Abstract
Access to safe, clean drinking water is a critical challenge across many resource-constrained settings, especially in developing economies. Large-scale water treatment technologies are often available in urban areas, whereas such centralized systems are unavailable in rural and remote areas due to high infrastructure costs, rugged terrains, and maintenance challenges. To address this challenge, point-of-use (PoU) water treatment systems can fill this critical gap. This study critically evaluates the role low-cost PoU water treatment solutions play as a promising alternative to address water access and quality aspects in remote rural areas. The study explores the present state of global water sources, the challenges of water scarcity and pollution, and the limitations of existing large-scale treatment technologies. It highlights the motivation behind PoU systems and provides an in-depth analysis of various low-cost technologies, including operational principles, performance efficiency, and economic viability. Embedded in this study is a concise evaluation of the sustainability of these solutions in addressing water access and quality challenges in resource-limited regions. Finally, the study proposes solutions and perspectives on improving PoU systems and scale-up of the systems for large-scale applications to facilitate increased access to clean and safe water.
1. Introduction
The provision of safe drinking water is a fundamental human right, yet more than two billion people worldwide still lack reliable access to it, particularly in low-income regions often with limited resources and infrastructure [1]. Water is not only essential for human health and survival but also plays a crucial role in economic development and poverty reduction. Sustainable Development Goal (SDG) 6 underscores the need for access to clean water and sanitation for all. However, the quality and availability of water are increasingly compromised by pollution, overuse, and changing climatic patterns [2]. In regions with scarce resources, the challenges of providing clean water are further aggravated by financial constraints, poor infrastructure, and geographical isolation [3]. These challenges, alongside the ever-growing demand for water, have led to a global water crisis, placing millions at risk of consuming unsafe water. Water contamination is aggravated by discharge of industrial effluent, agricultural runoff, and poor sanitation practices. These contaminate the water sources with harmful pathogens, chemicals, and heavy metals [4]. This widespread contamination of water supplies is linked to serious health risks, including waterborne diseases such as diarrhea, cholera, typhoid, and dysentery, leading to high morbidity and mortality, particularly in children under five years of age [5].
To address water quality challenges, large-scale centralized water treatment plants are widely used in urban areas. These systems have proven effective at delivering clean water to populations with robust infrastructure and financial resources [6]. However, such systems are often impractical in remote or rural regions, which constitute resource-limited settings. These areas have limited resources and therefore cannot afford the high costs of installation, operation, and maintenance, skilled plant operators, and reliable energy supplies. Additionally, the infrastructure required to distribute treated water from central plants to individual households is often lacking, leaving vulnerable populations dependent on untreated or contaminated water sources [7].
To address these limitations, point-of-use (PoU) water treatment technologies have gained considerable attention as a potential solution for improving local water quality at the household or community level [8,9]. Unlike large-scale systems, PoU technologies focus on treating water immediately before consumption, ensuring that contaminants are removed just before use. These systems are useful in remote areas where centralized treatment facilities are limited or non-existent. PoU systems include a range of low-cost technologies, such as ceramic filters, biosand filters, solar disinfection (SODIS), and chlorine tablets, all of which aim to provide safe drinking water with minimal infrastructure requirements [10]. PoU systems are primarily advantageous due to their affordability, simplicity, and adaptability to various environmental and economic settings. Most of these technologies are designed to be user-friendly, requiring little to no technical expertise to operate and maintain [11,12]. They are also typically low maintenance, often requiring only periodic cleaning or simple filter replacements. Moreover, PoU systems can be rapidly deployed in emergency situations, such as during natural disasters or disease outbreaks, where immediate access to clean water is critical [13]. Despite their promise, however, the effectiveness and sustainability of low-cost PoU technologies are still a subject of ongoing research and debate. The consistent removal of contaminants and potential environmental impacts are the key areas of concern regarding these systems [14]. A few studies have assessed the cost and environmental sustainability of four PoU water disinfection technologies through techno-economic and life cycle analyses. One such study reported that chlorination and silver-nanoparticle ceramic filters were the most affordable and sustainable options for rural and low-income settings, while UV-based systems were costly and energy-intensive [14]. Further research is necessary to determine possible ways to enhance the performance of PoU technologies in field applications [15].
Although several studies have demonstrated the performance of PoU technologies, comprehensive reviews of the various low-cost PoU water purification systems are lacking. The present study endeavored to fill this gap. Firstly, the study provides an overview of global water sources, scarcity, and pollution issues. Then it critically evaluates the current large-scale treatment technologies and their limitations. It thereafter delves into the different types of low-cost PoU technologies, examining their principles of operation, performance efficiency, economic feasibility, and sustainability. Finally, the study proposes new approaches and considers future perspectives on improving PoU systems and scaling them up to significantly expand access to potable water. As such, the application of PoU makes a crucial contribution to the attainment of SDG 6 (clean water and sanitation) and SDG 3 (good health and wellbeing), among other global development goals. Relevant data for the past 10 years (2015 to 2025) on Scopus search engines were obtained for this review. A comprehensive search was conducted to assess relevant information for this review. During the search, appropriate keywords and phrases such as “point of use”, “household water treatment”, “adsorption”, “water filtration”, “limited water resource”, and “water disinfection” were used. The study did not consider sources of water (surface water, groundwater, rainwater, etc.) in the inclusion criteria. The selected PoU systems are discussed in Section 4.
2. Water Sources, Scarcity, and Pollution
2.1. Global Water Sources
Water, the most abundant resource on Earth, is characterized by a highly unequal distribution. Freshwater, which is essential for drinking, agriculture, and industry, accounts for only about 3% of the Earth’s total water, and the remaining amount is mainly saline [16]. Groundwater constitutes 30.1% of the total available freshwater globally [17] and is mainly used in areas where surface water is either scarce or contaminated. High dependence on groundwater has resulted in challenges regarding the over-extraction and depletion of these vital resources, particularly in regions affected by recurrent droughts [18,19]. Surface water accounts for 10% of the available freshwater worldwide and is crucial for supporting agriculture, urban water supply, and industrial activities [18]. However, in most developing economies, surface water sources are often affected by pollution and overuse, further straining their sustainability [20]. Atmospheric water constitutes only 0.04% of the Earth’s total water and plays an essential role in the hydrological cycle by replenishing surface and groundwater through precipitation [21]. However, arid and semi-arid lands constitute nearly 41% of the world’s total land size and face variable rainfall patterns, which often lead to prolonged droughts and water scarcity [22]. Therefore, developing countries in sub-Saharan Africa and parts of Asia face significant challenges in managing their water resources, exacerbated by climate change and increasing population. The uneven distribution of freshwater resources, coupled with growing demand, highlights the urgent need for sustainable water management practices to ensure reliable access to clean water.
2.2. Water Scarcity
Water scarcity is a critical issue in many developing countries. In many parts of the world, many people still lack clean drinking water, particularly in arid and semi-arid regions, with limited water sources and inadequate infrastructure [23], leading to physical and economic water scarcity [24]. Emile and co-workers reported physical and economic water scarcity in developing regions such as sub-Saharan Africa, South Asia, and parts of the Middle East [25]. This is attributed to erratic rainfall patterns, and prolonged droughts, exacerbated by climate change, have strained already limited water resources, making physical scarcity a persistent issue. Economic water scarcity is evident in rural and underserved communities, where water infrastructure is either outdated or nonexistent, leading to unreliable access to clean water. In areas that lack surface water, over-reliance on groundwater has led to its depletion in many areas, adding further stress to her water systems [23,26]. Addressing water affordability in developing countries is essential to combat the broader issue of water scarcity. This requires a combination of improved water management practices, sustainable infrastructure development, and climate adaptation strategies to enable provision of equitable and reliable water access.
2.3. Water Pollution
Water pollution remains a critical challenge in many developing nations due to factors such as rapid urbanization, industrialization, and insufficient waste management systems. In many developing countries in sub-Saharan Africa and South-East Asia, poor infrastructure and weak regulatory enforcement have led to widespread pollution of water bodies, severely impacting public health and the environment [27,28]. Microbial contamination from untreated sewage is a major concern in developing nations, contributing to waterborne diseases like cholera, diarrhea, and typhoid [1,29]. Agricultural runoff, laden with fertilizers and pesticides, results in eutrophication, which depletes oxygen in water bodies and damages aquatic ecosystems [30]. Additionally, industrial pollutants such as heavy metals and toxic chemicals are often discharged into rivers and lakes without proper treatment, further degrading water quality [31]. Improper storage practices further exacerbate water contamination. Clean water, after treatment, is often re-contaminated during storage in open or unclean containers, exposing it to pathogens and environmental contaminants [32]. Poor hygiene during water handling and biofilm formation inside storage containers also contribute to this problem [33].
PoU water treatment systems offer a practical solution to addressing water contamination, especially in resource-limited settings. These systems treat water at the point of use, making them an effective way to remove pollutants from water already compromised by storage issues or environmental exposure. The flexibility of PoU systems allows them to address specific contaminants depending on the technology used, including microbial pathogens, chemical pollutants, and heavy metals. PoU systems are cost-effective, user-friendly, and scalable, making them suitable for developing countries that lack centralized water treatment infrastructure. Their effectiveness in improving water quality at the household level, even in areas with significant pollution, makes them a vital part of the solution to water contamination in these regions.
3. Water Treatment Technologies
3.1. Existing Large-Scale Technologies and Their Limitations
Large-scale water treatment technologies are essential for providing safe drinking water, especially in urban areas where the population density is high, and distribution networks are well established. These systems are effective but face several limitations, particularly in resource-limited settings. The centralized water treatment system involves processes such as screening, coagulation and flocculation, sedimentation/clarification, advanced treatment (reverse osmosis, ultrafiltration, adsorption), disinfection, storage, and distribution. The limitations of large-scale water treatment technologies are presented in Table 1.

Table 1.
Key unit operations in centralized water treatment systems and limitations.
3.2. The Motivation for Point-of-Use Systems
Point-of-use (PoU) water treatment systems are becoming increasingly popular, particularly in underdeveloped countries where access to clean and safe water remains a significant challenge. These systems treat water at the point where it is consumed, such as in homes or small communities, rather than through large, centralized water treatment plants. Several factors motivate the adoption of PoU systems, and they are discussed below.
- (a)
- Addressing Limited Access to Centralized Water Treatment
In many regions, particularly rural and peri-urban areas of developing countries, centralized water treatment plants are often absent or unreliable due to infrastructure challenges, high costs, and limited government capacity. PoU systems provide a viable solution by allowing households to treat their own water directly from local sources such as rivers, lakes, or wells [43]. This helps ensure that even in areas far from urban centers, people can have access to treated drinking water.
- (b)
- Affordability and Low Infrastructure Requirements
Unlike large-scale water treatment facilities, PoU systems typically require minimal infrastructure and can be implemented at a lower cost. Technologies such as ceramic filters, solar disinfection (SODIS), and biosand filters are relatively affordable and require little maintenance, making them accessible to low-income households [44]. This cost-effectiveness is crucial in resource-limited settings, where budgets for water infrastructure development are often constrained.
- (c)
- Flexibility and Adaptability
PoU systems offer flexibility in their application, allowing them to fit specific needs and available resources of a community. For example, regions with high solar exposure may benefit from solar-powered water disinfection systems, while areas with abundant clay materials may prefer ceramic filters. This adaptability ensures that communities can select the most appropriate technology based on their local environmental and socio-economic conditions [45].
- (d)
- Reduction of Waterborne Diseases
In regions with poor sanitation and water quality, the prevalence of waterborne diseases like cholera, diarrhea, and typhoid remains high. PoU systems effectively reduce microbial contaminants at the point of consumption, helping to lower the incidence of these diseases. Studies have shown that PoU technologies, such as chlorine dispensers and ceramic filters, can reduce diarrheal diseases by up to 35% in low-income areas [46].
- (e)
- Minimizing Water Contamination during Storage
One of the key challenges in water distribution, particularly in developing countries, is contamination during storage and handling. Even if water is initially treated, it can become contaminated by pathogens or chemicals during transportation or in storage containers [47]. PoU systems help mitigate this by allowing water to be treated just before consumption, ensuring its quality is maintained until it reaches the end user.
- (f)
- Sustainability and Environmentally Friendly Solutions
Many PoU systems, such as biosand filters and solar disinfection, rely on natural processes and require little to no external energy, making them environmentally friendly and sustainable. These technologies can be used for extended periods with minimal maintenance or energy costs, unlike large-scale water treatment plants, which often rely on electricity and chemicals [48]. This sustainability aspect makes PoU systems particularly appealing for communities seeking long-term, eco-friendly solutions to their water challenges.
- (g)
- Empowering Local Communities
Implementing PoU systems empowers local communities to take ownership of their water supply and decrease their reliance on centralized authorities. This can be particularly important in areas where government resources are stretched thin or where centralized systems are prone to inefficiencies and failures. By managing their own water treatment solutions, communities can ensure that their specific needs are met, and that water quality is maintained at a household level [49,50].
4. Low-Cost Point-of-Use Water Treatment Systems
4.1. Types of PoU Technologies, Principle of Operation, and Performance Efficiency
Point-of-use technologies treat water at the exact place where it is used or consumed. On-site drinking water treatment systems are installed near water outlets, showers, and dispensers so that water can be purified just before being used for cooking, bathing, or drinking. The systems are designed for easy operation and require minimal maintenance. Key accessible technologies include filtration, disinfection, flocculation, coagulation, reverse osmosis, and ion exchange units; these are discussed further. Common PoU systems and their applications are illustrated in Figure 1.
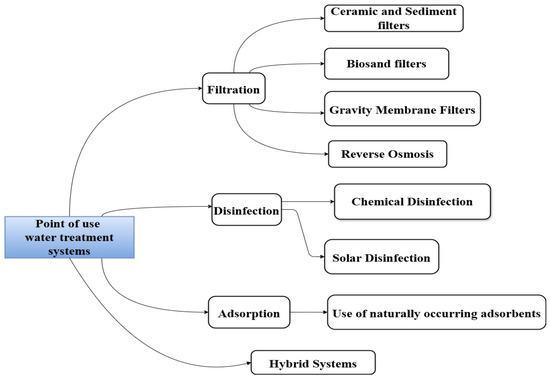
Figure 1.
Categories of point-of-use water treatment systems.
4.1.1. Filtration Systems
Filtration is a physical process that removes suspended solids, colloids, and pathogens by size exclusion, whereby particles larger than the pore size of the filter are retained within the system while the fluid passes through. Filtration systems can function in two different modes of operation: dead-end filtration and cross-flow filtration.
Dead-end filtration is the most common mode used in gravity-driven membrane (GDM) filters. In this process, the feed stream flows perpendicularly to the membrane surface, passing through it as filtrate/permeate (Figure 2). Particles and aggregates that are blocked by the membrane accumulate to form a filter cake. Dead-end filtration is widely used in point-of-use water treatment systems, including household water purifiers and community-scale ultrafiltration units, due to its simplicity, low energy requirement, and effectiveness in removing pathogens.
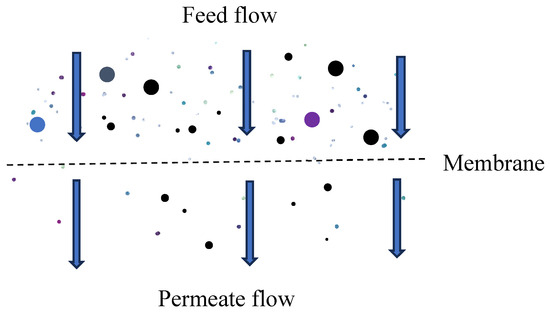
Figure 2.
Illustration of dead-end filtration.
In cross-flow filtration, the feed solution flows parallel to the membrane surface (Figure 3). This allows a portion of it to pass through as purified water (permeate) while the remaining water carries away retained particles in a separate stream (concentrate). This continuous flow reduces the buildup of contaminants on the membrane surface, thereby enhancing long-term performance.
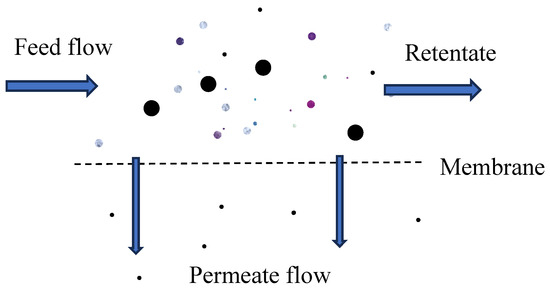
Figure 3.
Illustration of cross-flow filtration.
There are several PoU filtration technologies, which include the following:
- (a)
- Ceramic filters and sediment cartridges
Ceramic filtration is one of the water filtration methods that utilizes a porous ceramic material to trap and remove contaminants from water. The ceramic filters have tiny pores that trap and remove bacteria, sediment, and other microorganisms from water. Water passes through the ceramic filter by gravity. Similarly, a sediment filter is another form of physical filtration that removes suspended solids from water, such as insoluble impurities, thereby reducing water turbidity. Ceramic pot filters produced from locally available materials have been used to reduce pathogenic organisms such as E. coli from water. The study concluded that high-flow ceramic pot filters exhibit high filtration rates and significant log reduction values (LRV) for E. coli [51]. Ceramic disc filters coated with nano-TiO2 showed effectiveness in the removal of E. coli from water [52]. Meanwhile, biochar-clay composite ceramic filter (BCF), fabricated from a mixture of clay and sawdust, have demonstrated effective removal of total hardness (42.5%), total dissolved solids (45.8%), and turbidity (67%) compared to clay-made ceramic filter (14.8, 17.6, and 56%), respectively. Ceramic filters made from recycled paper fiber (green fiber) and those made from combustible material (starch and rice husk) depicted higher water flow rates than the flow rates of regular ceramic filters currently in use [53]. Figure 4 illustrates ceramic and sediment cartridge filters.
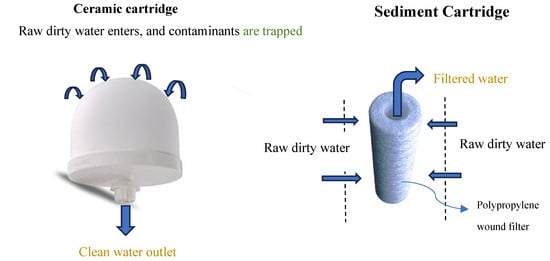
Figure 4.
Illustration of ceramic filter and sediment cartridge filter.
Bacterial log reduction values (LRVs) are used to quantify bacterial removal efficiency, to determine the performance of ceramic filters. LRV is calculated using the equation below:
where and (CFU/mL) represent the E. coli concentrations in the untreated and treated water, respectively.
- (b)
- Biosand Filters
A biosand filter (BSF) is a modern take on traditional slow sand filters, designed to remove pathogens and suspended solids from water through biological and physical processes that take place in a sand column covered with a biofilm. Biosand filters improved by the addition of iron-sand mixture have been found to have the highest removal efficiency for contaminants. Household slow sand filters present average turbidity removal efficiencies of 75% or greater, often reaching removals above 90% while bacteria removal is an average LRV between 1 and 2 [11,54,55]. Although the BSF has been widely used and studied, its effectiveness can be improved by, (i) integration of nanotechnology such as incorporating silver nanoparticles into the sand layer to enhance the disinfection capabilities; and (ii) the use of alternative porous materials instead of sand that have large surface area to volume ration resulting in improved pollutant removal and reduced filter clogging.
- (c)
- Gravity-based membrane systems
Gravity-based membrane filtration is a decentralized water treatment technology designed to harness the force of gravity to filter water through a filter membrane. The central component of the GDM filter is a membrane with a pore size of 20–40 nm. Water is filtered through the membrane at a very low pressure (10–150 mbar) and remarkably, this system does not require backflushing, cleaning, or electricity to allow sustainable operation without clogging [56]. Low-pressure membrane systems like microfiltration and ultrafiltration have been employed as PoU systems in areas that lack electricity or where electricity costs are prohibitive (Figure 5). For such areas, gravity-driven membrane systems are a suitable alternative for sustainable water purification [57,58]. The effectiveness of gravity-driven membrane (GDM) filters is typically evaluated based on flux and removal efficiency. The key formula used for flux calculation is:
where J represents the permeate rate (L m−2 h−1), V is the permeate volume (L), A is the filtration area (m2), and t is the duration of permeate collection (h).
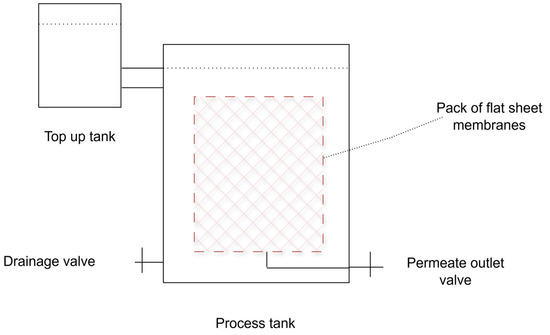
Figure 5.
Schematic representation of the cross-section of a gravity based flat sheet membrane system, adapted from [59].
Permeability is often used to compare membrane performance under different transmembrane pressure (TMP). The permeate flow through the membranes can be described by Darcy’s law, which explains that the water flow (flux) through the membrane is directly proportional to the applied pressure.
where k is the permeability constant containing structural factors such as porosity and viscosity, and ΔP is the applied transmembrane pressure (TMP).
For gravity-driven systems, TMP is determined by hydrostatic pressure:
where ρ is water density (kg/m3;), is gravitational acceleration (m/s2), and h is water column height (m).
Contaminant removal efficiency is calculated using Equation (5)
where and denote the initial and final concentration of the contaminant (mg L−1).
- (d)
- Reverse Osmosis
A point-of-use reverse osmosis (RO) system is a water filtration unit that is connected to a single fixture (such as under the kitchen sink) and uses the process of reverse osmosis to remove contaminants from the water supplied to that fixture. The system employs reverse osmosis where pressure forces water through a semi-permeable membrane, resulting in a stream of treated water, called permeate, and a stream of rejected water called concentrate or brine. Moreira and co-workers studied the use of recycled RO membranes as the alternative for groundwater treatment at the POU [60] and reported that after the membranes were cleaned by chemical oxidation (NaOCl), permeability increased from 4.5 to 50.4 L.m−2.h−1.bar−1 and salinity rejection decreased from 99.4 to 11.6%. The performance of a reverse osmosis (RO) membrane is evaluated using several key parameters, including flux, salt rejection, and recovery rate. The flux or permeation rate of the membrane can be determined using Equation (2). Salt rejection indicates how effectively the membrane removes dissolved salts as shown in Equation (6).
where SR represents the salt rejection (%), is the feed water concentration (ppm or mg/L), and is the permeate concentration (ppm or mg/L)
The recovery rate measures the percentage of feed water that has been converted into permeate and is given by Equation (7).
where RR denotes the recovery rate (%), represents the volume of permeate (L), and represents the volume of feed water (L).
4.1.2. Adsorption Systems
Adsorption is one of the methods that can be used to remove a wide range of dissolved pollutants, such as toxic metal ions, pesticides, disinfection by-products, and industrial solvents from water. The contaminants, such as dissolved organic and inorganic substances, accumulate on the surface of a solid (adsorbent), forming a molecular or atomic film (adsorbate). The adsorption can either be physical or chemical adsorption, also known as physisorption and chemisorption, depending on the forces of attraction between the adsorbent and the adsorbate. Adsorbents such as biosorbents [61], activated carbon and biochar [62,63], clays and minerals [64], polymers, nanoparticles, and composites [65] with high adsorption uptake capacity have been successfully employed to remove hazardous pollutants from wastewater. Low-cost adsorbents such as rice husk, ash, and activated carbon, and fruit peels such as banana peels, lemon peels, and pomegranate peels, have shown ability to capture new pollutants such as pharmaceuticals and personal care products (PPCPs), pesticides, dyes, and heavy metals [66]. The adsorption capacity () on the adsorbent can be calculated as,
where V is the solution volume (L), and represent initial and equilibrium concentration of the adsorbate (mg L−1), and m is the adsorbent mass (g).
4.1.3. Disinfection Systems
Disinfection is the process of inactivation, elimination, or killing of pathogenic microorganisms in water to make it safe for human consumption. Processes of water disinfection include chemical disinfection and solar disinfection by UV and are discussed further.
- (a)
- Chemical disinfection
Chemical disinfection is the process of eliminating pathogens such as viruses and parasites in water to make it portable. The process involves adding a chemical to water to react with organic matter and harmful microorganisms. Chemicals that are mostly used for chemical disinfection are chlorine, chlorine dioxide, chloramines, and ozone. Chlorination of water is achieved at the household level using chlorine-releasing tablets such as Waterguard and Aquatabs tablets. These tablets release free chlorine in water, hence destroying bacteria and viruses. A key benefit of free chlorine is that it disinfects the water and maintains chlorine residuals that will remain in the water as it travels through the distribution system. Ozone disinfection, on the other hand, occurs by damaging the cell membrane and protoplasm of microorganisms, which impedes cell re-activation in bacteria, coliform, viruses, and protozoa. Ozone is best used as a primary disinfectant prior to chlorination [67].
- (b)
- Solar disinfection
Solar disinfection (SoDis) is a low-cost and efficacious disinfection technology that involves exposure of water to UV radiation and thermal heating from sunlight to inactivate microbes in contaminated water. The procedure typically entails the containment of water within polyethylene terephthalate (PET) bottles and exposure to sunlight for 6–48 h. Solar disinfection can be enhanced using visible light active photocatalysts to enhance the disinfection efficiency [68]. Numerous studies have proven the effectiveness of SoDiS in improving water quality. SoDiS application shows potential for the remediation of contaminants of emerging concern (CECs) such as pharmaceutically active compounds, personal care products, and pesticides in groundwater matrices, with removal efficiencies higher than 90% [15]. The study also confirmed that the addition of TiO2 as a photocatalyst significantly enhances the performance of SoDis by up to three orders of magnitude because of increased adsorption and photocatalytic degradation.
4.1.4. Hybrid Water Treatment Systems
Hybrid water treatment involves the combination of two or more water treatment systems, for instance, pairing physical filtration methods, such as ceramic filters, with chemical disinfection like chlorination or UV treatment to ensure microbial safety while maintaining overall system efficiency. Electrocoagulation systems have been used in tandem with UV, O3, and H2O2 to remove various organic and inorganic pollutants and to ensure that the effluent is of high quality prior to discharge into the aquatic environment. By combining electrochemical and advanced oxidation technologies, a hybrid system has been developed. Hybrid systems such as ozone-assisted electrocoagulation, for instance, peroxi-electrocoagulation photo-electrocoagulation, and ozone-photo-Fenton, were applied to improve the quality of wastewater [69]. Figure 6 depicts hybrid water treatment systems and the advantages of their use, and Table 2 provides a summary of PoU systems, mechanisms of operation, strengths, and limitations.
Figure 6.
Benefits of hybrid water treatment systems.

Table 2.
Comparison of the point of use of water treatment technologies.
4.2. Economic Viability and Sustainability of the Systems
In low-income nations, PoU water treatment methods serve as a practical solution in areas lacking piped water networks or where existing water treatment and delivery systems are inadequate [74,75]. Cost-effective inorganic substances, such as mineral-based or silicate waste materials, show potential as suitable alternatives in producing affordable ceramic membranes, thus notably lowering material expenses. Compared to centralized water treatment setups, ceramic pot filters have been proven to be more economical and environmentally friendly [76].
Disinfection solutions or tablets that release active agents represent one of the most efficient and low-cost mobile options for water purification [77]. Chlorination delivers a lasting disinfectant barrier, crucial for preserving water safety during household storage, where recontamination risks exist. A key issue highlighted in multiple studies is the formation of various disinfection by-products (DBPs) during chlorination, which may pose health hazards. Despite chlorine’s affordability, it requires constant replenishment, leading to recurring household expenses. Moreover, user preferences influence chlorination adoption, as some individuals may favor more convenient methods that avoid frequent purchases [78].
Biochar, made from pyrolysis of agricultural and forest residues, is economically viable as waste biomass is converted into carbon-rich filtration media with a potential for water decontamination [79]. The use of biochar economically at scale requires that conversion systems such as gasifiers are located near the contaminated water sources to reduce transportation costs. Additionally, its low cost, high adsorption capacity, environmental benefits, and ability to be regenerated and reused make it sustainable and potentially suitable for continuous large-scale water treatment.
Gravity-driven filtration avoids the need for pumps or chemical additives, prolongs membrane lifespan, and minimizes energy consumption for filtration, ensuring continued operation during periods of limited resources [80]. Implementing gravity-based filtration at centralized plants is both technically and economically viable under multiple conditions, dependent on energy pricing, stable flow rates, and membrane durability. In such settings, traditional UF is replaced with gravity filtration, thereby boosting the reliability of centralized drinking water systems.
Adsorption is widely regarded as one of the most effective pollutant removal techniques for wastewater due to its adaptable nature, easy system design, reuse of materials, regeneration potential, low expense, and environmentally friendly attributes, making it both well-researched and extensively implemented [81]. These adsorbents can also be derived from eco-friendly sources, offering additional sustainability advantages. Table 3 highlights the efficiency levels of various PoU water treatment technologies.

Table 3.
Performance efficiencies of different point-of-use water treatment systems.
4.3. Social Acceptance of PoU Technologies
The social acceptance of point-of-use (PoU) water treatment technologies in rural communities varies significantly depending on factors such as awareness, cultural beliefs, affordability, and trust in the technology. Despite PoU systems such as chlorine disinfection becoming widely accepted in many developing regions due to their ability to provide safe drinking water without reliance on centralized infrastructure, adoption rates are currently inconsistent. Studies indicate that community engagement, empowerment, and a sense of ownership enhance the acceptance and sustainability of these PoU technologies [86]. Another recent study done in Nigeria highlighted the need for education on safe water treatment including chlorination and its benefits [87]. Table 4 provides a summary of the social acceptance of PoU systems.

Table 4.
Social acceptance of the different POU systems.
5. Outlook and Future Perspectives
Point-of-use (PoU) water treatment technologies offer strong potential in tackling global water quality issues, particularly in isolated and underserved regions. With the growing need for safe water, technological progress, and creative solutions are anticipated to be key in delivering clean drinking water. The enhancements outlined below can significantly improve both the efficiency and practicality of PoU systems.
5.1. Metal–Organic Frameworks and Nanomaterials
The creation and utilization of customized materials with enhanced properties, such as nanomaterials and metal–organic frameworks (MOFs), show great potential. These materials exhibit high efficiency in eliminating pollutants like heavy metals and harmful microorganisms. Their outstanding adsorption abilities and photocatalytic features contribute to the development of more efficient filtration technologies. The MOFs possess remarkable traits, including large surface area, adjustable pore dimensions, and high porosity, which make them suitable for water purification; however, their broad application is hindered by costly production and complex synthesis processes [95]. Future investigations should emphasize affordable production methods, eco-friendly ligands, and scalable approaches such as mechanochemical and microwave-assisted synthesis. Enhancing automation and integrating hybrid processes could improve production efficiency, positioning MOFs as key components in clean water solutions. At present, nanotechnology applications in water treatment are mostly confined to lab or pilot-scale due to financial constraints, technical challenges, high setup costs, and potential risks to health and the environment. Another major hurdle is the insufficient understanding of the underlying mechanisms of nanomaterials used in water purification.
5.2. Enhancing the Efficiency and Affordability of Ceramic Water Filters
Reducing costs and enhancing treatment effectiveness are key future trends for expanding ceramic membrane applications in water treatment. It is essential to design not only newer, more affordable ceramic membrane variants that offer comparable or improved performance, but also to establish more process strategies that boost efficiency, supporting broader implementation. Creating ceramic filters that maintain steady water flow while ensuring ongoing contaminant removal remains highly desirable yet challenging. Fouling is an unavoidable issue in ceramic water filters (CWFs) after prolonged usage, primarily due to sediment accumulation and/or particle blockage. Extended performance evaluation of CWFs made with refined formulations should be carried out and analyzed under actual environmental and regional conditions. As water pollutants grow more intricate, additional studies are necessary to grasp their behavior, the formation of by-products, associated toxicity, and risk analysis during point-of-use (POU) treatment, along with potential improvements to enhance the field-level performance of these technologies.
5.3. Integrating Advanced Technologies for Enhanced PoU Systems
Incorporation of cutting-edge technologies such as nanofiltration, improved oxidation processes, and membrane bioreactors is predicted to boost the effectiveness and efficiency of PoU systems. This equipment offers improved removal of contaminants such as pathogens, heavy metals, and emerging impurities. For instance, advanced oxidation processes (AOPs) have shown great potential in degrading persistent organic pollutants and pathogens, making them a promising option for PoU applications. The AOPs are promising technologies for treating water containing pollutants with extreme chemical stability and/or low biodegradability.
5.4. Advancing PoU Systems Through Integrated and Sustainable Approaches
The integration of PoU systems to combat physical, chemical, and biological treatment pollutants is a growing trend. For instance, systems integrating adsorption and photocatalysis demonstrate improved removal rates for a variety of pollutants, including emerging contaminants like pharmaceuticals and perfluoroalkyl and polyfluoroalkyl substances (PFAs). More studies are needed exploring natural coagulants and bio-based adsorbents as low-cost, biodegradable alternatives. These materials contribute to reducing environmental footprints while maintaining performance.
5.5. Integration of IoT-Enabled Devices in Water Treatment
Embracing modern technologies such as IoT-enabled devices for real-time water quality monitoring and data analytics are being integrated into PoU systems. This includes the use of sensors and smart devices to monitor and manage water quality and water resources in real time. This facilitates timely maintenance and troubleshooting, user confidence, and enhances operational efficiency. The use of IoT devices enhances smart water management and leads to efficient water use.
5.6. Application of Point-of-Use Technologies for Emerging Contaminants
The future of point-of-use (PoU) water treatment is likely to shift considerably as emerging contaminants such as pharmaceuticals, microplastics, and nanoparticles become more rampant in water sources. Existing PoU technologies have primarily focused on removing common pathogens and chemical impurities, but the increasing detection of these complex contaminants is pushing researchers towards more advanced point-of-use water treatment solutions such as magnetic nanoparticle filtration, electrochemical disinfection and solar photocatalytic systems.
6. Conclusions
PoU water treatment systems are essential in the global effort to provide potable water. This review has examined various technologies used in PoU systems, including biosand filters, ultraviolet (UV) purifiers, ceramic filters, reverse osmosis (RO) systems, and activated carbon filters, along with emerging nanofiltration and advanced oxidation processes. These systems have shown significant effectiveness in contaminant removal. However, there is a need to integrate different PoU systems, particularly through hybrid approaches, to maximize elimination efficiencies for a wide range of contaminants, reduce energy consumption, promote sustainable water management, and enhance water recovery rates. The accessibility and affordability of PoU systems are central to their extensive implementation, especially in undeveloped regions in which centralized and advanced water treatment facilities and technology may be limited. Innovations in low-cost materials and scalable manufacturing processes are vital to bridge this gap. Future research should focus on integrating PoU systems with smart technologies that provide real-time monitoring and improved management, ensuring consistent water quality and system performance. Sustainability is another critical aspect of future PoU systems. Utilizing environmentally friendly materials, energy-cost-effective designs, and renewable energy sources minimizes environmental pollution and supports SDG, which focuses on clean water and sanitation, as well as SDG 13, which deals with climate Action. Finally, major advancements have been made in the enhancement and deployment of PoU water treatment systems; ongoing research, innovation, and collaboration are necessary to solve the evolving limitations of water contamination and scarcity. By leveraging PoU technologies, we can make sure that safe and potable water is accessible to all, thereby enhancing public health and quality of life worldwide.
Author Contributions
D.C.S.: Data curation, visualization, writing—original draft preparation; C.A.M.: Conceptualization, writing—reviewing and editing, supervision, visualization; M.N.C.: Validation, writing—reviewing and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data is available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PoU | Point of use |
| RO | Reverse osmosis |
| DBP | Disinfection by-products |
| UV | Ultraviolet |
References
- WHO. Global Strategy on Digital Health 2020–2025; WHO: Geneva, Switzerland, 2021; Available online: https://iris.who.int/handle/10665/344249 (accessed on 16 February 2025).
- UNICEF. For Every Child, Reim.Agine; UNICEF Annual Report, 2019; UNICEF: New York, NY, USA, 2020. [Google Scholar]
- Bose, D.; Bhattacharya, R.; Kaur, T.; Banerjee, R.; Bhatia, T.; Ray, A.; Batra, B.; Mondal, A.; Ghosh, P.; Mondal, S. Overcoming water, sanitation, and hygiene challenges in critical regions of the global community. Water-Energy Nexus 2024, 7, 277–296. [Google Scholar] [CrossRef]
- Abanyie, S.K.; Apea, O.B.; Abagale, S.A.; Amuah, E.E.Y.; Sunkari, E.D. Sources and factors influencing groundwater quality and associated health implications: A review. Emerg. Contam. 2023, 9, 100207. [Google Scholar] [CrossRef]
- Mutono, N.; Wright, J.A.; Mutembei, H.; Muema, J.; Thomas, M.L.; Mutunga, M.; Thumbi, S.M. The nexus between improved water supply and water-borne diseases in urban areas in Africa: A scoping review. AAS Open Res. 2021, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Shemer, H.; Wald, S.; Semiat, R. Challenges and Solutions for Global Water Scarcity. Membranes 2023, 13, 612. [Google Scholar] [CrossRef] [PubMed]
- Lawrencia, D.; Maniam, G.; Chuah, L.H.; Poh, P.E. Critical review of household water treatment in Southeast Asian countries. Wiley Interdiscip. Rev. Water 2023, 10, 1–24. [Google Scholar] [CrossRef]
- WHO; UNICEF. Progress on Drinking Water, Sanitation and Hygiene: 2017 Update and SDG Baseline; WHO: Geneva, Switzerland, 2017; p. 34. Available online: https://data.unicef.org/resources/progress-drinking-water-sanitation-hygiene-2017-update-sdg-baselines/ (accessed on 16 February 2025).
- Hill, C.L.; McCain, K.; Nyathi, M.E.; Edokpayi, J.N.; Kahler, D.M.; Operario, D.J.; Taylor, D.D.J.; Wright, N.C.; Smith, J.A.; Guerrant, R.L.; et al. Impact of low-cost point-of-use water treatment technologies on enteric infections and growth among children in limpopo, South Africa. Am. J. Trop. Med. Hyg. 2020, 103, 1405–1415. [Google Scholar] [CrossRef]
- Chaukura, N.; Moyo, W.; Kajau, T.A.; Muleja, A.A.; Mamba, B.B.; Nkambule, T.T. Low-cost ceramic filtration for point-of-use water treatment in low-income countries. Water Secur. 2023, 20, 100145. [Google Scholar] [CrossRef]
- Freitas, B.L.S.; Terin, U.C.; Fava, N.M.N.; Maciel, P.M.F.; Garcia, L.A.T.; Medeiros, R.C.; Oliveira, M.; Fernandez-Ibañez, P.; Byrne, J.A.; Sabogal-Paz, L.P. A critical overview of household slow sand filters for water treatment. Water Res. 2022, 208, 117870. [Google Scholar] [CrossRef]
- Patil, R.; Ahmad, D.; Balkundae, P.; Kausley, S.; Malhotra, C. Development of low cost point-of-use (POU) interventions for instant decontamination of drinking water in developing countries. J. Water Process Eng. 2020, 37, 101435. [Google Scholar] [CrossRef]
- Lindmark, M.; Cherukumilli, K.; Crider, Y.S.; Marcenac, P.; Lozier, M.; Voth-Gaeddert, L.; Lantagne, D.S.; Mihelcic, J.R.; Zhang, Q.M.; Just, C.; et al. Passive In-Line Chlorination for Drinking Water Disinfection: A Critical Review. Environ. Sci. Technol. 2022, 56, 9164–9181. [Google Scholar] [CrossRef]
- Elijah, B.C.; Ahmad, A.; Li, Y.; Plazas-Tuttle, J.; Rowles, L.S. Assessing the Relative Sustainability of Point-of-Use Water Disinfection Technologies for Off-Grid Communities. ACS Environ. Au 2024, 4, 248–259. [Google Scholar] [CrossRef]
- K’oreje, K.; Okoth, M.; Van Langenhove, H.; Demeestere, K. Occurrence and point-of-use treatment of contaminants of emerging concern in groundwater of the Nzoia River basin, Kenya. Environ. Pollut. 2022, 297, 118725. [Google Scholar] [CrossRef] [PubMed]
- Musie, W.; Gonfa, G. Fresh water resource, scarcity, water salinity challenges and possible remedies: A review. Heliyon 2023, 9, e18685. [Google Scholar] [CrossRef]
- Algailani, E.; Hayder, G. Assessment and spatial mapping of water quality parameters using QGIS: Creating a dynamic map. Water Qual. Res. J. 2025, 60, 109–123. [Google Scholar] [CrossRef]
- Mishra, R.K. Fresh Water availability and It’s Global challenge. J. Mar. Sci. Res. 2023, 2, 1–3. [Google Scholar] [CrossRef]
- Innocent, O.N.; James, K.; Susan, M.; Salome, G. Groundwater pollution and climate change vulnerability in Kenya: A review. Int. J. Water Resour. Environ. Eng. 2024, 16, 17–31. [Google Scholar] [CrossRef]
- Gaye, C.B.; Tindimugaya, C. Review: Challenges and opportunities for sustainable groundwater management in Africa. Hydrogeol. J. 2019, 27, 1099–1110. [Google Scholar] [CrossRef]
- Reichle, D.E. The Global Carbon Cycle and the Biosphere; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar] [CrossRef]
- Morante-Carballo, F.; Montalván-Burbano, N.; Quiñonez-Barzola, X.; Jaya-Montalvo, M.; Carrión-Mero, P. What Do We Know about Water Scarcity in Semi-Arid Zones? A Global Analysis and Research Trends. Water 2022, 14, 2685. [Google Scholar] [CrossRef]
- Mulwa, F.; Li, Z.; Fangninou, F.F. Water Scarcity in Kenya: Current Status, Challenges and Future Solutions. OALib 2021, 8, 96. [Google Scholar] [CrossRef]
- Gleick, P.H.; Cooley, H. Freshwater Scarcity. Annu. Rev. Environ. Resour. 2021, 46, 319–348. [Google Scholar] [CrossRef]
- Emile, R.; Clammer, J.R.; Jayaswal, P.; Sharma, P. Addressing water scarcity in developing country contexts: A socio-cultural approach. Humanit. Soc. Sci. Commun. 2022, 9, 144. [Google Scholar] [CrossRef]
- Climate Ventures. 2022. Available online: https://www.climateventures.co/?utm_campaign=newsletter_novembro&utm_medium=email&utm_source=RD+Station (accessed on 14 May 2025).
- Omohwovo, E.J. Wastewater Management in Africa: Challenges and Recommendations. Environ. Health Insights 2024, 18, 11786302241289681. [Google Scholar] [CrossRef] [PubMed]
- Ngasala, T.M.; Masten, S.J.; Gasteyer, S.P. System-wide approaches to mitigate environmental and health impacts of water contamination. Water Policy 2022, 24, 192–211. [Google Scholar] [CrossRef]
- Kitole, F.A.; Ojo, T.O.; Emenike, C.U.; Khumalo, N.Z.; Elhindi, K.M.; Kassem, H.S. The Impact of Poor Waste Management on Public Health Initiatives in Shanty Towns in Tanzania. Sustainability 2024, 16, 10873. [Google Scholar] [CrossRef]
- Dhanker, R.; Khatana, K.; Verma, K.; Singh, A.; Heena; Kumar, R.; Mohamed, H.I. An integrated approach of algae-bacteria mediated treatment of industries generated wastewater: Optimal recycling of water and safe way of resource recovery. Biocatal. Agric. Biotechnol. 2023, 54, 102936. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, M.; Lan, J.; Huang, Y.; Zhang, J.; Huang, S.; Yang, Y.; Ru, J. Water Quality Degradation Due to Heavy Metal Contamination: Health Impacts and Eco-Friendly Approaches for Heavy Metal Remediation. Toxics 2023, 11, 828. [Google Scholar] [CrossRef]
- Luvhimbi, N.; Tshitangano, T.G.; Mabunda, J.T.; Olaniyi, F.C.; Edokpayi, J.N. Water quality assessment and evaluation of human health risk of drinking water from source to point of use at Thulamela municipality, Limpopo Province. Sci. Rep. 2022, 12, 6059. [Google Scholar] [CrossRef]
- Whitley, L.; Hutchings, P.; Cooper, S.; Parker, A.; Kebede, A.; Joseph, S.; Butterworth, J.; Van Koppen, B.; Mulejaa, A. A framework for targeting water, sanitation and hygiene interventions in pastoralist populations in the Afar region of Ethiopia. Int. J. Hyg. Environ. Health 2019, 222, 1133–1144. [Google Scholar] [CrossRef]
- Tahraoui, H.; Toumi, S.; Boudoukhani, M.; Touzout, N.; Sid, A.N.E.H.; Amrane, A.; Belhadj, A.E.; Hadjadj, M.; Laichi, Y.; Aboumustapha, M.; et al. Evaluating the Effectiveness of Coagulation–Flocculation Treatment Using Aluminum Sulfate on a Polluted Surface Water Source: A Year-Long Study. Water 2024, 16, 400. [Google Scholar] [CrossRef]
- Aragaw, T.A.; Bogale, F.M. Role of coagulation/flocculation as a pretreatment option to reduce colloidal/bio-colloidal fouling in tertiary filtration of textile wastewater: A review and future outlooks. Front. Environ. Sci. 2023, 11, 1142227. [Google Scholar] [CrossRef]
- Kim, J.; Park, K.; Yang, D.; Hong, S. A comprehensive review of energy consumption of seawater reverse osmosis desalination plants. Appl. Energy 2019, 254, 113652. [Google Scholar] [CrossRef]
- Matin, A.; Laoui, T.; Falath, W.; Farooque, M. Fouling control in reverse osmosis for water desalination & reuse: Current practices & emerging environment-friendly technologies. Sci. Total Environ. 2021, 765, 142721. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Kim, J.; Yang, D.R.; Hong, S. Towards a low-energy seawater reverse osmosis desalination plant: A review and theoretical analysis for future directions. J. Memb. Sci. 2020, 595, 117607. [Google Scholar] [CrossRef]
- Mecha, A.C. Applications of Reverse and Forward Osmosis Processes in Wastewater Treatment: Evaluation of Membrane Fouling; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Shoshaa, R.; Ashfaq, M.Y.; Al-Ghouti, M.A. Recent developments in ultrafiltration membrane technology for the removal of potentially toxic elements, and enhanced antifouling performance: A review. Environ. Technol. Innov. 2023, 31, 103162. [Google Scholar] [CrossRef]
- Hossen, A.; Ahmed, F.; Saha, S.S.; Mondal, M.I.H. Advantages of ozone disinfection method for water purification over chlorine disinfection. Nat. Resour. Conserv. Res. 2023, 6, 2090. [Google Scholar] [CrossRef]
- Srinivasan, N.R.; Kamaraj, M.; Prabhu, S.V. Strategies and Limitations of Water Treatment Methods for Point-of-Use Application BT—Strategies and Tools for Pollutant Mitigation: Avenues to a Cleaner Environment; Aravind, J., Kamaraj, M., Prashanthi Devi, M., Rajakumar, S., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 117–133. [Google Scholar] [CrossRef]
- Odwori, E.O. Assessment of Point of Use Household Water Treatment Technologies in Nzoia River Basin. Int. Res. J. Adv. Eng. Sci. 2019, 4, 478–486. [Google Scholar]
- Erhuanga, E.; Banda, M.M.; Kiakubu, D.; Kashim, I.B.; Ogunjobi, B.; Jurji, Z.; Ayoola, I.; Soboyejo, W. Potential of ceramic and biosand water filters as low-cost point-of-use water treatment options for household use in nigeria. J. Water Sanit. Hyg. Dev. 2021, 11, 126–140. [Google Scholar] [CrossRef]
- Tyagi, R.D.; Pandey, A.; Drogui, P.; Yadav, B.; Pilli, S.; Wong, J.W.C. Decentralized Sanitation and Water Treatment; CRC Press: Boca Raton, FL, USA, 2024. [Google Scholar] [CrossRef]
- Verhougstraete, M.; Reynolds, K.A.; Pearce-Walker, J.; Gerba, C. Cost-benefit analysis of point-of-use devices for health risks reduction from pathogens in drinking water. J. Water Health 2020, 18, 968–982. [Google Scholar] [CrossRef]
- Pichel, N.; Vivar, M.; Fuentes, M. The problem of drinking water access: A review of disinfection technologies with an emphasis on solar treatment methods. Chemosphere 2019, 218, 1014–1030. [Google Scholar] [CrossRef]
- Bhojwani, S.; Topolski, K.; Mukherjee, R.; Sengupta, D.; El-Halwagi, M.M. Technology review and data analysis for cost assessment of water treatment systems. Sci. Total Environ. 2019, 651, 2749–2761. [Google Scholar] [CrossRef]
- Lantagne, D.; Saltori, R.; Shaylor, E.; String, G.; Wise, T.; Quick, R.; Ramos, M. Comment on “Adoption of Point-of-Use Chlorination for Household Drinking Water Treatment: A Systematic Review”. Environ. Health Perspect. 2023, 131, 016001. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, S.; Camacho-Galván, M.N.; Castelán-Martínez, H.E.; Galdos-Balzategui, A.; Reygadas, F. A Hybrid Centralized-Point-of-Use Drinking Water Treatment System in a Rural Community in Chiapas, Mexico. Environ. Eng. Sci. 2021, 38, 418–429. [Google Scholar] [CrossRef]
- van Halem, D.; van der Laan, H.; Soppe, A.I.A.; Heijman, S.G.J. High flow ceramic pot filters. Water Res. 2017, 124, 398–406. [Google Scholar] [CrossRef]
- He, Y.; Huang, G.; An, C.; Huang, J.; Zhang, P.; Chen, X.; Xin, X. Reduction of Escherichia Coli using ceramic disk filter decorated by nano-TiO2: A low-cost solution for household water purification. Sci. Total Environ. 2018, 616–617, 1628–1637. [Google Scholar] [CrossRef]
- Yang, H.; Min, X.; Xu, S.; Bender, J.; Wang, Y. Development of Effective and Fast-Flow Ceramic Porous Media for Point-of-Use Water Treatment: Effect of Pore Size Distribution. ACS Sustain. Chem. Eng. 2020, 8, 2531–2539. [Google Scholar] [CrossRef]
- Pooi, C.K.; Ng, H.Y. Review of low-cost point-of-use water treatment systems for developing communities. npj Clean Water 2018, 1, 11. [Google Scholar] [CrossRef]
- Singer, S.; Skinner, B.; Cantwell, R.E. Impact of surface maintenance on BioSand filter performance and flow. J. Water Health 2017, 15, 262–272. [Google Scholar] [CrossRef]
- Peter, M.; Derksen, S.; Johnston, R.; Meierhofer, R.; Flückiger, C.; Kage, F.; Owino, J.; Were, E.; Mwango, C.; Sodis, F.; et al. Eawag: Das Wasserforschungs-Institut des ETH-Bereichs Gravity-Driven Membrane Disinfection for Household Water Treatment Partners and Organizations Eawag-Swiss Federal Institute of Aquatic Science and technology, Department of Water and Sanitation in Deve. 2015. Available online: https://www.eawag.ch/fileadmin/Domain1/Abteilungen/eng/projekte/trinkwasser/gdm/Ab_6796.GDMD_final_report_Peter_032015.pdf (accessed on 20 March 2025).
- Mecha, C.A.; Pillay, V.L. Development and evaluation of woven fabric microfiltration membranes impregnated with silver nanoparticles for potable water treatment. J. Memb. Sci. 2014, 458, 149–156. [Google Scholar] [CrossRef]
- Mecha, A.C.; Otieno, F.A.O.; Pillay, V.L. Long-term disinfection performance of silver nanoparticles impregnated membranes. Desalin. Water Treat. 2016, 57, 4906–4912. [Google Scholar] [CrossRef]
- Achisa, M. Evaluation of Silver Nanoparticles Impregnated Woven Fabric Microfiltration. 2013. Available online: https://openscholar.dut.ac.za/server/api/core/bitstreams/9f9c8c8b-901b-472f-adc9-73efd533cf77/content (accessed on 20 February 2025).
- Moreira, V.R.; Lebron, Y.A.R.; de Souza Santos, L.V.; Amaral, M.C.S. Low-cost recycled end-of-life reverse osmosis membranes for water treatment at the point-of-use. J. Clean. Prod. 2022, 362, 132495. [Google Scholar] [CrossRef]
- Costa de Souza, E.; Pimenta, A.; Silva, A.; Nascimento, P.; Ighalo, J.O. Oxidized eucalyptus charcoal: A renewable biosorbent for removing heavy metals from aqueous solutions. Biomass Convers. Biorefinery 2021, 13, 4105–4119. [Google Scholar] [CrossRef]
- Zhang, W.; Song, J.; He, Q.; Wang, H.; Lyu, W.; Feng, H.; Xiong, W.; Guo, W.; Wu, J.; Chen, L. Novel pectin based composite hydrogel derived from grapefruit peel for enhanced Cu(II) removal. J. Hazard. Mater. 2020, 384, 121445. [Google Scholar] [CrossRef] [PubMed]
- Satyam, S.; Patra, S. Innovations and challenges in adsorption-based wastewater remediation: A comprehensive review. Heliyon 2024, 10, e29573. [Google Scholar] [CrossRef]
- Khan, S.; Ajmal, S.; Hussain, T.; Rahman, M.U. Clay-based materials for enhanced water treatment: Adsorption mechanisms, challenges, and future directions. J. Umm Al-Qura Univ. Appl. Sci. 2023, 11, 219–234. [Google Scholar] [CrossRef]
- Ogunfowora, L.A.; Iwuozor, K.O.; Ighalo, J.O.; Igwegbe, C.A. Trends in the treatment of aquaculture effluents using nanotechnology. Clean. Mater. 2021, 2, 100024. [Google Scholar] [CrossRef]
- Varsha, M.; Senthil Kumar, P.; Senthil Rathi, B. A review on recent trends in the removal of emerging contaminants from aquatic environment using low-cost adsorbents. Chemosphere 2022, 287, 132270. [Google Scholar] [CrossRef]
- Mecha, A.C.; Onyango, M.S.; Ochieng, A.; Momba, M.N.B. Modelling inactivation kinetics of waterborne pathogens in municipal wastewater using ozone. Environ. Eng. Res. 2020, 25, 890–897. [Google Scholar] [CrossRef]
- Mecha, A.C.; Onyango, M.S.; Ochieng, A.; Momba, M.N.B. UV and solar photocatalytic disinfection of municipal wastewater: Inactivation, reactivation and regrowth of bacterial pathogens. Int. J. Environ. Sci. Technol. 2019, 16, 3687–3696. [Google Scholar] [CrossRef]
- Mojiri, A.; Bashir, M.J.K. Wastewater Treatment: Current and Future Techniques. Water 2022, 14, 448. [Google Scholar] [CrossRef]
- Mukherjee, D.; Ghosh, S. Ceramic Membranes in Water Treatment: Potential and Challenges for Technology Development. In Sustainable Water Treatment; Wiley: Hoboken, NJ, USA, 2022; pp. 325–381. [Google Scholar] [CrossRef]
- Rathi, B.S.; Kumar, P.S. Application of adsorption process for effective removal of emerging contaminants from water and wastewater. Environ. Pollut. 2021, 280, 116995. [Google Scholar] [CrossRef]
- El Messaoudi, N.; Franco, D.S.P.; Gubernat, S.; Georgin, J.; Şenol, Z.M.; Ciğeroğlu, Z.; Allouss, D.; El Hajam, M. Advances and future perspectives of water defluoridation by adsorption technology: A review. Environ. Res. 2024, 252, 118857. [Google Scholar] [CrossRef]
- Suresh, R.; Rajoo, B.; Chenniappan, M.; Palanichamy, M. Experimental analysis on the synergistic effect of combined use of ozone and UV radiation for the treatment of dairy industry wastewater. Environ. Eng. Res. 2021, 26, 200375. [Google Scholar] [CrossRef]
- Shushu, U.P.; Komakech, H.C.; Dodoo-Arhin, D.; Ferras, D.; Kansal, M.L. Managing non-revenue water in Mwanza, Tanzania: A fast-growing sub-Saharan African city. Sci. Afr. 2021, 12, e00830. [Google Scholar] [CrossRef]
- Komba, F.E.; Fabian, C.; Elimbinzi, E.; Shao, G.N. Efficiency of common filters for water treatment in Tanzania. Bull. Natl. Res. Cent. 2022, 46, 208. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, H.; Yang, F.; Gray, S. Cost and efficiency perspectives of ceramic membranes for water treatment. Water Res. 2022, 220, 118629. [Google Scholar] [CrossRef]
- Nielsen, A.M.; Garcia, L.A.T.; Silva, K.J.S.; Sabogal-Paz, L.P.; Hincapié, M.M.; Montoya, L.J.; Galeano, L.; Galdos-Balzategui, A.; Reygadas, F.; Herrera, C.; et al. Chlorination for low-cost household water disinfection—A critical review and status in three Latin American countries. Int. J. Hyg. Environ. Health 2022, 244, 114004. [Google Scholar] [CrossRef]
- Ercumen, A.; Naser, A.M.; Unicomb, L.; Arnold, B.F.; Colford, J.M., Jr.; Luby, S.P. Effects of Source- versus Household Contamination of Tubewell Water on Child Diarrhea in Rural Bangladesh: A Randomized Controlled Trial. PLoS ONE 2015, 10, e0121907. [Google Scholar] [CrossRef]
- Trivedi, Y.; Sharma, M.; Mishra, R.K.; Sharma, A.; Joshi, J.; Gupta, A.B.; Achintya, B.; Shah, K.; Vuppaladadiyamd, A.K. Biochar potential for pollutant removal during wastewater treatment: A comprehensive review of separation mechanisms, technological integration, and process analysis. Desalination 2025, 600, 118509. [Google Scholar] [CrossRef]
- Pronk, W.; Ding, A.; Morgenroth, E.; Derlon, N.; Desmond, P.; Burkhardt, M.; Wu, B.; Fane, A.G. Gravity-driven Membrane Filtration for Water and Wastewater Treatment: A Review. Water Res. 2018, 149, 553–565. [Google Scholar] [CrossRef]
- Aibinu, A.M.; Folorunso, T.A.; Saka, A.A.; Ogunfowora, L.A.; Iwuozor, K.O.; Ighalo, J.O. Green synthesis of CuO nanocomposite from watermelon (Citrullus lanatus) rind for the treatment of aquaculture effluent. Reg. Stud. Mar. Sci. 2022, 52, 102308. [Google Scholar] [CrossRef]
- Tiwari, S.S.K.; Schmidt, W.P.; Darby, J.; Kariuki, Z.G.; Jenkins, M.W. Intermittent slow sand filtration for preventing diarrhoea among children in Kenyan households using unimproved water sources: Randomized controlled trial. Trop. Med. Int. Heal. 2009, 14, 1374–1382. [Google Scholar] [CrossRef]
- Budeli, P.; Moropeng, R.C.; Momba, M.N.B. Improvement of biosand filtration systems using silver-impregnated clay granules. J. Water Process Eng. 2021, 41, 102049. [Google Scholar] [CrossRef]
- Bailey, E.S.; Beetsch, N.; Wait, D.A.; Oza, H.H.; Ronnie, N.; Sobsey, M.D. Methods, protocols, guidance and standards for performance evaluation for point-of-use water treatment technologies: History, current status, future needs and directions. Water 2021, 13, 1094. [Google Scholar] [CrossRef]
- Chaukura, N.; Chiworeso, R.; Gwenzi, W.; Motsa, M.M.; Munzeiwa, W.; Moyo, W.; Chikurunhe, I.; Nkambule, T.T.I. A new generation low-cost biochar-clay composite ‘biscuit’ ceramic filter for point-of-use water treatment. Appl. Clay Sci. 2020, 185, 105409. [Google Scholar] [CrossRef]
- Oliveira, P.A.D.; Matos, P.G. Review of Community-Managed Water Supply—Factors Affecting Its Long-Term Sustainability. Water 2022, 14, 2209. [Google Scholar]
- Idigbe, I.; Cherian, M.; Salako, A.O.; Adewale, B.; Salako, B.L.; Maffioli, E.M. Safe water treatment practices: A qualitative study on point-of-use chlorination in Nigeria. J. Glob. Health 2024, 14, 04178. [Google Scholar] [CrossRef]
- Ngasala, T.M.; Masten, S.J.; Cohen, C.; Ravitz, D.; Mwita, E.J. Implementation of point-of-use water treatment methods in a rural tanzanian community: A case study. J. Water Sanit. Hyg. Dev. 2020, 10, 1012–1018. [Google Scholar] [CrossRef]
- Afitiri, A. Households’ Willingness to Use Water from a Solar Water Disinfection Treatment System for Household Purposes. World 2024, 5, 1181–1193. [Google Scholar] [CrossRef]
- Halperin, M.; Paz-Soldán, V.A.; Quispe, V.; Paxton, A.; Gilman, R.H. Sustainability of solar disinfection to provide safe drinking water in rural Peru. Public Health Rep. 2011, 126, 762–767. [Google Scholar] [CrossRef]
- Tamas, A.; Mosler, H.-J. Why do people stop treating contaminated drinking water with Solar Water Disinfection (SODIS)? Health Educ. Behav. 2011, 38, 357–366. [Google Scholar] [CrossRef]
- Swearingen, C.; Alsouss, N.; Babic, M.; Clark, J.; Dwyer, C.; Hudock, C.; Julien, L.; McGraw, P.; Mekhel, R.; Mekhel, V.; et al. Efficacy of water filters and training interventions in mitigating gastrointestinal symptoms in rural Honduras. Water Pract. Technol. 2025, 20, 362–374. [Google Scholar] [CrossRef]
- Sudiyani, Y.; Widmer, K.; Andreas, A.; Tasfiyati, A.N.; Athaillah, Z.A.; Muryanto, M.; Aziz, A.A.; Lee, E.Y.; Lee, Y.; Kang, S. Impact of Gravity-Driven Membrane Filtration Water Treatment Systems on a Rural School in Indonesia. Sustainability 2022, 14, 13733. [Google Scholar] [CrossRef]
- Sepe, M.B.; Sagadal, J.N.; Lange, R.D.; Porras, J.C. Impact of Biosand Filter on Access to Safe Drinking Water in the Rural Communities of the Philippines. UIC Res. J. 2013, 17, 1–22. [Google Scholar] [CrossRef]
- Kiteto, M.; Vidija, B.; Mecha, C.A.; Mrosso, R.; Chollom, M.N. Advances in metal–organic frameworks as adsorbents, photocatalysts and membranes: A new frontier in water purification. Discov. Water 2024, 4, 54. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).