkova, Y.; Dubinin, Y.; Yazykov, N.; Yakovlev, V. Thermal effects investigation during biomass slow pyrolysis in a fixed bed reactor. Biomass Bioenergy 2019, 126, 26–33. [Google Scholar] [CrossRef]
- Embaye, T.M.; Zhou, A.; Li, R.; Ahmed, M.B.; Ruan, R.; Wu, D.; Deng, N.; Wang, X. Assessment of heavy metals distribution and environmental risks in biochar from co-pyrolysis of sewage sludge and mixed municipal waste. Process Saf. Environ. Prot. 2025, 193, 1332–1342. [Google Scholar] [CrossRef]
- Ruiz-Gómez, N.; Quispe, V.; Ábrego, J.; Atienza-Martínez, M.; Murillo, M.B.; Gea, G. Co-pyrolysis of sewage sludge and manure. Waste Manag. 2017, 59, 211–221. [Google Scholar] [CrossRef]
- Wang, X.; Wei-Chung Chang, V.; Li, Z.; Song, Y.; Li, C.; Wang, Y. Co-pyrolysis of sewage sludge and food waste digestate to synergistically improve biochar characteristics and heavy metals immobilization. Waste Manag. 2022, 141, 231–239. [Google Scholar] [CrossRef]
- Yin, Q.; Liu, M.; Ren, H. Biochar produced from the co-pyrolysis of sewage sludge and walnut shell for ammonium and phosphate adsorption from water. J. Environ. Manag. 2019, 249, 109410. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Lyu, Z.; Zheng, Y.; Gao, H.; Wang, J. Comprehensive analysis of bio-syngas production via the co-pyrolysis of sewage sludge and cotton stalk: Products, optimal conditions, kinetics, and synergistic effects. J. Anal. Appl. Pyrolysis 2025, 186, 106980. [Google Scholar] [CrossRef]
- Duan, X.Y.; Cao, Y.; Liu, T.Z.; Li, L.; Wang, B.; Wang, X.D. Nutrient stability and sorption of sewage sludge biochar prepared from co-pyrolysis of sewage sludge and stalks/mineral materials. Environ. Pollut. Bioavailab. 2020, 32, 12–18. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, J.; Wang, M.; Naidu, R.; Liu, Y.; Man, Y.B.; Liang, X.; Wong, M.H.; Christie, P.; Zhang, Y.; et al. Co-pyrolysis of sewage sludge and rice husk/bamboo sawdust for biochar with high aromaticity and low metal mobility. Environ. Res. 2020, 191, 110034. [Google Scholar] [CrossRef]
- Ma, M.; Xu, D.; Gong, X.; Diao, Y.; Feng, P.; Kapusta, K. Municipal sewage sludge product recirculation catalytic pyrolysis mechanism from a kinetic perspective. Renew. Energy 2023, 215, 118955. [Google Scholar] [CrossRef]
- Qiu, Z.; Zhai, Y.; Li, S.; Liu, X.; Liu, X.; Wang, B.; Liu, Y.; Li, C.; Hu, Y. Catalytic co-pyrolysis of sewage sludge and rice husk over biochar catalyst: Bio-oil upgrading and catalytic mechanism. Waste Manag. 2020, 114, 225–233. [Google Scholar] [CrossRef]
- Shrestha, P.; Chun, D.D.; Kang, K.; Simson, A.E.; Klinghoffer, N.B. Role of Metals in Biochar Production and Utilization in Catalytic Applications: A Review. Waste Biomass Valorization 2022, 13, 797–822. [Google Scholar] [CrossRef]
- Liu, Z.; Li, P.; Chang, C.; Wang, X.; Song, J.; Fang, S.; Pang, S. Influence of metal chloride modified biochar on products characteristics from biomass catalytic pyrolysis. Energy 2022, 250, 123776. [Google Scholar] [CrossRef]
- Shao, J.; Yan, R.; Chen, H.; Yang, H.; Lee, D.H. Catalytic effect of metal oxides on pyrolysis of sewage sludge. Fuel Process. Technol. 2010, 91, 1113–1118. [Google Scholar] [CrossRef]
- Cai, R.; Pei, X.; Pan, H.; Wan, K.; Chen, H.; Zhang, Z.; Zhang, Y. Biomass Catalytic Pyrolysis over Zeolite Catalysts with an Emphasis on Porosity and Acidity: A State-of-the-Art Review. Energy Fuels 2020, 34, 11771–11790. [Google Scholar] [CrossRef]
- Csutoras, B.; Miskolczi, N. Thermo-catalytic pyrolysis of sewage sludge and techno-economic analysis: The effect of synthetic zeolites and natural sourced catalysts. Bioresour. Technol. 2024, 400, 130676. [Google Scholar] [CrossRef]
- Valizadeh, S.; Valizadeh, B.; Kang, B.S.; Shim, H.; Park, Y.K. Enhanced generation of aromatic-enriched bio-oil from sewage sludge pyrolysis using zeolite-based catalysts under H2-rich conditions. Energy Convers. Manag. 2024, 311, 118530. [Google Scholar] [CrossRef]
- Czerski, G.; Śpiewak, K.; Makowska, D.; Grycova, B. Study on steam co-gasification of waste tire char and sewage sludge. Energies 2023, 16, 2156. [Google Scholar] [CrossRef]
- Bayat, D.; Sharifian, M.; Khajehpour, H. Comprehensive 4E (energy, exergy, exergoeconomic, and exergoenvironmental) analysis and optimization of wastewater sludge gasification: A case study in a resource-constrained area. Energy 2025, 317, 134633. [Google Scholar] [CrossRef]
- Li, H.; Li, M.; Wang, H.; Tan, M.; Zhang, G.; Huang, Z.; Yuan, X. A review on migration and transformation of nitrogen during sewage sludge thermochemical treatment: Focusing on pyrolysis, gasification and combustion. Fuel Process. Technol. 2023, 240, 107562. [Google Scholar] [CrossRef]
- Quan, L.M.; Kamyab, H.; Yuzir, A.; Ashokkumar, V.; Hosseini, S.E.; Balasubramanian, B.; Kirpichnikova, I. Review of the application of gasification and combustion technology and waste-to-energy technologies in sewage sludge treatment. Fuel 2022, 316, 123199. [Google Scholar] [CrossRef]
- Dai, F.; Zhang, S.; Luo, Y.; Wang, K.; Liu, Y.; Ji, X. Recent progress on hydrogen-rich syngas production from coal gasification. Processes 2023, 11, 1765. [Google Scholar] [CrossRef]
- Tremel, A.; Becherer, D.; Fendt, S.; Gaderer, M.; Spliethoff, H. Performance of entrained flow and fluidised bed biomass gasifiers on different scales. Energy Convers. Manag. 2013, 69, 95–106. [Google Scholar] [CrossRef]
- Kuttin, K.W.; Ding, L.; Yu, G. Numerical and experimental assessment of parametric effect on steam-carbon dioxide co-gasification of sewage sludge and coal in an updraft reactor. Int. J. Hydrogen Energy 2024, 91, 204–218. [Google Scholar] [CrossRef]
- Emami Taba, L.; Irfan, M.F.; Wan Daud, W.A.M.; Chakrabarti, M.H. The effect of temperature on various parameters in coal, biomass and CO-gasification: A review. Renew. Sustain. Energy Rev. 2012, 16, 5584–5596. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, M.; Wang, H.; Kong, J.; Xie, W.; Wang, J.; Chang, L.; Bao, W. Effect of temperature and gasification gas from char on the reactions of volatiles generated from rapid pyrolysis of a low rank coal. Fuel Process. Technol. 2021, 212, 106601. [Google Scholar] [CrossRef]
- Galiwango, E.; Butler, J.; Lotfi, S. A review of catalyst integration in hydrothermal gasification. Fuels 2024, 5, 375–393. [Google Scholar] [CrossRef]
- Chen, Y.; Yi, L.; Li, S.; Yin, J.; Jin, H. Catalytic gasification of sewage sludge in near and supercritical water with different catalysts. Chem. Eng. J. 2020, 388, 124292. [Google Scholar] [CrossRef]
- Kang, B.S.; Farooq, A.; Valizadeh, B.; Lee, D.; Seo, M.W.; Jung, S.C.; Hussain, M.; Kim, Y.M.; Khan, M.A.; Jeon, B.H.; et al. Valorization of sewage sludge via air/steam gasification using activated carbon and biochar as catalysts. Int. J. Hydrogen Energy 2024, 54, 284–293. [Google Scholar] [CrossRef]
- Alper, D.; Babayiğit, E.; Zengin, G.E.; Okutan, H.C.; Sarıoğlan, A. The catalytic influence of low-cost natural minerals on sewage sludge gasification for hydrogen production. Int. J. Hydrogen Energy 2025, in press. [CrossRef]
- Shahbaz, M.; Al-Ansari, T.; Inayat, M.; Sulaiman, S.A.; Parthasarathy, P.; McKay, G. A critical review on the influence of process parameters in catalytic co-gasification: Current performance and challenges for a future prospectus. Renew. Sustain. Energy Rev. 2020, 134, 110382. [Google Scholar] [CrossRef]
- Gabbrielli, R.; Frigo, S.; Bressan, L. Oxy-steam co-gasification of sewage sludge and woody biomass for biomethane production: An experimental and numerical approach. E3S Web Conf. 2021, 238, 01006. [Google Scholar] [CrossRef]
- Yan, J.; Yan, Y.; Lai, J.; Jia, D.; Jiao, Y.; Zhao, X.; Yang, L. Co-gasification of municipal sewage sludge and cotton stalk enhanced by metal-enriched texture dyeing sludge additives for syngas production. Fuel 2023, 341, 127669. [Google Scholar] [CrossRef]
- Zhou, A.; Ma, L. Thermogravimetric analysis on co-gasification characteristics of sludge and straw under CO2 atmosphere. Processes 2023, 11, 1402. [Google Scholar] [CrossRef]
- Rey, J.R.C.; Longo, A.; Rijo, B.; Pedrero, C.M.; Tarelho, L.A.C.; Brito, P.S.D.; Nobre, C. A review of cleaning technologies for biomass-derived syngas. Fuel 2024, 377, 132776. [Google Scholar] [CrossRef]
- Chen, D.; Yin, L.; Wang, H.; He, P. Pyrolysis technologies for municipal solid waste: A review. Waste Manag. 2014, 34, 2466–2486. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).