1. Introduction
Balneotherapy is a health intervention that refers to the use of natural mineral waters, muds, or gases under medical supervision and is conducted in specialized facilities, such as sanatoriums and wellness or spa centers. According to Gutembruner et al., “the use of natural mineral waters, gases and peloids in many countries is called balneotherapy, but other (equivalent) terms exist. Substances used for balneotherapy are medical mineral waters, medical peloids, and natural gases” [
1].
According to Fioravanti et al., “balneotherapy, traditionally involves either immersion in mineral and/or thermal waters from natural springs and/or balneological interventions with natural gases or peloids (mud)" [
2].
The World Health Organization has not provided a specific definition of balneotherapy but has identified it as an effective component of its strategy to combat non-communicable diseases and reduce premature mortality by 30% by 2030 [
3].
In many European countries (France, Italy, Spain, Romania, etc.), balneotherapy is widely accepted and integrated into public health systems and is often reimbursed by national health insurance.
In Romania, the use of natural therapeutic factors refers to a therapeutic intervention performed as a course in a thermal station/resort, using one or more natural therapeutic factors according to procedures methodologically defined in balneal law [
4], the norms for the application of balneal law, and the framework contract with the National Insurance House, which is renewed every year.
Techirghiol Lake is located on the Romanian coast of the Black Sea. The lake’s water is rich in salts (66–86 g/L sodium chloride and sulfates). The mud deposit at the bottom of the lake is organic (9.6 g/L organic substances), strongly hydrated (71.24% content in water), and rich in minerals (28.73 g/L) (
Table 1).
Mud is a complex mixture of organic and inorganic substances with unique biophysical properties and biochemical compositions, making it a distinct natural therapeutic agent [
5,
6].
The topical application of mud and natural mineral water (cold, warm, or hot) induces skin temperature modifications that lead to physiological changes in the dermis and muscles to maintain thermal homeostasis.
The skin responds to biophysical stimuli, such as temperature, pressure, density, and buoyancy, primarily through the peripheral blood circulation [
7]. Biochemical compounds in mud, including hydrogen sulfide, ions, humic acids, and amino acids, penetrate the skin and disseminate within bodily fluids and organs. Although the skin barrier is impermeable, compared with other body membranes, some substances in the mud can cross it via capillarity, absorption, or chemical inter-reactions (trans-epidermal passage), or the ducts of hair follicles, sebaceous glands, and sweat glands (trans-follicular passage) [
8].
Some studies have linked the therapeutic effects of balneotherapy to inorganic components [
9,
10,
11].
The dermis and muscles are well supplied with blood vessels. The peripheral circulation is concerned with the transport of blood, the blood flow distribution, the exchange between blood and tissues, and the storage of blood. Its function is to alter the blood distribution to meet the needs of the tissues to maintain homeostasis. In the dermis, vascularization far exceeds the skin's maximum biological needs, playing a crucial role in thermoregulation [
12,
13].
A direct application (topical application) on the skin increases the tissue temperature and local blood flow. For instance, studies have shown that applying a heating pad at 40 °C to the lower-back region increased the deep-muscle tissue temperature by 5 °C, 3.5 °C, and 2 °C at depths of 19 mm, 28 mm, and 38 mm below the skin surface, respectively [
14].
Similarly, applying heat to the knees increased the popliteal artery's blood flow by 29%, 94%, and 200% with heating pad temperatures of 38 °C, 40 °C, and 43 °C, respectively [
15].
There is a well-established linear relationship between increases in the local temperature (skin and muscle) and corresponding increases in the local blood flow [
16].
Animal model studies under experimental conditions have demonstrated capillary growth and an increased blood vessel density in response to increased blood flow [
17,
18].
This pilot prospective study used classic histological methods to assess the response of skin and muscle tissues to heat stimulation (hyperthermal, thermal neutral, and thermal contrast) with hypersaline water and mud from Techirghiol Lake in real-life conditions, following established therapeutic protocols. The dermis and muscles are involved in the thermoregulatory function via their microvascular structure. We investigated whether 10 days of responses to heat stimulation by the microvascular component in the dermis and muscle could produce evident histological changes under a light microscope. Up to the time of this study, the database search did not reveal any histological studies conducted under these conditions.
2. Materials and Methods
2.1. Materials
- -
Natural therapeutic factors specific to the Romania Techirghiol ecosystem, including mud from the bottom of the lake and salted water of Techirghiol Lake, as described in balneal law [
4].
- -
The facilities for treatment in Techirghiol Balneal and Rehabilitation Sanatorium, Romania, like bathtubs, mud pack beds, the swimming pool, the solarium (a beach on the front side of the lake and its facilities), as described in methodological norms [
11] and applied according to therapeutic protocols.
- -
Laboratory reagents, fixators, solvents, specific dyestuffs, common microscopic blades, counting microscopic blades with Neubauer counting chambers (with a precisely engraved grid of 100 × 100, visible under a microscope), a microtome, a research microscope, equipped with an automatic expometer and video camera, and the image analysis software LUCIA© G.
- -
An IBM computer with the SPSS statistics software, version 23.
- -
The facilities of the emergency unit of the Constanta University Emergency Clinical Hospital.
2.2. Study Design
This study was a prospective pilot study using classic histological methods to assess the response of the dermis and muscle tissues after two weeks of balneotherapy with salted water and mud from Techirghiol Lake.
2.3. Therapeutic Intervention
Mud was applied according to the regulations stipulated by balneal law, the norms of its application, and sanatorium working protocols: outdoors, as a cold mud ointment in the summer season, and indoors, all year long, as a mud pack (hyperthermal mud therapy at 42 °C for 20–30 min) or a mud bath (thermal neutral therapy at 38 °C for 20–30 min) alternatively with a thermal neutral salted water bath (37 °C) for ten or twelve days of staying in a thermal station.
Admission to a balneal course is regulated by the framework contract with the National Insurance House. A referral and medical recommendation containing the diagnosis of the main disease (primary diagnosis) for which the person received the recommendation and the known comorbidities/polypathology (secondary diagnosis) are needed for admission.
2.4. Inclusion Criteria
The study plots included patients who fulfilled the sanatorium’s criteria for admission at the balneal course for chronic rheumatological conditions (osteoarthritis) and post-traumatic conditions after fractures, sprains, and dislocations.
2.5. Exclusion Criteria
The following participants were not included in the study plots:
- -
Those using medications that could impact muscles and microvascular histological aspects, such as vasodilators, muscle relaxants, etc.;
- -
Those with cardiovascular conditions (e.g., recent myocardial infarction or cardiovascular surgery), heart failure, and uncontrolled blood hypertension;
- -
Those with skin conditions (e.g., psoriasis or eczema);
- -
Those with chronic inflammatory conditions (e.g., rheumatoid arthritis, ankylosing spondylitis, lupus, etc.);
- -
Those with neurological conditions (e.g., multiple sclerosis or hemiparesis after a recent stroke);
- -
Those with muscular conditions (e.g., Duchenne muscular dystrophy or juvenile dermatomyositis);
- -
Those with chronic failure of different organs (e.g., the kidneys or lungs);
- -
Those with any clinical and/or biological signs of inflammation.
2.6. Cropping, Sample Numbering, and Fixation
Biopsy collection was conducted in the belly of the deltoid muscle, twenty-four hours after the end of the balneal cure. The cropping was performed by a surgeon in the surgery room at the emergency unit of the Constanta University Clinical Hospital, respecting all the requirements of a small surgical intervention/biopsy. The tegument and muscle fragment were taken via a perpendicular incision on the tegument. The cropping was performed under contact anesthesia (spray), and the incision was closed with a stitch and covered with a compress. First, the skin was cropped up to the muscle fascia, and then the muscle fragment. The tissues had dimensions of 1.5/0.5/0.5 cm. Immediately after cropping, the fragments were introduced into a test glass tube with a bung that contained the fixative solution. The volumetric report between the piece for fixing and the fixative solution was 1/10–1/15 and 10–15 times greater than the fragment, to prevent the tissues' alteration. Each test glass was noted and indexed according to the type of application and the number given to each patient.
2.7. Processing Assays for Histological Study
For the microtome sectioning, after fixation in aldehydes, the samples were dehydrated in ethanol, clarified with xylene, and embedded in paraffin. The paraffin blocks were sectioned with a microtome, and the fragments were transferred and glued to glass blades. The serial sections were colored with routine (hematoxylin–eosin) and specific (Masson’s trichrome and GS trichrome) staining [
19,
20]. Then, they were examined with a Nikon E–600 microscope and acquired with a Sony video camera. For histometric measurements, we used a 40x lens that was calibrated using a Carl Zeiss Jena standard blade. Eight to ten blades were obtained for each patient, of which two or three were dedicated to histometry using counting blades. A blade was taken from the blade collection of each patient to analyze its histologic general aspect, and another to count elements zigzagging the section (similar to a leucogram). The label of each blade contained the blade number, the patient’s identification number, and the type of intervention.
Although the histopathologist was not blinded to the treatment allocation, the evaluation relied on reproducible morphological criteria and histometric counts. Given the exploratory nature of this first histological study in balneotherapy and the lack of prior reference standards, the subjective influence on interpretation was minimized.
2.8. Statistical Analysis
The statistical analysis was performed using the IBM SPSS statistics software, version 23.
Nonparametric tests were used for comparisons between the categories of the group variable when the numerical variables did not meet the condition of a normal distribution (p < 0.05 in the Shapiro–Wilk test).
Kruskal–Wallis tests and the median were used for hypothesis testing. The significance level was set at α = 0.05 and α’ = 0.05/4 = 0.0125 (adjustment was available for post hoc tests).
The Mann–Whitney U test was used to evaluate the difference in the number of blood vessels between the dermis and muscles.
The data are presented as follows:
- -
Means ± standard deviations (SDs) for continuous variables in the case of symmetric distributions;
- -
Medians and interquartile ranges (IQR: P75-P25) for continuous variables in the case of skewed distributions;
- -
Percentages for categorical variables.
2.9. Participants
A current physician allocated the patients within the study groups after the complete clinical examination and the evaluation of co-morbidities and daily chronic medication, following the sanatorium's protocol and the inclusion criteria. The indications and contraindications of mud application are provided in Balneal Law 343/31st May 2002, as amended in the national therapeutic guidelines for balneotherapy. A small portion of the patients opted only for balneotherapy, which they considered very valuable, and did not want other procedures (e.g., massage, electrotherapy, or kinesiotherapy). In this regard, the risk of bias was almost nonexistent.
4. Discussion
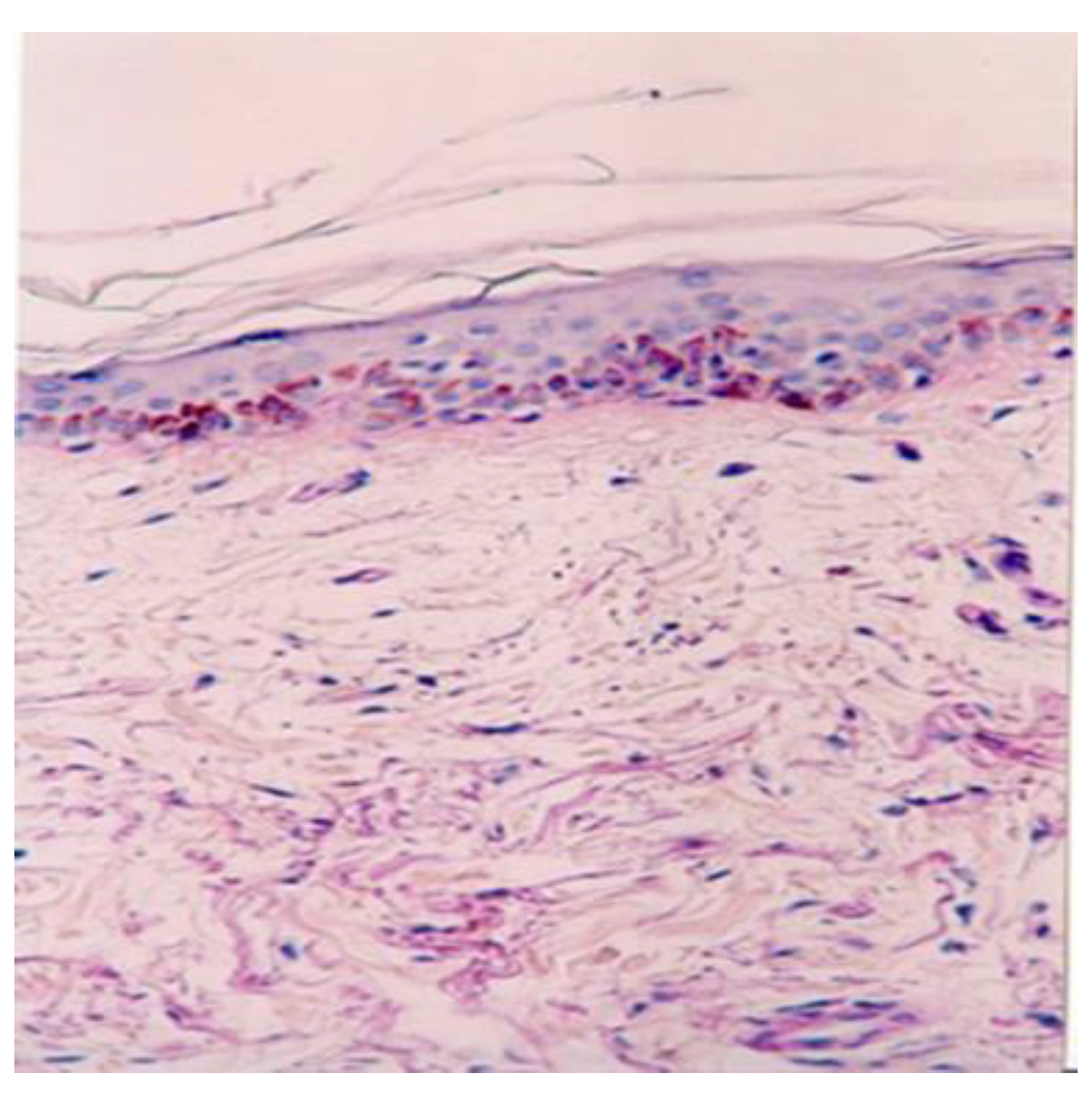
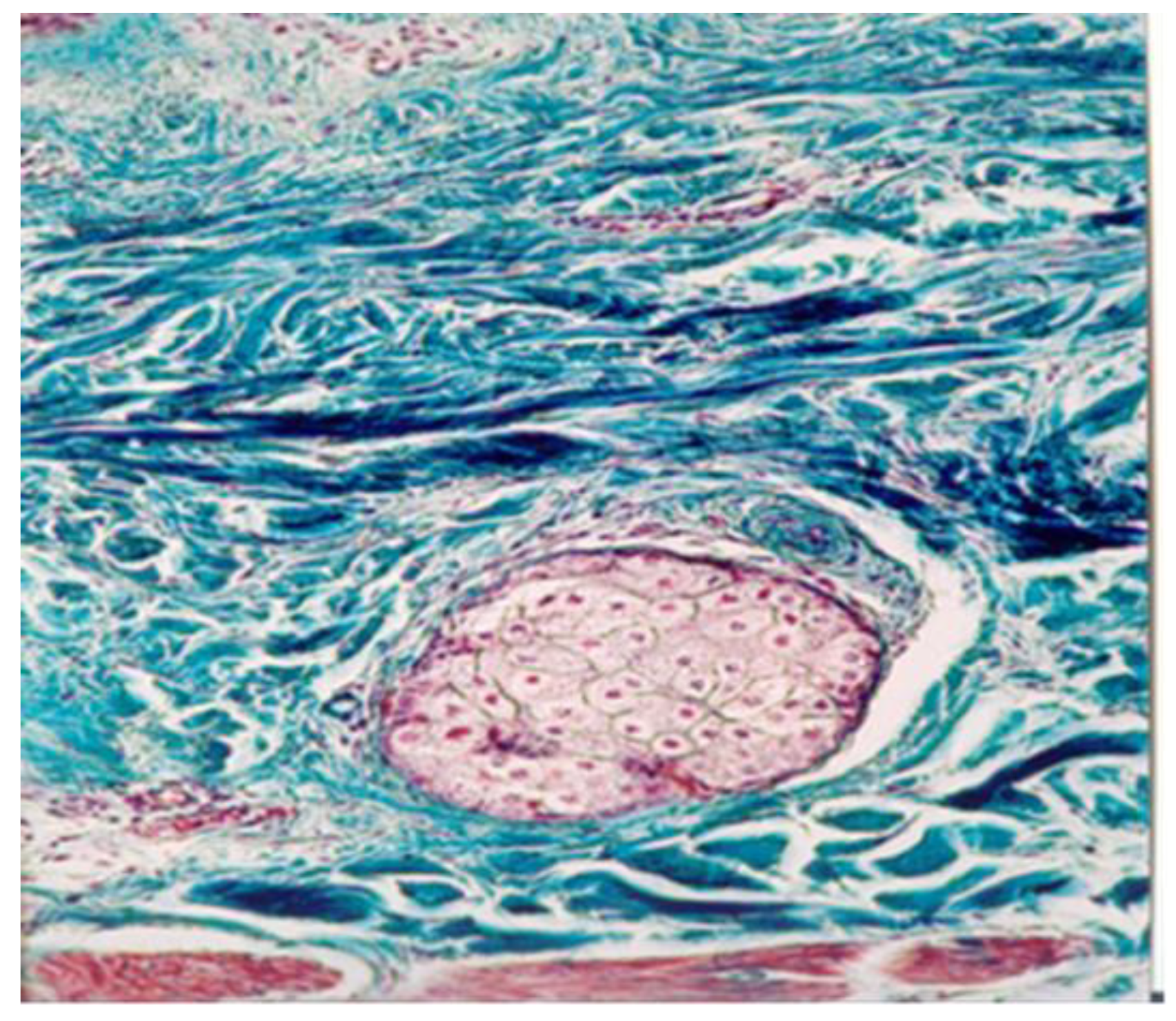
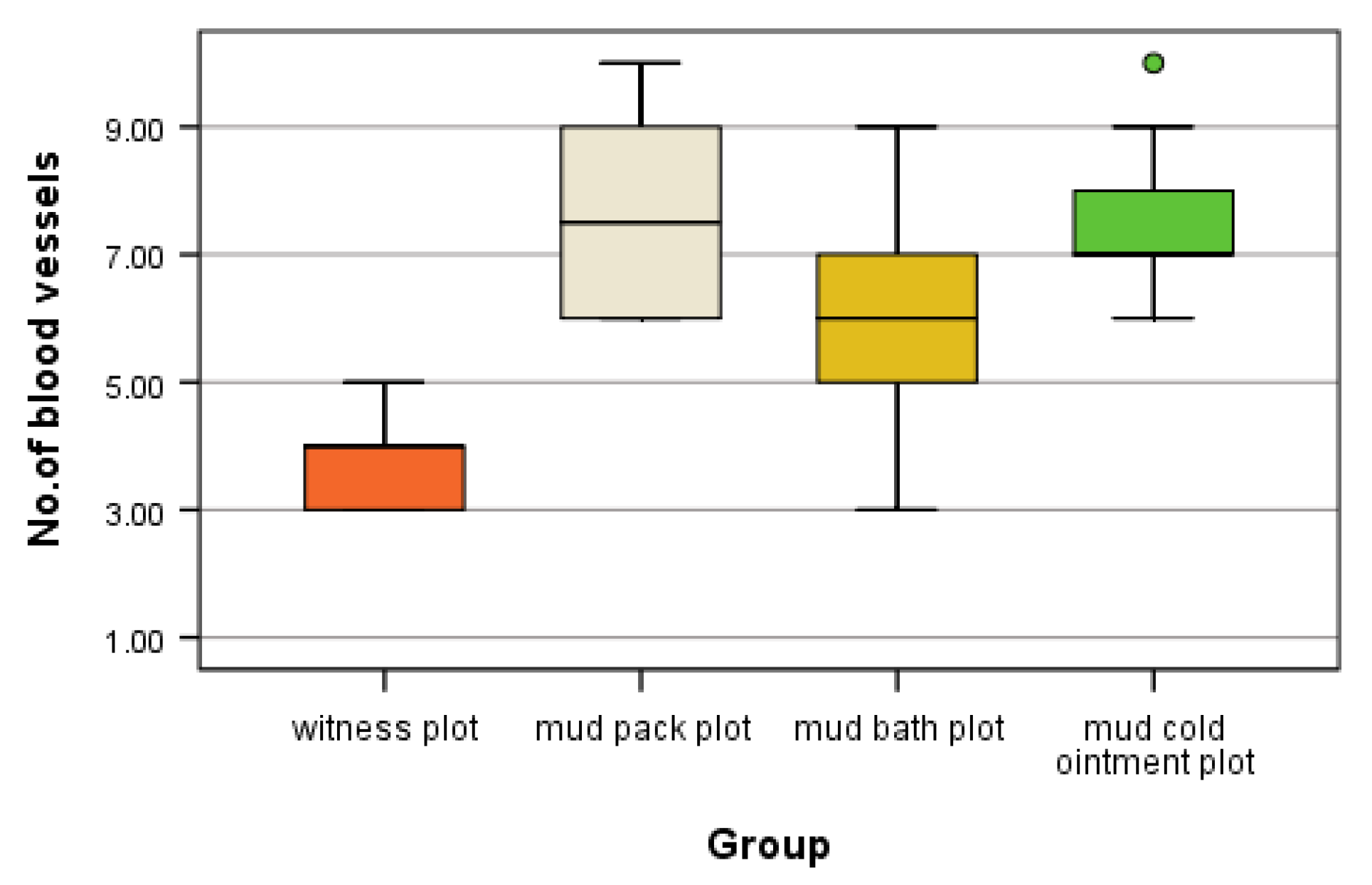
Angiogenesis—the process of new capillary growth from existing vasculature—is enhanced in conditions involving increased local and central temperatures and the abolishment of sweating in a humid environment, as observed in mud pack therapy. Under optic microscopy, they appear as an increased number of thin-walled capillaries and poorly formed, disorganized, and leaky vessels.
We hypothesized that an increased vascular network is necessary to store and dissipate heat effectively to maintain thermal homeostasis, including the opening of capillary shunts and the formation of new blood vessels via angiogenesis, which is stimulated by the exogenous hydrogen sulfide present in mud.
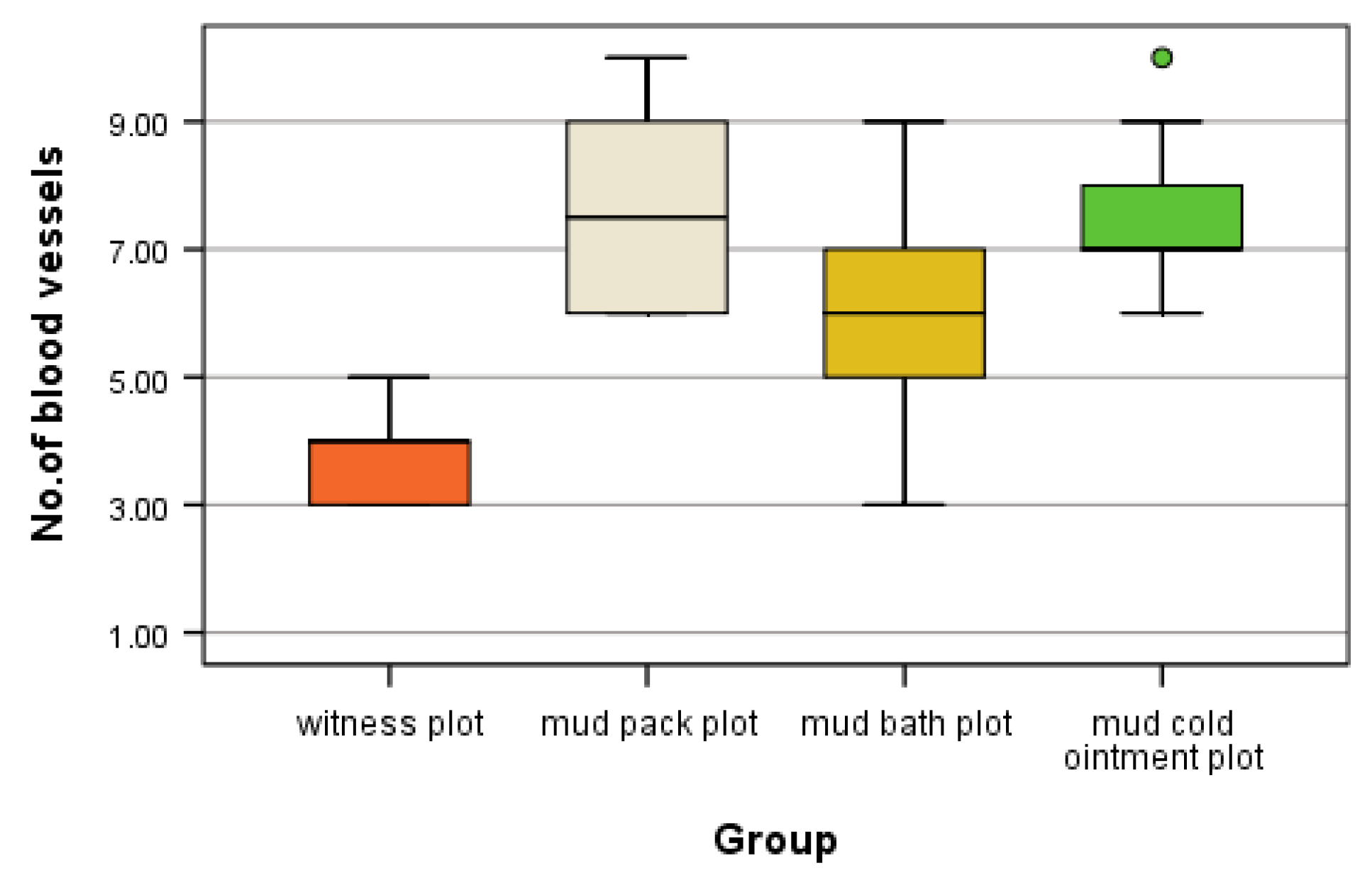
This study provides novel insights into the microvascular changes induced by different mud therapy applications in both dermal and muscle tissues. Our findings demonstrate statistically significant increases in angiogenesis following various mud treatments, with distinct patterns observed between different application methods and tissue types.
4.1. Key Findings and Interpretation
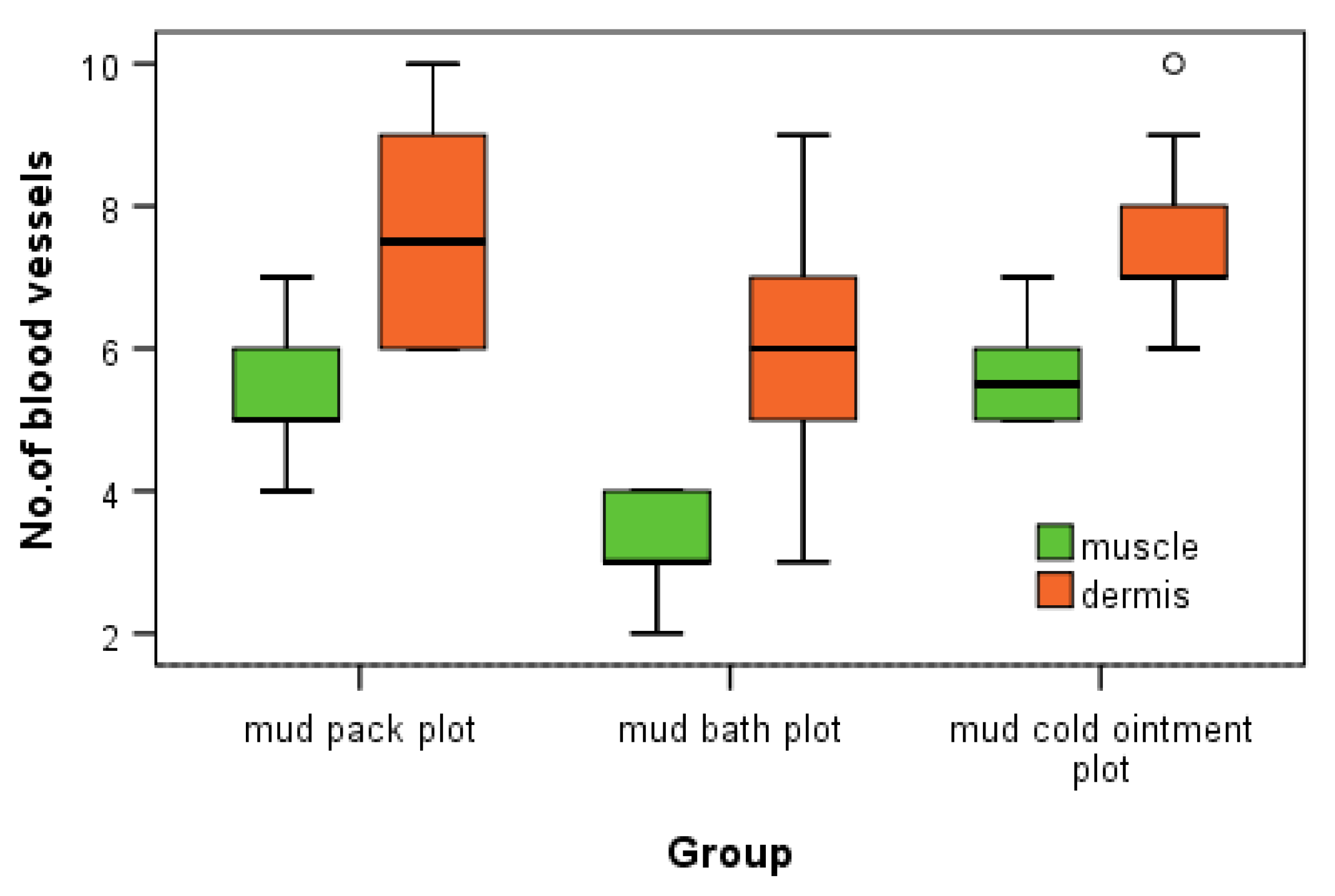
In the dermis, we observed the highest number of angiogenesis vessels in the mud pack and cold mud ointment groups. The vasodilation and increased skin blood flow, along with sweating, play crucial roles in heat dissipation. However, the application of mud over the entire skin surface (excluding the head) abolishes sweat evaporation and disrupts thermoregulation. During exposure to cold temperatures, skin vasoconstriction reduces the heat loss from the body to prevent hypothermia. Any alteration in the control of the skin's blood flow can significantly impair the body’s ability to maintain a normal temperature [
20].
Pericytes—which are located around capillaries, post-capillary venules, and angiogenesis vessels—perform crucial functions for vascular homeostasis, including regulating blood flow, vessel formation, and controlling vascular growth and function. The molecular mechanisms underlying these processes have yet to be fully elucidated [
21]. In the analyzed biopsy samples, pericytes participated in the occurrence, growth, and regulation of new vessels. This increase in dermal angiogenesis may be attributed to the thermal effects of mud therapy, particularly in the case of hyperthermal mud packs, which create a humid environment that abolishes sweating and potentially stimulates vascular adaptation.
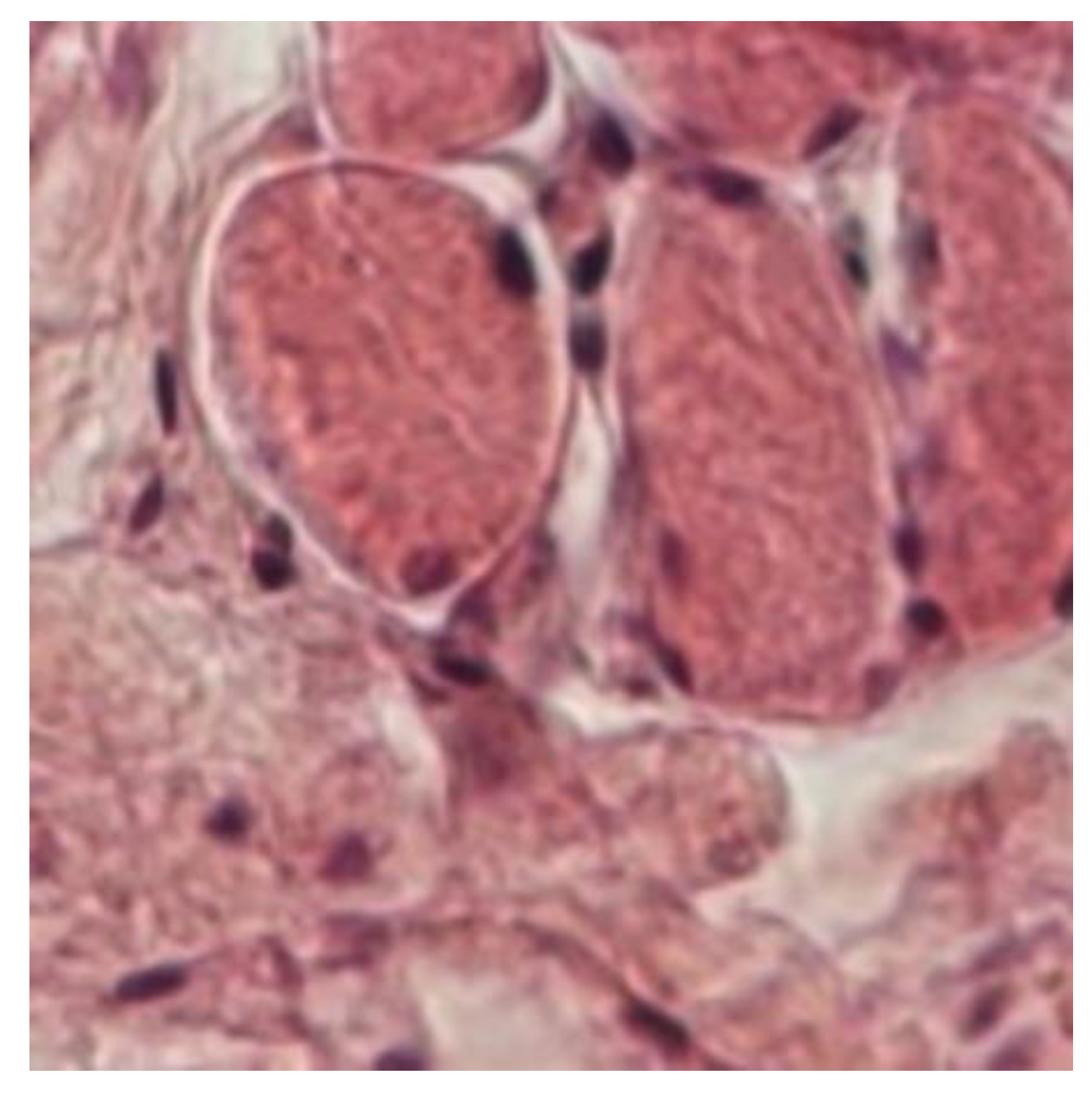
In muscle tissue, the cold mud ointment group exhibited the highest mean number of angiogenesis vessels, closely followed by the mud pack group. The skeletal muscle is highly vascularized and exhibits adaptive responses in microvessels based on the muscle demand. This finding suggests that the rapid alternations between heating and cooling in cold mud ointment therapy may be particularly effective in stimulating angiogenesis in deeper tissues. The contrast in temperature could promote swift transitions from vasodilation to vasoconstriction, potentially triggering adaptive responses in the muscular microvasculature.
The mud bath group, while showing increased angiogenesis compared with the control group, demonstrated lower values than the mud pack and cold mud ointment groups in both the dermis and muscles. This difference may be due to the thermal neutral nature of mud baths, which exert less extreme thermal stress on the tissues.
The increased number of angiogenesis vessels in both the dermis and muscles following mud therapy demonstrated statistical significance, with variations influenced by several factors: the thermal regimen (significant for hyperthermal and contrast thermal therapy), the tissue depth and temperature (significant in the dermis for the mud pack treatment and in the muscles for the cold mud ointment), and the abolishment of sweating in a humid environment (significant for the mud pack treatment). The number of angiogenesis vessels in the dermis after a mud bath may be influenced by the abolishment of sweating in a humid environment and the participation of hydrogen sulfide. Heat plays a critical role in opening capillary shunts and increasing the capillary caliber.
This indicates that mud pack and mud cold ointment therapies elicit distinct microvascular responses compared with thermal neutral applications and each other.
Thermotherapy enhances local peripheral tissue perfusion via thermosensitive mechanisms that increase microvascular blood flow, correlating linearly with the muscle temperature. This cascade includes an increased muscle temperature, vasodilation, and enhanced oxygen consumption, which can trigger metabolically induced vasodilation [
16]. The regulation of the skeletal muscle blood flow involves various compounds sourced from different cells, ensuring vital oxygen delivery to contracting skeletal muscles [
21].
Hydrogen sulfide—which is present in the biochemical composition of Techirghiol mud—has been recognized as a third gas signaling transmitter alongside nitric oxide and carbon monoxide, despite its historical classification as a toxic gas. Endogenous hydrogen sulfide modulates numerous physiological and pathological processes, such as inflammation, oxidative stress, and cell apoptosis, crucial for vascular function [
22,
23].
Exogenous hydrogen sulfide from mineral/thermal waters and mud (including Techirghiol mud) can be absorbed through the skin and integrated into biological systems. Research in Romania has demonstrated that an increased mud temperature enhances the skin's permeability to soluble substances from the liquid phase of mud, facilitating the passage of sulfur compounds into the skin [
24,
25]. Hydrogen sulfide has been shown to exert proangiogenic effects and improve regional blood flow [
26]. Recent studies have highlighted its vasculoprotective properties, influencing various cellular pathways involved in endothelial cell proliferation, migration, apoptosis, oxidative stress, and inflammation within the vasculature. Despite its recognized benefits, the detailed molecular mechanisms underlying these pathways require further exploration [
27].
4.2. Comparison with Existing Literature
Our findings align with previous research demonstrating the beneficial effects of balneotherapy on vascular function. Some authors reported on the vasodilatory effects of sulfurous mineral waters, which are similar to the mud used in our study [
7]. The increased angiogenesis observed in our study may be partially attributed to the presence of hydrogen sulfide in the mud, which has been shown to have proangiogenic effects.
The differential responses observed between the dermis and muscle tissues in our study contribute to the growing body of evidence suggesting that balneotherapy can induce tissue-specific adaptations. This is consistent with other works, which reviewed the effects of hydrotherapy on various body systems and noted differential responses based on treatment parameters and target tissues [
28].
4.3. Strengths and Limitations
A key strength of this study was its use of human subjects in a real-world therapeutic setting, providing clinically relevant insights into the effects of mud therapy. The comparison of multiple mud application methods and the histological analysis of both the dermis and muscle tissues offer a comprehensive view of the microvascular changes induced by balneotherapy.
However, our study had several limitations. The sample size was relatively small, which may limit the generalizability of our findings. Additionally, we did not conduct a long-term follow-up, so the duration of the observed angiogenic effects remains unknown. The absence of temperature measurements during treatments was another limitation, as precise thermal data could have provided further insights into the mechanisms underlying the observed changes.
4.4. Implications and Future Directions
Our findings have potential implications for clinical practice, suggesting that different mud therapy applications may be tailored to target specific tissues. For instance, mud packs might be preferentially used when targeting dermal angiogenesis, while a cold mud ointment could be more effective for muscular adaptations.
Future research should focus on elucidating the long-term effects of repeated balneotherapy interventions and investigating the molecular mechanisms underlying the observed angiogenesis. The potential role of hydrogen sulfide and other mud components in mediating these effects warrants further investigation. Additionally, studies combining balneotherapy with other treatment modalities could explore potential synergistic effects.
In cold mud ointment therapy, rapid alternations between local and central temperature changes via heating and cooling promote swift transitions from vasodilation to vasoconstriction. This mechanism ensures an adequate muscle blood flow by locally adjusting the number of open capillaries and facilitating non-shivering thermoregulation.
The presence of pericytes in the dermis and muscle tissue at twenty-four hours after completing the treatment suggests that morphological changes continue even after the cessation of treatment.
5. Conclusions
This study suggested that different mud therapy applications can induce significant and tissue-specific angiogenic responses in both the dermis and muscles. These findings contribute to our understanding of the physiological effects of balneotherapy and may support the development of more targeted therapeutic protocols. Further research is needed to fully elucidate the mechanisms underlying these effects and to optimize their clinical application.
This histological approach provided a visual representation of morphological changes induced in the dermis and muscles following a two-week balneotherapy regimen with hypersaline water and mud, helping to elucidate the empirical aspects surrounding balneology and balneotherapy.
Our study demonstrated that natural therapeutic factors are pivotal in eliciting histological modifications via functional stimulation. Repeated topical heat treatments under various thermal regimens (hyperthermal, thermal neutral, and thermal contrast), with options for sweating or its abolishment, and the penetration of biochemical compounds (such as hydrogen sulfide and sulfur species) from hypersaline water and mud during a balneotherapy intervention led to statistically significant histological modifications in both the dermis and muscle tissues, persisting for at least twenty-four hours post-treatment.