Potential of Textile Wastewater Decolorization Using Cation Exchange Membrane Electrolysis Coupled with Magnesium Salt Precipitation (CEM-MSP)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
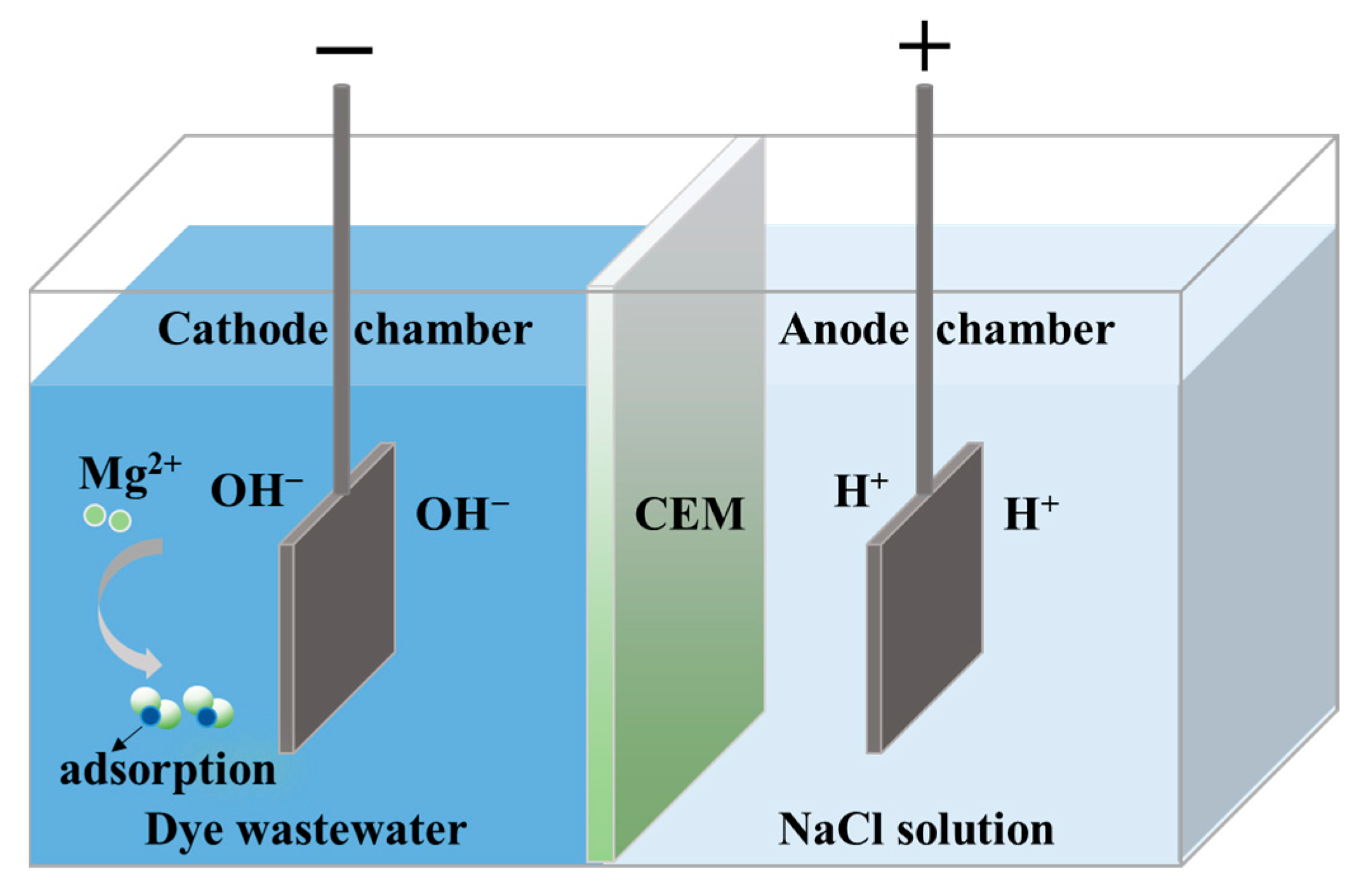
2.2. Experimental Procedures and Methods
2.3. Instrumental Analysis
2.4. Adsorption Kinetics Model Fitting
3. Results and Discussion
3.1. Dye DB86 Adsorption Mechanism
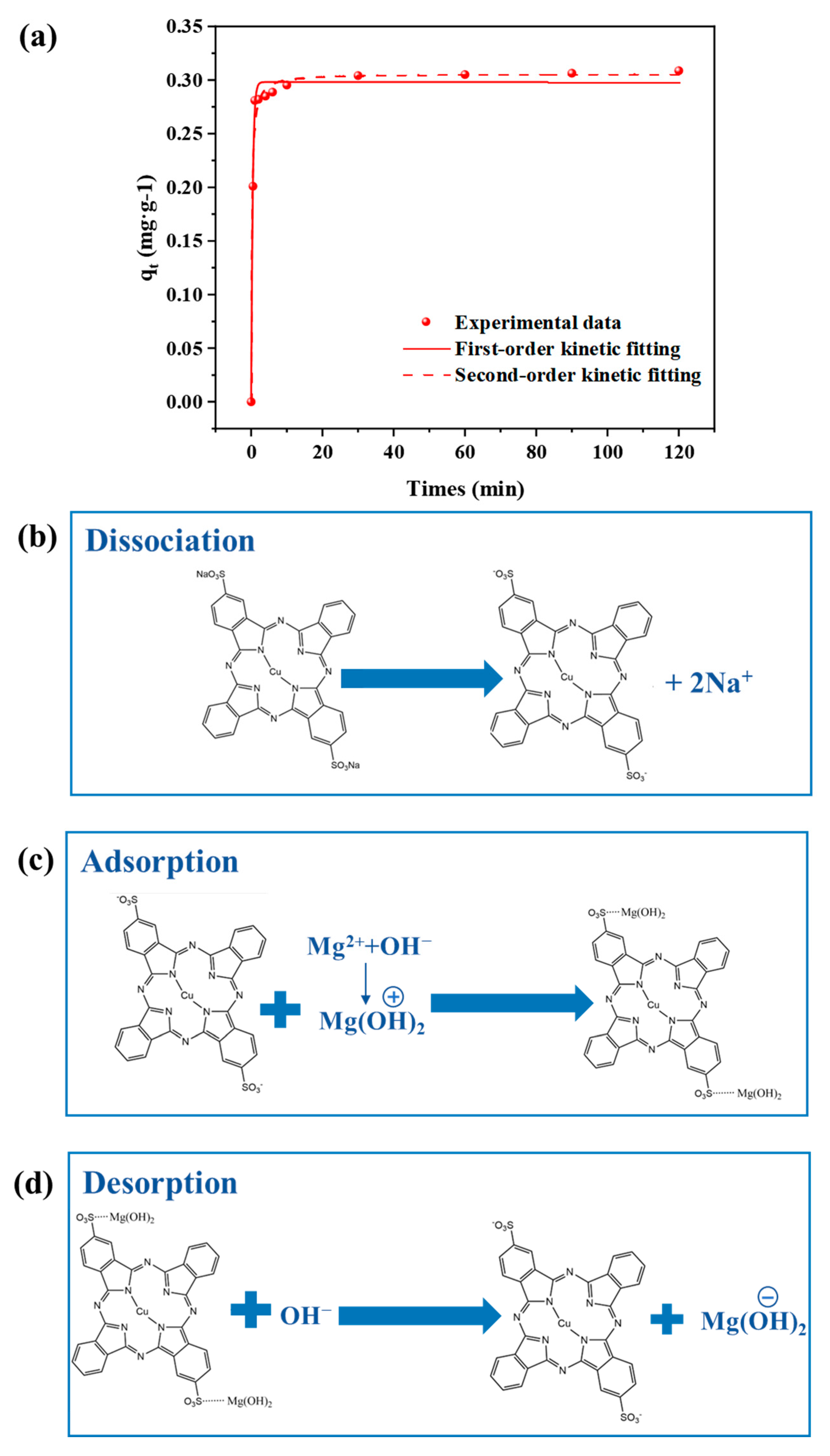
3.1.1. Kinetic Analysis of Dye DB86 Adsorption by Mg(OH)2
3.1.2. DB86 Adsorption Principle by Mg(OH)2
3.2. Dye Decolorization Performance
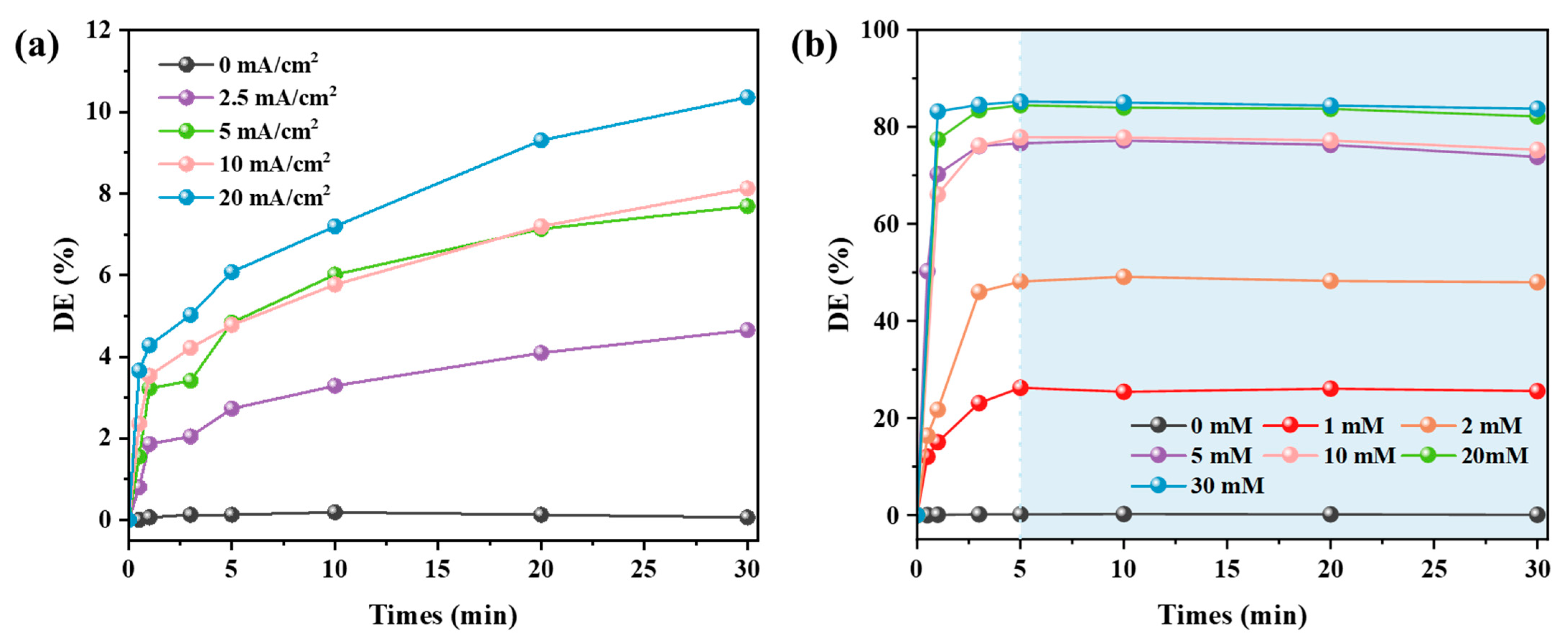
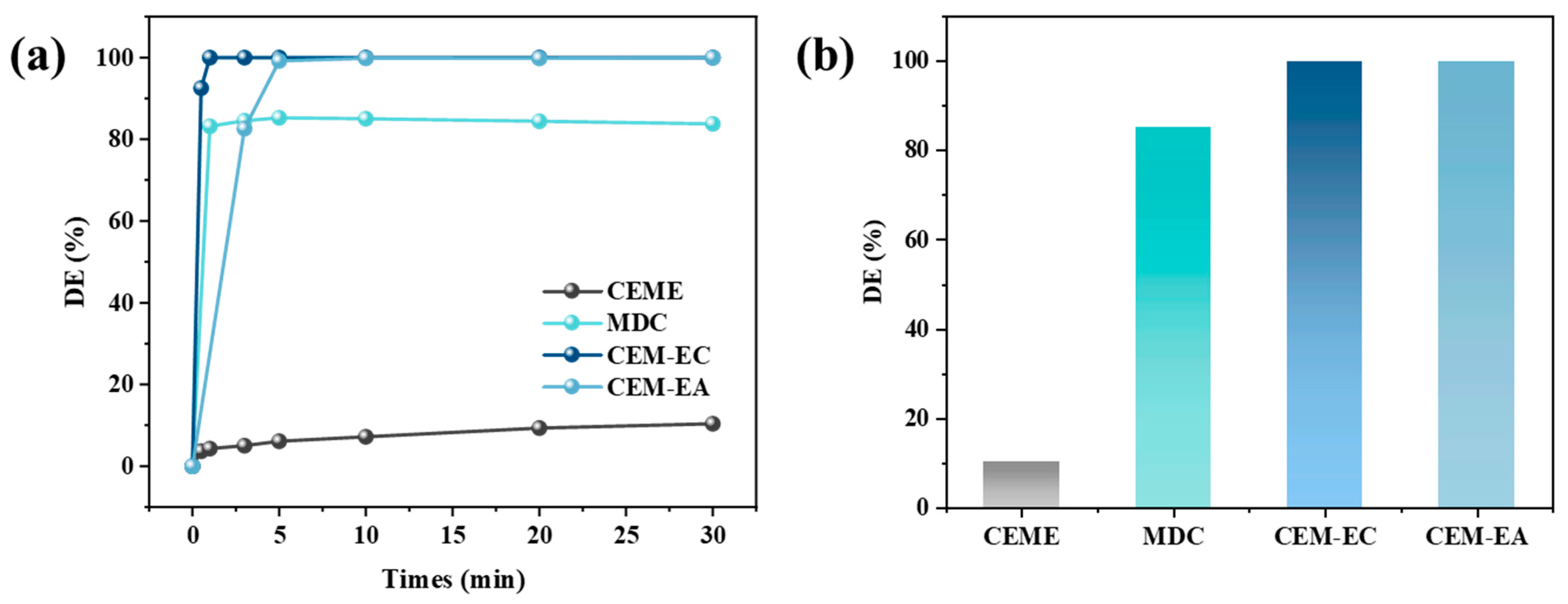
3.2.1. Dye Decolorization Performance of Systems CEME and MDC
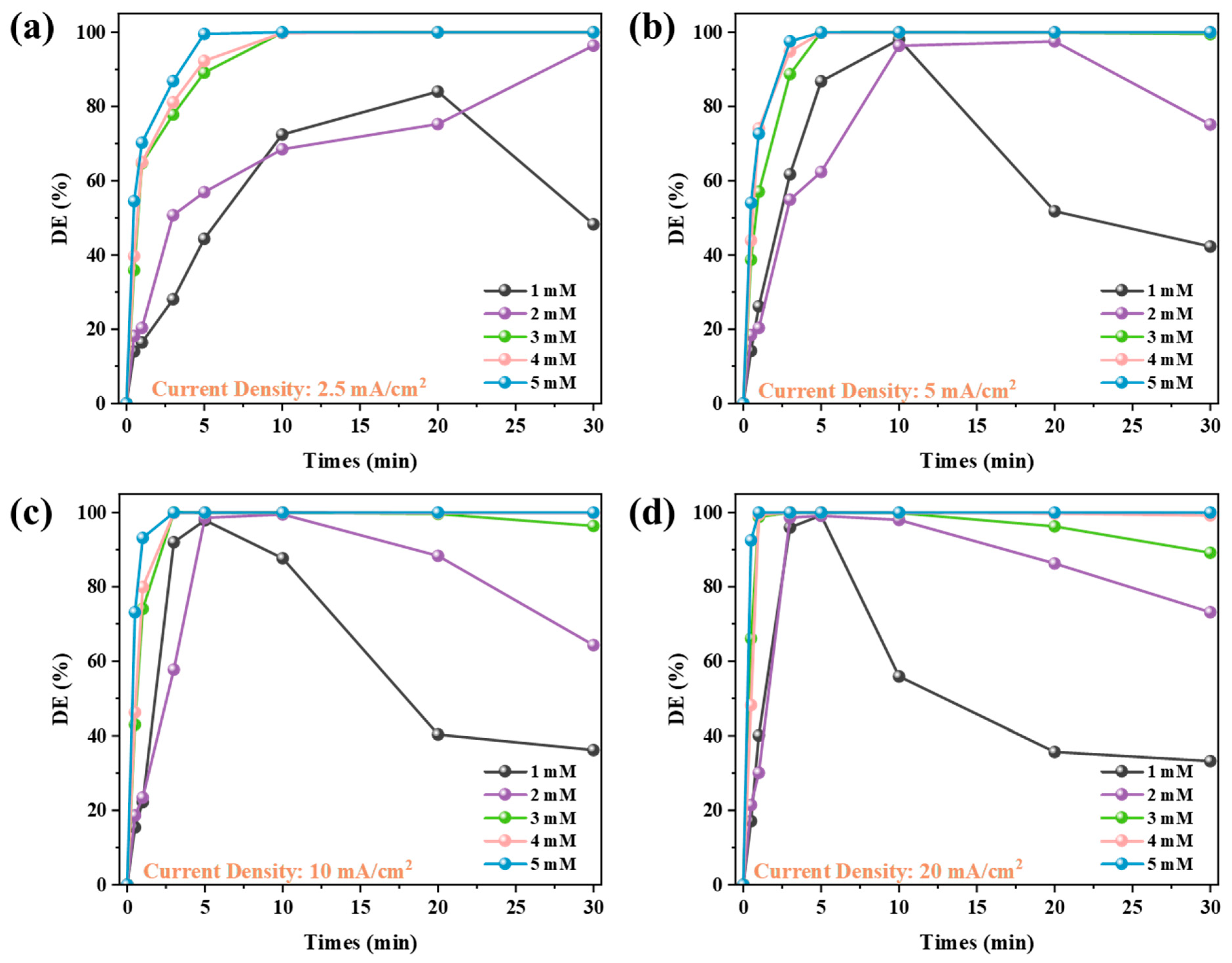
3.2.2. Dye Decolorization Performance of System CEM-EC
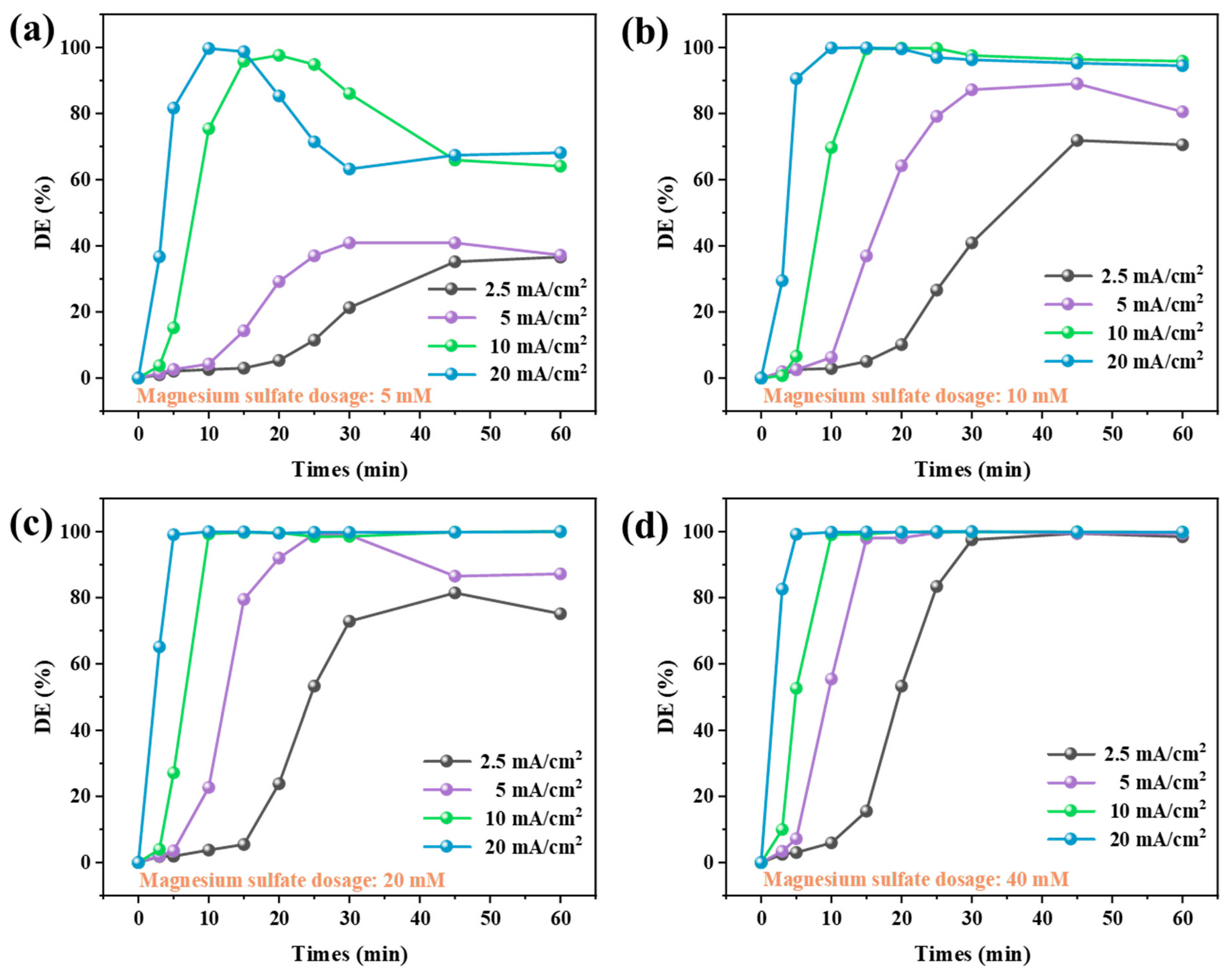
3.2.3. Dye Decolorization Performance of System CEM-EA
3.2.4. Comparison of Decolorization Performance of Four Different Systems
3.3. Precipitates Analysis and Cation Exchange Membranes Fouling
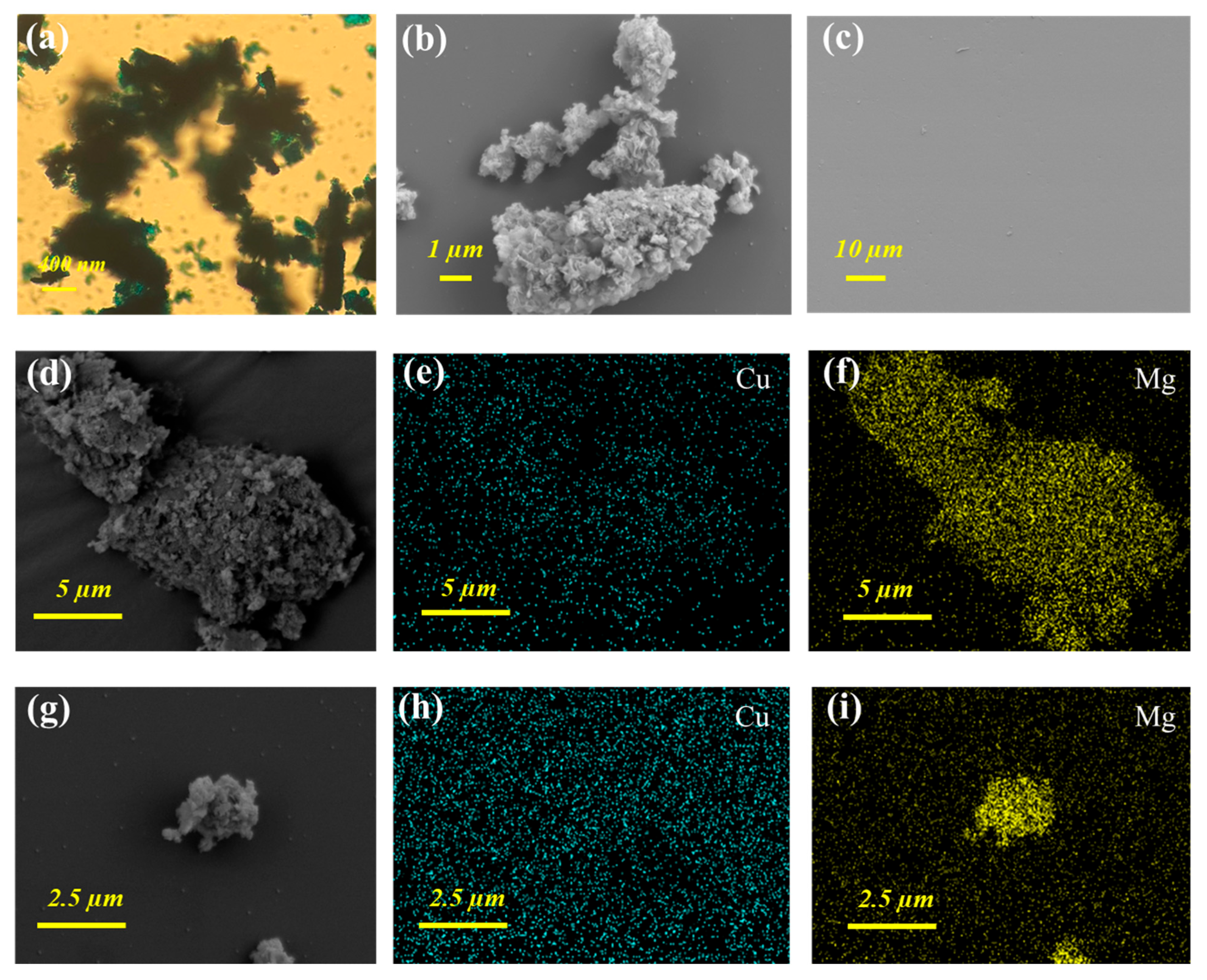
3.3.1. Morphology Characterizations of Mg(OH)2 Precipitates
3.3.2. Morphology Characterizations of the Fouling Cation Exchange Membrane
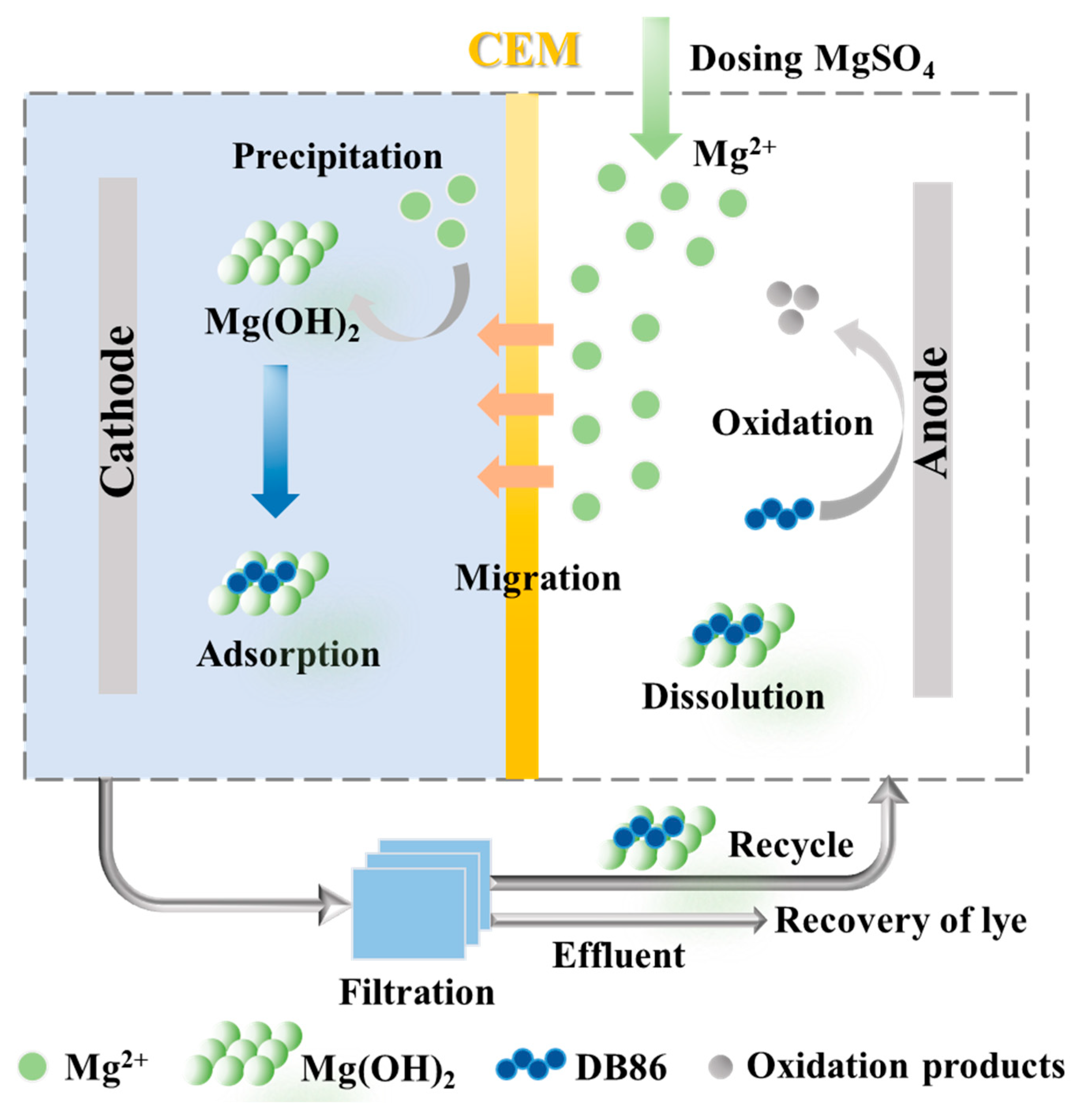
3.4. A Prospective Technology for Simultaneous Dye Degradation, Magnesium Salt Recovery and Lye Reuse
4. Conclusions
- (1)
- The adsorption of the dye DB86 by Mg (OH)2 matches the first-order kinetic model, as a process dominated by electrostatic interactions. The adsorption equilibrium is promptly reached within 5 min, demonstrating the feasibility of DB86 removal by Mg(OH)2 precipitation adsorption. During membrane electrolysis, the Mg(OH)2 colloid is positively and then negatively charged due to the pH variation in the cathode chamber. Hence, the adsorption process of DB86 by Mg(OH)2 might be followed by the desorption process.
- (2)
- Four different experimental systems were designed to investigate and compare the dye decolorization performance on alkaline textile wastewater. Without external Mg2+ addition, the max decolorization rate of CEME only reached 10.36%. The MDC achieved limited decolorization efficiency (max 85.24%) due to insufficient alkalizing agents in the single magnesium addition system. Remarkably, the CEM-EC achieved beyond 99% dye removal at 5 mM MgSO4, indicating exceptional treatment performance. Meanwhile, increasing MgSO4 dosage significantly enhanced decolorization efficiency, demonstrating a clear dose-dependent performance. Compared with CEM-EC, the CEM-EA enabled a reduction in the Mg2+ in the effluent water while guaranteeing the decolorization efficiency. The decolorization efficiency peak of CEM-EA reached its peak with a time delay due to the requirement for the transmembrane transport of Mg2+.
- (3)
- The morphology characterizations of Mg(OH)2 precipitates formed in the cathode chamber were observed to prove the adsorption co-precipitation process of dyes. The formed Mg(OH)2 particles were dense agglomerates of copper blue color, whose surface presented a thin lamellar layer facilitating dye adsorption. Additionally, only slight lumpy fouling was observed on the surface of the CEM after several electrolysis cycles. Analysis of contaminated CEM illustrated the stable decolorization efficiency of the CEM-EA system.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, X.X.; Tan, Y.H.; Huang, J.W.; Zhu, G.Z.; Yin, R.; Tao, X.M.; Tian, X. Industrialization of open- and closed-loop waste textile recycling towards sustainability: A review. J. Clean. Prod. 2024, 436, 140676. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Ding, X. Textile supply chain waste management in China. J. Clean. Prod. 2021, 289, 125147. [Google Scholar] [CrossRef]
- Halepoto, H.; Gong, T.; Memon, H. Current status and research trends of textile wastewater treatments-A bibliometric-based study. Front. Environ. Sci. 2022, 10, 1042256. [Google Scholar] [CrossRef]
- Islam, T.; Repon, M.R.; Islam, T.; Sarwar, Z.; Rahman, M.M.M. Impact of textile dyes on health and ecosystem: A review of structure, causes, and potential solutions. Environ. Sci. Pollut. R 2023, 30, 9207–9242. [Google Scholar] [CrossRef]
- Mukimin, A.; Vistanty, H.; Harihastuti, N.; Setianingsih, N.I.; Djayanti, S.; Nilawati; Astuti, Y. Hybrid Fenton-electrochemical reactor and system as post-treatment of textile wastewater. J. Water Process. Eng. 2024, 59, 105028. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, T.; Zhang, X.; Chen, R.; Zhu, N.; Li, L.; Zhao, L.; Li, Z.; Wang, Y.; Jiang, G. Occurrence and Ecological Risk Assessment of Highly Toxic Halogenated Byproducts during Chlorination Decolorization of Textile Printing and Dyeing Wastewater. Environ. Sci Technol. 2024, 58, 17970–17978. [Google Scholar] [CrossRef]
- Raman, C.D.; Kanmani, S. Textile dye degradation using nano zero valent iron: A review. J. Environ. Manag. 2016, 177, 341–355. [Google Scholar] [CrossRef]
- Chung, K.-T. Azo dyes and human health: A review. J. Environ. Sci. Health Part C-Environ. Carcinog. Ecotoxicol. Rev. 2016, 34, 233–261. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Periyasamy, A.P. Recent Advances in the Remediation of Textile-Dye-Containing Wastewater: Prioritizing Human Health and Sustainable Wastewater Treatment. Sustainability 2024, 16, 495. [Google Scholar] [CrossRef]
- Pazdzior, K.; Bilinska, L.; Ledakowicz, S. A review of the existing and emerging technologies in the combination of AOPs and biological processes in industrial textile wastewater treatment. Chem. Eng. J. 2019, 376, 120597. [Google Scholar] [CrossRef]
- Egbuikwem, P.N.; Mierzwa, J.C.; Saroj, D.P. Evaluation of aerobic biological process with post-ozonation for treatment of mixed industrial and domestic wastewater for potential reuse in agriculture. Bioresour. Technol. 2020, 318, 124200. [Google Scholar] [CrossRef]
- Shoukat, R.; Khan, S.J.; Jamal, Y. Hybrid anaerobic-aerobic biological treatment for real textile wastewater. J. Water Process. Eng. 2019, 29, 100804. [Google Scholar] [CrossRef]
- Donkadokula, N.Y.; Kola, A.K.; Naz, I.; Saroj, D. A review on advanced physico-chemical and biological textile dye wastewater treatment techniques. Rev. Environ. Sci. Bio-Technol. 2020, 19, 543–560. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abba, A.; Miino, M.C.; Damiani, S. Treatments for color removal from wastewater: State of the art. J. Environ. Manag. 2019, 236, 727–745. [Google Scholar] [CrossRef]
- Su, Y.-Y.; Yan, X.; Chen, Y.; Guo, X.-J.; Chen, X.-F.; Lang, W.-Z. Facile fabrication of COF-LZU1/PES composite membrane via interfacial polymerization on microfiltration substrate for dye/salt separation. J. Membr. Sci. 2021, 618, 118706. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, S.; Wang, D.; Zhang, N.; Shen, Y.; Wang, Z. N-Oxide Zwitterionic-Based Antifouling Loose Nanofiltration Membranes with Superior Water Permeance and Effective Dye/Salt Separation. Environ. Sci. Technol. 2025, 59, 5856–5865. [Google Scholar] [CrossRef]
- Hameed, B.H.; Lee, T.W. Degradation of malachite green in aqueous solution by Fenton process. J. Hazard Mater. 2009, 164, 468–472. [Google Scholar] [CrossRef]
- Hien, S.A.; Trellu, C.; Oturan, N.; Assemian, A.S.; Briton, B.G.H.; Drogui, P.; Adouby, K.; Oturan, M.A. Comparison of homogeneous and heterogeneous electrochemical advanced oxidation processes for treatment of textile industry wastewater. J. Hazard Mater. 2022, 437, 129326. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Wang, W.; Li, K.; Yang, W.; Lee, J.; Xie, B.; Wu, B.; Ren, H.; Hong, S.; Zhan, M. Controlling of irreversible fouling and mechanism in a hybrid ceramic membrane bioreactor (CMBR)-reverse osmosis (RO) process for textile wastewater reclamation. Desalination 2024, 586, 117914. [Google Scholar] [CrossRef]
- Punzi, M.; Anbalagan, A.; Borner, R.A.; Svensson, B.-M.; Jonstrup, M.; Mattiasson, B. Degradation of a textile azo dye using biological treatment followed by photo-Fenton oxidation: Evaluation of toxicity and microbial community structure. Chem. Eng. J. 2015, 270, 290–299. [Google Scholar] [CrossRef]
- Tan, B.H.; Teng, T.T.; Omar, A.K.M. Removal of dyes and industrial dye wastes by magnesium chloride. Water Res. 2000, 34, 597–601. [Google Scholar] [CrossRef]
- Pang, F.M.; Teng, S.P.; Teng, T.T.; Omar, A.K.M. Heavy Metals Removal by Hydroxide Precipitation and Coagulation-Flocculation Methods from Aqueous Solutions. Water Qual. Res. J. Can. 2009, 44, 174–182. [Google Scholar] [CrossRef]
- Tolonen, E.-T.; Ramo, J.; Lassi, U. The effect of magnesium on partial sulphate removal from mine water as gypsum. J. Environ. Manag. 2015, 159, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, L.F.; Salgueiro, A.A.; Melo, J.L.d.S.; Chiavone-Filho, O. Coagulation of indigo blue present in dyeing wastewater using a residual bittern. Sep. Purif. Technol. 2013, 104, 246–249. [Google Scholar] [CrossRef]
- Rasilingwani, T.E.; Gumbo, J.R.; Masindi, V.; Foteinis, S. Removal of Congo red dye from industrial effluents using metal oxide-clay nanocomposites: Insight into adsorption and precipitation mechanisms. Water Resour. Ind. 2024, 31, 100253. [Google Scholar] [CrossRef]
- Shen, C.; Pan, Y.; Wu, D.; Liu, Y.; Ma, C.; Li, F.; Ma, H.; Zhang, Y. A crosslinking-induced precipitation process for the simultaneous removal of poly(vinyl alcohol) and reactive dye: The importance of covalent bond forming and magnesium coagulation. Chem. Eng. J. 2019, 374, 904–913. [Google Scholar] [CrossRef]
- Vassallo, F.; La Corte, D.; Cancilla, N.; Tamburini, A.; Bevacqua, M.; Cipollina, A.; Micale, G. A pilot-plant for the selective recovery of magnesium and calcium from waste brines. Desalination 2021, 517, 115231. [Google Scholar] [CrossRef]
- Fontana, D.; Forte, F.; Pietrantonio, M.; Pucciarmati, S.; Marcoaldi, C. Magnesium recovery from seawater desalination brines: A technical review. Environ. Dev. Sustain. 2023, 25, 13733–13754. [Google Scholar] [CrossRef]
- Paidar, M.; Fateev, V.; Bouzek, K. Membrane electrolysis-History, current status and perspective. Electrochim. Acta 2016, 209, 737–756. [Google Scholar] [CrossRef]
- Lalia, B.S.; Hashaikeh, R. Electrochemical precipitation to reduce waste brine salinity. Desalination 2021, 498, 114796. [Google Scholar] [CrossRef]
- Wang, J.; Tang, X.; Liang, H.; Bai, L.; Xie, B.; Xing, J.; Wang, T.; Zhao, J.; Li, G. Efficient recovery of divalent metals from nanofiltration concentrate based on a hybrid process coupling single-cation electrolysis (SCE) with ultrafiltration (UF). J. Membr. Sci. 2020, 602, 117953. [Google Scholar] [CrossRef]
- Aghdam, M.A.; Zraick, F.; Simon, J.; Farrell, J.; Snyder, S.A. A novel brine precipitation process for higher water recovery. Desalination 2016, 385, 69–74. [Google Scholar] [CrossRef]
- Wang, J.; Jiao, J.; Duan, J.; Zheng, C.; Wu, C.; Luo, J.; Wang, H.; Zhang, H.; Tang, X.; Liang, H. Potential of divalent ion recovery from nanofiltration concentrate using hybrid ion exchange membrane processes with ettringite mineral transition: Separation performance, recovered precipitates and membrane fouling. Resour. Conserv. Recycl. 2024, 209, 107745. [Google Scholar] [CrossRef]
- Zaslavschi, I.; Shemer, H.; Hasson, D.; Semiat, R. Electrochemical CaCO3 scale removal with a bipolar membrane system. J. Membr. Sci. 2013, 445, 88–95. [Google Scholar] [CrossRef]
- Rogener, F.; Sartor, M.; Ban, A.; Buchloh, D.; Reichardt, T. Metal recovery from spent stainless steel pickling solutions. Resour. Conserv. Recycl. 2012, 60, 72–77. [Google Scholar] [CrossRef]
- Ahmed, D.N.; Naji, L.A.; Faisal, A.A.H.; Al-Ansari, N.; Naushad, M. Waste foundry sand/MgFe-layered double hydroxides composite material for efficient removal of Congo red dye from aqueous solution. Sci. Rep. 2020, 10, 2042. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.X.; Wang, L. Adsorption of dyes using magnesium hydroxide-modified diatomite. Desalination Water Treat. 2009, 8, 263–271. [Google Scholar] [CrossRef]
- Semerjian, L.; Ayoub, G.M. High-pH magnesium coagulation-flocculation in wastewater treatment. Adv. Environ. Res. 2003, 7, 389–403. [Google Scholar] [CrossRef]
- Candido, L.; Ponciano Gomes, J.A.C. Evaluation of anode materials for the electro-oxidation of ammonia and ammonium ions. Mater. Chem. Phys. 2011, 129, 1146–1151. [Google Scholar] [CrossRef]
- Yuan, Y.; Yin, W.; Huang, Y.; Feng, A.; Chen, T.; Qiao, L.; Cheng, H.; Liu, W.; Li, Z.; Ding, C.; et al. Intermittent electric field stimulated reduction-oxidation coupled process for enhanced azo dye biodegradation. Chem. Eng. J. 2023, 451, 138732. [Google Scholar] [CrossRef]
- Lopez-Espejel, M.; Gomez-Trevino, A.; Munoz-Flores, B.M.; Treto-Suarez, M.A.; Schott, E.; Paez-Hernandez, D.; Zarate, X.; Jimenez-Perez, V.M. Organotin Schiff bases as halofluorochromic dyes: Green synthesis, chemio-photophysical characterization, DFT, and their fluorescent bioimaging in vitro. J. Mater. Chem. B 2021, 9, 7698–7712. [Google Scholar] [CrossRef]
- Zeng, K.; Zhang, D. Recent progress in alkaline water electrolysis for hydrogen production and applications (vol 36, pg 307, 2010). Prog. Energy Combust. Sci. 2011, 37, 631. [Google Scholar] [CrossRef]
- Wei, X.; Kakimoto, T.; Umehara, Y.; Nakajima, H.; Ito, K.; Inagaki, H.; Mori, S. Improvement of the critical current density of alkaline water electrolysis based on the hydrodynamic similarity between boiling and water electrolysis. Int. J. Heat Mass Transf. 2023, 214, 124420. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, S.; Jin, W.; Mu, Y. A novel method based on near-infrared imaging spectroscopy and graph-learning to evaluate the dyeing uniformity of polyester yarn. Eng. Appl. Artif. Intell. 2024, 131, 107912. [Google Scholar] [CrossRef]
- Zou, G.; Liu, R.; Chen, W. Highly textural lamellar mesostructured magnesium hydroxide via a cathodic electrodeposition process. Mater. Lett. 2007, 61, 1990–1993. [Google Scholar] [CrossRef]
- Cifuentes-Araya, N.; Astudillo-Castro, C.; Bazinet, L. Mechanisms of mineral membrane fouling growth modulated by pulsed modes of current during electrodialysis: Evidences of water splitting implications in the appearance of the amorphous phases of magnesium hydroxide and calcium carbonate. J. Colloid Interface Sci. 2014, 426, 221–234. [Google Scholar] [CrossRef]
- Xu, T.; Wu, B.; Hou, L.; Zhu, Y.; Sheng, F.; Zhao, Z.; Dong, Y.; Liu, J.; Ye, B.; Li, X.; et al. Highly Ion-Permselective Porous Organic Cage Membranes with Hierarchical Channels. J. Am. Chem. Soc. 2022, 144, 10220–10229. [Google Scholar] [CrossRef]
- Ren, H.; Wang, Q.; Zhang, X.; Kang, R.; Shi, S.; Cong, W. Membrane fouling caused by amino acid and calcium during bipolar membrane electrodialysis. J. Chem. Technol. Biotechnol. 2008, 83, 1551–1557. [Google Scholar] [CrossRef]
- Aquino, J.M.; Rodrigo, M.A.; Rocha-Filho, R.C.; Saez, C.; Canizares, P. Influence of the supporting electrolyte on the electrolyses of dyes with conductive-diamond anodes. Chem. Eng. J. 2012, 184, 221–227. [Google Scholar] [CrossRef]
| Systems | Temperature (K) | Electrolysis Time (min) | Current Densities (mA/cm2) | MgSO4 Dosages (mM) |
|---|---|---|---|---|
| CEME | 298 | 30 | 2.5, 5, 10, 20 | - |
| MDC | 30 | - | 1, 2, 5, 10, 20, 40 | |
| CEM-EC | 30 | 2.5, 5, 10, 20 | 1, 2, 3, 4, 5 | |
| CEM-EA | 60 | 2.5, 5, 10, 20 | 5, 10, 20, 40 |
| First-Order Kinetic Fitting | Second-Order Kinetic Fitting | |||||
|---|---|---|---|---|---|---|
| Qe (mg/g) | k1 | R2 | Qe (mg/g) | k2 | k2qe | R2 |
| 308.7 | 2.3552 | 0.9896 | 308.7 | 7.900 | 0.2981 | 0.9865 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Cheng, N.; Jiang, R.; Jiao, J.; Chen, C.; Liang, J.; Hu, L.; Wang, H.; Wang, J. Potential of Textile Wastewater Decolorization Using Cation Exchange Membrane Electrolysis Coupled with Magnesium Salt Precipitation (CEM-MSP). Water 2025, 17, 1785. https://doi.org/10.3390/w17121785
Zhao Y, Cheng N, Jiang R, Jiao J, Chen C, Liang J, Hu L, Wang H, Wang J. Potential of Textile Wastewater Decolorization Using Cation Exchange Membrane Electrolysis Coupled with Magnesium Salt Precipitation (CEM-MSP). Water. 2025; 17(12):1785. https://doi.org/10.3390/w17121785
Chicago/Turabian StyleZhao, Yujing, Nuo Cheng, Ruihan Jiang, Jian Jiao, Chen Chen, Jiahao Liang, Longfeng Hu, Hesong Wang, and Jinlong Wang. 2025. "Potential of Textile Wastewater Decolorization Using Cation Exchange Membrane Electrolysis Coupled with Magnesium Salt Precipitation (CEM-MSP)" Water 17, no. 12: 1785. https://doi.org/10.3390/w17121785
APA StyleZhao, Y., Cheng, N., Jiang, R., Jiao, J., Chen, C., Liang, J., Hu, L., Wang, H., & Wang, J. (2025). Potential of Textile Wastewater Decolorization Using Cation Exchange Membrane Electrolysis Coupled with Magnesium Salt Precipitation (CEM-MSP). Water, 17(12), 1785. https://doi.org/10.3390/w17121785







