Microfluidic Electrochemical Desalination Systems: A Review
Abstract
1. Introduction
2. Fundamentals of Microfluidics
- Direct manufacturing: this approach can be further classified into mechanical methods that use mechanical forces to remove excess material to shape the device and energy-assisted methods that use beams of energy (e.g., lasers, electron beams, or focused ion beams) to either add or remove layers directly, often guided by masks to create precise micro- or nanostructures [24].
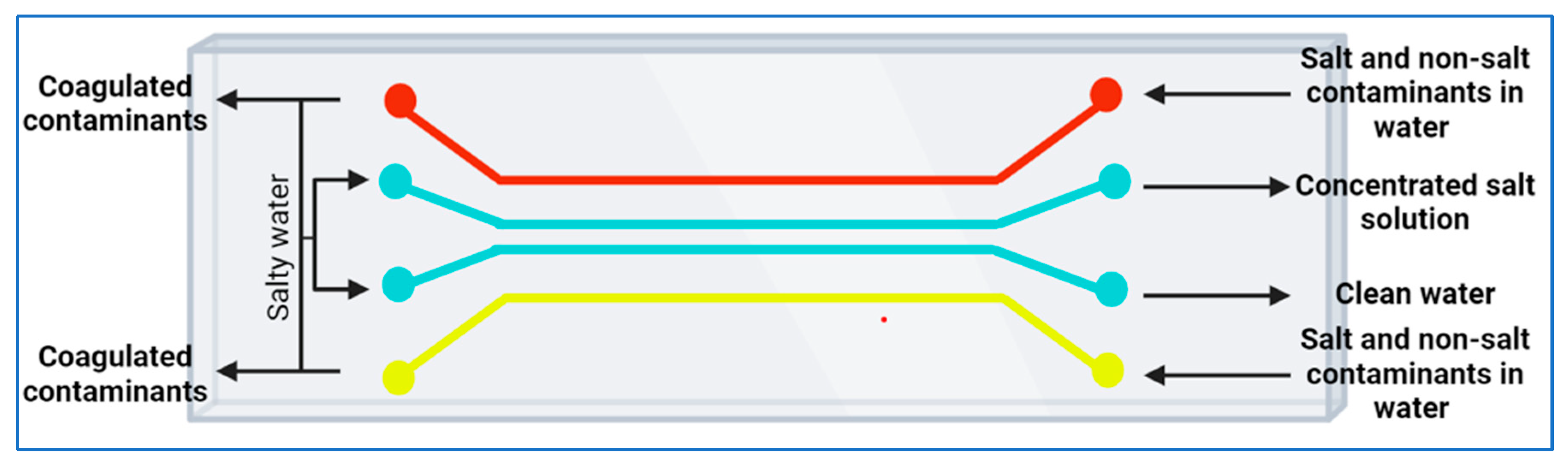
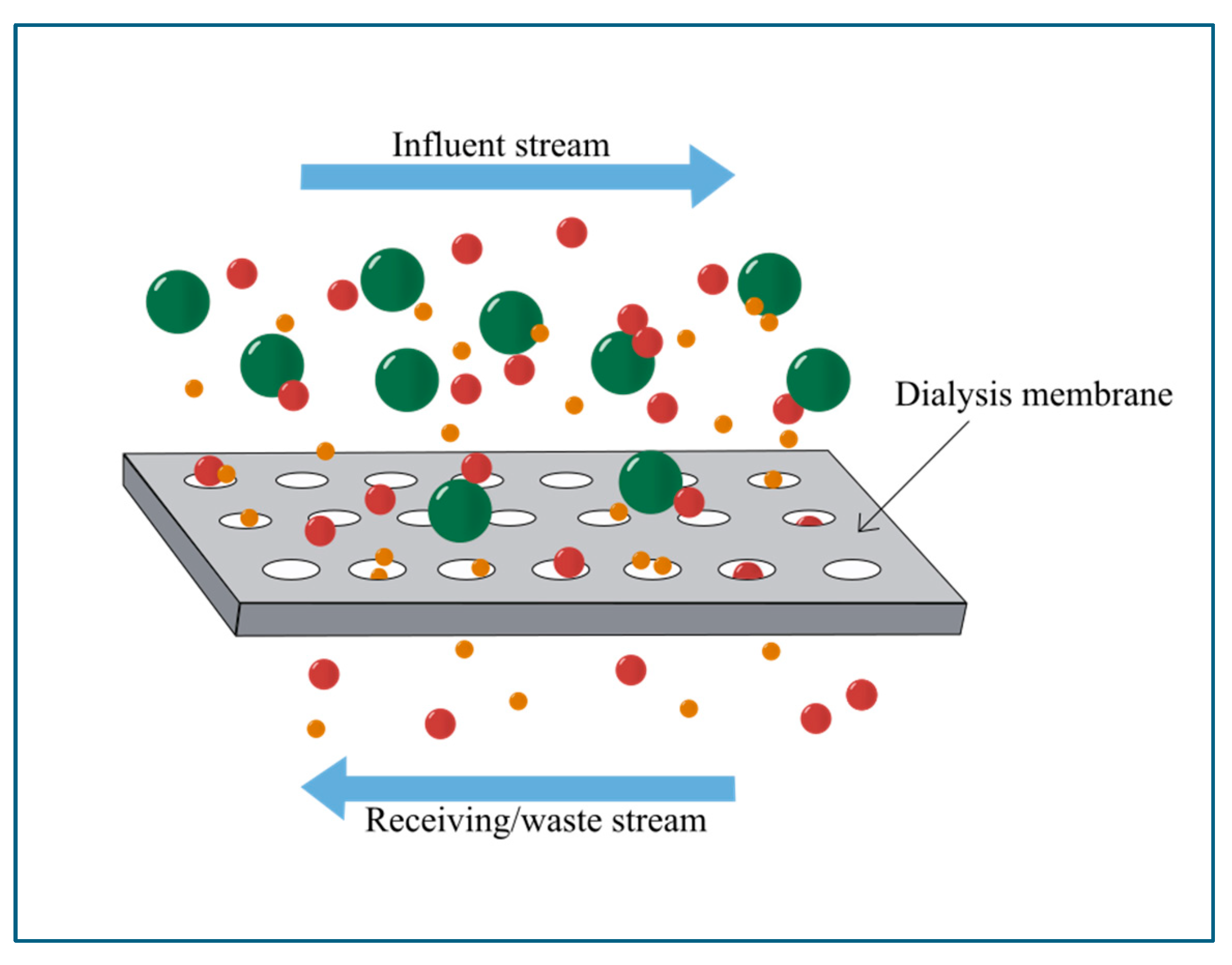
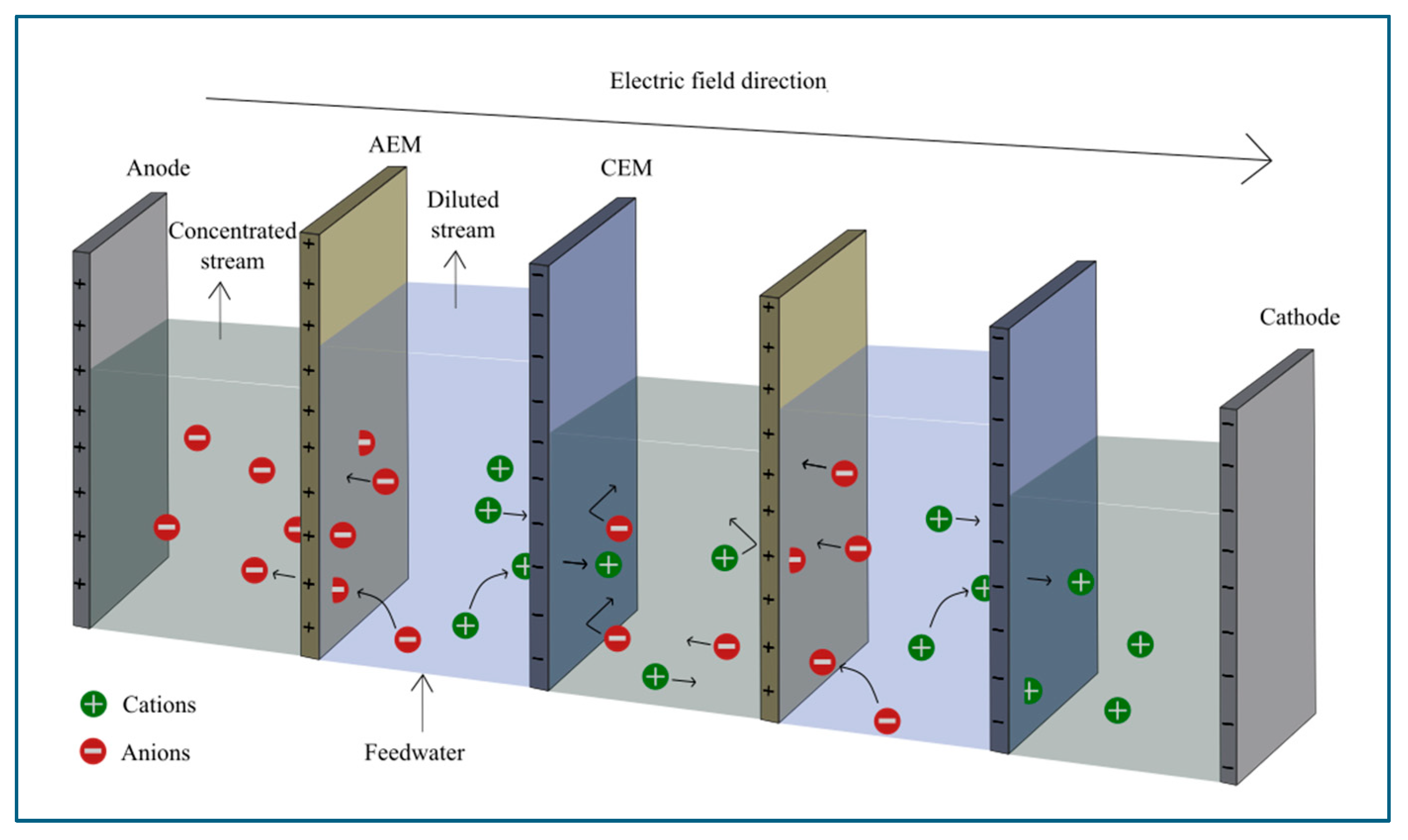
3. Overview of Dialysis and Electrodialysis
Microfluidic Dialysis and Electrodialysis
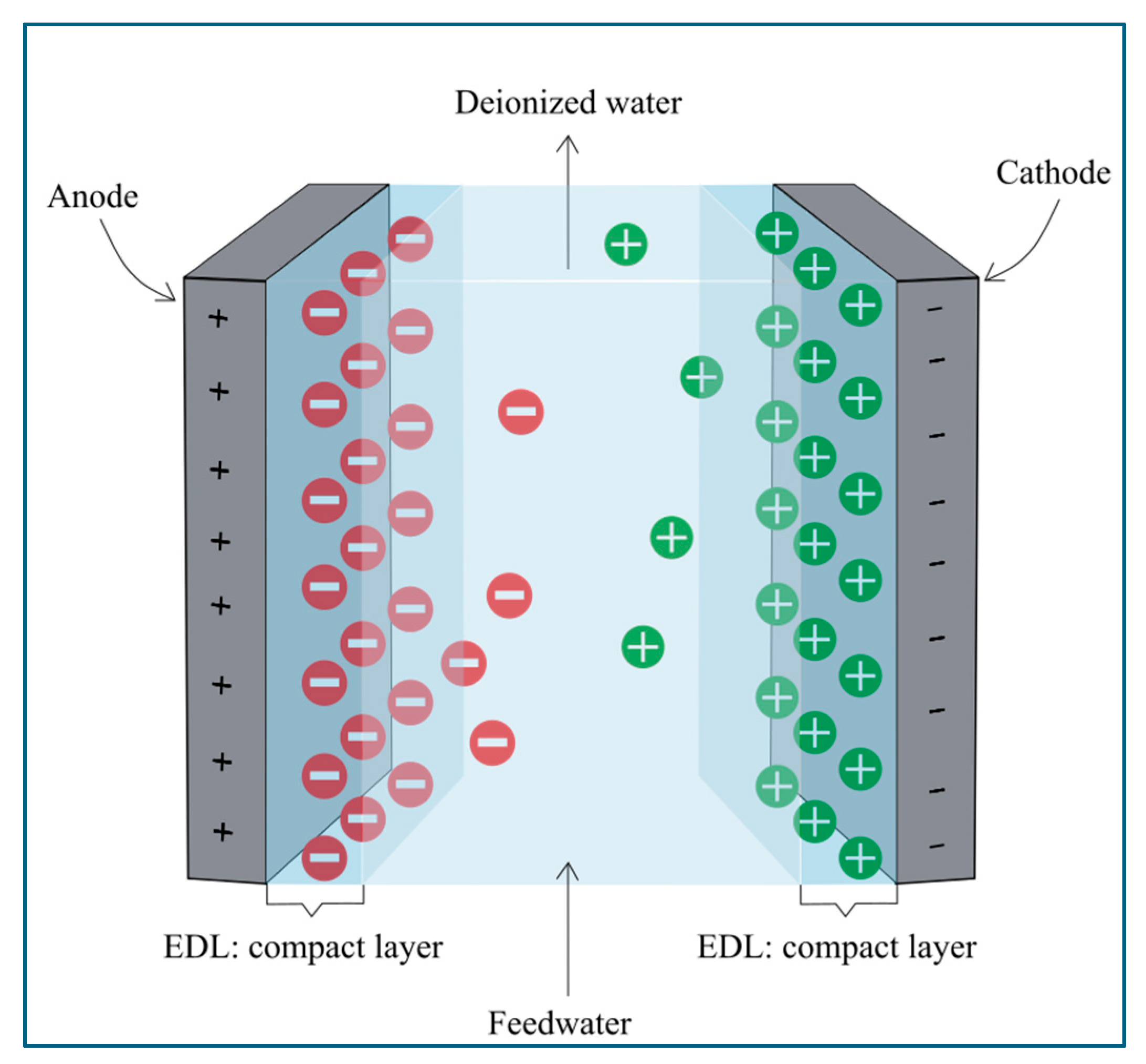
4. Overview of Capacitive Deionization
- The first type involves anodic oxidation reactions. These reactions include oxidizing carbon electrodes, chloride ions, water, and other contaminants, such as inorganic ions and organic matter. Among these, carbon oxidation has been a primary focus due to its detrimental impact on the CDI system. Carbon oxidation can damage the electrode’s porous structure, reduce its mass, and significantly decrease both the electrode’s lifespan and the overall performance of the CDI system [51,56].
- The second type of faradaic reaction is cathodic reduction reactions. These reactions mainly include the reduction of oxygen, leading to the production of by-products such as hydrogen peroxide (H2O2) [51]. While cathodic reduction can cause an asymmetric potential distribution between the anode and cathode, which accelerates the unwanted carbon oxidation at the anode, it also offers potential benefits. For instance, the hydrogen peroxide generated can be utilized for water disinfection and the degradation of organic contaminants. Additionally, cathodic reduction reactions contribute to the removal of heavy metals from water by depositing them on the electrode surface [57].
- The third type is the faradaic ion storage, where reversible redox reactions enable ion storage through pseudocapacitive/intercalation effects. Unlike traditional electrostatic storage, which confines ions to the electrode surface, this process allows ions to penetrate and be stored within the electrode’s internal structure. For instance, in electrode materials like sodium transition metal oxides, cations intercalate into the electrode’s layered structure during reduction and deintercalate during oxidation when the voltage is reversed in the regeneration process. Similarly, conductive polymers and metal halides can reversibly bind anions. This approach significantly enhances the ion storage capacity by utilizing the electrode’s bulk rather than just its surface. Moreover, the reversibility of the redox reactions allows for repeated cycling, enabling efficient desalination while maintaining the structural stability of the electrodes [56,58].
Microfluidic CDI
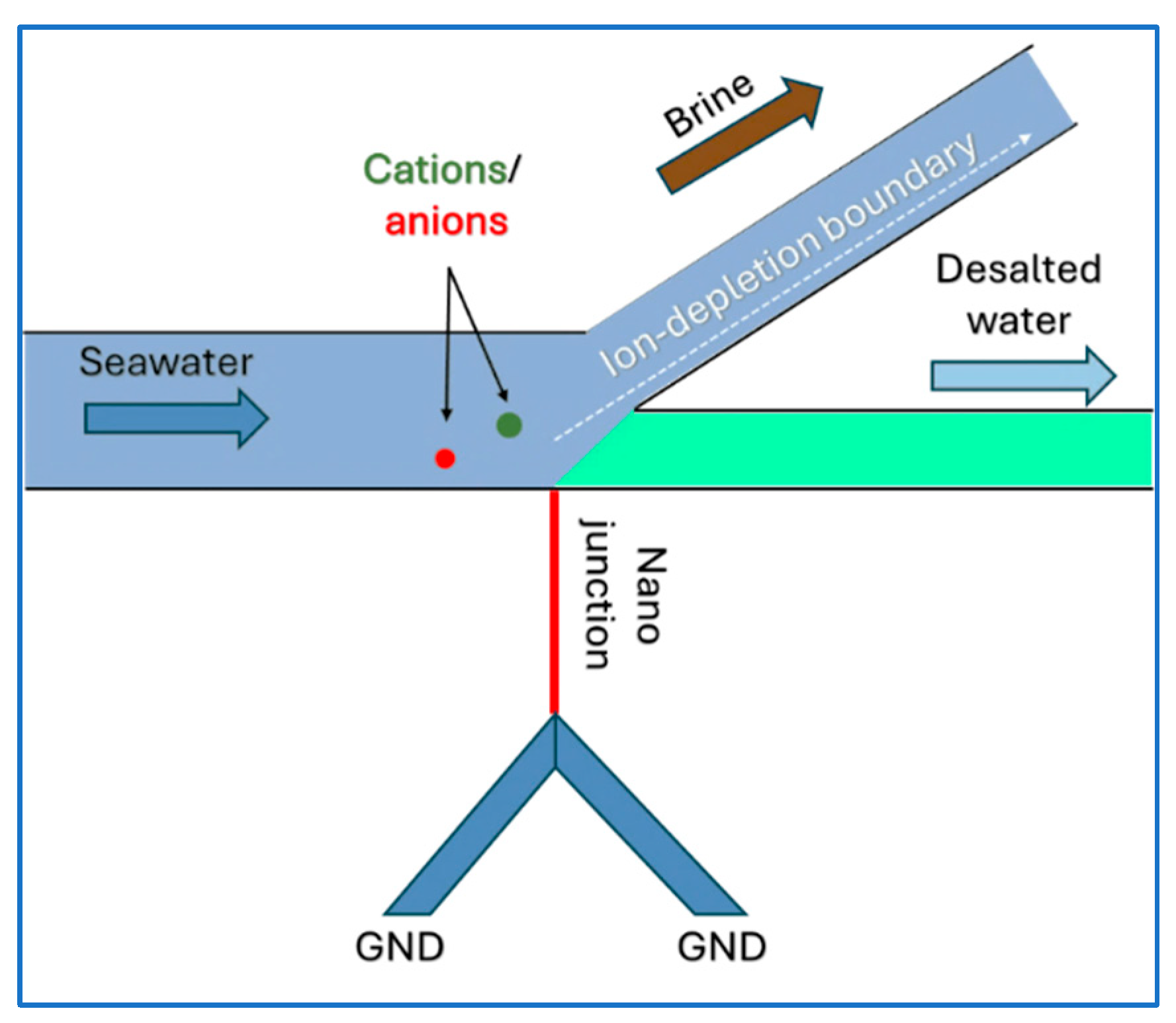
5. Overview of Ion Concentration Polarization
Microfluidic Ion Concentration Polarization Desalination
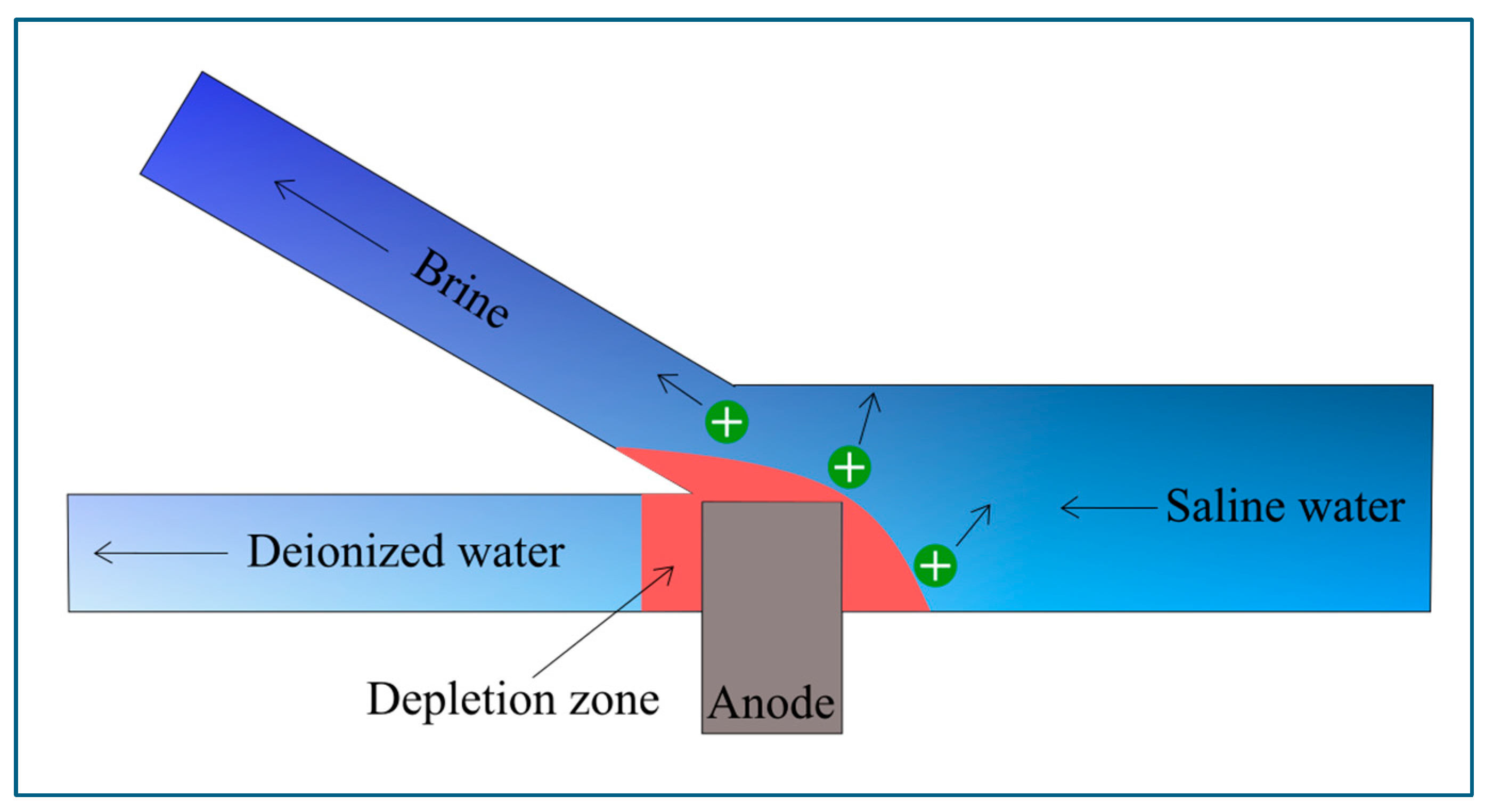
6. Overview of Electrochemical Desalination
Microfluidic Electrochemical Desalination
7. Desalination Membrane Materials
- Polymeric membranes, including those made out of cellulose acetate (CA), polyethersulfone (PES), polypropylene (PP), poly(vinyl alcohol) (PVA), as well as polyamide (PA) and its derivatives. Early membranes made out of CA polymers suffered from low water permeance, a narrow pH operating range, and poor biodegradation resistance. Limitations of CA membranes led to their replacement by thin-film composite (TFC) polyamide membranes. TFC polyamide membranes offer higher water permeance, broader pH tolerance, and excellent salt rejection but face a trade-off between permeability and selectivity due to structural factors like pore size distribution and crosslinking density [104].
- Inorganic or ceramic membranes, typically made from silica, alumina, zirconia, or a mixture of these materials. Inorganic membranes, featuring a macro-porous support-layer and a meso- or micro-porous active layer, offer the advantage of durability in harsh environments, e.g., high temperatures, highly contaminated feeds, and/or corrosive environments where polymeric membranes might fail [105]. Zeolites, MXenes, and molybdenum disulfide (MoS2) are additional examples of inorganic materials used to make desalination membranes. Zeolite membranes are made from natural or synthetic crystalline aluminosilicates with Group I and Group II cations, and the oxygen ring size defines their pore structure. In contrast, the Si/Al ratio controls properties like wettability and surface charge [106]. MXenes have a general formula of Mn+1Xn, where M denotes the transition metal and X denotes the carbon, nitrogen, or carbon–nitrogen that makes up the MXene material. The formula of a MXene could also be Mn+1XnTx, where T denotes a functional group such as -O, -OH, or -F. In addition, MoS2 membranes offer high hydrophilicity, chemical stability, and stronger interactions between MoS2 nanosheets, leading to a more stable laminar structure. Although these membranes have shown superior separation performance, they tend to be more expensive and more challenging to fabricate as a continuous, defect-free layer than polymeric membranes [11,101,106].
- Composite or mixed matrix membranes (MMMs), which consist of polymers embedded with nanoparticles “fillers” to enhance the physicochemical properties of the membrane and, in turn, its separation performance. A well-designed composite membrane should ensure the stability and homogeneous distribution of the filler material in the polymer-solution system, as well as the interfacial compatibility between the polymer and the filler material [103]. One of the main advantages of MMMs is their high surface-to-volume ratio, which can provide high porosity on the surface and in the membrane bulk. Moreover, incorporating the nanomaterials into the polymeric matrix can enhance desalination performance by modifying membrane properties, leading to improved permeability and selectivity, increased hydrophilicity, better mechanical strength, enhanced antifouling and antibacterial properties, and greater thermal stability [106].
7.1. Membrane Fabrication and Synthesis Techniques
- Solution casting: this technique involves dissolving the polymer and any necessary additives in a suitable solvent until homogeneity is attained. The polymer solution is then spread onto a flat surface (e.g., a flat glass plate), followed by evaporation or phase inversion to remove the solvent. Once dry, the membrane is peeled off from the support plate, and a flat-sheet membrane is formed.
- Stretching: this is a solvent-free technique used to fabricate porous membranes. The polymer is first heated to its melting point, followed by extrusion and stretching to achieve the desired thickness and structure. The membrane is first stretched under cooler conditions to create nucleate structures. This is then followed by stretching at high temperatures, e.g., 130–140 °C, to further expand and refine the pores.
- Solution coating: a method suitable for making composite membranes as it involves depositing a thin layer of the polymer solution on the microporous substrate.
- Spray coating: here, a polymer solution is atomized and deposited onto a substrate, forming a thin, uniform membrane layer after solvent evaporation.
- Hollow fiber spinning: In this technique a polymer solution is extruded through a spinneret with a coaxial flow of bore fluid, forming continuous hollow fibers with a porous or dense selective layer. The solvent is then removed through phase inversion or thermal processing, allowing the fibers to solidify and create high-surface-area membranes.
- Interfacial polymerization: a technique that involves dissolving two reactive monomers separately in immiscible phases, one in an aqueous phase and the other in an organic phase. When these two phases come into contact, polymerization occurs at the interface, forming an ultrathin selective membrane layer. This technique is commonly used in TFC membranes for desalination.
- Electrospinning: an electrodynamic process in which the polymer solution is expelled through a needle under a high-voltage electric field. The ejected droplets then stretch and elongate, forming ultrafine fibers that are collected as a porous nanofibrous membrane with high surface area and tunable properties.
7.2. Enhancing Membrane Selectivity, Permeability, and Stability
- Optimization of fabrication methods, where adjusting monomer concentrations and reaction conditions can help create more uniform polymeric membranes and improve salt rejection.
- Modification and post-modification of membrane materials: As mentioned above, incorporating nanomaterials into polymeric membranes can help improve selectivity, thermal, chemical, and mechanical stability. In addition, adding or grafting functional groups (e.g., carboxylate) onto the polymer can help increase water affinity. Acid- and alcohol-based surface modifications have also improved hydrophilicity, surface charge, and water flux. Improving membrane hydrophilicity has also been achieved by coating the membrane surface with more hydrophilic compounds, e.g., PVA and poly(N,N-dimethylaminoethyl methacrylate). These coatings have proven useful because they help improve resistance to chlorine attacks and reduce fouling effects [105]. Other surface modification techniques used to graft useful monomers on the surfaces of membranes include free-radical-, photochemical-, radiation-, plasma-induced grafting, and gas plasma treatment [104,105]. For instance, oxygen plasma treatment can help improve water permeability by introducing the hydrophilic carboxylate group. In contrast, argon plasma treatment can improve resistance to chlorine attacks by increasing the cross-linking at the nitrogen sites [105].
- Post-treatment of the membranes with acids and/or organic solvents [112]: post-treatment with solvents, known as solvent activation, is a promising method for improving the performance of desalination membranes. Aliphatic and aromatic alcohols, polar aprotic solvents (e.g., NMP, DMF, DMSO), and ionic liquids have been tested as activating solvents. While weak solvents (e.g., aliphatic alcohols) were able to provide minor permeability improvements, strong solvents (e.g., NMP, DMF, DMSO) were found to increase water flux significantly but compromised salt rejection and damaged membrane supports [112,113].
8. Important Advances in Microfluidic Desalination
8.1. Evaluation of Solar-Energy-Powered Seawater Desalination Processes
8.2. Nanofluids in Microfluidic Desalination
9. Challenges and Future Directions
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AQP | Aquaporins |
| AEM | Anion-exchange membrane |
| AGMD | Air gap membrane distillation |
| BPE | Bipolar electrode |
| BSA | Bovine serum albumin |
| BODIPY | Boron-dipyrromethene |
| CGMD | Conductive gap membrane distillation |
| CSP | Concentrated solar power |
| CA | Cellulose acetate |
| CU | Current utilization |
| CNT | Carbon nanotubes |
| CNC | Cellulose nanocrystals |
| CNF | Cellulose nanofiber |
| CEM | Cation-exchange membrane |
| CDI | Capacitive deionization |
| DNA | Deoxyribonucleic acid |
| DEP | Dielectrophoresis |
| DSBA | Dodecylbenzene sulfonic acid |
| ECDI | Electrochemical capacitive deionization |
| ECD | Electrochemical desalination |
| EDL | Electric double layer |
| ED | Electrodialysis |
| ESI-MS | Electrospray ionization mass spectrometry |
| EOF | Electroosmotic flow |
| FT | Flow-through |
| FB | Flow-by |
| FCDI | Flow-electrode capacitive deionization |
| FITC-dextran | Fluorescein isothiocyanate–dextran |
| GO | Graphene oxide |
| HEA | 2-Hydroxyethyl acrylate |
| ICP | Ion concentration polarization |
| IHC | Intercalation host compounds |
| ICC | Isolated closed cycle |
| IE | Inter-electrode |
| MD | Membrane desalination |
| MWCO | Molecular weight cut-off |
| MFB | Membrane flow-by |
| MFECDI | Microfluidic flow-electrode capacitive deionization |
| MMM | Mixed matrix membrane |
| NASICON | Sodium (Na) super ionic conductor |
| NiHCF | Nickel hexacyanoferrate |
| OFC | Open flow channels |
| PDMS | Polydimethylsiloxane |
| PET | Polyethylene terephthalate |
| PPy | Polypyrrole |
| PVDF | Polyvinylidene difluoride |
| PES | Polyethersulfone |
| PANI | Polyaniline |
| PP | Polypropylene |
| PVA | Polyvinyl alcohol |
| PA | Polyamide |
| PV | Photovoltaic |
| PEM | Polymer electrolyte membrane |
| PGMD | Permeate gap membrane distillation |
| RO | Reverse osmosis |
| SIM | Silica isoporous membrane |
| SEM | Scanning electron microscopy |
| TFC | Thin-film composite |
| WHEG | Wood-based hydroelectric generator |
References
- Campione, A.; Gurreri, L.; Ciofalo, M.; Micale, G.; Tamburini, A.; Cipollina, A. Electrodialysis for water desalination: A critical assessment of recent developments on process fundamentals, models and applications. Desalination 2018, 434, 121–160. [Google Scholar] [CrossRef]
- Jones, E.; Qadir, M.; Van Vliet, M.T.; Smakhtin, V.; Kang, S.M. The state of desalination and brine production: A global outlook. Sci. Total Environ. 2019, 657, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Baten, R.; Stummeyer, K. How sustainable can desalination be? Desalin. Water Treat. 2013, 51, 44–52. [Google Scholar] [CrossRef]
- Camacho, L.M.; Dumée, L.; Zhang, J.; Li, J.D.; Duke, M.; Gomez, J.; Gray, S. Advances in membrane distillation for water desalination and purification applications. Water 2013, 5, 94–196. [Google Scholar] [CrossRef]
- Goh, P.S.; Kang, H.S.; Ismail, A.F.; Hilal, N. The hybridization of thermally-driven desalination processes: The state-of-the-art and opportunities. Desalination 2021, 506, 115002. [Google Scholar] [CrossRef]
- Feria-Díaz, J.J.; López-Méndez, M.C.; Rodríguez-Miranda, J.P.; Sandoval-Herazo, L.C.; Correa-Mahecha, F. Commercial thermal technologies for desalination of water from renewable energies: A state of the art review. Processes 2021, 9, 262. [Google Scholar] [CrossRef]
- Subramani, A.; Jacangelo, J.G. Emerging desalination technologies for water treatment: A critical review. Water Res. 2015, 75, 164–187. [Google Scholar] [CrossRef]
- AlSawaftah, N.; Abuwatfa, W.; Darwish, N.; Husseini, G.A. A Review on Membrane Biofouling: Prediction, Characterization, and Mitigation. Membranes 2022, 12, 1271. [Google Scholar] [CrossRef]
- Abdulbari, H.A.; Basheer, E. Microfluidic Desalination: A New Era Towards Sustainable Water Resources. ChemBioEng 2021, 8, 121–133. [Google Scholar] [CrossRef]
- de Aguiar, I.B.; Schroën, K. Microfluidics used as a tool to understand and optimize membrane filtration processes. Membranes 2020, 10, 316. [Google Scholar] [CrossRef]
- de Jong, J.; Lammertink, R.G.H.; Wessling, M. Membranes and microfluidics: A review. Lab Chip 2006, 6, 1125–1139. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.T.; Park, J.E.; Jung, S.Y.; Kang, T.G. Fouling mitigation via chaotic advection in a flat membrane module with a patterned surface. Membranes 2021, 11, 724. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, R. When Microfluidic Devices Go Bad. Anal. Chem. 2005, 77, 429A–432A. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Cheng, D.; Zhao, L. Microfluidics: Fundamental, Devices and Applications; Wiley-VCH: Weinheim, Germany, 2018. [Google Scholar]
- Convery, N.; Gadegaard, N. 30 years of microfluidics. Micro Nano Eng. 2019, 2, 76–91. [Google Scholar] [CrossRef]
- Abdelrasoul, A.; Doan, H.; Lohi, A.; Cheng, C.-H. Mass Transfer Mechanisms and Transport Resistances in Membrane Separation Process. In Mass Transfer—Advancement in Process Modelling; InTech: London, UK, 2015. [Google Scholar] [CrossRef]
- Gervais, T.; Jensen, K.F. Mass transport and surface reactions in microfluidic systems. Chem. Eng. Sci. 2006, 61, 1102–1121. [Google Scholar] [CrossRef]
- You, I.; Yun, N.; Lee, H. Surface-tension-confined microfluidics and their applications. ChemPhysChem 2013, 14, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Sajdera, N. Control, Analysis, and Testing SURFACE TENSION. [Online]. Available online: www.kocour.net (accessed on 20 May 2025).
- Godwin, L.A.; Deal, K.S.; Hoepfner, L.D.; Jackson, L.A.; Easley, C.J. Measurement of microchannel fluidic resistance with a standard voltage meter. Anal. Chim. Acta 2013, 758, 101–107. [Google Scholar] [CrossRef]
- Scott, S.M.; Ali, Z. Fabrication methods for microfluidic devices: An overview. Micromachines 2021, 12, 319. [Google Scholar] [CrossRef]
- Niculescu, A.G.; Chircov, C.; Bîrcă, A.C.; Grumezescu, A.M. Fabrication and applications of microfluidic devices: A review. Int. J. Mol. Sci. 2021, 22, 2011. [Google Scholar] [CrossRef]
- Choi, S.; Kim, B.; Han, J. Integrated pretreatment and desalination by electrocoagulation (EC)-ion concentration polarization (ICP) hybrid. Lab Chip 2017, 17, 2076–2084. [Google Scholar] [CrossRef]
- Naderi, A.; Bhattacharjee, N.; Folch, A. Digital Manufacturing for Microfluidics. Annu. Rev. Biomed. Eng. 2019, 21, 325–364. [Google Scholar] [CrossRef] [PubMed]
- Waldbaur, A.; Rapp, H.; Länge, K.; Rapp, B.E. Let there be chip—Towards rapid prototyping of microfluidic devices: One-step manufacturing processes. Anal. Methods 2011, 3, 2681–2716. [Google Scholar] [CrossRef]
- Hwang, J.; Cho, Y.H.; Park, M.S.; Kim, B.H. Microchannel Fabrication on Glass Materials for Microfluidic Devices. Int. J. Precis. Eng. Manuf. 2019, 20, 479–495. [Google Scholar] [CrossRef]
- Mesquita, P.; Gong, L.; Lin, Y. Low-cost microfluidics: Towards affordable environmental monitoring and assessment. Front. Lab Chip Technol. 2022, 1, 1074009. [Google Scholar] [CrossRef]
- Roelofs, S.H.; Van Den Berg, A.; Odijk, M. Microfluidic desalination techniques and their potential applications. Lab Chip 2015, 15, 3428–3438. [Google Scholar] [CrossRef]
- Perozziello, G.; Candeloro, P.; Gentile, F.; Coluccio, M.L.; Tallerico, M.; De Grazia, A.; Nicastri, A.; Perri, A.M.; Parrotta, E.; Pardeo, F.; et al. A microfluidic dialysis device for complex biological mixture SERS analysis. Microelectron. Eng. 2015, 144, 37–41. [Google Scholar] [CrossRef]
- Al-Amshawee, S.; Yunus, M.Y.B.M.; Azoddein, A.A.M.; Hassell, D.G.; Dakhil, I.H.; Hasan, H.A. Electrodialysis desalination for water and wastewater: A review. Chem. Eng. J. 2020, 380, 122231. [Google Scholar] [CrossRef]
- Juve, J.M.A.; Christensen, F.M.S.; Wang, Y.; Wei, Z. Electrodialysis for metal removal and recovery: A review. Chem. Eng. J. 2022, 435, 134857. [Google Scholar] [CrossRef]
- Tibavinsky, I.A.; Kottke, P.A.; Fedorov, A.G. Microfabricated ultrarapid desalting device for nanoelectrospray ionization mass spectrometry. Anal. Chem. 2015, 87, 351–356. [Google Scholar] [CrossRef]
- Song, S.; Singh, A.K.; Shepodd, T.J.; Kirby, B.J. Microchip Dialysis of Proteins Using in Situ Photopatterned Nanoporous Polymer Membranes. Anal. Chem. 2004, 76, 2367–2373. [Google Scholar] [CrossRef]
- Xu, N.; Lin, Y.; Hofstadler, S.A.; Matson, D.; Call, C.J.; Smith, R.D. A Microfabricated Dialysis Device for Sample Cleanup in Electrospray Ionization Mass Spectrometry. Anal. Chem. 1998, 70, 3553–3556. [Google Scholar] [CrossRef]
- Eijkel, J.C.T.; Bomer, J.G.; van den Berg, A. Osmosis and pervaporation in polyimide submicron microfluidic channel structures. Appl. Phys. Lett. 2005, 87, 114103. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, D.; Chen, K.; Zhou, P.; Zhao, M.; Qiao, L.; Su, B. Highly Efficient Desalting by Silica Isoporous Membrane-Based Microfluidic Chip for Electrospray Ionization Mass Spectrometry. Anal. Chem. 2018, 90, 14395–14401. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, L.; Ajami, N.J.; Adachi, J.A.; Gascoyne, P.R.C.; Petrosino, J.F. Isolation and concentration of bacteria from blood using microfluidic membraneless dialysis and dielectrophoresis. Lab Chip 2017, 17, 1340–1348. [Google Scholar] [CrossRef]
- Strathmann, H. Electrodialysis, a mature technology with a multitude of new applications. Desalination 2010, 264, 268–288. [Google Scholar] [CrossRef]
- Kwak, R.; Guan, G.; Peng, W.K.; Han, J. Microscale electrodialysis: Concentration profiling and vortex visualization. Desalination 2013, 308, 138–146. [Google Scholar] [CrossRef]
- Rubinstein, I.; Warshawsky, A.; Schechtman, L.; Kedem, O. Elimination of acid-base generation (‘water-splitting’) in electrodialysis. Desalination 1984, 51, 55–60. [Google Scholar] [CrossRef]
- Nikonenko, V.V.; Kovalenko, A.V.; Urtenov, M.K.; Pismenskaya, N.D.; Han, J.; Sistat, P.; Pourcelly, G. Desalination at overlimiting currents: State-of-the-art and perspectives. Desalination 2014, 342, 85–106. [Google Scholar] [CrossRef]
- Rubinstein, I.; Zaltzman, B. Convective diffusive mixing in concentration polarization: From Taylor dispersion to surface convection. J. Fluid Mech. 2013, 728, 239–278. [Google Scholar] [CrossRef]
- Kim, N.; Jeong, S.; Go, W.; Kim, Y. A Na+ ion-selective desalination system utilizing a NASICON ceramic membrane. Water Res. 2022, 215, 118250. [Google Scholar] [CrossRef]
- Jashni, E.; Hosseini, S.M.; Shen, J.N.; Van der Bruggen, B. Electrochemical characterization of mixed matrix electrodialysis cation exchange membrane incorporated with carbon nanofibers for desalination. Ionics 2019, 25, 5595–5610. [Google Scholar] [CrossRef]
- Chialvo, A.A.; Crisalle, O.D. On the Transition-State theory approach to the Jones-Dole’s viscosity B-coefficient: A novel molecular-based interpretation, assessment of its implications, and experimental evidence. J. Mol. Liq. 2023, 379, 121548. [Google Scholar] [CrossRef]
- Kinart, Z.; Adam, B.; Domańska, A. Viscosity coefficients of KCl, NaCl, NaI, NaBr, KNO3, LiNO3, AgNO3, NaClO4, NaBPh4, Bu4NI and Et4NI in rich of water binary mixtures containing propan-1-ol at 298.15 K. Phys. Chem. Liq. 2016, 54, 14–26. [Google Scholar] [CrossRef]
- Smith, K.C. Theoretical evaluation of electrochemical cell architectures using cation intercalation electrodes for desalination. Electrochim. Acta 2017, 230, 333–341. [Google Scholar] [CrossRef]
- Dydek, E.V.; Zaltzman, B.; Rubinstein, I.; Deng, D.S.; Mani, A.; Bazant, M.Z. Overlimiting Current in a Microchannel. Phys. Rev. Lett. 2011, 107, 118301. [Google Scholar] [CrossRef] [PubMed]
- Benneker, A.M.; Gumuscu, B.; Derckx, E.G.H.; Lammertink, R.G.H.; Eijkel, J.C.T.; Wood, J.A. Enhanced ion transport using geometrically structured charge selective interfaces. Lab Chip 2018, 18, 1652–1660. [Google Scholar] [CrossRef]
- Porada, S.; Zhao, R.; Van Der Wal, A.; Presser, V.; Biesheuvel, P.M. Review on the science and technology of water desalination by capacitive deionization. Prog. Mater. Sci. 2013, 58, 1388–1442. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Tewari, S. Capacitive deionization: Processes, materials and state of the technology. J. Electroanal. Chem. 2018, 813, 178–192. [Google Scholar] [CrossRef]
- Zhang, D.; Yan, T.; Shi, L.; Peng, Z.; Wen, X.; Zhang, J. Enhanced capacitive deionization performance of graphene/carbon nanotube composites. J. Mater. Chem. 2012, 22, 14696–14704. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Liu, C.-F.; Hsu, C.-C.; Hu, C.-C. An integrated strategy for improving the desalination performances of activated carbon-based capacitive deionization systems. Electrochim. Acta 2019, 302, 277–285. [Google Scholar] [CrossRef]
- Zhao, R.; Biesheuvel, P.M.; Miedema, H.; Bruning, H.; van der Wal, A. Charge Efficiency: A Functional Tool to Probe the Double-Layer Structure Inside of Porous Electrodes and Application in the Modeling of Capacitive Deionization. J. Phys. Chem. Lett. 2010, 1, 205–210. [Google Scholar] [CrossRef]
- Mossad, M.; Zou, L. Evaluation of the salt removal efficiency of capacitive deionisation: Kinetics, isotherms and thermodynamics. Chem. Eng. J. 2013, 223, 704–713. [Google Scholar] [CrossRef]
- Salari, K.; Zarafshan, P.; Khashehchi, M.; Chegini, G.; Etezadi, H.; Karami, H.; Szulżyk-Cieplak, J.; Łagód, G. Knowledge and Technology Used in Capacitive Deionization of Water. Membranes 2022, 12, 459. [Google Scholar] [CrossRef]
- He, D.; Wong, C.E.; Tang, W.; Kovalsky, P.; Waite, T.D. Faradaic Reactions in Water Desalination by Batch-Mode Capacitive Deionization. Environ. Sci. Technol. Lett. 2016, 3, 222–226. [Google Scholar] [CrossRef]
- Sun, K.; Tebyetekerwa, M.; Wang, C.; Wang, X.; Zhang, X.; Zhao, X.S. Electrocapacitive Deionization: Mechanisms, Electrodes, and Cell Designs. Adv. Funct. Mater. 2023, 33, 2213578. [Google Scholar] [CrossRef]
- Brousse, T.; Bélanger, D.; Long, J.W. To Be or Not To Be Pseudocapacitive? J. Electrochem. Soc. 2015, 162, A5185–A5189. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, J. Definitions of Pseudocapacitive Materials: A Brief Review. Energy Environ. Mater. 2019, 2, 30–37. [Google Scholar] [CrossRef]
- Suss, M.E.; Presser, V. Water Desalination with Energy Storage Electrode Materials. Joule 2018, 2, 10–15. [Google Scholar] [CrossRef]
- Chen, K.-Y.; Shen, Y.-Y.; Wang, D.-M.; Hou, C.-H. Carbon nanotubes/activated carbon hybrid as a high-performance suspension electrode for the electrochemical desalination of wastewater. Desalination 2022, 522, 115440. [Google Scholar] [CrossRef]
- Chang, L.M.; Duan, X.Y.; Liu, W. Preparation and electrosorption desalination performance of activated carbon electrode with titania. Desalination 2011, 270, 285–290. [Google Scholar] [CrossRef]
- Bokov, D.; Turki Jalil, A.; Chupradit, S.; Suksatan, W.; Javed Ansari, M.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by Sol-Gel Method: Synthesis and Application. Adv. Mater. Sci. Eng. 2021, 2021, 5102014. [Google Scholar] [CrossRef]
- Anderson, M.A.; Cudero, A.L.; Palma, J. Capacitive deionization as an electrochemical means of saving energy and delivering clean water. Comparison to present desalination practices: Will it compete? Electrochim. Acta 2010, 55, 3845–3856. [Google Scholar] [CrossRef]
- AlMarzooqi, F.A.; Al Ghaferi, A.A.; Saadat, I.; Hilal, N. Application of Capacitive Deionisation in water desalination: A review. Desalination 2014, 342, 3–15. [Google Scholar] [CrossRef]
- Suss, M.E.; Biesheuvel, P.M.; Baumann, T.F.; Stadermann, M.; Santiago, J.G. In Situ Spatially and Temporally Resolved Measurements of Salt Concentration between Charging Porous Electrodes for Desalination by Capacitive Deionization. Environ. Sci. Technol. 2014, 48, 2008–2015. [Google Scholar] [CrossRef]
- Demirer, O.N.; Hidrovo, C.H. Laser-induced fluorescence visualization of ion transport in a pseudo-porous capacitive deionization microstructure. Microfluid. Nanofluid. 2014, 16, 109–122. [Google Scholar] [CrossRef]
- Roelofs, S.H.; van Soestbergen, M.; Odijk, M.; Eijkel, J.C.T.; van den Berg, A. Effect of pH waves on capacitive charging in microfluidic flow channels. Ionics 2014, 20, 1315–1322. [Google Scholar] [CrossRef][Green Version]
- Roelofs, S.H.; Kim, B.; Eijkel, J.C.T.; Han, J.; van den Berg, A.; Odijk, M. Capacitive deionization on-chip as a method for microfluidic sample preparation. Lab Chip 2015, 15, 1458–1464. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.-H.; Tai, Y.-C.; Xu, J.-Y.; Yang, Y.-H.; Huang, H.-Y.; Huang, J.-H.; Hu, C.-C. Highly efficient water purification devices utilizing the microfluidic electrochemical deionization technique. Desalination 2022, 538, 115928. [Google Scholar] [CrossRef]
- Kim, S.; Kim, C.; Lee, J.; Kim, S.; Lee, J.; Kim, J.; Yoon, J. Hybrid Electrochemical Desalination System Combined with an Oxidation Process. ACS Sustain. Chem. Eng. 2018, 6, 1620–1626. [Google Scholar] [CrossRef]
- De Valença, J.C.; Wagterveld, R.M.; Lammertink, R.G.H.; Tsai, P.A. Dynamics of microvortices induced by ion concentration polarization. Phys. Rev. E 2015, 92, 031003(R). [Google Scholar] [CrossRef]
- Han, W.; Chen, X. A review: Applications of ion transport in micro-nanofluidic systems based on ion concentration polarization. Chem. Technol. Biotechnol. 2020, 95, 1622–1631. [Google Scholar] [CrossRef]
- Kwak, R.; Kim, S.J.; Han, J. Continuous-flow biomolecule and cell concentrator by ion concentration polarization. Anal. Chem. 2011, 83, 7348–7355. [Google Scholar] [CrossRef]
- Kim, S.J.; Song, Y.-A.; Han, J. Nanofluidic concentration devices for biomolecules utilizing ion concentration polarization: Theory, fabrication, and applications. Chem. Soc. Rev. 2010, 39, 912–922. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, I.; Shtilman, L. Voltage against current curves of cation exchange membranes. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1979, 75, 231–246. [Google Scholar] [CrossRef]
- Gong, L.; Ouyang, W.; Li, Z.; Han, J. Force fields of charged particles in micro-nanofluidic preconcentration systems. AIP Adv. 2017, 7, 125020. [Google Scholar] [CrossRef]
- Kim, B.; Kwak, R.; Kwon, H.J.; Pham, V.S.; Kim, M.; Al-Anzi, B.; Lim, G.; Han, J. Purification of High Salinity Brine by Multi-Stage Ion Concentration Polarization Desalination. Sci. Rep. 2016, 6, 31850. [Google Scholar] [CrossRef]
- Pandurangappa, M.; Kempegowda, G. Chemically Modified Carbon Nanotubes: Derivatization and Their Applications. In Carbon Nanotubes Applications on Electron Devices; InTech: London, UK, 2011. [Google Scholar] [CrossRef]
- Shen, L.; Huang, Z.; Liu, Y.; Li, R.; Xu, Y.; Jakaj, G.; Lin, H. Polymeric Membranes Incorporated with ZnO Nanoparticles for Membrane Fouling Mitigation: A Brief Review. Front. Chem. 2020, 8, 224. [Google Scholar] [CrossRef]
- Jashni, E.; Hosseini, S.M.; Shen, J. A new approach to providing heterogeneous cation-exchange membrane with enhanced electrochemical and desalination performance by incorporation of Fe3O4/PVP composite nanoparticles. Ionics 2020, 26, 861–874. [Google Scholar] [CrossRef]
- Wang, K.; Xu, L.; Li, K.; Liu, L.; Zhang, Y.; Wang, J. Development of polyaniline conductive membrane for electrically enhanced membrane fouling mitigation. J. Memb. Sci. 2019, 570–571, 371–379. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, J.; Liu, X.; Wang, Y.; Lin, J.; Peng, N.; Li, J.; Zhao, F. Performance enhancement of polyvinyl chloride ultrafiltration membrane modified with graphene oxide. J. Colloid Interface Sci. 2016, 480, 1–8. [Google Scholar] [CrossRef]
- Shen, L.; Feng, S.; Li, J.; Chen, J.; Li, F.; Lin, H.; Yu, G. Surface modification of polyvinylidene fluoride (PVDF) membrane via radiation grafting: Novel mechanisms underlying the interesting enhanced membrane performance. Sci. Rep. 2017, 7, 2721. [Google Scholar] [CrossRef] [PubMed]
- Knust, K.N.; Sheridan, E.; Anand, R.K.; Crooks, R.M. Dual-channel bipolar electrode focusing: Simultaneous separation and enrichment of both anions and cations. Lab Chip 2012, 12, 4107–4114. [Google Scholar] [CrossRef]
- Kim, S.J.; Ko, S.H.; Kang, K.H.; Han, J. Direct seawater desalination by ion concentration polarization. Nat Nanotechnol 2010, 5, 297–301. [Google Scholar] [CrossRef]
- Kwak, R.; Pham, V.S.; Kim, B.; Chen, L.; Han, J. Enhanced Salt Removal by Unipolar Ion Conduction in Ion Concentration Polarization Desalination. Sci. Rep. 2016, 6, 25349. [Google Scholar] [CrossRef]
- Tang, J.; Gong, L.; Jiang, J.; Li, Z.; Han, J. Numerical simulation of electrokinetic desalination using microporous permselective membranes. Desalination 2020, 477, 114262. [Google Scholar] [CrossRef]
- Ko, S.H.; Kim, S.J.; Cheow, L.F.; Li, L.D.; Kang, K.H.; Han, J. Massively parallel concentration device for multiplexed immunoassays. Lab Chip 2011, 11, 1351–1358. [Google Scholar] [CrossRef]
- Kwon, H.J.; Kim, B.; Lim, G.; Han, J. A multiscale-pore ion exchange membrane for better energy efficiency. J. Mater. Chem. A 2018, 6, 7714–7723. [Google Scholar] [CrossRef]
- Suresh, A.; Hill, G.T.; Hoenig, E.; Liu, C. Electrochemically mediated deionization: A review. Mol. Syst. Des. Eng. 2021, 6, 25–51. [Google Scholar] [CrossRef]
- Knust, K.N.; Hlushkou, D.; Anand, R.K.; Tallarek, U.; Crooks, R.M. Electrochemically Mediated Seawater Desalination. Angew. Chem. 2013, 125, 8265–8268. [Google Scholar] [CrossRef]
- Ghimire, U.; Heili, M.K.; Gude, V.G. Electrochemical desalination coupled with energy recovery and storage. Desalination 2021, 503, 114929. [Google Scholar] [CrossRef]
- Scialdone, O.; Guarisco, C.; Galia, A. Oxidation of organics in water in microfluidic electrochemical reactors: Theoretical model and experiments. Electrochim. Acta 2011, 58, 463–473. [Google Scholar] [CrossRef]
- Grygolowicz-Pawlak, E.; Sohail, M.; Pawlak, M.; Neel, B.; Shvarev, A.; De Marco, R.; Bakker, E. Coulometric Sodium Chloride Removal System with Nafion Membrane for Seawater Sample Treatment. Anal. Chem. 2012, 84, 6158–6165. [Google Scholar] [CrossRef] [PubMed]
- Fighera, M.; van der Wal, P.D.; Shea, H. Microfluidic Platform for Seawater Desalination by Coulometric Removal of Chloride Ions through Printed Ag Electrodes. J. Electrochem. Soc. 2017, 164, H836. [Google Scholar] [CrossRef]
- Srimuk, P.; Husmann, S.; Presser, V. Low voltage operation of a silver/silver chloride battery with high desalination capacity in seawater. RSC Adv. 2019, 9, 14849–14858. [Google Scholar] [CrossRef]
- Santana, H.S.; Silva, J.L., Jr.; Aghel, B.; Ortega-Casanova, J. Review on microfluidic device applications for fluids separation and water treatment processes. SN Appl. Sci. 2020, 2, 395. [Google Scholar] [CrossRef]
- Folaranmi, G.; Bechelany, M.; Sistat, P.; Cretin, M.; Zaviska, F. Towards electrochemical water desalination techniques: A review on capacitive deionization, membrane capacitive deionization and flow capacitive deionization. Membranes 2020, 10, 96. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, J.; Zhang, X.; Chen, L.; Zhang, Q.; Ma, L. Recent advances in membrane-based materials for desalination and gas separation. J. Clean. Prod. 2023, 387, 135845. [Google Scholar] [CrossRef]
- Yang, Z.; Ma, X.-H.; Tang, C.Y. Recent development of novel membranes for desalination. Desalination 2018, 434, 37–59. [Google Scholar] [CrossRef]
- Prihatiningtyas, I.; Van der Bruggen, B. Nanocomposite pervaporation membrane for desalination. Chem. Eng. Res. Des. 2020, 164, 147–161. [Google Scholar] [CrossRef]
- Wu, S.; Peng, L.E.; Yang, Z.; Sarkar, P.; Barboiu, M.; Tang, C.Y.; Fane, A.G. Next-Generation Desalination Membranes Empowered by Novel Materials: Where Are We Now? Nano-Micro Lett. 2024, 17, 91. [Google Scholar] [CrossRef]
- Lee, K.P.; Arnot, T.C.; Mattia, D. A review of reverse osmosis membrane materials for desalination—Development to date and future potential. J. Memb. Sci. 2011, 370, 1–22. [Google Scholar] [CrossRef]
- Fard, A.K.; McKay, G.; Buekenhoudt, A.; Al Sulaiti, H.; Motmans, F.; Khraisheh, M.; Atieh, M. Inorganic membranes: Preparation and application for water treatment and desalination. Materials 2018, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Sanaeepur, H.; Amooghin, A.E.; Shirazi, M.M.A.; Pishnamazi, M.; Shirazian, S. Water desalination and ion removal using mixed matrix electrospun nanofibrous membranes: A critical review. Desalination 2022, 521, 115350. [Google Scholar] [CrossRef]
- Jaspal, D.; Malviya, A.; El Allaoui, B.; Zari, N.; Bouhfid, R.; Kacem Qaiss, A.E.; Bhagwat, S. Emerging advances of composite membranes for seawater pre-treatment: A review. Water Sci. Technol. 2023, 88, 408–429. [Google Scholar] [CrossRef]
- Katare, A.; Kumar, S.; Kundu, S.; Sharma, S.; Kundu, L.M.; Mandal, B. Mixed Matrix Membranes for Carbon Capture and Sequestration: Challenges and Scope. ACS Omega 2023, 8, 17511–17522. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, S.R.; Venkatachalam, C.D.; Sengottian, M.; Sekar, S.; Ramasamy, B.S.; Narayanan, M.; Gopalakrishnan, A.V.; Kandasamy, S.; Raja, R. A review on fabrication, characterization of membrane and the influence of various parameters on contaminant separation process. Chemosphere 2022, 306, 135629. [Google Scholar] [CrossRef]
- Porter, C.J.; Werber, J.R.; Zhong, M.; Wilson, C.J.; Elimelech, M. Pathways and Challenges for Biomimetic Desalination Membranes with Sub-Nanometer Channels. ACS Nano 2020, 14, 10894–10916. [Google Scholar] [CrossRef]
- Shin, M.G.; Seo, J.Y.; Park, H.; Park, Y.-I.; Lee, J.-H. Overcoming the permeability-selectivity trade-off of desalination membranes via controlled solvent activation. J. Memb. Sci. 2021, 620, 118870. [Google Scholar] [CrossRef]
- Wan, H.; Yan, X.; Yang, J.; Yan, G.; Zhang, G. A facile solvent activation process manipulates the structure and filtration performance of oxidized poly (arylene sulfide sulfone) TFC membrane. Desalination 2024, 588, 117971. [Google Scholar] [CrossRef]
- Zhu, R.; Liu, M.; Hou, Y.; Wang, D.; Zhang, L.; Wang, D.; Fu, S. Mussel-inspired photothermal synergetic system for clean water production using full-spectrum solar energy. Chem. Eng. J. 2021, 423, 129099. [Google Scholar] [CrossRef]
- He, H.; Song, Z.; Lan, Y.; Huang, M.; Wu, S.; Ben, C.; He, D.; Hou, X.; Song, X.M.; Zhang, Y. Photocorrosion-Based BiOCl Photothermal Materials for Synergistic Solar-Driven Desalination and Photoelectrochemistry Energy Storage and Release. ACS Appl. Mater. Interfaces 2023, 15, 17947–17956. [Google Scholar] [CrossRef] [PubMed]
- Tawalbeh, M.; Shomope, I.; Al-Othman, A. Comprehensive review on non-Newtonian nanofluids, preparation, characterization, and applications. Int. J. Thermofluids 2024, 22, 100705. [Google Scholar] [CrossRef]
- Gonçalves, I.; Souza, R.; Coutinho, G.; Miranda, J.; Moita, A.; Pereira, J.E.; Moreira, A.; Lima, R. Thermal conductivity of nanofluids: A review on prediction models, controversies and challenges. Appl. Sci. 2021, 11, 2525. [Google Scholar] [CrossRef]
- Yao, S.; Teng, Z. Effect of nanofluids on boiling heat transfer performance. Appl. Sci. 2019, 9, 2818. [Google Scholar] [CrossRef]
- Al-Obaidi, M.A.; Zubo, R.H.A.; Rashid, F.L.; Dakkama, H.J.; Abd-Alhameed, R.; Mujtaba, I.M. Evaluation of Solar Energy Powered Seawater Desalination Processes: A Review. Energies 2022, 15, 6562. [Google Scholar] [CrossRef]
- Arunachalam, M.; Han, D.S. Efficient solar-powered PEM electrolysis for sustainable hydrogen production: An integrated approach. Emergent Mater. 2024, 7, 1401–1415. [Google Scholar] [CrossRef]
- Wani, T.A.; Kaith, P.; Garg, P.; Bera, A. Microfluidic Salinity Gradient-Induced All-Day Electricity Production in Solar Steam Generation. ACS Appl. Mater. Interfaces 2022, 14, 35802–35808. [Google Scholar] [CrossRef]
- Kaur, M.; Nagao, T. Minireview on Solar Desalination and Hydropower Generation by Water Evaporation: Recent Challenges and Perspectives in Materials Science. Energy Fuels 2022, 36, 11443–11456. [Google Scholar] [CrossRef]
- Luo, Z.; Hu, Y.; Cao, L.; Li, S.; Liu, X.; Fan, R. Enhanced Separation Performance of Graphene Oxide Membrane through Modification with Graphitic Carbon Nitride. Water 2024, 16, 967. [Google Scholar] [CrossRef]
- Iqbal, A.; Mahmoud, M.S.; Sayed, E.T.; Elsaid, K.; Abdelkareem, M.A.; Alawadhi, H.; Olabi, A.G. Evaluation of the nanofluid-assisted desalination through solar stills in the last decade. J. Environ. Manag. 2021, 277, 111415. [Google Scholar] [CrossRef]
- Singh, T.; Atieh, M.A.; Al-Ansari, T.; Mohammad, A.W.; McKay, G. The role of nanofluids and renewable energy in the development of sustainable desalination systems: A review. Water 2020, 12, 2002. [Google Scholar] [CrossRef]
- Parmar, H.B.; Juybari, H.F.; Yogi, Y.S.; Nejati, S.; Jacob, R.M.; Menon, P.S.; Warsinger, D.M. Nanofluids improve energy efficiency of membrane distillation. Nano Energy 2021, 88, 106235. [Google Scholar] [CrossRef]
- Čížek, J.; Cvejn, P.; Marek, J.; Tvrzník, D. Desalination performance assessment of scalable, multi-stack ready shock electrodialysis unit utilizing anion-exchange membranes. Membranes 2020, 10, 347. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Wang, E.; Chen, P.-W.; Tsai, Y.-H.; Langouche, L.; Lo, Y.-H. A microfluidic design for desalination and selective removal and addition of components in biosamples. Biomicrofluidics 2019, 13, 024109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wu, J.; Wang, L.; Xiao, K.; Wen, W. A simple method for fabricating multi-layer PDMS structures for 3D microfluidic chips. Lab Chip 2010, 10, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
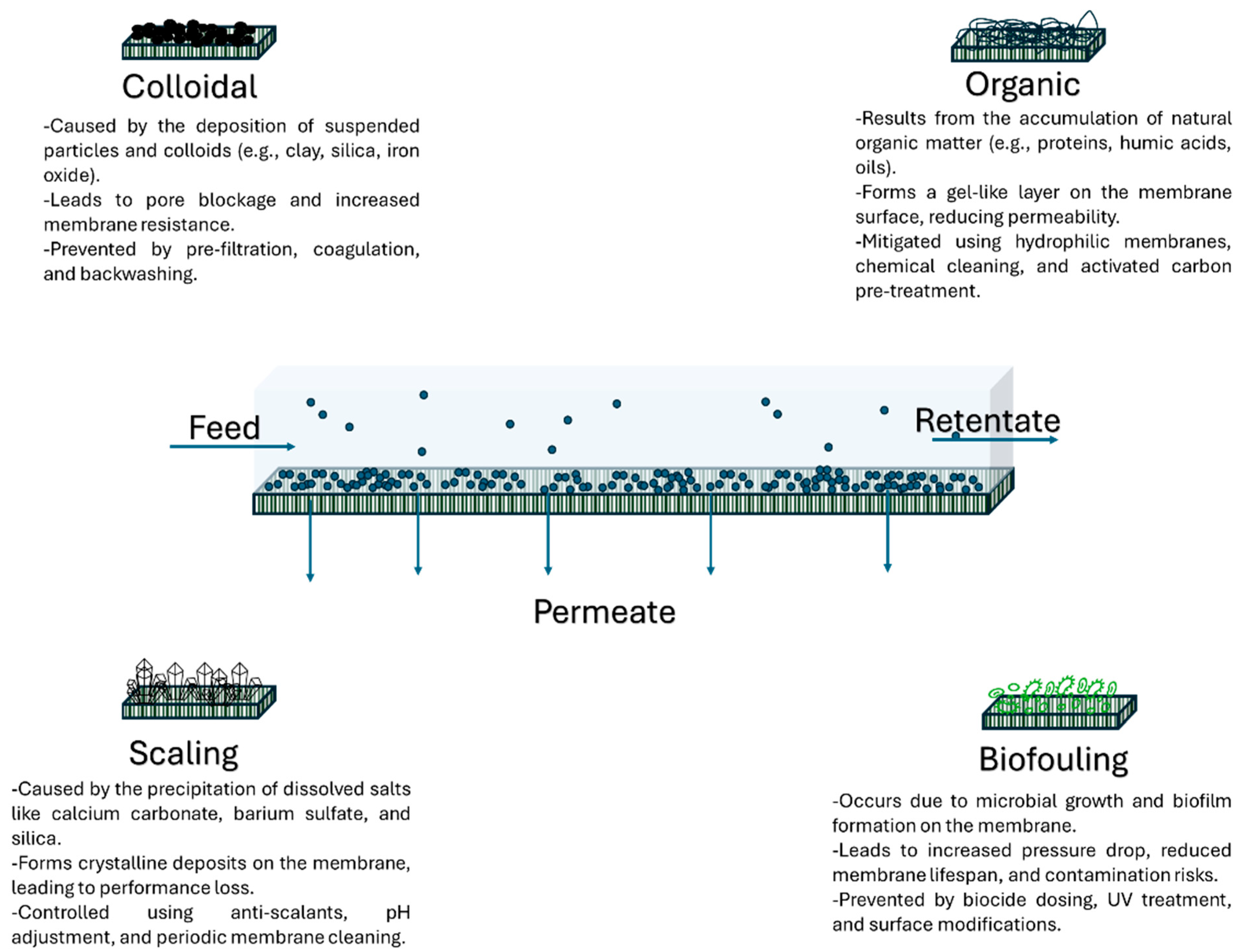
- Alsawaftah, N.; Abuwatfa, W.; Darwish, N.; Husseini, G. A comprehensive review on membrane fouling: Mathematical modelling, prediction, diagnosis, and mitigation. Water 2021, 13, 1327. [Google Scholar] [CrossRef]
- Cirillo, A.I.; Tomaiuolo, G.; Guido, S. Membrane fouling phenomena in microfluidic systems: From technical challenges to scientific opportunities. Micromachines 2021, 12, 820. [Google Scholar] [CrossRef]
- Abuwatfa, W.H.; AlSawaftah, N.; Darwish, N.; Pitt, W.G.; Husseini, G.A. A Review on Membrane Fouling Prediction Using Artificial Neural Networks (ANNs). Micromachines 2021, 12, 820. [Google Scholar] [CrossRef]
- Ige, E.O.; Arun, R.K.; Singh, P.; Gope, M.; Saha, R.; Chanda, N.; Chakraborty, S. Water desalination using graphene oxide-embedded paper microfluidics. Microfluid. Nanofluid. 2019, 23, 80. [Google Scholar] [CrossRef]
- Nguyen, H.-T.; Massino, M.; Keita, C.; Salmon, J.-B. Microfluidic dialysis using photo-patterned hydrogel membranes in PDMS chips. Lab Chip 2020, 20, 2383–2393. [Google Scholar] [CrossRef]
- Tripathy, S.; Tripathy, D.K.; Samantaray, S. Chapter 3—Recent advances on 3D printing for wastewater treatment and process optimization using artificial intelligence and machine learning: Updates and perspectives. In 3D Printing Technology for Water Treatment Applications; Pandey, J.K., Manna, S., Patel, R.K., Qian, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 55–82. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Ginn, M.; Rastogi, V. A review of 3D printing techniques for environmental applications. Curr. Opin. Chem. Eng. 2020, 28, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Balogun, H.A.; Sulaiman, R.; Marzouk, S.S.; Giwa, A.; Hasan, S.W. 3D printing and surface imprinting technologies for water treatment: A review. J. Water Process Eng. 2019, 31, 100786. [Google Scholar] [CrossRef]
- Ghandehari, S.; Montazer-Rahmati, M.M.; Asghari, M. A comparison between semi-theoretical and empirical modeling of cross-flow microfiltration using ANN. Desalination 2011, 277, 348–355. [Google Scholar] [CrossRef]
- Panagopoulos, A. Assessing the energy footprint of desalination technologies and minimal/zero liquid discharge (mld/zld) systems for sustainable water protection via renewable energy integration. Energies 2025, 18, 962. [Google Scholar] [CrossRef]

| Technique | Mechanical | Chemical | Energy-Assisted |
|---|---|---|---|
| Material removing methods |
|
|
|
| Material depositing methods |
|
|
|
| Enhancement Strategy | Targeted Performance Indicator | Outcome | Practical Implications | Limitations | Ref. |
|---|---|---|---|---|---|
| Incorporation of Fe3O4/PVP composite nanoparticles | Electrochemical properties and desalination performance | Significant enhancement in electrochemical properties, including reduced electrical resistance, improved hydrophilicity, increased membrane potential transport number and permselectivity, and enhanced ion flux. | Fe3O4/PVP composite nanoparticles can significantly improve the efficiency of cation-exchange membranes for desalination through electrodialysis | The study does not extensively discuss the modified membranes’ long-term stability and performance under varied operational conditions. | [82] |
| Modification with GO via phase inversion method | Hydrophilicity, water flux, and mechanical properties | Hydrophilicity and water flux significantly increased; pure water flux increased to 430.0 L/(m²·h·bar), and tensile strength to 305.3 cN with 0.1 wt% GO. | Demonstrates the effectiveness of GO in enhancing the performance of PVC ultrafiltration membranes for wastewater treatment, offering a cost-effective solution with improved efficiency. | The study primarily focuses on improving hydrophilicity and mechanical properties without extensive discussion on long-term stability and fouling resistance under various water qualities. | [84] |
| Incorporation of DBSA into PANI membranes via non-solvent-induced phase separation | Antifouling performance and conductivity | The application of external voltage significantly mitigated BSA fouling, demonstrating enhanced antifouling performance with increased voltage. | This approach offers a promising strategy for fabricating conductive membranes capable of electrically enhanced fouling mitigation, potentially improving the longevity and efficiency of membrane filtration systems. | The study primarily focuses on BSA as a model foulant, suggesting further research to evaluate performance with diverse foulants and in real wastewater treatment scenarios. | [83] |
| Incorporation of ZnO nanoparticles into polymeric membranes (e.g., PVDF, PES) | Hydrophilicity, photocatalytic self-cleaning capabilities, and antimicrobial activities | Enhanced antifouling performance, improved water permeability, and stability; introduction of photocatalytic self-cleaning and antimicrobial properties | Offers a promising approach for reducing membrane fouling in water treatment applications, potentially extending membrane lifespan and efficiency | The review calls for further research on overcoming ZnO nanoparticles’ aggregation and leakage and understanding long-term performance in practical applications. | [81] |
| Radiation grafting of HEA onto PVDF membrane | Hydrophilicity, pH-dependent water flux, and antifouling performance | Enhanced hydrophilicity and pH-responsive water flux; improved antifouling performance with a novel observation of higher BSA flux than water flux | Presents a novel approach for creating pH-sensitive and antifouling PVDF membranes, with potential applications in controlled drug release and water treatment | The study mainly focuses on the characterization and performance evaluation of modified membranes at the laboratory scale, with limited discussion on large-scale applicability and long-term operational stability. | [85] |
| Incorporation of CNFs into membrane matrix | Surface hydrophilicity, potential transport number, permselectivity, ionic conductivity, and sodium flux | Enhanced electrochemical properties and sodium flux, with optimal performance observed at 0.5wt% and 2–8wt%. CNF ratios. | Demonstrates the effectiveness of CNFs in enhancing the performance of CEMs for electrodialysis, offering a scalable approach for improved desalination processes. | The study focuses on a specific range of CNF concentrations, suggesting further research is needed to explore the full potential and practicality of CNF incorporation across a broader range of membrane formulations. | [44] |
| Applications | Feed | Feed Channel Width | Feed Channel Height | Flow Rate | Efficiency | Advantages | Potential Improvements | Ref | |
|---|---|---|---|---|---|---|---|---|---|
| Dialysis | Cleanup of biological samples for ESI-MS | 5 µM horse heart myoglobin in 500 mM NaCl, 100 mM Tris, and 10 mM EDTA | 160 µm | 60 µm | 0.3 µL/min | Signal-to-noise ratio increased by a factor of 40 |
|
| [34] |
| Salt removal from biological samples prior to ESI-MS | 40 μM cytochrome-c in 100 mM KCl | 100 µm | 6 µm | 30–150 µL/h | 95% salt removal in 1 s |
|
| [32] | |
| ESI-MS | Cytochrome dissolved in 154 mM NaCl | 0.5 mm | 89 µm | 1 µL/min | ~99% |
|
| [36] | |
| Isolation and concentration of bacteria from blood | 100 µL of blood and an E coli suspension in 500 µL dielectrophoresis buffer | 250 µm | 3 mm | 0.6 mL/h | 79 ± 3% of E. coli separated 78 ± 2% of Staphylococcus aureus separated |
|
| [37] | |
| ED | Investigating and optimizing ED systems | 10 mM NaCl 10 µM Rhodamine 6G | 1 mm | 200 µm | 10 µL/min | 90% salt removal |
|
| [39] |
| Usage of Na super ionic conductor (NASICON) in ED | Seawater | - | - | 500 mL/min | Achieved up to 98% of Na+ removal |
|
| [43] | |
| Investigating the effect of hydrogel geometry on ion transport in electrodialysis | 0.1 mM NaCl | 700 µm | 75 µm | 3 µm/min |
|
| [49] | ||
| CDI | In situ concentration mapping | 50 or 80 mM KCl 5mM 6-methoxy-N-(3-sulfopropyl)quinolinium (SPQ) | 1.5 mm | 5 mm | 0 | 10% within 25 s |
|
| [67] |
| Laser-induced fluorescence visualization of ion transport in a pseudo-porous CDI microstructure | 0.7 mM solution of Sulforhodamine B (cationic dye) and Fluorescein (anionic dye) | 0.2 mm | 0.1 mm | 0 | 60% within 60 s |
|
| [68] | |
| Visualization of pH waves | 10 mM NaCl Dyes: BODIPY and fluorescein | 97.5 µm | ~7 µm | 0 |
|
| [69] | ||
| Sample clean up to concentrate FITC-dextran | 10 mM NaCl with FITC-dextran (model protein) | 1.5 mm | 0.4 mm | 1–10 µL/min | 23% salt removal within 30 min |
|
| [51] | |
| Water purification | Brackish water (8 mM) seawater (600 mM) | 2 mm | 1.5 mm | 0.5–2 mL/min | 88% ion removal in a single pass |
|
| [71] | |
| Hybrid electrochemical desalination with integrated oxidation | 3–35 g/L NaCl | Desalination: batch mode Oxidation: 2 mL/min | 28 mg Na+ deionized/g Na0.44MnO2 (electrode material) |
|
| [72] | |||
| ICP | Brine desalination | 0.1–1.7 M NaCl | 1.5–2.0 mm | 0.2–0.6 mm | 20 μL/min | Up to 70% salt rejection |
|
| [79] |
| Simultaneous separation and enrichment of anions and cations in a single microchannel | 0.5 M TrisH+ and acetate buffers | 50 µm | 8.7 µm | Pressure-driven counter-flow |
|
| [86] | ||
| Seawater desalination | Seawater (~500 mM salinity) | 1 µm (simulation-based) | 1 m (simulation-based) | 0.56 m/s | Salt removal > 98% |
|
| [89] | |
| ECD | Electrochemical desalination with energy recovery and storage | Seawater | - | 30.3% salt removal during a 12-h discharge |
|
| [94] | ||
| Electrochemical removal of NaCl from seawater samples | 0.6 mM NaCl | 30 µm | 40 µL/min | 90% salt removal within 90 s |
|
| [96] |
| Method | Driving Mechanism | Salt Removal Efficiency | Scalability | Fouling Risk | Energy Requirement | Key Advantages | Limitations |
|---|---|---|---|---|---|---|---|
| Dialysis/ED | Concentration gradient/Electric field | Moderate to High (up to ~95%) | Moderate (parallelization needed) | Moderate (membrane-based) | Moderate | Established method; high selectivity; modular | Membrane fouling; limited flow rates; mechanical stability limits |
| CDI/ECDI | Electric double layer or redox ion storage | Moderate to High (~60–90%) | Moderate (surface area dependent) | Low to Moderate | Low to Moderate | Low energy consumption; modular; reusable electrodes | Electrode degradation (faradaic); co-ion effects; limited for high salinity |
| ICP | Electrokinetic ion transport at nano/membrane interface | High (up to ~99%) | Low (single chip = low throughput) | Low | Low to Moderate | No membrane fouling; high salt rejection; suitable for portable use | Limited throughput; needs extreme parallelization to scale; energy at high current |
| ECD | Redox reactions and local electric fields | Moderate (up to ~90%) | High (via parallelization) | Low | Very Low (~25 mWh/L reported) | Membraneless; simple setup; renewable integration possible | Electrode material consumption; slower desalination rate; stability issues |
| Membrane Material | Polymeric | Inorganic | Composite |
|---|---|---|---|
| Characteristics |
|
|
|
| Advantages |
|
|
|
| Disadvantages |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abuwatfa, W.H.; Taleb, H.; AlSawaftah, N.; Chahrour, K.; Husseini, G.A.; Darwish, N. Microfluidic Electrochemical Desalination Systems: A Review. Water 2025, 17, 1601. https://doi.org/10.3390/w17111601
Abuwatfa WH, Taleb H, AlSawaftah N, Chahrour K, Husseini GA, Darwish N. Microfluidic Electrochemical Desalination Systems: A Review. Water. 2025; 17(11):1601. https://doi.org/10.3390/w17111601
Chicago/Turabian StyleAbuwatfa, Waad H., Haya Taleb, Nour AlSawaftah, Khaled Chahrour, Ghaleb A. Husseini, and Naif Darwish. 2025. "Microfluidic Electrochemical Desalination Systems: A Review" Water 17, no. 11: 1601. https://doi.org/10.3390/w17111601
APA StyleAbuwatfa, W. H., Taleb, H., AlSawaftah, N., Chahrour, K., Husseini, G. A., & Darwish, N. (2025). Microfluidic Electrochemical Desalination Systems: A Review. Water, 17(11), 1601. https://doi.org/10.3390/w17111601






