Seasonal Distribution of Microbial Community and n-Alkane Functional Genes in Diesel-Contaminated Groundwater: Influence of Water Table Fluctuation
Abstract
1. Introduction
2. Materials and Methods
2.1. Diesel Transient Leakage and Seasonal Water Table Fluctuation Simulation
2.2. Groundwater Sampling
2.3. Chemical and Geochemical Analysis
2.4. Molecular Analysis
3. Results
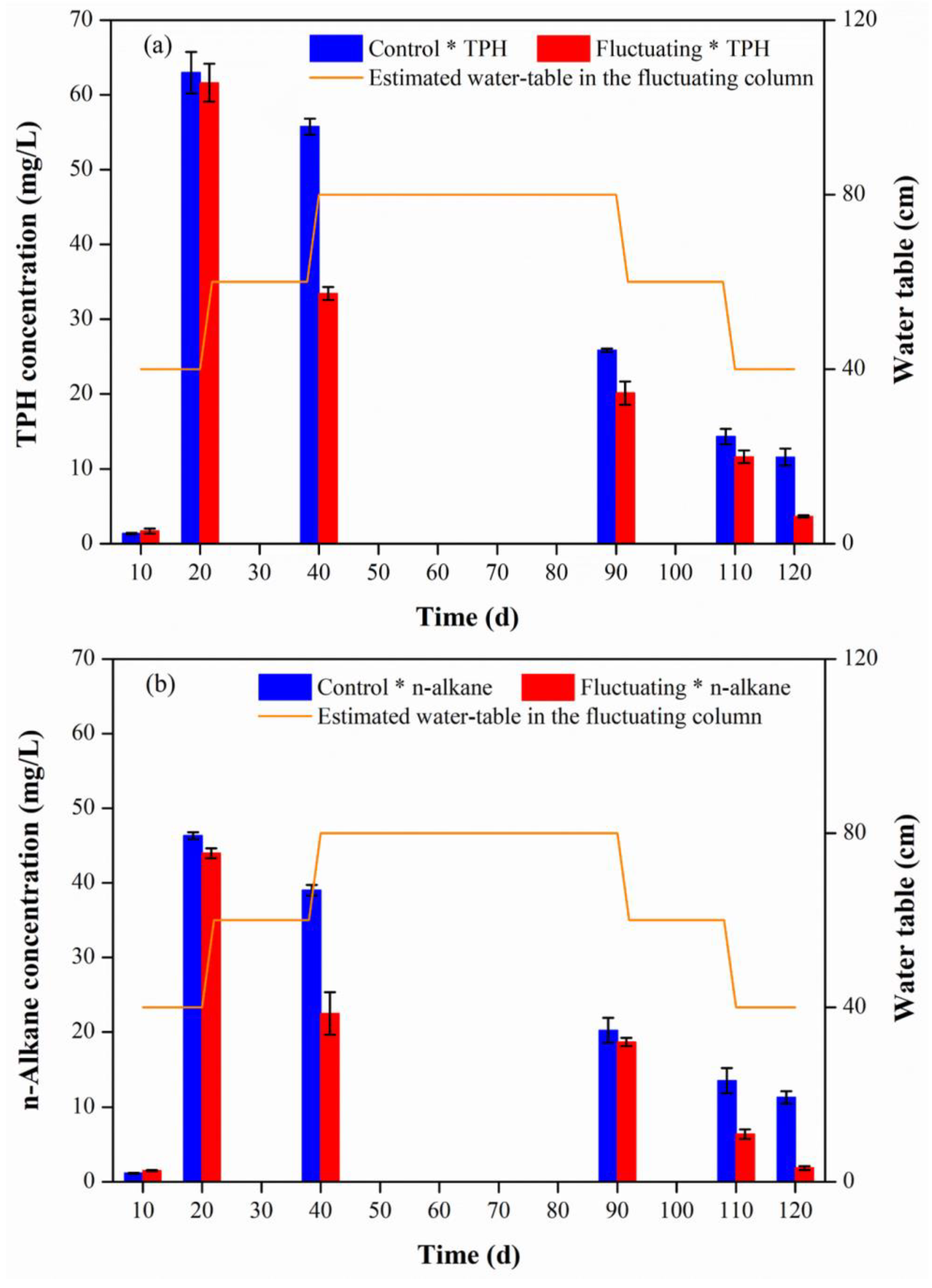
3.1. n-Alkane Concentration
3.2. Geochemical Properties
3.2.1. Temperature (T), pH
3.2.2. Dissolved Organic Carbon (DOC)
3.2.3. Dissolved Oxygen (DO), NO3− and SO42−
3.3. Bacterial Community Structure
3.4. n-Alkane Biodegradation Gene Abundance
3.5. Correlation Among Environmental Factors, Bacterial Communities and Functional Genes
4. Discussion
4.1. n-Alkane Attenuation of the Leakage Point During Water Table Fluctuation
4.2. Geochemical Footprint of the Leakage Point During Water Table Fluctuation
4.3. Temporal Distribution of Bacterial Community Structure
4.4. Variations in Composition of Functional Genes in Response to Surrounding Environment
4.5. Identifying Main Factors Driving Succession of Functional Potential
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nelson, R.K.; Aeppli, C.; Arey, J.S.; Chen, H.; de Oliveira, A.H.; Eiserbeck, C.; Frysinger, G.S.; Gaines, R.B.; Grice, K.; Gros, J. Applications of comprehensive two-dimensional gas chromatography (GC × GC) in studying the source, transport, and fate of petroleum hydrocarbons in the environment. In Handbook of Oil Spill Environmental Forensics: Fingerprinting and Source Identification, 2nd ed.; Stout, S., Wang, Z.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 399–448. [Google Scholar]
- Haider, F.U.; Ejaz, M.; Cheema, S.A.; Khan, M.I.; Zhao, B.; Liqun, C.; Salim, M.A.; Naveed, M.; Khan, N.; Núñez-Delgado, A.; et al. Phytotoxicity of petroleum hydrocarbons: Sources, impacts and remediation strategies. Environ. Res. 2021, 197, 111031. [Google Scholar] [CrossRef]
- Stafford, B.P.; Rixey, W.G. Distribution of fuel-grade ethanol near a dynamic water table. Ground Water Monit. Remediat. 2011, 31, 55–60. [Google Scholar]
- Haberer, C.M.; Roy, J.W.; Smith, J.E. Patterns of entrapped air dissolution in a two-dimensional pilot-scale synthetic aquifer. Groundwater 2015, 53, 271–281. [Google Scholar]
- Borer, B.; Tecon, R.; Or, D. Spatial organization of bacterial populations in response to oxygen and carbon counter-gradients in pore networks. Nat. Commun. 2018, 9, 769. [Google Scholar] [CrossRef]
- Ning, Z.; Zhang, M.; He, Z.; Cai, P.P.; Guo, C.J.; Wang, P. Spatial pattern of bacterial community diversity formed in different groundwater field corresponding to electron donors and acceptors distributions at a petroleum-contaminated site. Water 2018, 10, 842. [Google Scholar] [CrossRef]
- Gkorezis, P.; Daghio, M.; Franzetti, A.; van Hamme, J.D.; Sillen, W.; Vangronsveld, J. The interaction between plants and bacteria in the remediation of petroleum hydrocarbons: An environmental perspective. Front. Microbiol. 2016, 7, 1836. [Google Scholar] [CrossRef]
- Kloos, K.; Munch, J.C.; Schloter, M. A new method for the detection of alkanemonooxygenase homologous genes (alkB) in soils based on PCR-hybridization. J. Microbiol. Methods 2006, 66, 486–496. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Seo, Y.; Ha, M.; Lee, J.; Yang, H.; Cho, K.S. Evaluation of rhizoremediation and methane emission in diesel-contaminated soil cultivated with tall fescue (Festuca arundinacea). Environ. Res. 2021, 194, 110606. [Google Scholar] [CrossRef]
- Tourova, T.P.; Sokolova, D.S.; Semenova, E.M.; Shumkova, E.S.; Korshunova, A.V.; Babich, T.L.; Poltaraus, A.B.; Nazina, T.N. Detection of n-alkane biodegradation genes alkB and ladA in thermophilic hydrocarbon-oxidizing bacteria of the genera Aeribacillus and Geobacillus. Microbiology 2016, 85, 693–707. [Google Scholar] [CrossRef]
- Jurelevicius, D.; Alvarez, V.M.; Peixoto, R.; Rosado, A.S.; Seldin, L. The use of a combination of alkB primers to better characterize the distribution of alkanedegrading bacteria. PLoS ONE 2013, 8, e66565. [Google Scholar] [CrossRef]
- Ji, J.H.; Liu, Y.F.; Zhou, L.; Mbadinga, S.M.; Pan, P.; Chen, J.; Liu, J.F.; Yang, S.Z.; Sand, W.; Gu, J.D.; et al. Methanogenic degradation of long n-alkanes requires fumarate-dependent activation. Appl. Environ. Microbiol. 2019, 85, e00985-19. [Google Scholar] [CrossRef]
- Ma, H.; Yan, W.; Xiao, X.; Shi, G.; Li, Y.; Sun, B.; Dou, Y.; Zhang, Y. Ex situ culturing experiments revealed psychrophilic hydrogentrophic methanogenesis being the potential dominant methane-producing pathway in subglacial sediment in Larsemann Hills. Antarctic. Front. Microbiol. 2018, 9, 237. [Google Scholar] [CrossRef] [PubMed]
- Rezanezhad, F.; Couture, R.M.; Kovac, R.; O’Connell, D.; van Cappellen, P. Water table fluctuations and soil biogeochemistry: An experimental approach using an automated soil column system. J. Hydrol. 2014, 509, 245–256. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Ding, A.Z.; Sun, Y.J.; Xia, X.F.; Zhang, D.Y. Impacts of n-alkane concentration on soil bacterial community structure and alkane monooxygenase genes abundance during bioremediation processes. Front. Environ. Sci. Eng. 2018, 12, 3. [Google Scholar] [CrossRef]
- Rühle, F.A.; Von Netzer, F.; Lueders, T.; Stumpp, C. Response of transport parameters and sediment microbiota to water table fluctuations in laboratory columns. Vadose Zone J. 2015, 14, 12. [Google Scholar] [CrossRef]
- Xia, X.F.; Stewart, D.I.; Cheng, L.R.; Wang, K.; Li, J.; Zhang, D.; Ding, A.Z. Changes in groundwater bacterial community during cyclic groundwater-table variations. Hydrol. Process. 2020, 34, 4973–4984. [Google Scholar] [CrossRef]
- Tu, F.; Zhang, Y.N.; Xu, S.K.; Yang, X.T.; Zhou, L.; Ge, X.N.; Han, j.; Guo, X.; Yang, H.C. Detection of pseudorabies virus with a real-time recombinase-aided amplification assay. Transbound Emerg. Dis. 2022, 69, 2266–2274. [Google Scholar] [CrossRef]
- Yin, F.B.; Dong, H.M.; Zhang, W.Q.; Zhu, Z.P.; Shang, B. Antibiotic degradation and microbial community structures during acidification and methanogenesis of swine manure containing chlortetracycline or oxytetracycline. Bioresour. Technol. 2018, 250, 247–255. [Google Scholar] [CrossRef]
- Edgar, R.C. SEARCH_16S: A new algorithm for identifying 16S ribosomal RNA genes in contigs and chromosomes. bioRxiv 2017, 124131. [Google Scholar]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Zhang, D.; Cui, R.Y.; Fu, B.; Yang, Y.X.; Wang, P.L.; Mao, Y.T.; Chen, A.Q.; Lei, B.K. Shallow groundwater table fluctuations affect bacterial communities and nitrogen functional genes along the soil profile in a vegetable field. Appl. Soil Ecol. 2020, 146, 103–368. [Google Scholar] [CrossRef]
- Xi, H.; Shen, J.L.; Qu, Z.; Yang, D.Y.; Liu, S.M.; Nie, X.H.; Zhu, L.F. Effects of long-term cotton continuous cropping on soil microbiome. Sci. Rep. 2019, 9, 18297. [Google Scholar] [CrossRef]
- Xia, X.F.; Stewart, D.I.; Cheng, L.R.; Liu, Y.Q.; Wang, Y.Y.; Ding, A.Z. Variation of bacterial community and alkane monooxygenase gene abundance in diesel n-alkane contaminated subsurface environment under seasonal water table fluctuation. J. Contam. Hydrol. 2022, 248, 104017. [Google Scholar] [CrossRef]
- Kechavarzi, C.; Soga, K.; Illangasekare, T.H. Two-dimensional laboratory simulation of LNAPL infiltration and redistribution in the vadose zone. J. Contam. Hydrol. 2005, 76, 211–233. [Google Scholar] [CrossRef]
- Powers, S.E.; Anckner, W.H.; Seacord, T.F. Wettability of NAPL-contaminated sands. J. Environ. Eng. 1996, 122, 889–896. [Google Scholar] [CrossRef]
- Oppong-Anane, A.B.; Quinones, K.Y.D.; Harris, W.; Townsend, T.; Bonzongo, J.C.J. Iron reductive dissolution in vadose zone soils: Implication for groundwater pollution in landfill impacted sites. Appl. Geochem. 2018, 94, 21–27. [Google Scholar] [CrossRef]
- Malik, A.; Gleixner, G. Importance of microbial soil organic matter processing in dissolved organic carbon production. FEMS Microbiol. Ecol. 2013, 86, 139–148. [Google Scholar] [CrossRef]
- Abdelrady, A.; Bachwenkizi, J.; Sharma, S.; Sefelnasr, A.; Kennedy, M. The fate of heavy metals during bank filtration: Effect of dissolved organic matter. J. Water Process Eng. 2020, 38, 101563. [Google Scholar] [CrossRef]
- Huang, W.; Hall, S.J. Elevated moisture stimulates carbon loss from mineral soils by releasing protected organic matter. Nat. Commun. 2017, 8, 1774. [Google Scholar] [CrossRef]
- Robinson, C. Microbial respiration, the engine of ocean deoxygenation. Front. Mar. Sci. 2019, 5, 533. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, J.; Mostofa, K.M.G.; Zheng, W.; Liu, C.Q.; Senesi, N.; Senesi, G.S.; Vione, D.; Yuan, J.; Liu, Y.; et al. Sulfur-mediated transformation, export and mineral complexation of organic and inorganic C, N, P and Si in dryland soils. Sci. Rep. 2025, 15, 9850. [Google Scholar] [CrossRef]
- Marwanto, S.; Watanabe, T.; Iskandar, W.; Sabiham, S.; Funakawa, S. Effects of seasonal rainfall and water table movement on the soil solution composition of tropical peatland. Soil Sci. Plant Nutr. 2018, 64, 386–395. [Google Scholar] [CrossRef]
- Wei, Y.Q.; Chen, Y.L.; Cao, X.D.; Xiang, M.H.; Huang, Y.; Li, H. A critical review of groundwater table fluctuation: Formation, effects on multifields, and contaminant behaviors in a soil and aquifer system. Environ. Sci. Technol. 2024, 58, 2185–2203. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, X.B.; Li, C.C.; Luo, W.T.; Wang, Y.X.; Luo, X.S. Seasonal dynamics and interaction of shallow groundwater geochemical properties and microbial community patterns. Chem. Geol. 2023, 638, 121703. [Google Scholar] [CrossRef]
- Ehiosun, K.I.; Godin, S.; Urios, L.; Lobinski, R.; Grimaud, R. Degradation of long-chain alkanes through biofilm formation by bacteria isolated from oil-polluted soil. Int. Biodeterior. Biodegrad. 2022, 175, 105508. [Google Scholar] [CrossRef]
- Santana-Pereira, A.L.R.; Moen, F.S.; Severance, B.; Liles, M.R. Influence of soil nutrients on the presence and distribution of CPR bacteria in a long-term crop rotation experiment. Front. Microbiol. 2023, 14, 1114548. [Google Scholar] [CrossRef]
- Pavlova, O.N.; Izosimova, O.N.; Chernitsyna, S.M.; Ivanov, V.G.; Pogodaeva, T.V.; Khabuev, A.V.; Gorshkov, A.G.; Zemskaya, T.I. Anaerobic oxidation of petroleum hydrocarbons in enrichment cultures from sediments of the Gorevoy Utes natural oil seep under methanogenic and sulfate-reducing conditions. Microb. Ecol. 2022, 83, 899–915. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Wang, M.H.; Wang, L.S.; Zheng, W.T.; Zeng, Q.N.; Wang, K. Comparison of the basic processes of aerobic, anaerobic, and aerobic-anaerobic coupling composting of Chinese medicinal herbal residues. Bioresour. Technol. 2023, 379, 128996. [Google Scholar] [CrossRef]
- Sutton, N.B.; Maphosa, F.; Morillo, J.A.; Abu Al-Soud, W.; Langenhoff, A.A.M.; Grotenhuis, T.; Rijnaarts, H.H.M.; Smidt, H. Impact of long-term diesel contamination on soil microbial community structure. Appl. Environ. Microbiol. 2013, 79, 619–630. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, Y.Y.; Cho, K.S. Effect of Novosphingobium sp. CuT1 inoculation on the rhizoremediation of heavy metal- and diesel-contaminated soil planted with tall fescue. Environ. Sci. Pollut. Res. 2023, 30, 16612–16625. [Google Scholar] [CrossRef]
- Neethu, C.S.; Saravanakumar, C.; Purvaja, R.; Robin, R.S.; Ramesh, R. Oil-spill triggered shift in indigenous microbial structure and functional dynamics in different marine environmental matrices. Sci. Rep. 2019, 9, 1354. [Google Scholar] [CrossRef]
- Huang, Y.L.; Lu, Z.J.; Jiang, T.T.; Zeng, Y.H.; Zeng, Y.H.; Chen, B.L. Oxygen availability affects the synthesis of quorum sensing signal in the facultative anaerobe Novosphingobium Pentaromativorans US6-1. Appl. Microbiol. Biot. 2021, 105, 1191–1201. [Google Scholar] [CrossRef]
- Blees, J.; Niemann, H.; Wenk, C.B.; Zopfi, J.; Schubert, C.J.; Kirf, M.K.; Veronesi, M.L.; Hitz, C.; Lehmann, M.F. Micro-aerobic bacterial methane oxidation in the chemocline and anoxic water column of deep south-Alpine Lake Lugano (Switzerland). Limnol. Oceanogr. 2014, 59, 311–324. [Google Scholar] [CrossRef]
- Brunhoferova, H.; Venditti, S.; Laczny, C.C.; Lebrun, L.; Hansen, J. Bioremediation of 27 Micropollutants by Symbiotic Microorganisms of Wetland Macrophytes. Sustainability 2022, 14, 3944. [Google Scholar] [CrossRef]
- Iturbe-Espinoza, P.; Brown, D.M.; Weedon, J.T.; Braster, M.; Brandt, B.W.; Bonte, M. Microbial communities associated with landfarming amendments during bioremediation of crude oil in Niger Delta soils. Appl. Soil Ecol. 2023, 191, 105058. [Google Scholar] [CrossRef]
- Garrido-Sanz, D.; Redondo-Nieto, M.; Guirado, M.; Jiménez, O.P.; Millán, R.; Martin, M.; Rivilla, R. Metagenomic insights into the bacterial functions of a diesel-degrading consortium for the rhizoremediation of diesel-polluted soil. Genes 2019, 10, 456. [Google Scholar] [CrossRef]
- Yang, S.; Wen, X.; Zhao, L.; Shi, Y.; Jin, H. Crude oil treatment leads to shift of bacterial communities in soils from the deep active layer and upper permafrost along the China-Russia crude oil pipeline route. PLoS ONE 2014, 9, e96552. [Google Scholar] [CrossRef]
- Cébron, A.; Borreca, A.; Beguiristain, T.; Biache, C.; Faure, P. Taxonomic and functional trait-based approaches suggest that aerobic and anaerobic soil microorganisms allow the natural attenuation of oil from natural seeps. Sci. Rep. 2022, 12, 7245. [Google Scholar]
- Deng, Y.; Deng, C.; Yang, J.; Li, B.; Wang, E.; Yuan, H. Novel butane-oxidizing bacteria and diversity of bmoX genes in Puguang gas field. Front. Microbiol. 2018, 9, 1576. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X.; Li, Y.; Song, G.; Zhao, L. Understanding the variation of bacteria in response to summertime oxygen depletion in water column of Bohai Sea. Front. Microbiol. 2022, 13, 890973. [Google Scholar] [CrossRef]
- Humbert, J.F.; Dorigo, U. Biodiversity and aquatic ecosystem functioning: A mini review. Aquat. Ecosyst. Health Manag. 2005, 8, 367–374. [Google Scholar] [CrossRef]
- Cavelan, A.; Faure, P.; Lorgeoux, C.; Colombano, S.; Deparis, J.; Davarzani, D. An experimental multi-method approach to better characterize the LNAPL fate in soil under fluctuating groundwater levels. J. Contam. Hydrol. 2024, 262, 104319. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Sun, Y.J.; Yu, J.S.; Xia, X.F.; Ding, A.Z.; Zhang, D.Y. Impacts of groundwater level fluctuation on soil microbial community, alkane degradation efficiency and alkane-degrading gene diversity in the critical zone: Evidence from an accelerated water table fluctuation simulation. Environ. Sci. Pollut. Res. 2022, 29, 83060–83070. [Google Scholar] [CrossRef]
- Kundu, A.; Harrisson, O.; Ghoshal, S. Impacts of Arctic diesel contamination on microbial community composition and degradative gene abundance during hydrocarbon biodegradation with and without nutrients: A case study of seven sub-Arctic soils. Sci. Total Environ. 2023, 871, 161777. [Google Scholar] [CrossRef]
- Pérez-de-Mora, A.; Engel, M.; Schloter, M. Abundance and diversity of n-alkane-degrading bacteria in a forest soil co-contaminated with hydrocarbons and metals: A molecular study on alkB homologous genes. Microb. Ecol. 2011, 62, 959–972. [Google Scholar] [CrossRef]
- Harindintwali, J.D.; Xiang, L.; Wang, F.; Chang, S.X.; Zhao, Z.; Mei, Z.; Tiedje, J.M. Syntrophy of bacteria and archaea in the anaerobic catabolism of hydrocarbon contaminants. Crit. Rev. Environ. Sci. Technol. 2022, 53, 1331–1357. [Google Scholar] [CrossRef]
- Kane, E.S.; Chivers, M.R.; Turetsky, M.R.; Treat, C.C.; Petersen, D.G.; Waldrop, M.; Harden, J.W.; McGuire, A.D. Response of anaerobic carbon cycling to water table manipulation in an Alaskan rich fen. Soil Biol. Biochem. 2013, 58, 50–60. [Google Scholar] [CrossRef]
- Qiu, H.S.; Liu, J.Y.; Boorboori, M.R.; Li, D.; Chen, S.; Ma, X. Effect of biochar application rate on changes in soil labile organic carbon fractions and the association between bacterial community assembly and carbon metabolism with time. Sci. Total Environ. 2023, 855, 158876. [Google Scholar] [CrossRef]
- Li, W.; Wang, L.Y.; Duan, R.Y.; Liu, J.F.; Gu, J.D.; Mu, B.Z. Microbial community characteristics of petroleum reservoir production water amended with n-alkanes and incubated under nitrate-, sulfate-reducing and methanogenic conditions. Int. Biodeterior. Biodegrad. 2012, 69, 87–96. [Google Scholar] [CrossRef]
- Xu, J.L.; Xu, L.; Qiao, X.; Zheng, Y.Y.; Xie, Y.L.; Yang, Z.L. Stimulated biodegradation of all alkanes in soil. Chemosphere 2021, 278, 130444. [Google Scholar] [CrossRef]
- She, Z.X.; Wang, J.; He, C.; Pan, X.; Shi, Q.; Shao, R. New insights into microbial interactions with dissolved organic matter in acid mine drainage with the integration of microbial community and chemical composition analysis. ACS EST Water 2022, 2, 278–287. [Google Scholar] [CrossRef]
- Samkov, A.A.; Volchenko, N.N.; Musorina, T.N.; Kruglova, M.N.; Samkova, S.M.; Khudokormov, A.A. Biodegradation of n-alkanes in oil-contaminated bottom sediments under bioelectrochemical stimulation. Microbiology 2024, 93, 314–323. [Google Scholar] [CrossRef]
- Kuhn, E.; Bellicanta, G.S.; Pellizari, V.H. New alk genes detected in Antarctic marine sediments. Environ. Microbiol. 2009, 11, 669–673. [Google Scholar] [CrossRef]
- Marchant, R.; Sharkey, F.H.; Banat, I.M.; Rahman, T.J.; Perfumo, A. The degradation of n-hexadecane in soil by thermophilic geobacilli. FEMS Microbiol. Ecol. 2006, 56, 44–54. [Google Scholar] [CrossRef]
- Wang, X.Z.; Zhao, X.H.; Li, H.B.; Jia, J.L.; Liu, Y.Q.; Ejenavi, O.; Ding, A.; Sun, Y.; Zhang, D. Separating and characterizing functional alkane degraders from crude-oil-contaminated sites via magnetic nanoparticle mediated isolation. Res. Microbiol. 2016, 167, 731–744. [Google Scholar] [CrossRef]
- Oberding, L.K.; Gieg, L.M. Methanogenic Paraffin Biodegradation: Alkylsuccinate Synthase Gene Quanttification and Dicarboxylic Acid Production. Appl. Environ. Microbiol. 2018, 84, e01773-17. [Google Scholar] [CrossRef]
- Johnson, J.M.; Wawrik, B.; Isom, C.; Boling, W.B.; Callaghan, A.V. Interrogation of Chesapeake Bay sediment microbial communities for intrinsic alkane-utilizing potential under anaerobic conditions. FEMS Microbiol. Ecol. 2015, 91, 1–14. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, X.; Jia, W.; Wang, K.; Ding, A. Seasonal Distribution of Microbial Community and n-Alkane Functional Genes in Diesel-Contaminated Groundwater: Influence of Water Table Fluctuation. Water 2025, 17, 1710. https://doi.org/10.3390/w17111710
Xia X, Jia W, Wang K, Ding A. Seasonal Distribution of Microbial Community and n-Alkane Functional Genes in Diesel-Contaminated Groundwater: Influence of Water Table Fluctuation. Water. 2025; 17(11):1710. https://doi.org/10.3390/w17111710
Chicago/Turabian StyleXia, Xuefeng, Wenjuan Jia, Kai Wang, and Aizhong Ding. 2025. "Seasonal Distribution of Microbial Community and n-Alkane Functional Genes in Diesel-Contaminated Groundwater: Influence of Water Table Fluctuation" Water 17, no. 11: 1710. https://doi.org/10.3390/w17111710
APA StyleXia, X., Jia, W., Wang, K., & Ding, A. (2025). Seasonal Distribution of Microbial Community and n-Alkane Functional Genes in Diesel-Contaminated Groundwater: Influence of Water Table Fluctuation. Water, 17(11), 1710. https://doi.org/10.3390/w17111710




