Study on the Characteristics of TPH in Groundwater and Its Biodegradation Mechanism in Typical Petrochemical Enterprises in Jiangbei New Area, Nanjing
Abstract
1. Introduction
2. Materials and Methods
2.1. Regional Hydrogeological Conditions
2.2. Oil Storage Enterprises Overview
2.3. Sample Collection and Analysis
2.4. Factor Analysis
2.5. Correlation Analysis
2.6. Relative Contribution Rate Analysis
3. Results and Discussion
3.1. Spatial Distribution Characteristics of TPH in Groundwater in the Study Area
3.2. Groundwater Chemical Characteristics
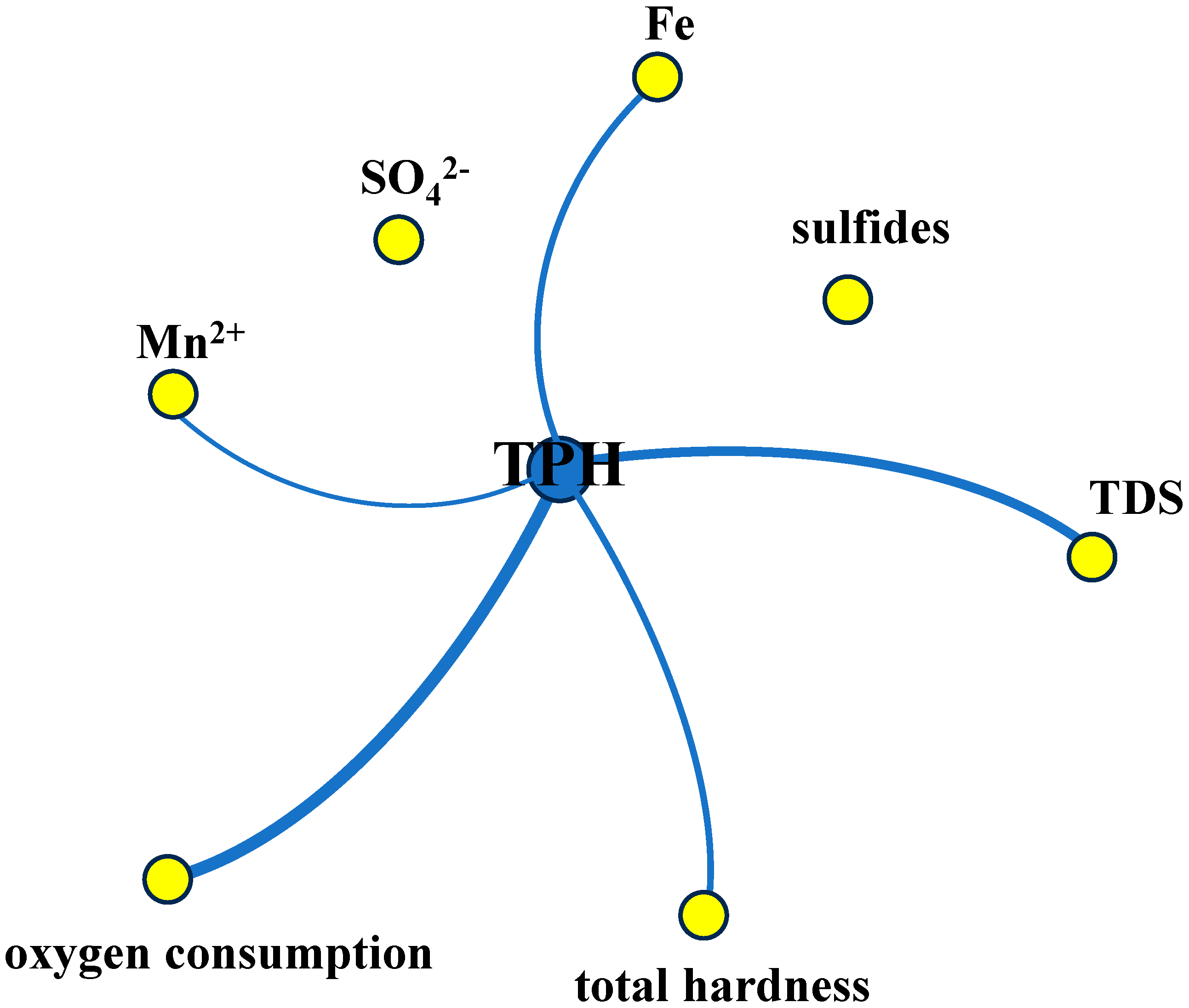
3.3. Groundwater Factor Analysis
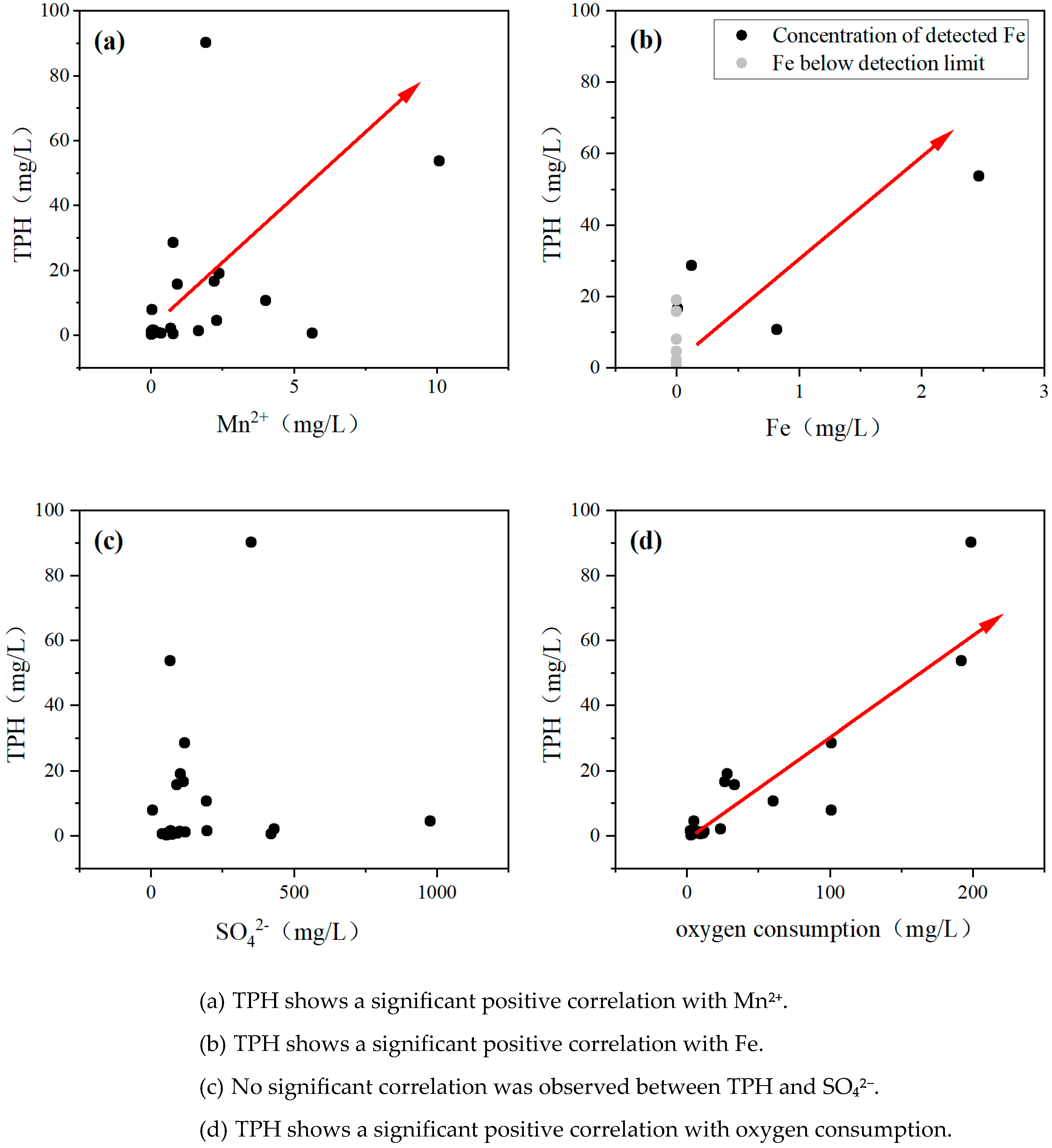
3.4. Influence of Groundwater Chemical Indicators on TPH
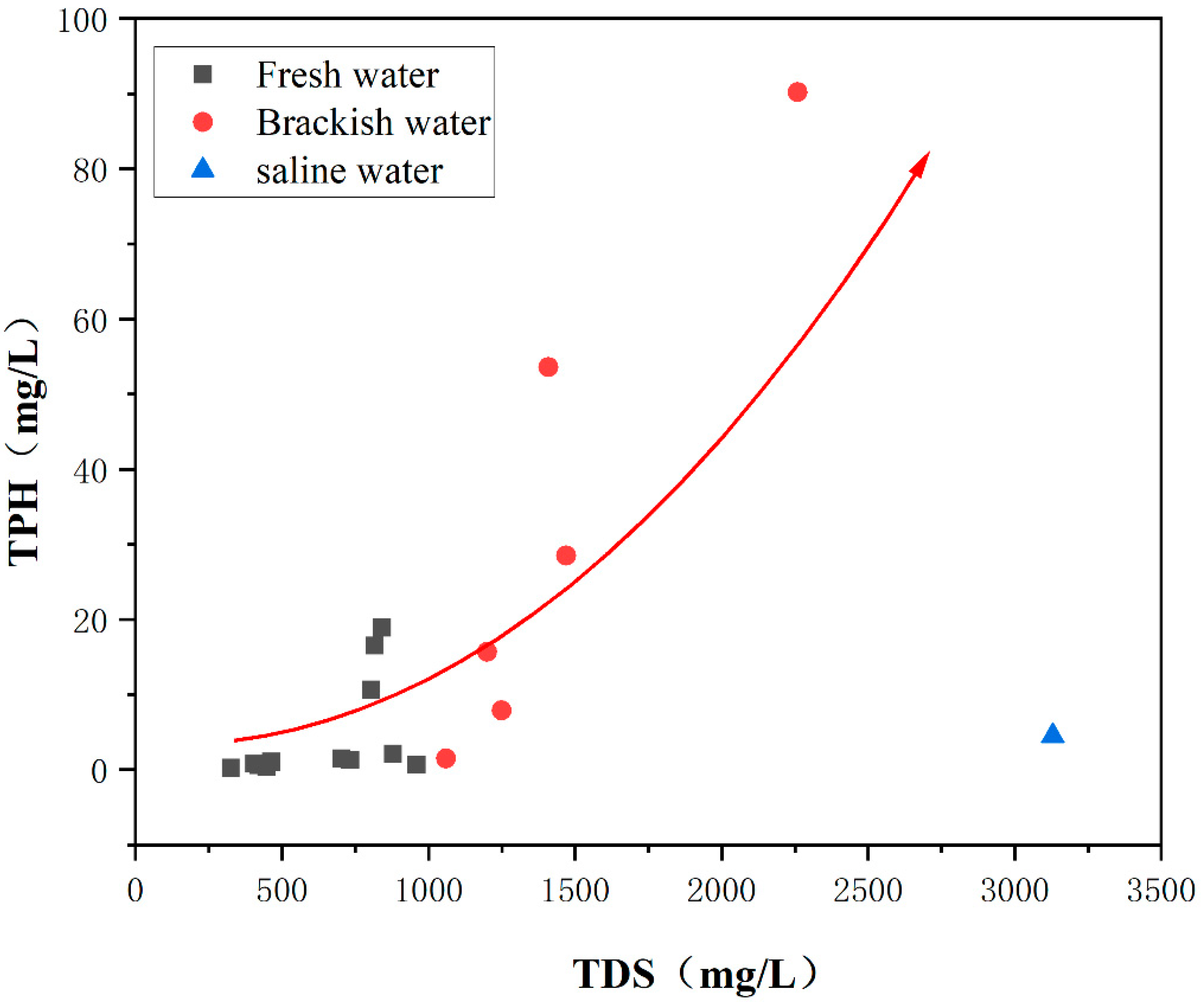
3.5. Influence of Salinity on TPH Degradation
3.6. TPH Biodegradation and Electron Acceptor Contribution Rate
4. Conclusions
- (1)
- Using factor analysis, two principal factors (F1, F2) with eigenvalues greater than one were selected. F1 includes Fe, oxygen consumption, TPH, Mn2+, and sulfides, representing that TPH may have undergone biodegradation via iron–manganese reduction, while also indicating that ion adsorption has influenced the chemical composition of the groundwater. F2 includes SO42−, TDS, and total hardness, representing that TPH may have undergone biodegradation via sulfate reduction;
- (2)
- In the study area, the strongest correlation between TPH and oxygen consumption indicates that the microbial degradation of TPH is predominantly aerobic, a process that consumes substantial amounts of oxygen. There is a significant positive correlation between TPH and TDS in groundwater. In freshwater environments, as TDS concentrations increase, TPH concentrations rise slowly. However, in brackish water environments, the rate of TPH concentration increase gradually accelerates with rising TDS levels. This indicates that biodegradation rates slow down with increasing mineralization. In freshwater environments, microbial growth is more favorable, promoting TPH degradation, whereas in brackish and saline water environments, microbial activity decreases, leading to reduced TPH degradation efficiency;
- (3)
- Through a multiple regression analysis, the relative contribution rates of electron acceptors (Mn, Fe, SO42−) utilized by microorganisms in the biodegradation of TPH were determined as follows: iron reduction (90.62%) > manganese reduction (9.35%) > sulfate reduction (0.032%). This indicates that the biodegradation of TPH in the study area’s groundwater is primarily driven by iron–manganese reduction;
- (4)
- It is recommended that petrochemical enterprises monitor the TDS levels in groundwater to prevent excessive mineralization and create a favorable environment for microbial growth;
- (5)
- Although this study has confirmed that iron–manganese reduction is the predominant pathway for TPH biodegradation in groundwater, the influence of different microbial communities and their underlying mechanisms remain unclear and require further investigation.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kang, L.; Sun, C.J.; Yang, Q.; Chen, X.; Zhao, Z.; Chen, M. Characteristics of Pollutants from Underground Storage Tank Leakage. Environ. Sci. Manag. 2014, 39, 53–57. [Google Scholar]
- Wu, Q.; Zhang, X.; Zhang, Q. Current situation and control measures of groundwater pollution in gas station. IOP Conf. Ser. Earth Environ. Sci. 2017, 94, 012005. [Google Scholar] [CrossRef]
- Bai, X.; Song, K.; Liu, J.; Mohamed, A.K.; Mou, C.; Liu, D. Health Risk Assessment of Groundwater Contaminated by Oil Pollutants Based on Numerical Modeling. Int. J. Environ. Res. Public Health 2019, 16, 3245. [Google Scholar] [CrossRef]
- Zuo, H.; Jin, W.; Gao, J. Investigation on Corrosion Status and Anti-Corrosion Strategies for Storage Tanks in Karamay Petrochemical Company. J. Xinjiang Pet. Inst. 2002, 14, 70–73. [Google Scholar]
- Li, C.; Wu, Q. Research Advances in Organic Contamination of Groundwate. Geotech. Investig. Surv. 2007, 7, 27–30. [Google Scholar]
- Jiao, X.; Su, X.; Lü, X. Geochemical indicators of intrinsic biodegradation in a petroleum hydrocarbon-contaminated aquifer. Chin. J. Geol. 2012, 47, 499–506. [Google Scholar]
- Borden, R.C.; Gomez, C.A.; Becker, M.T. Geochemical indicators of intrinsic bioremediation. Ground Water 1995, 33, 180–189. [Google Scholar] [CrossRef]
- Lu, Y. Mechanisms of Biodegradation for Petroleum Hydrocarbons in Shallow Groundwater Systems. Ph.D Thesis, Jilin University, Changchun, China, 2013. [Google Scholar]
- Schreiber, M.; Carey, G.; Feinstein, D.; Bahr, J. Mechanisms of electron acceptor utilization: Implications for simulating anaerobic biodegradation. J. Contam. Hydrol. 2004, 73, 99–127. [Google Scholar] [CrossRef] [PubMed]
- Heron, G.; Crouzet, C.; Bourg, A.C.M.; Christensen, T.H. Speciation of Fe(II) and Fe(III) in Contaminated Aquifer Sediments Using Chemical Extraction Techniques. Environ. Sci. Technol. 1994, 28, 1698–1705. [Google Scholar] [CrossRef]
- Zhao, Q.; Su, X.; Zuo, E.; Wu, X.; Ma, F. TPH pollution characteristics in vadose zone and water-bearing medium at a petroleum contaminated site. Sci. Technol. Rev. 2015, 33, 25–29. [Google Scholar]
- Su, X.; Lü, H.; Zhang, W.; Zhang, Y.; Jiao, X. 13C and 34S Isotope Evidence for Biodegradation of a Petroleum Hydrocarbon-Contaminated Aquifer in the Northeast of China. J. Jilin Univ. (Earth Sci. Ed.) 2011, 41, 847–854. [Google Scholar] [CrossRef]
- Kukharova, T.; Maltsev, P.; Novozhilov, I. Development of a Control System for Pressure Distribution During Gas Production in a Structurally Complex Field. Appl. Syst. Innov. 2025, 8, 51. [Google Scholar] [CrossRef]
- Dang, J. Variation Characteristics of Water Environmental Elements and Enhanced bioremediation of Slightly Petroleum Contaminated Groundwater. Master’s Thesis, Jilin University, Changchun, China, 2015. [Google Scholar]
- Báez-Cazull, S.E.; McGuire, J.T.; Cozzarelli, I.M.; Voytek, M.A. Determination of dominant biogeochemical processes in a contaminated aquifer-wetland system using multivariate statistical analysis. J. Environ. Qual. 2008, 37, 30–46. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Cheon, J.-Y.; Lee, K.-K.; Lee, S.-Y.; Lee, M.-H. Factors affecting the distribution of hydrocarbon contaminants and hydrogeochemical parameters in a shallow sand aquifer. J. Contam. Hydrol. 2001, 50, 139–158. [Google Scholar] [CrossRef]
- Lü, H.; Liu, M.; Su, X.; Lin, G. Determination of biogeochemical processes in oil-contaminatedaquifer using principal component analysis. J. Cent. South Univ. (Sci. Technol.) 2013, 44, 3552–3560. [Google Scholar]
- Farnham, I.M.; Johannesson, K.H.; Singh, A.K.; Hodge, V.F.; Stetzenbach, K.J. Factor analytical approaches for evaluating groundwater trace element chemistry data. Anal. Chim. Acta 2003, 490, 123–138. [Google Scholar] [CrossRef]
- Wu, C.; Su, X.; Guo, J.; Dong, W. Multivariate statistical analysis of hydrogeochemical evolution of groundwater in Cretaceous aquifer Ordos desert plateau. Glob. Geol. 2011, 30, 244–253. [Google Scholar]
- Liu, C.W.; Lin, K.H.; Kuo, Y.M. Application of factor analysis in the assessment of groundwater quality in a blackfoot disease area in Taiwan. Sci. Total Environ. 2003, 313, 77–89. [Google Scholar] [CrossRef]
- Yuan, J.; Deng, G.; Xu, F.; Tang, Y.; Li, P. The multivariate statistical analysis of chemical characteristics and influencing factors of karst groundwater in the northern part of Bijie City, Guizhou Province. Geol. China 2016, 43, 1446–1456. [Google Scholar]
- Cui, J.Q.; Li, X.Y.; Shi, H.B.; Sun, Y.N.; An, H.J.; Xing, J.P. Chemical Evolution and Formation Mechanism of Groundwater in Hetao Irrigation Area. Environ. Sci. 2020, 41, 4011–4020. [Google Scholar] [CrossRef]
- Gao, Y. Spatio-Temporal Evolution of Hydrochemical Components and Human Health Risk Assessment of Groundwater in Guanzhong Plain. Ph.D. Thesis, Chang’an University, Xi’an, China, 2020. [Google Scholar]
- Li, Z.; Chen, Y.; Yang, J.; Wang, Y. Potential evapotranspiration and its attribution over the past 50 years in the arid region of Northwest China. Hydrol. Process. 2014, 28, 1025–1031. [Google Scholar] [CrossRef]
- Xuena, L.; Haiming, L.; Mengdi, L.; Weihua, Z.; Han, X. Pollution characteristics and biodegradation mechanism of petroleum hydrocarbons in gas station groundwater in the Tianjin Plain. Earth Sci. Front. 2022, 29, 227–238. [Google Scholar] [CrossRef]
- Dong, J. Study of Landfill Leachate Redox Zones and Degradation Mechanisms of Pollutants in the Subsurface Environment. Ph.D. Thesis, Jilin University, Changchun, China, 2006. [Google Scholar]
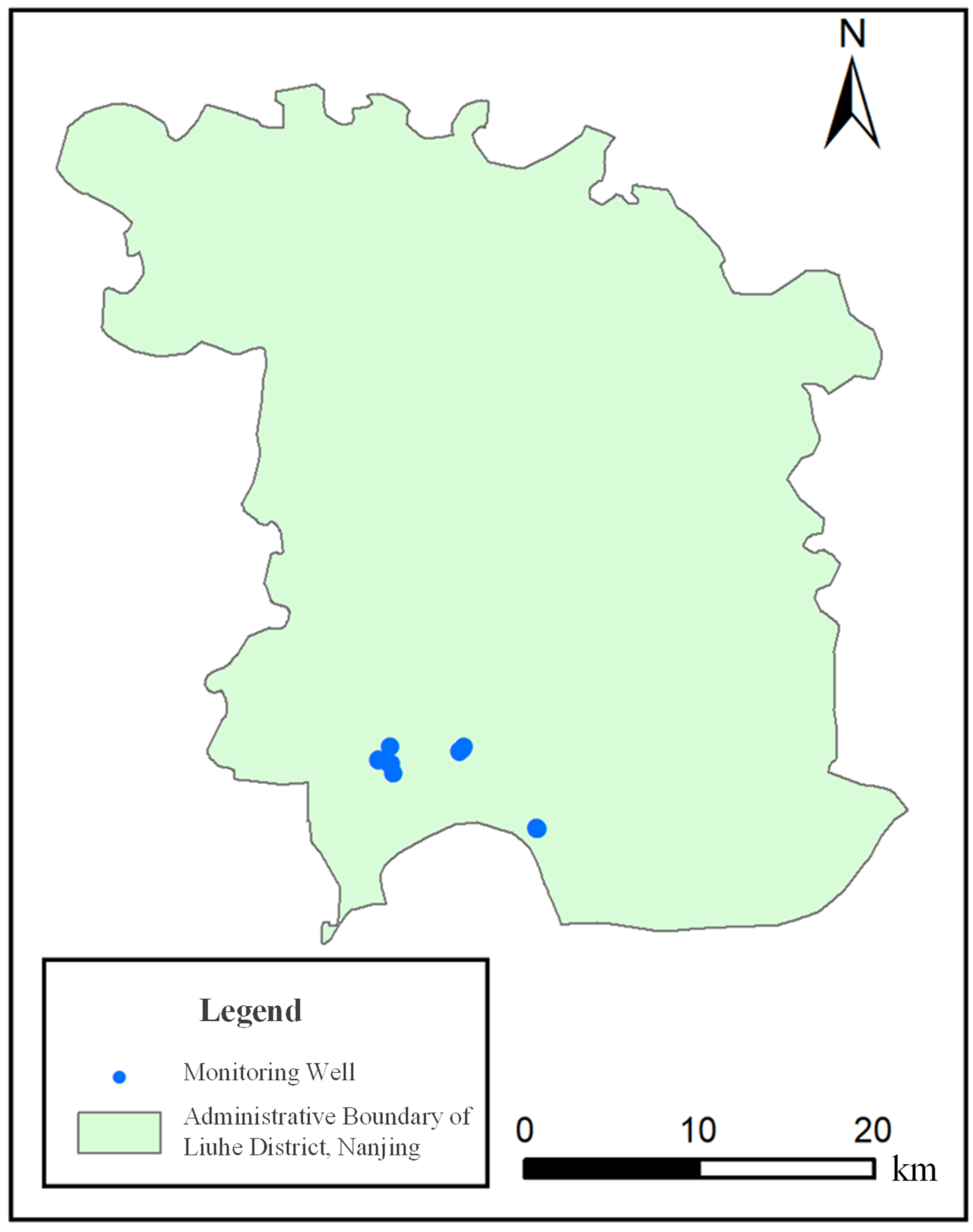
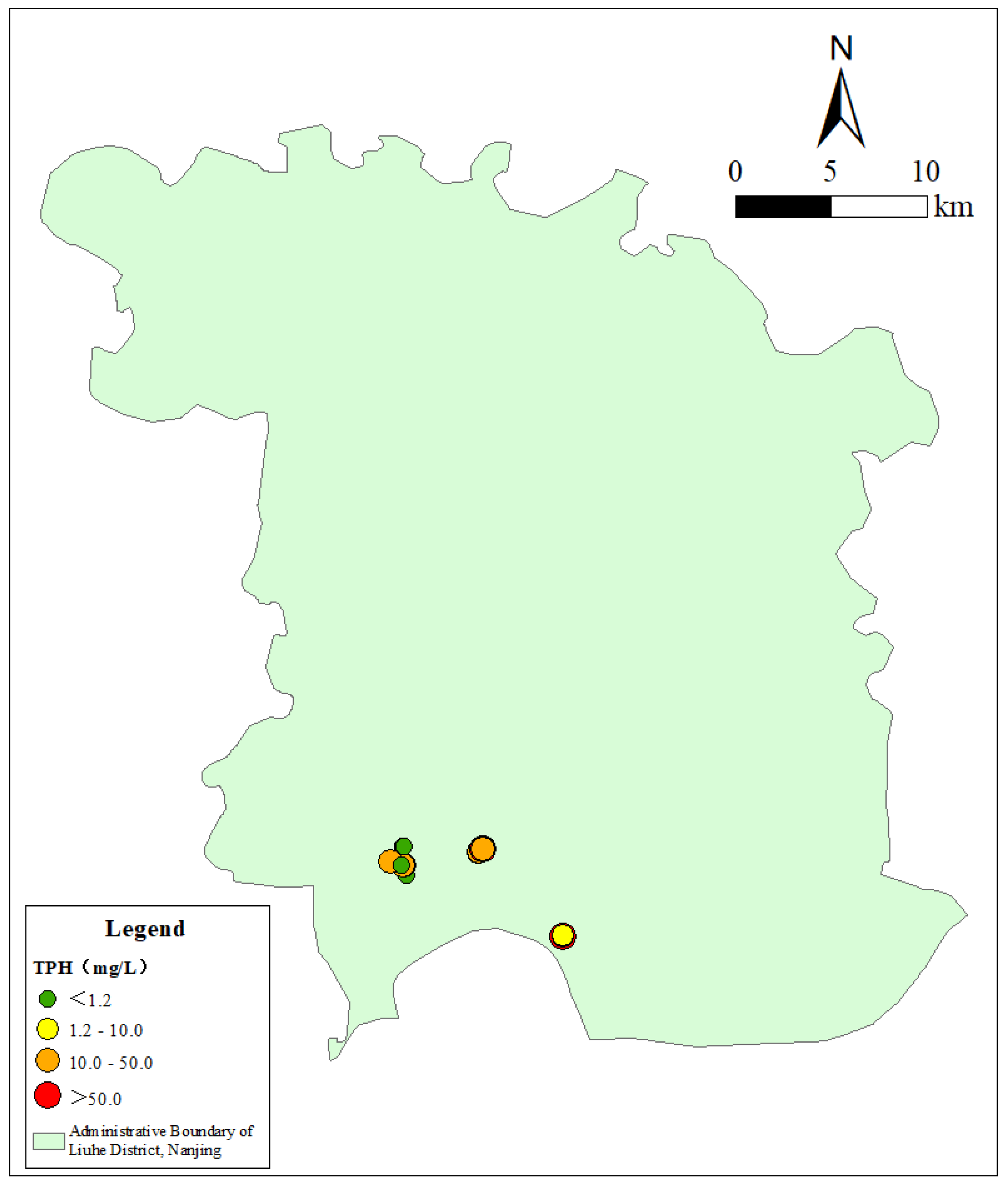
| No. | Abbreviation | Basic Information | Number of Monitoring Wells |
|---|---|---|---|
| 1 | TD Corporation | ① Business scope: Chemical products, fuel oil sales; ② Tank conditions: 8 vertical tanks (empty), 5 horizontal tanks (2# horizontal tank stores about 45 cubic meters of diesel, the rest are empty); ③ Total site area: 5794 m2; ④ Material situation: Stored 45 cubic meters of diesel (now transported to storage units, tanks on site are empty). | 2 |
| 2 | TH Corporation | ① Business scope: Chemical product sales; ② Tank conditions: 7 vertical tanks (empty), 6 horizontal tanks (3# vertical tank stores 6 tons of ethylene tar, the rest are empty); ③ Total site area: 2882.52 m2; ④ Material situation: Stored ethylene tar and carbon nine (currently all tanks are empty). | 2 |
| 3 | SP Corporation | ① Business scope: Wholesale of hazardous chemicals; ② Tank conditions: 2 vertical tanks (1# tank stores about 6 tons of carbon nine, 2# tank stores about 7 tons of carbon nine), 11 horizontal tanks (2# stores about 4 tons of carbon nine, 3# stores about 5 tons of ethylene tar, 6# stores about 2 tons of ethylene tar, the rest are empty); ③ Total site area: 2134.4 m2. | 1 |
| 4 | ZY Corporation | ① Business scope: Chemical products, fuel oil sales; ② Tank conditions: 2 vertical tanks (1# stores about 30 tons of mixed oil, 2# stores about 30 tons of mixed oil), 7 horizontal tanks (2# stores about 50 tons of mixed oil, 4# stores about 40 tons of mixed oil, 8# stores about 60 tons of mixed oil, 7# stores about 30 tons of mixed oil, 9# stores about 60 tons of mixed oil), 1 ground pool; ③ Total site area: 2668 m2. | 2 |
| 5 | DT Corporation | ① Business scope: Petroleum products and chemical product sales; ② Tank conditions: 13 horizontal tanks (3# tank stores about 5 tons of ethylene tar, 5# tank stores about 20 tons of ethylene tar, 6# tank stores about 30 tons of ethylene tar, 7# tank stores about 2 tons of carbon nine, the rest are empty); ③ Total site area: 2668 m2. | 3 |
| 6 | HY Corporation | ① Business scope: Chemical product sales (excluding hazardous chemicals); ② Tank conditions: 19 horizontal tanks (empty); ③ Total site area: 5336 m2. | 5 |
| 7 | PW Corporation | ① Business scope: Petroleum products and chemical product sales; ② Tank conditions: Used for storage and transportation of petroleum products (carbon nine, fuel oil, mixed aromatics, ethylene tar); 3 vertical tanks and 15 horizontal tanks, as well as office areas; ③ Total site area: 2133 m2. | 2 |
| 8 | YP Corporation | ① Business scope: Petroleum products and chemical product sales; ② Tank conditions: 2 vertical tanks, 9 horizontal tanks, and 3 ground tanks (buried ground pools). The results show that the site previously had 2 vertical tanks, each with a volume of 200 cubic meters. 9 horizontal tanks, including 5 with a volume of 65 cubic meters, 3 with a volume of 35 cubic meters, and 1 with a volume of 30 cubic meters. The vertical and horizontal tanks stored carbon nine and ethylene tar. The 3 ground tanks each had a volume of 170 cubic meters, storing ethylene tar. 2000 tons/year of carbon nine, 3000 tons/year of ethylene tar; ③ Total site area: 1267 m2. | 2 |
| 9 | ZL Corporation | ① Business scope: Petroleum products and chemical product sales; ② Tank conditions: Storage and transportation of petroleum products (carbon nine, carbon ten, ethylene tar, fuel oil). The site has 6 vertical tanks, including 4 with a volume of 400 cubic meters and 2 with a volume of 1000 cubic meters; 6 horizontal tanks, including 4 with a volume of 90 cubic meters and 2 with a volume of 30 cubic meters; ③ Total site area: 3246 m2. | 2 |
| No. | Indicator Category | Specific Indicators |
|---|---|---|
| 1 | Conventional Indicators | pH, color, turbidity, odor and taste, visible matter, total hardness (as CaCO3), total dissolved solids, anionic surfactants, sulfate (SO42−), chloride (Cl−), sulfide, volatile phenols, permanganate index, ammonia nitrogen, arsenic, cadmium, copper, lead, mercury, nickel, zinc, iron, manganese, sodium, aluminum, hexavalent chromium |
| 2 | Volatile Organic Compounds (VOCs) | Chloromethane, vinyl chloride, 1,1-dichloroethylene, dichloromethane, trans-1,2-dichloroethylene, 1,1-dichloroethane, chloroprene, cis-1,2-dichloroethylene, 2,2-dichloropropane, bromochloromethane, chloroform, 1,1,1-trichloroethane, 1,1-dichloropropene, carbon tetrachloride, benzene, 1,2-dichloroethane, trichloroethylene, 1,2-dichloropropane, dibromomethane, bromodichloromethane, epichlorohydrin, cis-1,3-dichloropropene, toluene, trans-1,3-dichloropropene, 1,1,2-trichloroethane, tetrachloroethylene, 1,3-dichloropropane, dibromochloromethane, 1,2-dibromoethane, chlorobenzene, 1,1,1,2-tetrachloroethane, ethylbenzene, m,p-xylene, o-xylene, styrene, bromoform, isopropylbenzene, 1,1,2,2-tetrachloroethane, bromobenzene, 1,2,3-trichloropropane, n-propylbenzene, 2-chlorotoluene, 1,3,5-trimethylbenzene, 4-chlorotoluene, tert-butylbenzene, 1,2,4-trimethylbenzene, sec-butylbenzene, 1,3-dichlorobenzene, 4-isopropyltoluene, 1,4-dichlorobenzene, n-butylbenzene, 1,2-dichlorobenzene, 1,2-dibromo-3-chloropropane, 1,2,4-trichlorobenzene, 1,2,3-trichlorobenzene |
| 3 | Semi-Volatile Organic Compounds (SVOCs) | N-Nitrosodimethylamine, phenol, aniline, bis(2-chloroethyl) ether, 2-chlorophenol, 2-methylphenol, bis(2-chloroisopropyl) ether, 4-methylphenol, N-Nitrosodi-n-propylamine, hexachloroethane, nitrobenzene, isophorone, 2-nitrophenol, 2,4-dimethylphenol, bis(2-chloroethoxy)methane, 2,4-dichlorophenol, naphthalene, 4-chloroaniline, hexachlorobutadiene, 4-chloro-3-methylphenol, 2-methylnaphthalene, hexachlorocyclopentadiene, 2,4,6-trichlorophenol, 2,4,5-trichlorophenol, 2-chloronaphthalene, 2-nitroaniline, dimethyl phthalate, 2,6-dinitrotoluene, acenaphthylene, 3-nitroaniline, acenaphthene, 2,4-dinitrophenol, 4-nitrophenol, dibenzofuran, 2,4-dinitrotoluene, diethyl phthalate, 4-chlorophenyl phenyl ether, fluorene, 4-nitroaniline, 4,6-dinitro-2-methylphenol, azobenzene, 4-bromodiphenyl ether, hexachlorobenzene, pentachlorophenol, phenanthrene, anthracene, carbazole, di-n-butyl phthalate, fluoranthene, pyrene, butyl benzyl phthalate, benzo(a)anthracene, chrysene, di(2-ethylhexyl) phthalate, di-n-octyl phthalate, benzo(b)fluoranthene, benzo(k)fluoranthene, benzo(a)pyrene, indeno(1,2,3-cd)pyrene, dibenzo(a,h)anthracene, benzo(ghi)perylene |
| 4 | Petroleum Hydrocarbons | Petroleum hydrocarbons (C6–C9), petroleum hydrocarbons (C10–C40) |
| No. | Testing Indicator | Testing Method | Detection Limit | Unit |
|---|---|---|---|---|
| 1 | pH | Water Quality—Determination of pH—Electrode Method (HJ 1147-2020) | / | Dimensionless |
| 2 | Color | Water Quality—Determination of Color (GB 11903-1989) | / | Degree |
| 3 | Turbidity | Standard Examination Methods for Drinking Water—Sensory and Physical Indicators—Visual Turbidity Method (GB/T 5750.4-2006, 2.2) | 1 | NTU |
| 4 | Odor and Taste | Standard Examination Methods for Drinking Water—Sensory and Physical Indicators—Odor and Taste Test (GB/T 5750.4-2006, 3.1) | / | / |
| 5 | Visible Particles | Standard Examination Methods for Drinking Water—Sensory and Physical Indicators—Direct Observation Method (GB/T 5750.4-2006, 4.1) | / | / |
| 6 | Total Hardness (as CaCO3) | Water Quality—Determination of Calcium and Magnesium—EDTA Titrimetric Method (GB/T 7477-1987) | 5 | mg/L |
| 7 | Total Dissolved Solids (TDS) | Standard Examination Methods for Drinking Water—Sensory and Physical Indicators—Gravimetric Method (GB/T 5750.4-2006, 8.1) | / | mg/L |
| 8 | Anionic Surfactants | Water Quality—Determination of Anionic Surfactants—Methylene Blue Spectrophotometric Method (GB/T 7494-1987) | 0.05 | mg/L |
| 9 | Sulfate (SO42−) | Water Quality—Determination of Inorganic Anions (F− Cl−, NO2−, Br−, NO3−, PO43−, SO32−, SO42−)—Ion Chromatography (HJ 84-2016) | 0.018 | mg/L |
| 10 | Chloride (Cl−) | Water Quality—Determination of Inorganic Anions (F− Cl−, NO2−, Br−, NO3−, PO43−, SO32−, SO42−)—Ion Chromatography (HJ 84-2016) | 0.007 | mg/L |
| 11 | Sulfide | Water Quality—Determination of Sulfide—Methylene Blue Spectrophotometric Method (GB/T 16489-1996) | 0.005 | mg/L |
| 12 | Volatile Phenols | Water Quality—Determination of Volatile Phenols—4-Aminoantipyrine Spectrophotometric Method (HJ 503-2009) | 0.003 | mg/L |
| 13 | Permanganate Index | Water Quality—Determination of Permanganate Index—Acidic Permanganate Method (GB/T 11892-1989) | 0.5 | mg/L |
| 14 | Ammonia Nitrogen | Water Quality—Determination of Ammonia Nitrogen—Nessler’s Reagent Spectrophotometry (HJ 535-2009) | 0.025 | mg/L |
| 15 | Arsenic (As) | Water Quality—Determination of 65 Elements—Inductively Coupled Plasma Mass Spectrometry (HJ 700-2014) | 0.12 | μg/L |
| 16 | Cadmium (Cd) | Water Quality—Determination of 65 Elements—Inductively Coupled Plasma Mass Spectrometry (HJ 700-2014) | 0.05 | μg/L |
| 17 | Copper (Cu) | Water Quality—Determination of 65 Elements—Inductively Coupled Plasma Mass Spectrometry (HJ 700-2014) | 0.08 | μg/L |
| 18 | Lead (Pb) | Water Quality—Determination of 65 Elements—Inductively Coupled Plasma Mass Spectrometry (HJ 700-2014) | 0.09 | μg/L |
| 19 | Mercury (Hg) | Water Quality—Determination of Mercury, Arsenic, Selenium, Bismuth, and Antimony—Atomic Fluorescence Spectrometry (HJ 694-2014) | 0.04 | μg/L |
| 20 | Nickel (Ni) | Water Quality—Determination of 65 Elements—Inductively Coupled Plasma Mass Spectrometry (HJ 700-2014) | 0.06 | μg/L |
| 21 | Zinc (Zn) | Water Quality—Determination of 65 Elements—Inductively Coupled Plasma Mass Spectrometry (HJ 700-2014) | 0.67 | μg/L |
| 22 | Iron (Fe) | Water Quality—Determination of 32 Elements—Inductively Coupled Plasma Optical Emission Spectrometry (HJ 776-2015) | 0.01 | mg/L |
| 23 | Manganese (Mn) | Water Quality—Determination of 32 Elements—Inductively Coupled Plasma Optical Emission Spectrometry (HJ 776-2015) | 0.01 | mg/L |
| 24 | Sodium (Na) | Water Quality—Determination of 65 Elements—Inductively Coupled Plasma Mass Spectrometry (HJ 700-2014) | 0.12 | mg/L |
| 25 | Aluminum (Al) | Water Quality—Determination of 32 Elements—Inductively Coupled Plasma Optical Emission Spectrometry (HJ 776-2015) | 1.15 | μg/L |
| 26 | Hexavalent Chromium (Cr6+) | Water Quality—Determination of Hexavalent Chromium—Diphenylcarbazide Spectrophotometric Method (GB/T 7467-1987) | 0.004 | mg/L |
| 27 | Petroleum Hydrocarbons (C6–C9) | Water Quality—Determination of Petroleum—Ultraviolet Spectrophotometry (Trial) (HJ 970-2018) | 0.01 | mg/L |
| 28 | Petroleum Hydrocarbons (C10–C40) | Water Quality—Determination of Extractable Petroleum Hydrocarbons (C10–C40)—Gas Chromatography (HJ 894-2017) | 0.01 | mg/L |
| 29 | Volatile Organic Compounds (VOCs) |
| 0.13 | μg/L |
| ||||
| 30 | Semi-Volatile Organic Compounds (SVOCs) | Water Quality—Determination of Semi-Volatile Organic Compounds—Liquid–Liquid Extraction/Gas Chromatography–Mass Spectrometry (HX-D067-2018, referencing US EPA 3510C-1996, 8270E-2018) | 1 | μg/L |
| No. | Indicator | Sample Size | Detection Rate | Minimum Value (mg/L) | Maximum Value (mg/L) | Mean Value (mg/L) | Standard Deviation (mg/L) | Coefficient of Variation |
|---|---|---|---|---|---|---|---|---|
| 1 | TPH | 20 | 100% | 0.26 | 90.24 | 12.87 | 22.48 | 174.72% |
| 2 | pH | 20 | 100% | 6.39 | 7.63 | 7.01 | 0.31 | 4.44% |
| 3 | sulfides | 20 | 75% | ND | 0.17 | 0.03 | 0.04 | 153.77% |
| 4 | SO42− | 20 | 100% | 5.04 | 977 | 183.46 | 222.92 | 121.51% |
| 5 | Cl− | 20 | 100% | 11.8 | 208 | 51.87 | 49.06 | 94.58% |
| 6 | TDS | 20 | 100% | 325 | 3130 | 1000.45 | 686.47 | 68.62% |
| 7 | total hardness | 20 | 100% | 149 | 1350 | 588.26 | 269.2 | 45.76% |
| 8 | Fe | 20 | 25% | ND | 2.47 | 0.25 | 0.64 | 260.92% |
| 9 | Mn2+ | 20 | 100% | 0.01 | 10.1 | 1.71 | 2.47 | 144.4% |
| 10 | Cu2+ | 20 | 75% | ND | 0.0028 | 0.001 | 0.00085 | 82.26% |
| 11 | Na+ | 20 | 100% | 18.93 | 87.1 | 46.49 | 20.07 | 43.17% |
| 12 | oxygen consumption | 20 | 100% | 2.33 | 199 | 41.9 | 60.34 | 143.99% |
| TPH | TDS | Total Hardness | Fe | Mn2+ | Oxygen Consumption | Sulfides | SO42− | |
|---|---|---|---|---|---|---|---|---|
| TPH | 1.000 | |||||||
| TDS | 0.520 | 1.000 | ||||||
| total hardness | 0.289 | 0.856 | 1.000 | |||||
| Fe | 0.791 | 0.334 | 0.299 | 1.000 | ||||
| Mn2+ | 0.430 | 0.274 | 0.398 | 0.756 | 1.000 | |||
| oxygen consumption | 0.908 | 0.469 | 0.363 | 0.854 | 0.521 | 1.000 | ||
| sulfides | 0.461 | 0.266 | 0.175 | 0.451 | 0.441 | 0.413 | 1.000 | |
| SO42− | 0.054 | 0.754 | 0.673 | −0.013 | 0.163 | −0.052 | 0.024 | 1.000 |
| Indicator | F1 | F2 |
|---|---|---|
| Fe | 0.940 | |
| oxygen consumption | 0.919 | 0.121 |
| TPH | 0.878 | 0.160 |
| Mn2+ | 0.710 | 0.201 |
| sulfides | 0.619 | |
| SO42− | −0.117 | 0.923 |
| TDS | 0.337 | 0.899 |
| total hardness | 0.255 | 0.885 |
| eigenvalues | 4.111 | 2.003 |
| contribution rates | 44.748% | 31.670% |
| cumulative variance contribution rates | 44.748% | 76.418% |
| Electron Acceptor | Biodegradation Reaction | Free Energy (kJ·mol−1) | Reaction Order |
|---|---|---|---|
| Mn4+ | CH2O + 3CO2 + 2MnO2 + H2O → 2Mn2+ + 4HCO3− | −349 | 1 |
| Fe3+ | CH2O + 7CO2 + 4Fe(OH)3 → 4Fe2+ + 8HCO3− + 3H2O | −114 | 2 |
| SO42− | 2CH2O + SO42− → H2S + 2HCO3− | −77 | 3 |
| Other Chemical Indicators | TDS | Total Hardness | Fe | Mn2+ | Oxygen Consumption | Sulfides | SO42− |
|---|---|---|---|---|---|---|---|
| TPH | 0.761 * | 0.696 * | 0.698 * | 0.515 * | 0.819 * | 0.337 | 0.277 |
| Category | Fresh Water | Brackish Water | Saline Water | Saltwater |
|---|---|---|---|---|
| TDS concentration | <1 g/L | 1~3 g/L | 3~10 g/L | >10 g/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Xia, B.; Ji, W.; Xie, Y.; Zhang, X.; Kong, L.; Zhao, C.; Yin, A.; Feng, Y.; Lu, Y.; et al. Study on the Characteristics of TPH in Groundwater and Its Biodegradation Mechanism in Typical Petrochemical Enterprises in Jiangbei New Area, Nanjing. Water 2025, 17, 1803. https://doi.org/10.3390/w17121803
Li Q, Xia B, Ji W, Xie Y, Zhang X, Kong L, Zhao C, Yin A, Feng Y, Lu Y, et al. Study on the Characteristics of TPH in Groundwater and Its Biodegradation Mechanism in Typical Petrochemical Enterprises in Jiangbei New Area, Nanjing. Water. 2025; 17(12):1803. https://doi.org/10.3390/w17121803
Chicago/Turabian StyleLi, Qi, Bing Xia, Wenbing Ji, Yufeng Xie, Xiaoyu Zhang, Lingya Kong, Caiyi Zhao, Aijing Yin, Yanhong Feng, Yuanyuan Lu, and et al. 2025. "Study on the Characteristics of TPH in Groundwater and Its Biodegradation Mechanism in Typical Petrochemical Enterprises in Jiangbei New Area, Nanjing" Water 17, no. 12: 1803. https://doi.org/10.3390/w17121803
APA StyleLi, Q., Xia, B., Ji, W., Xie, Y., Zhang, X., Kong, L., Zhao, C., Yin, A., Feng, Y., Lu, Y., Zhao, X., Li, T., Hu, Z., & Hu, R. (2025). Study on the Characteristics of TPH in Groundwater and Its Biodegradation Mechanism in Typical Petrochemical Enterprises in Jiangbei New Area, Nanjing. Water, 17(12), 1803. https://doi.org/10.3390/w17121803




