Multiscale Characterization of Anammox Granules and Microbial Migration Under Variable Nitrogen Loading Rates
Abstract
1. Introduction
2. Material and Methods
2.1. Experiment Setup
2.2. Reactor Operation
2.3. Synthetic Wastewater and Inoculum Sludge
2.4. Properties of AnGS with Different Particle Sizes
2.5. Analytical and Calculative Methods
2.6. Microbal Community Analysis
3. Results and Discussion
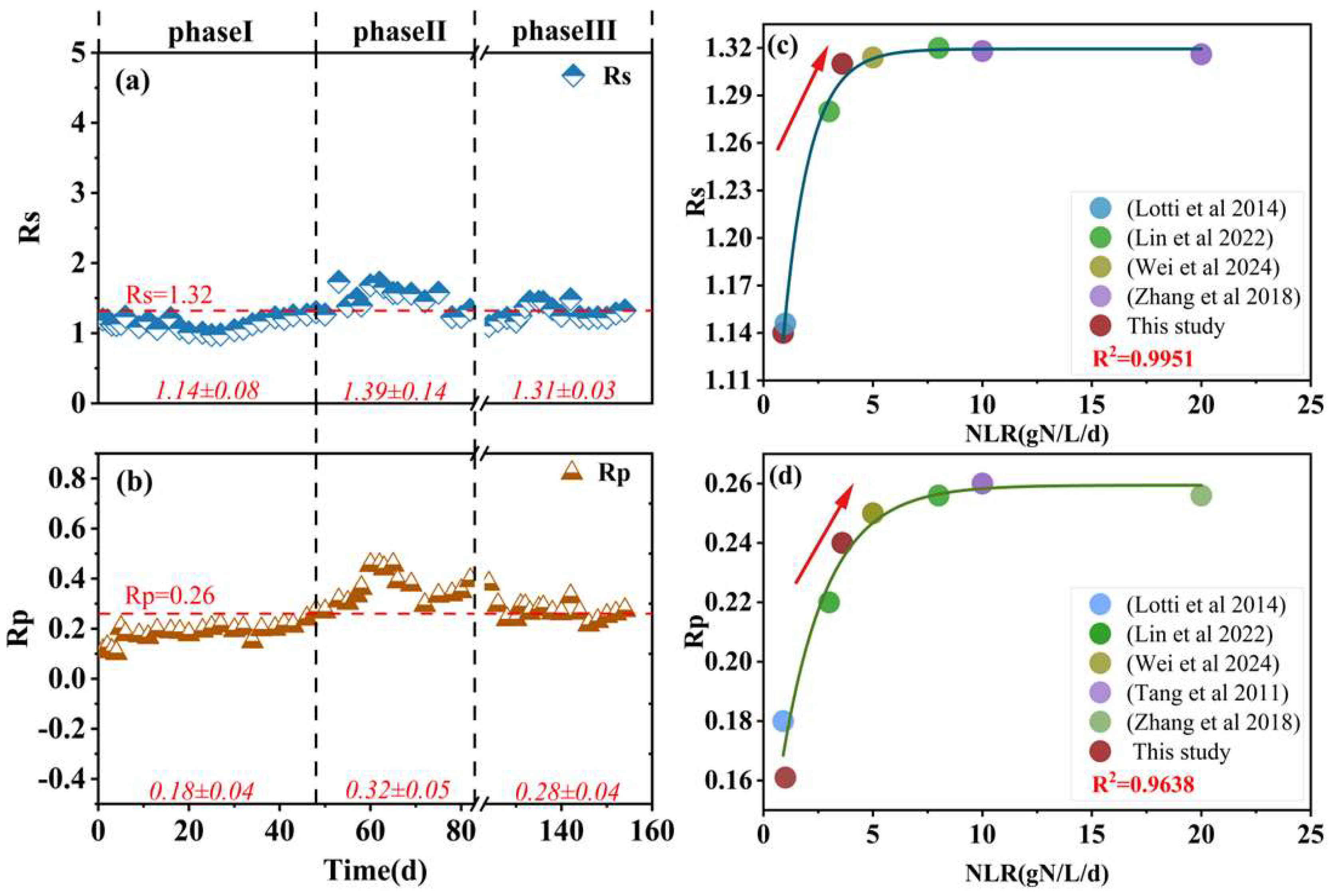
3.1. Overall Performance
3.2. Physicochemical Properties of AnGS
3.2.1. Morphology of AnGS at Different Sizes
3.2.2. Percentage of Granular Sludge
3.2.3. Sludge Settling Velocity
3.2.4. Specific Anammox Activity
3.3. Microbial Community
3.3.1. Taxonomic Results
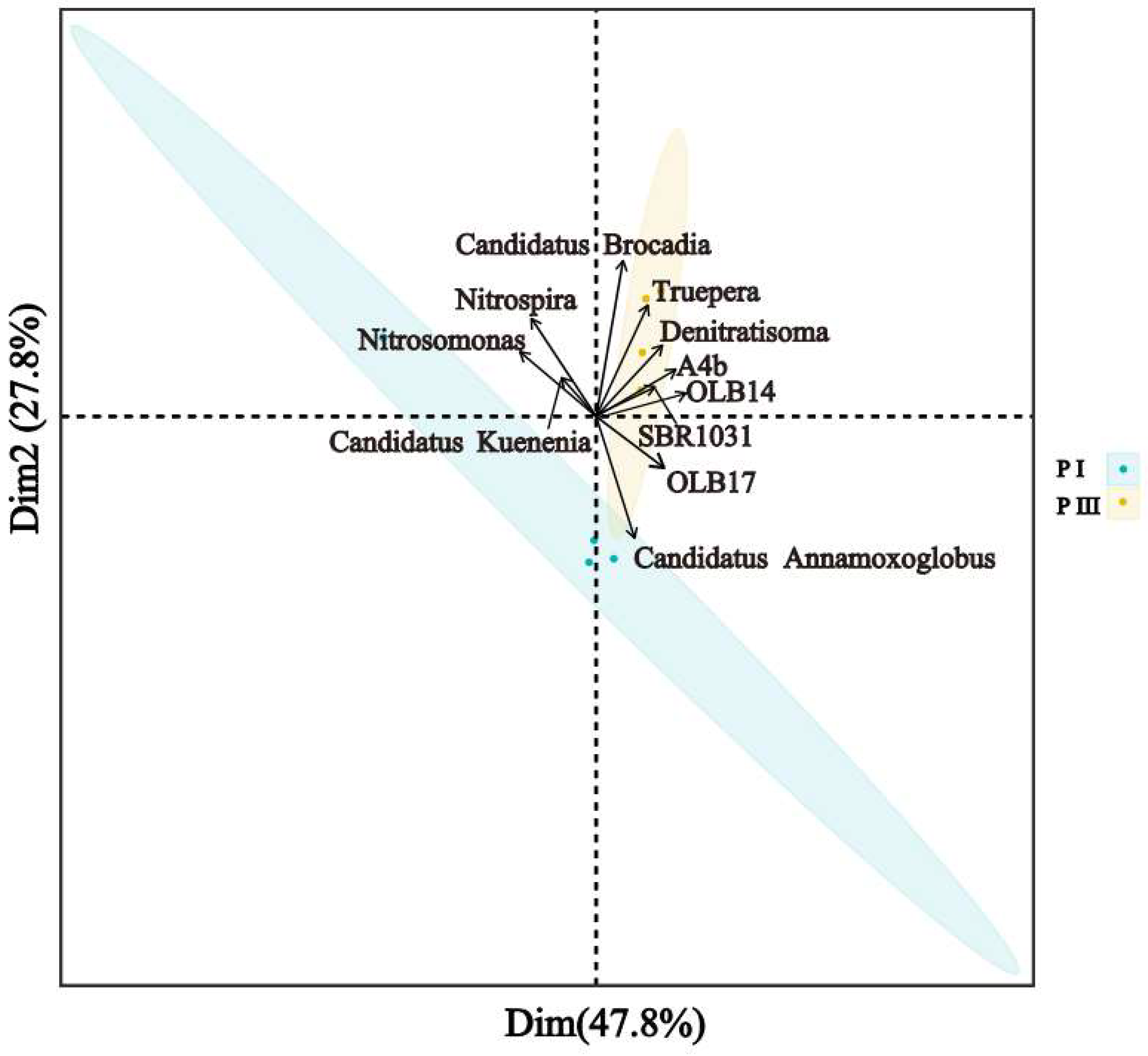
3.3.2. Interrelationships Between Major Microbial Communities
3.3.3. Pearson’s Correlation Analysis Between Genera and Environmental Factors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| VSS | volatile suspended solids |
| SS | suspended solids |
| HRT | hydraulic retention time |
| TN | total nitrogen |
| Anammox | anaerobic ammonium oxidation |
| AnAOB | anammox bacteria |
| NLR | nitrogen loading rate |
| HAP | hydroxyapatite |
| SAA | specific anammox activity |
| TNRE | total nitrogen removal efficiency |
| PCA | principal component analysis |
| SV | settling velocity |
References
- Yang, H. Let the Olympics serve as warning for water quality. Nat. Water 2024, 2, 912. [Google Scholar] [CrossRef]
- Yang, H.; Xie, P.; Ni, L.; Flower, R.J. Pollution in the Yangtze. Science 2012, 337, 410. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Wang, W.; Wang, X.; Yang, H.; Tang, J.; Li, Y.; Shi, J.; Wang, S. Performance and mechanism of nano Fe-Al bimetallic oxide enhanced constructed wetlands for the treatment of Cr(VI)-contaminated wastewater. Environ. Res. 2025, 271, 121154. [Google Scholar] [CrossRef]
- Li, J.; Peng, Y.; Zhang, Q.; Li, X.; Yang, S.; Li, S.; Zhang, L. Rapid enrichment of anammox bacteria linked to floc aggregates in a single-stage partial nitritation-anammox process: Providing the initial carrier and anaerobic microenvironment. Water Res. 2021, 191, 116807. [Google Scholar] [CrossRef]
- Tan, J.; Ye, M.; Lin, C.; Yu, B.; Li, Y.Y.; Liu, J. Assigning strategic COD flow to enhance denitrification coupled with anaerobic digestion and anammox for systematic upgradation of advanced nitrogen removal from food waste digestate. Chem. Eng. J. 2024, 493, 152740. [Google Scholar] [CrossRef]
- Wu, Y.J.; Weng, T.Y.; Yeh, T.Y.; Chou, P.J.; Whang, L.M. Nitrogen removal strategy for real swine wastewater by combining partial nitrification-denitrification process with anammox. Chemosphere 2024, 364, 143116. [Google Scholar] [CrossRef]
- Kumwimba, M.N.; Lotti, T.; Şenel, E.; Li, X.; Suanon, F. Anammox-based processes: How far have we come and what work remains? A review by bibliometric analysis. Chemosphere 2020, 238, 124627. [Google Scholar] [CrossRef]
- Qian, Y.; Guo, Y.; Shen, J.; Qin, Y.; Li, Y.Y. Biofilm growth characterization and treatment performance in a single stage partial nitritation/anammox process with a biofilm carrier. Water Res. 2022, 217, 118437. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.; Zhao, L.; Liu, W.; Chen, L.; Cai, T.; Ji, X.M. Microbial dynamics reveal the adaptation strategies of ecological niche in distinct anammox consortia under mainstream conditions. Environ. Res. 2022, 215, 114318. [Google Scholar] [CrossRef]
- Rong, C.; Song, Y.; Yan, W.; Zhang, T.; Li, Y.Y. Anaerobic membrane bioreactor and Anammox in municipal wastewater treatment: Mainstream versus side-stream, challenges, and prospects. Renew. Sust. Energ. Rev. 2025, 210, 115154. [Google Scholar] [CrossRef]
- Ni, S.Q.; Sun, N.; Yang, H.; Zhang, J.; Ngo, H.H. Distribution of extracellular polymeric substances in anammox granules and their important roles during anammox granulation. Biochem. Eng. J. 2015, 101, 126–133. [Google Scholar] [CrossRef]
- Lin, X.; Wang, Y. Microstructure of anammox granules and mechanismsendo-wing their intensity revealed by microscopic inspection and rheometry. Water Res. 2017, 120, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, H.; Su, Y.; Liu, X. Characteristics and mechanism of anammox granular sludge with different granule size in high load and low rising velocity sewage treatment. Bioresour. Technol. 2020, 312, 123608. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.M.; Jiang, X.W.; Sun, C.P.; Li, S.J.; Weng, X.; Peng, M.W.; Shen, Y. Exploring the physical disruptions of anammox granular sludge under propylene glycol stress: Implications for nitrogen removal long-term stability. J. Water Process Eng. 2025, 71, 107405. [Google Scholar] [CrossRef]
- Wei, L.; Hu, Y.; Xue, Y.; Tian, W.; Xue, Y.; Chen, R. Granular sludge characterization and microbial response in a hydroxyapatite (HAP)-anammox coupled process at different nitrogen loading rates. J. Water Process Eng. 2024, 64, 105582. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, H.; Chen, R.; Niu, Q.; Li, Y.Y. Stoichiometric variation and loading capacity of a high-loading anammox attached film expanded bed (AAEEB) reactor. Bioresour. Technol. 2018, 253, 130–140. [Google Scholar] [CrossRef]
- Lin, L.; Zhao, W.; Cui, S.; Song, Y.; Zhang, Y.; Li, Y.Y. Kuenenia-enriched hydroxyapatite granules enable stable high-rate nitrogen removal of high strength food waste permeate in an EGSB-anammox system: Insights into granule performance and process scalability. J. Water Process Eng. 2025, 72, 107642. [Google Scholar] [CrossRef]
- Liu, L.; Ji, M.; Wang, F. Microbial community shift and functional genes in response to nitrogen loading variations in an anammox biofilm reactor. J. Int. Biodeterior. Biodegrad. 2020, 153, 105023. [Google Scholar] [CrossRef]
- Chen, C.; Jiang, Y.; Zou, X.; Guo, M.; Liu, H.; Cui, M.; Zhang, T.C. Insight into the influence of particle sizes on characteristics and microbial community in the anammox granular sludge. J. Water Process Eng. 2021, 39, 101883. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, Y.; Luan, F. High loading start-Up and rapid loading increase of an Anammox UASB reactor generate superior anammox granules. Water Air Soil Poll. 2023, 234, 166. [Google Scholar] [CrossRef]
- Lu, H.; Ji, Q.; Ding, S.; Zheng, P. The morphological and settling properties of ANAMMOX granular sludge in high-rate reactors. Bioresour. Technol. 2013, 143, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, Y.Y. Hydroxyapatite crystallization-based phosphorus recovery coupling with the nitrogen removal through partial nitritation/anammox in a single reactor. Water Res. 2020, 187, 116444. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Zhang, W.; Wang, Y.; Yang, X.; Guo, J.; He, S. Insights into the influence of organic and salinity on the two-stage partial nitritation/anammox process in treating food waste digestate. Environ. Technol. 2024, 46, 2469–2484. [Google Scholar] [CrossRef]
- Lin, L.; Ishida, K.; Zhang, Y.; Usui, N.; Miyake, A.; Abe, N.; Li, Y.Y. Improving the biomass retention and system stability of the anammox EGSB reactor by adding a calcium silicate hydrate functional material. Sci. Total Environ. 2023, 857, 159719. [Google Scholar] [CrossRef]
- An, P.; Xu, X.; Yang, F.; Li, Z. Comparison of the characteristics of anammox granules of different sizes. Biotechnol. Bioproc. Eng. 2013, 18, 446–454. [Google Scholar] [CrossRef]
- Zhang, Y.; Niu, Q.; Ma, H.; He, S.; Kubota, K.; Li, Y.Y. Long-term operation performance and variation of substrate tolerance ability in an anammox attached film expanded bed (AAFEB) reactor. Bioresour. Technol. 2016, 211, 31–40. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Water Works Association and Water Environment Federation: Washington, DC, USA, 2005. [Google Scholar]
- Liu, Y.; Zhu, Y.; Deng, J.; Yan, B.; Zhan, J.; Wei, Y.; Gui, S. In Situ Enrichment of Anammox Bacteria from Pig Farm Anoxic Sludge Through Co-Cultivation with a Quorum-Sensing Functional Strain Pseudomonas aeruginosa. Fermentation 2024, 10, 548. [Google Scholar] [CrossRef]
- Van de Graaf, A.A.; de Bruijn, P.; Robertson, L.A.; Jetten, M.S.; Kuenen, J.G. Autotrophic growth of anaerobic ammonium-oxidizing micro-organisms in a fluidized bed reactor. Microbiology 1996, 142, 2187–2196. [Google Scholar] [CrossRef]
- Lin, L.; Luo, Z.; Ishida, K.; Urasaki, K.; Kubota, K.; Li, Y.Y. Fast formation of anammox granules using a nitrification-denitrification sludge and transformation of microbial community. Water Res. 2022, 221, 118751. [Google Scholar] [CrossRef]
- Sliekers, A.O.; Haaijer, S.C.; Stafsnes, M.H.; Kuenen, J.G.; Jetten, M.S. Competition and coexistence of aerobic ammonium-and nitrite-oxidizing bacteria at low oxygen concentrations. Appl. Microbiol. Biot. 2005, 68, 808–817. [Google Scholar] [CrossRef]
- Strous, M.; Heijnen, J.J.; Kuenen, J.G.; Jetten, M.S. The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidizing microorganisms. Appl. Microbiol. Biot. 1998, 50, 589–596. [Google Scholar] [CrossRef]
- Tang, C.J.; Zheng, P.; Wang, C.H.; Mahmood, Q.; Zhang, J.Q.; Chen, X.G.; Chen, J.W. Performance of high-loaded ANAMMOX UASB reactors containing granular sludge. Water Res. 2011, 45, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Lotti, T.; Kleerebezem, R.; Lubello, C.; van Loosdrecht, M.C. Physiological and kinetic characterization of a suspended cell anammox culture. Water Res. 2014, 60, 1–14. [Google Scholar] [CrossRef]
- Kartal, B.; Keltjens, J.T. Anammox biochemistry: A tale of heme c proteins. Trends Biochem. Sci. 2016, 41, 998–1011. [Google Scholar] [CrossRef]
- Kang, D.; Li, Y.; Xu, D.; Li, W.; Li, W.; Ding, A.; Zheng, P. Deciphering correlation between chromaticity and activity of anammox sludge. Water Res. 2020, 185, 116184. [Google Scholar] [CrossRef]
- Yu, H.Q.; Tay, J.H.; Fang, H.H. The roles of calcium in sludge granulation during UASB reactor start-up. Water Res. 2001, 35, 1052–1060. [Google Scholar] [CrossRef]
- Jiang, H.L.; Tay, J.H.; Liu, Y.; Tiong-Lee Tay, S. Ca2+ augmentation for enhancement of aerobically grown microbial granules in sludge blanket reactors. Biotechnol. Lett. 2003, 25, 95–99. [Google Scholar] [CrossRef]
- Cao, L.; Yang, Y.; Xue, Y.; Ma, H.; Li, Y.Y.; Hu, Y. A review of efficient nitrogen removal and phosphorus recovery by anammox-hydroxyapatite based processes: Challenges and opportunities. J. Environ. Chem. Eng. 2023, 11, 111103. [Google Scholar] [CrossRef]
- Xue, Y.; Ma, H.; Kong, Z.; Li, Y.Y. Formation mechanism of hydroxyapatite encapsulation in anammox-HAP coupled granular sludge. Water Res. 2021, 193, 116861. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, H.; Ji, J.; Li, Y.Y. A review of anaerobic membrane bioreactors for municipal wastewater treatment with a focus on multicomponent biogas and membrane fouling control. Environ. Sci-Wat Res. 2020, 6, 2641–2663. [Google Scholar] [CrossRef]
- Song, Y.; Lin, L.; Ni, J.; Ma, H.; Qi, W.K.; Li, Y.Y. Architecture of HAP-anammox granules contributed to high capacity and robustness of nitrogen removal under 7 °C. Water Res. 2021, 206, 117764. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Lin, L.; Qi, W.K.; Sasaki, O.; Li, Y.Y. Anammox-mediated hydroxyapatite granules: Physicochemical properties, 3D hierarchy, and biofilm thickness. Environ. Sci. Technol. 2023, 57, 10242–10251. [Google Scholar] [CrossRef] [PubMed]
- Pérez, J.; Laureni, M.; van Loosdrecht, M.C.; Persson, F.; Gustavsson, D.J. The role of the external mass transfer resistance in nitrite oxidizing bacteria repression in biofilm-based partial nitritation/anammox reactors. Water Res. 2020, 186, 116348. [Google Scholar] [CrossRef]
- Kang, P.; Liang, Z.; Zhang, Q.; Zheng, P.; Yu, G.; Cui, L.; Liang, Y. The optimum particle size of anaerobic ammonia oxidation granular sludge under different substrate concentrations. J. Environ. Manag. 2023, 328, 116992. [Google Scholar] [CrossRef]
- Xue, Y.; Ma, H.; Hu, Y.; Kong, Z.; Li, Y.Y. Microstructure and granulation cycle mechanisms of anammox-HAP coupled granule in the anammox EGSB reactor. Water Res. 2022, 210, 117968. [Google Scholar] [CrossRef]
- Lu, D.; Gong, H.; Diao, S.; Shi, W.; Yin, R.; Dai, X. Enhanced sludge settlement of two stage PN/Anammox for reject water treatment with respective diatomite addition. Sci. Total Environ. 2023, 877, 162784. [Google Scholar] [CrossRef]
- Xiao, W.; Hu, Y.; Li, S.; Shi, J.; Tian, W.; Xue, Y.; Chen, R. Operational characteristics and phosphorus recycling potential of a hydroxyapatite (HAP)-anammox process at high nitrogen loading rate. J. Environ. Chem. Eng. 2025, 13, 115759. [Google Scholar] [CrossRef]
- Lan, Y.; Li, X.; Du, R.; Fan, X.; Cao, S.; Peng, Y. Hydroxyapatite (HAP) formation in acetate-driven partial denitrification process: Enhancing sludge granulation and phosphorus removal. Sci. Total Environ. 2023, 903, 166659. [Google Scholar] [CrossRef]
- Fan, J.; Du, R.; Liu, Q.; Li, C.; Peng, Y. Insight into the microbial interactions of Anammox and heterotrophic bacteria in different granular sludge systems: Effect of size distribution and available organic carbon source. Bioresour. Technol. 2022, 364, 128055. [Google Scholar] [CrossRef]
- Shirazi, S.; Lin, C.J.; Chen, D. Inorganic fouling of pressure-driven membrane processes—A critical review. Desalination 2010, 250, 236–248. [Google Scholar] [CrossRef]
- Ali, M.; Okabe, S. Anammox-based technologies for nitrogen removal: Advances in process start-up and remaining issues. Chemosphere 2015, 141, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Xia, W.; Qian, Y.; Rong, C.; Ye, M.; Chen, Y.; Li, Y.Y. Revealing microbial compatibility of partial nitritation/Anammox biofilm from sidestream to mainstream applications: Origins, dynamics, and interrelationships. Bioresour. Technol. 2025, 418, 131963. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Feng, Y.; Chen, L.; Niu, Z.; Liu, S. Genome-centered omics insight into the competition and niche differentiation of Ca. Jettenia and Ca. Brocadia affiliated to anammox bacteria. Appl. Microbiol. Biot. 2019, 103, 8191–8202. [Google Scholar] [CrossRef]
- Wu, P.; Chen, J.; Garlapati, V.K.; Zhang, X.; Jenario, F.W.V.; Li, X.; Zhang, X. Novel insights into Anammox-based processes: A critical review. Chem. Eng. J. 2022, 444, 136534. [Google Scholar] [CrossRef]
- Lawson, C.E.; Wu, S.; Bhattacharjee, A.S.; Hamilton, J.J.; McMahon, K.D.; Goel, R.; Noguera, D.R. Metabolic network analysis reveals microbial community interactions in anammox granules. Nat. Commun. 2017, 8, 15416. [Google Scholar] [CrossRef]
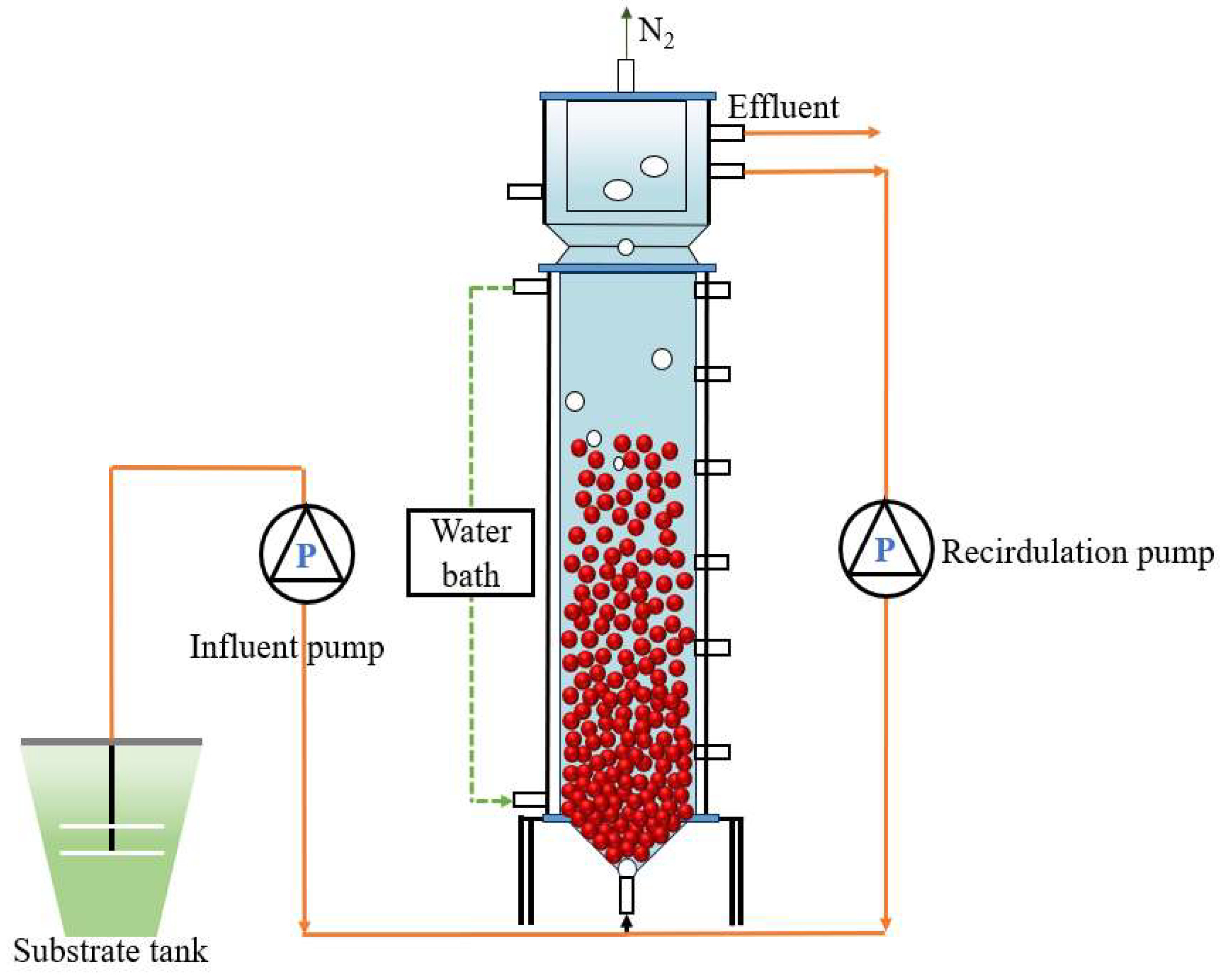

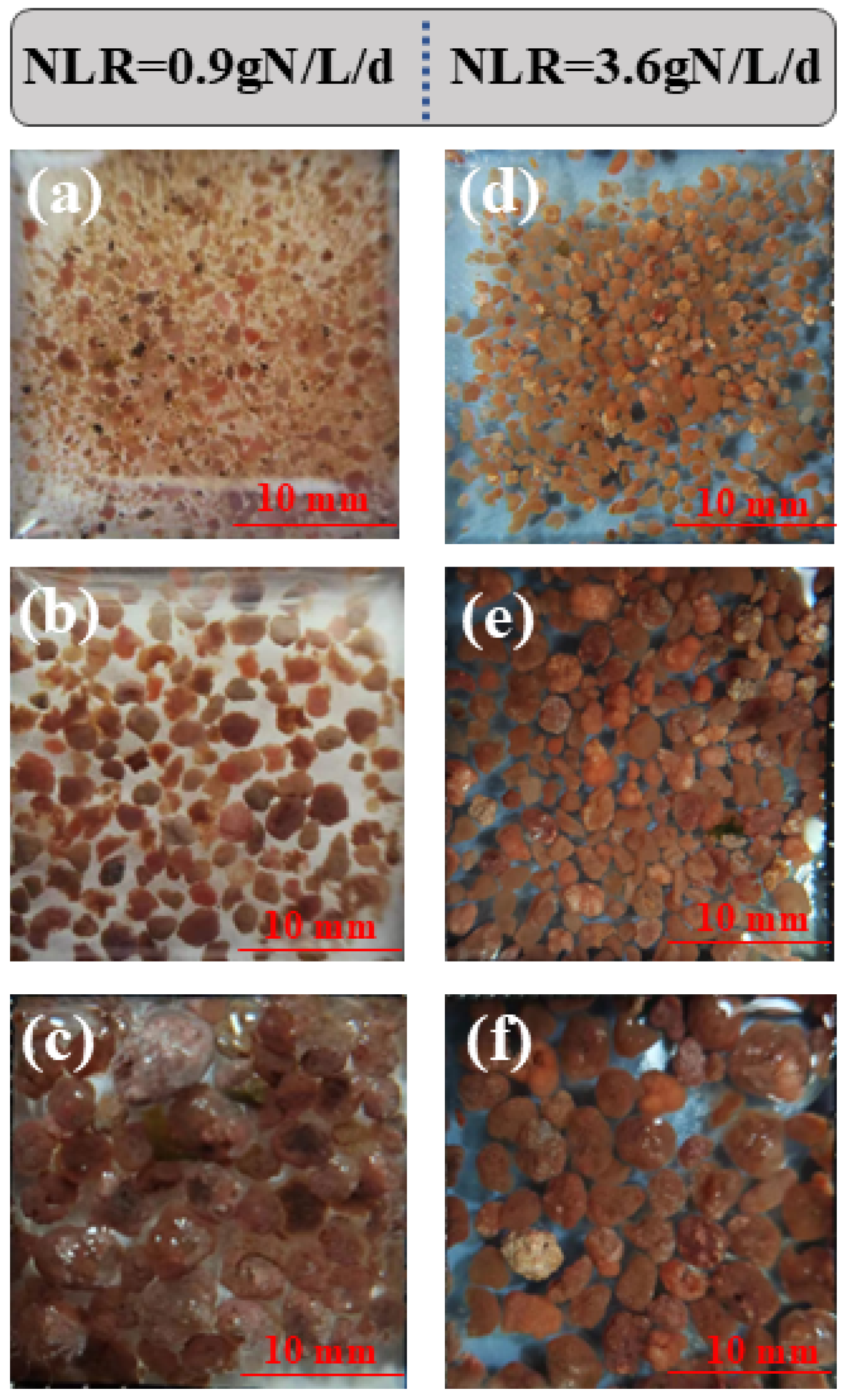
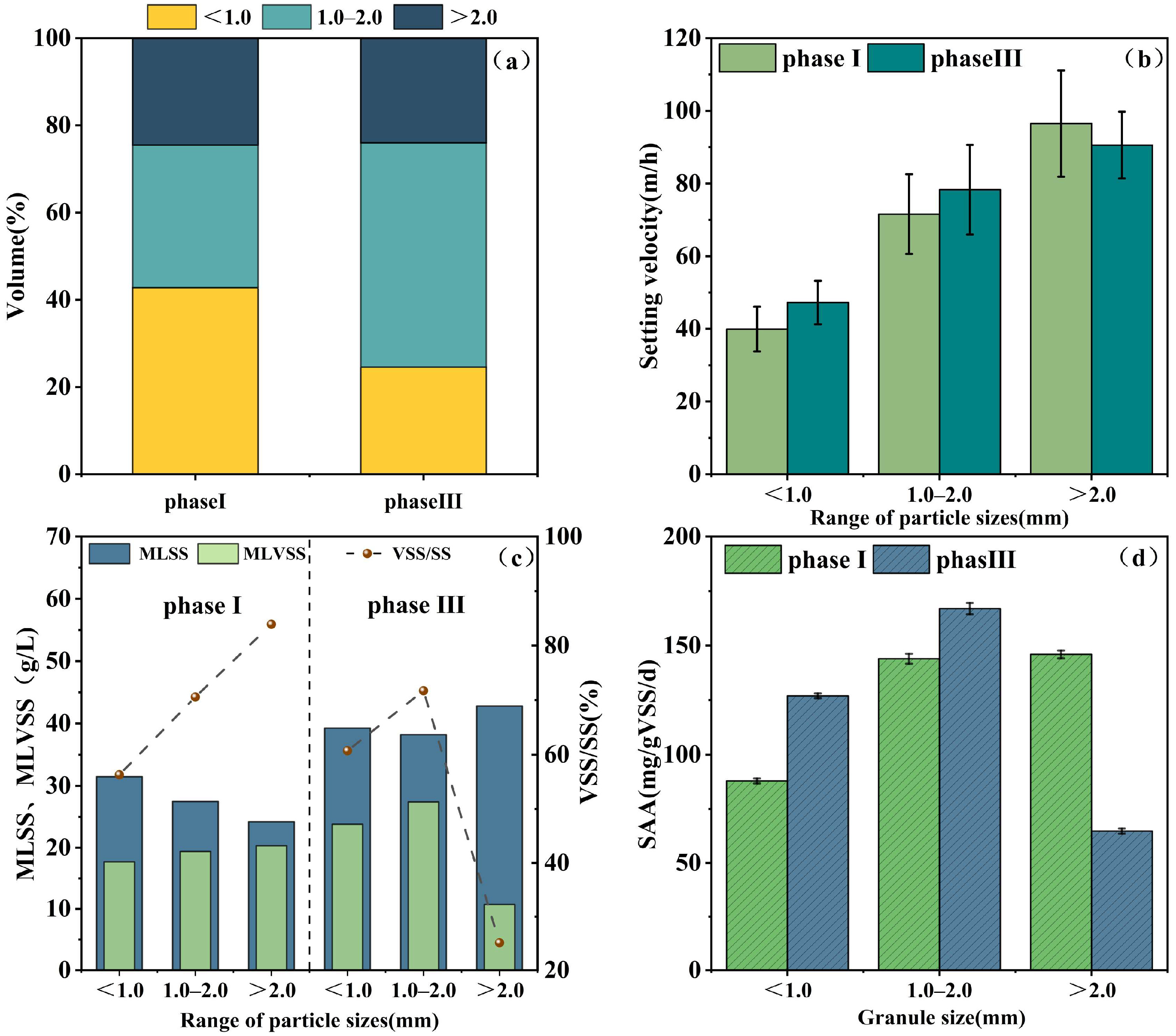


| Phase | Days (d) | Operation Conditions | Reactor Performance | ||||||
|---|---|---|---|---|---|---|---|---|---|
| TNin (mg/L) | NO2−in:NH4+in | HRT (h) | NLR (gN/L/d) | NH4+eff (mg/L) | NO2−eff (mg/L) | NO3−eff (mg/L) | TNRE (%) | ||
| I | 1–47 | 450 | 1:1 | 12 | 0.9 | 28.98 ± 11.91 | 10.26 ± 4.71 | 31.87 ± 7.44 | 82.69 ± 1.92 |
| II | 48–123 | 450 | 1.15:1 | 12 | 0.9 | 45.47 ± 16.90 | 9.11 ± 6.31 | 53.53 ± 9.37 | 79.28 ± 0.27 |
| III | 124–154 | 450 | 1.2:1 | 3 | 3.6 | 16.90 ± 7.976 | 20.03 ± 13.69 | 51.79 ± 3.17 | 83.64 ± 0.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, X.; Qian, Y.; Yang, X.; Wang, Y.; Yang, H.; He, S. Multiscale Characterization of Anammox Granules and Microbial Migration Under Variable Nitrogen Loading Rates. Water 2025, 17, 1653. https://doi.org/10.3390/w17111653
Fan X, Qian Y, Yang X, Wang Y, Yang H, He S. Multiscale Characterization of Anammox Granules and Microbial Migration Under Variable Nitrogen Loading Rates. Water. 2025; 17(11):1653. https://doi.org/10.3390/w17111653
Chicago/Turabian StyleFan, Xiaoliang, Yunzhi Qian, Xueying Yang, Yilin Wang, Hong Yang, and Shilong He. 2025. "Multiscale Characterization of Anammox Granules and Microbial Migration Under Variable Nitrogen Loading Rates" Water 17, no. 11: 1653. https://doi.org/10.3390/w17111653
APA StyleFan, X., Qian, Y., Yang, X., Wang, Y., Yang, H., & He, S. (2025). Multiscale Characterization of Anammox Granules and Microbial Migration Under Variable Nitrogen Loading Rates. Water, 17(11), 1653. https://doi.org/10.3390/w17111653