Responses of Soil Microbial Communities to Biogas Slurry Irrigation in Paddy Fields: Interactions with Environmental Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Soil Sampling
2.2. Soil Physicochemical Analyses
2.3. DNA Extraction and Illumina Sequencing
2.4. Statistical Analyses
3. Results and Discussion
3.1. Soil Properties and Heavy Metals
3.2. Soil Microbial Alpha Diversity
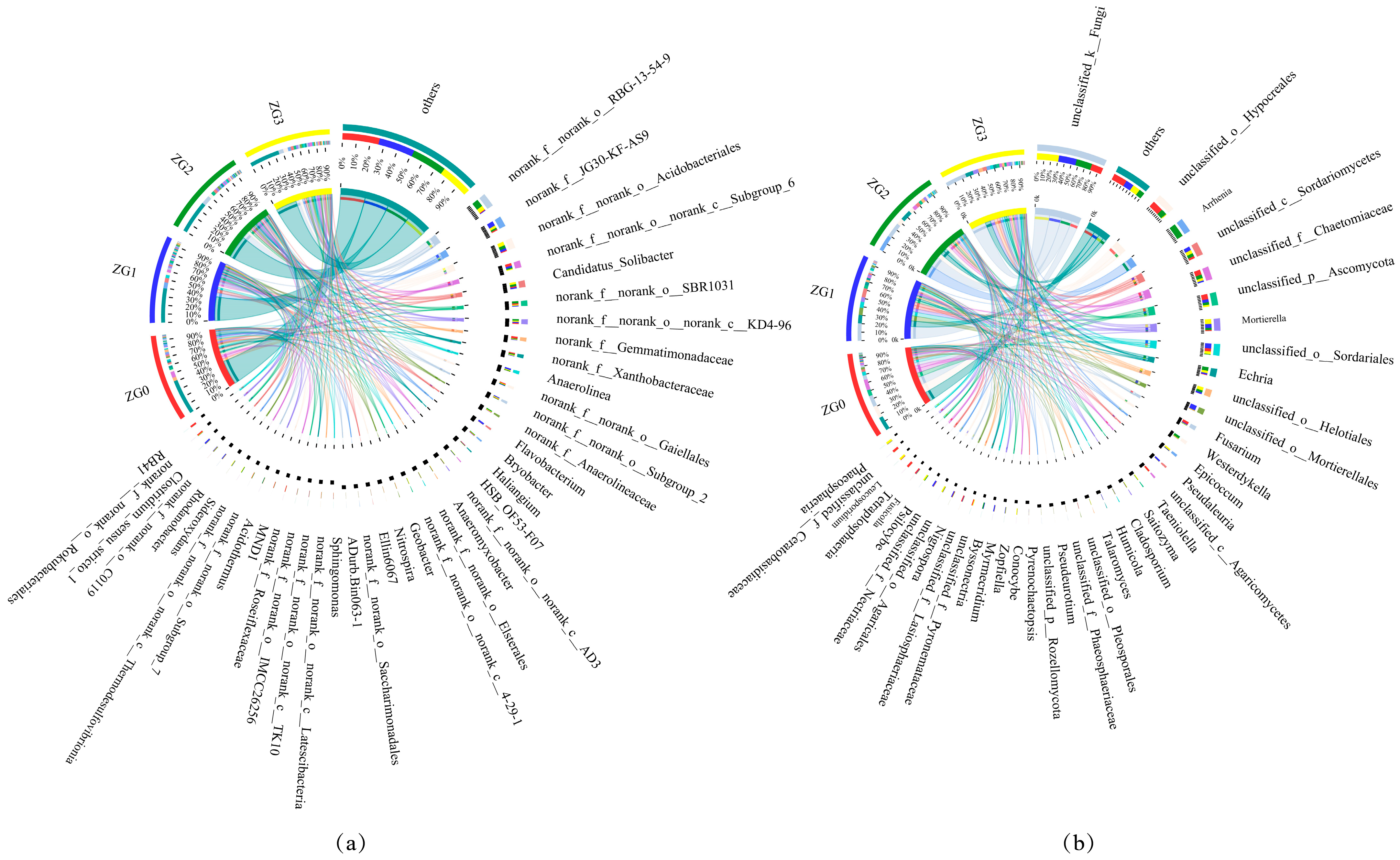
3.3. Soil Microbial Community Composition
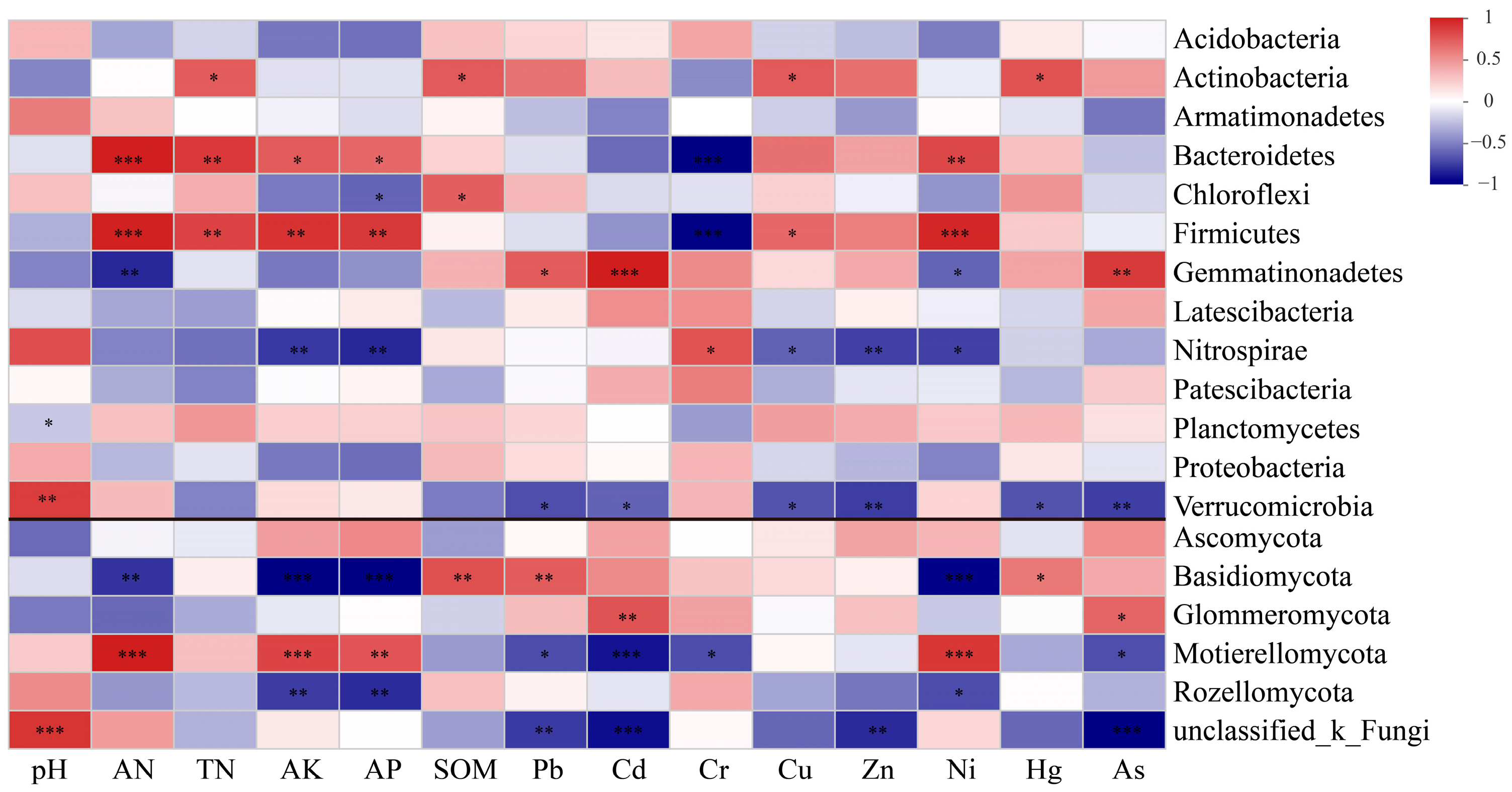
3.4. Relationship Between Soil Microbial Community Structure and Soil Environmental Factors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, Z.; Wang, Q. Soil microbial activity and community composition as influenced by application of pig biogas slurry in paddy field in southeast China. Paddy Water Environ. 2020, 18, 15–25. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, M. Replacement of mineral fertilizers with anaerobically digested pig slurry in paddy fields: Assessment of plant growth and grain quality. Environ. Sci. Pollut. Res. 2017, 24, 8916–8923. [Google Scholar] [CrossRef] [PubMed]
- Andlar, M.; Belskaya, H. Biogas production systems and upgrading technologies: A review. Food Technol. Biotechnol. 2021, 59, 387–412. [Google Scholar] [CrossRef]
- Liang, X.; Wang, H. Disentangling the impact of biogas slurry topdressing as a replacement for chemical fertilizers on soil bacterial and fungal community composition, functional characteristics, and co-occurrence networks. Environ. Res. 2023, 238, 117256. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, J. Long-term biogas slurry application increases microbial necromass but not plant lignin contribution to soil organic carbon in paddy soils as regulated by fungal community. Waste Manag. 2024, 175, 254–264. [Google Scholar] [CrossRef]
- Li, Y.; Liu, B. Flooding soil with biogas slurry suppresses root-knot nematodes and alters soil nematode communities. Heliyon 2024, 10, 30226. [Google Scholar] [CrossRef]
- Blagodatskaya, E.; Kuzyakov, Y. Active microorganisms in soil: Critical review of estimation criteria and approaches. Soil Biol. Biochem. 2013, 67, 192–211. [Google Scholar] [CrossRef]
- Bian, X.; Xiao, S. Comparative analysis of rhizosphere soil physiochemical characteristics and microbial communities between rusty and healthy ginseng root. Sci. Rep. 2020, 10, 15756. [Google Scholar] [CrossRef]
- Pan, X.; Yuzuak, S. Microbial community and antibiotic resistance gene distribution in food waste, anaerobic digestate, and paddy soil. Sci. Total Environ. 2023, 889, 164192. [Google Scholar] [CrossRef]
- Li, B.; Roley, S.S. Long-term excess nitrogen fertilizer increases sensitivity of soil microbial community to seasonal change revealed by ecological network and metagenome analyses. Soil Biol. Biochem. 2021, 160, 108349. [Google Scholar] [CrossRef]
- Lladó, S.; Baldrian, P. Community-level physiological profiling analyses show potential to identify the copiotrophic bacteria present in soil environments. PLoS ONE 2017, 12, e0171638. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, J. Soil chemical and microbial responses to biogas slurry amendment and its effect on Fusarium wilt suppression. Appl. Soil Ecol. 2016, 107, 116–123. [Google Scholar] [CrossRef]
- Li, M.; Wang, K. Metagenomics and network analysis decipher profiles and co-occurrence patterns of bacterial taxa in soils amended with biogas slurry. Sci. Total Environ. 2023, 877, 162911. [Google Scholar] [CrossRef] [PubMed]
- Mujakić, I.; Cabello-Yeves, P.J.; Villena-Alemany, C.; Piwosz, K.; Rodriguez-Valera, F.; Picazo, A.; Camacho, A.; Koblížek, M. Multi-environment ecogenomics analysis of the cosmopolitan phylum Gemmatimonadota. Microbiol. Spectr. 2023, 11, e01112-23. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Q. Fungal community composition change and heavy metal accumulation in response to the long-term application of anaerobically digested slurry in a paddy soil. Ecotox. Environ. Safe. 2020, 196, 110453. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Z. Influence of irrigation with microalgae-treated biogas slurry on agronomic trait, nutritional quality, oxidation resistance, and nitrate and heavy metal residues in Chinese cabbage. J. Environ. Manag. 2019, 244, 453–461. [Google Scholar] [CrossRef]
- Xu, M.; Xian, Y. Effect of biogas slurry addition on soil properties, yields, and bacterial composition in the rice-rape rotation ecosystem over 3 years. J. Soils Sediments 2019, 19, 2534–2542. [Google Scholar] [CrossRef]
- GB/T 36197; Soil Quality—Guidance on Sampling Techniques. National Standardization Administration: Beijing, China, 2018.
- Kimberly, A.K.; Janet, E.W. Comparison of commercially-available preservatives for maintaining the integrity of bacterial DNA in human milk. J. Microbiol. Meth. 2017, 141, 73–81. [Google Scholar]
- Bastian, F.; Bouziri, L. Impact of wheat straw decomposition on successional patterns of soil microbial community structure. Soil Biol. Biochem. 2009, 41, 262–275. [Google Scholar] [CrossRef]
- Olsen, S.; Sommers, L. 1982 Phosphorus. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; SSSA: Madison, WI, USA, 1982. [Google Scholar]
- Hao, Y.W.; Guan, W. Intestinal microbiome profiles in Oncomelania hupensis in mainland China. Acta Trop. 2020, 201, 105202. [Google Scholar] [CrossRef]
- Su, Y.; Lv, J.L. Long-term of decomposed straw return positively affects the soil microbial community. J. Appl. Microbiol. 2020, 128, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Ren, N.; Wang, Y.; Ye, Y.; Zhao, Y.; Huang, Y.; Fu, W.; Chu, X. Effects of continuous nitrogen fertilizer application on the diversity and composition of rhizosphere soil bacteria. Front. Microbiol. 2020, 11, 1948. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, J. Effects of over 30-year of different fertilization regimes on fungal community compositions in the black soils of northeast China. Agric. Ecosyst. Environ. 2017, 248, 113–122. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, J. Biochar decreased microbial metabolic quotient and shifted community composition four years after a single incorporation in a slightly acid rice paddy from southwest China. Sci. Total Environ. 2016, 571, 206–217. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X. Long-term nitrogen fertilization impacts soil fungal and bacterial community structures in a dryland soil of Loess Plateau in China. J. Soils Sediments 2017, 18, 1632–1640. [Google Scholar] [CrossRef]
- Yao, Q.; Liu, J. Three years of biochar amendment alters soil physiochemical properties and fungal community composition in a black soil of northeast China. Soil Biol. Biochem. 2017, 110, 56–67. [Google Scholar] [CrossRef]
- Hemme, C.L.; Deng, Y.; Gentry, T.J. Metagenomic insights into evolution of a heavy metal-contaminated groundwater microbial community. ISME J. 2010, 4, 660–672. [Google Scholar] [CrossRef]


| Shannon | Simpson | Chao1 | Coverage | |||||
|---|---|---|---|---|---|---|---|---|
| Bacteria | Fungi | Bacteria | Fungi | Bacteria | Fungi | Bacteria | Fungi | |
| ZG0 | 6.9571 ± 0.0462 b | 4.6176 ± 0.0616 d | 0.0023 ± 0.0001 a | 0.0365 ± 0.0080 b | 3759.9757 ± 150.5584 c | 1050.8603 ± 57.0251 c | 0.9687 ± 0.0114 a | 0.9982 ± 0.0003 a |
| ZG1 | 6.5355 ± 0.0192 a | 4.1278 ± 0.0429 a | 0.0046 ± 0.00003 c | 0.0537 ± 0.0027 c | 3549.9966 ± 119.4239 b | 684.0991 ± 15.4494 a | 0.9823 ± 0.0020 b | 0.9991 ± 0.0002 c |
| ZG2 | 6.5709 ± 0.0511 a | 4.4639 ± 0.0218 b | 0.0034 ± 0.0006 b | 0.0298 ± 0.0011 b | 3199.5067 ± 52.3983 a | 829.8786 ± 32.5932 b | 0.9789 ± 0.0020 ab | 0.9985 ± 0.0002 ab |
| ZG3 | 6.5847 ± 0.0484 a | 4.8571 ± 0.0285 c | 0.0037 ± 0.0001 b | 0.0167 ± 0.0004 a | 3654.2542 ± 66.7986 bc | 828.6809 ± 14.4812 b | 0.9787 ± 0.0008 ab | 0.9989 ± 0.0007 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, D.; Yu, M.; Qiao, Y.; Shang, Y.; Yan, Y.; Wang, S.; Chen, X. Responses of Soil Microbial Communities to Biogas Slurry Irrigation in Paddy Fields: Interactions with Environmental Factors. Water 2025, 17, 1577. https://doi.org/10.3390/w17111577
Hu D, Yu M, Qiao Y, Shang Y, Yan Y, Wang S, Chen X. Responses of Soil Microbial Communities to Biogas Slurry Irrigation in Paddy Fields: Interactions with Environmental Factors. Water. 2025; 17(11):1577. https://doi.org/10.3390/w17111577
Chicago/Turabian StyleHu, Die, Man Yu, Yuying Qiao, Yiping Shang, Yufei Yan, Shunyue Wang, and Xiaoyang Chen. 2025. "Responses of Soil Microbial Communities to Biogas Slurry Irrigation in Paddy Fields: Interactions with Environmental Factors" Water 17, no. 11: 1577. https://doi.org/10.3390/w17111577
APA StyleHu, D., Yu, M., Qiao, Y., Shang, Y., Yan, Y., Wang, S., & Chen, X. (2025). Responses of Soil Microbial Communities to Biogas Slurry Irrigation in Paddy Fields: Interactions with Environmental Factors. Water, 17(11), 1577. https://doi.org/10.3390/w17111577





