Effect of Inorganic Coagulant and Dissolved Organic Matter on the Toxicity of Nano-Zinc Oxide to Phosphorus-Accumulating Organisms in Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. nZnO, Coagulants, and Other Chemicals
2.2. Activation and Culture of Phosphorus-Accumulating Organisms
2.3. Toxicity Exposure Experiment
2.4. Analysis
3. Results
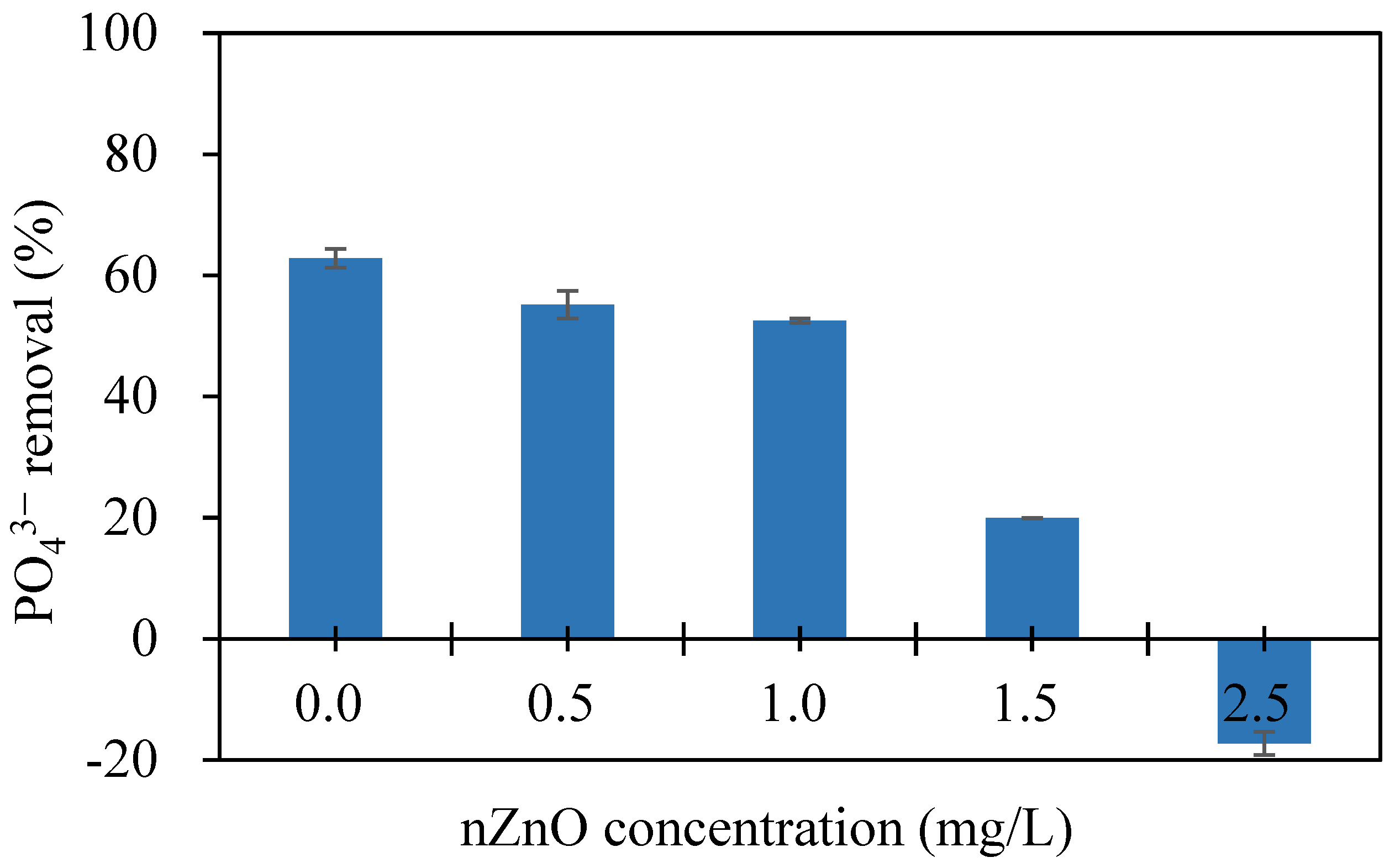
3.1. Effects of Individual nZnO, Coagulant, and DOM on PO43− Removal by PAOs
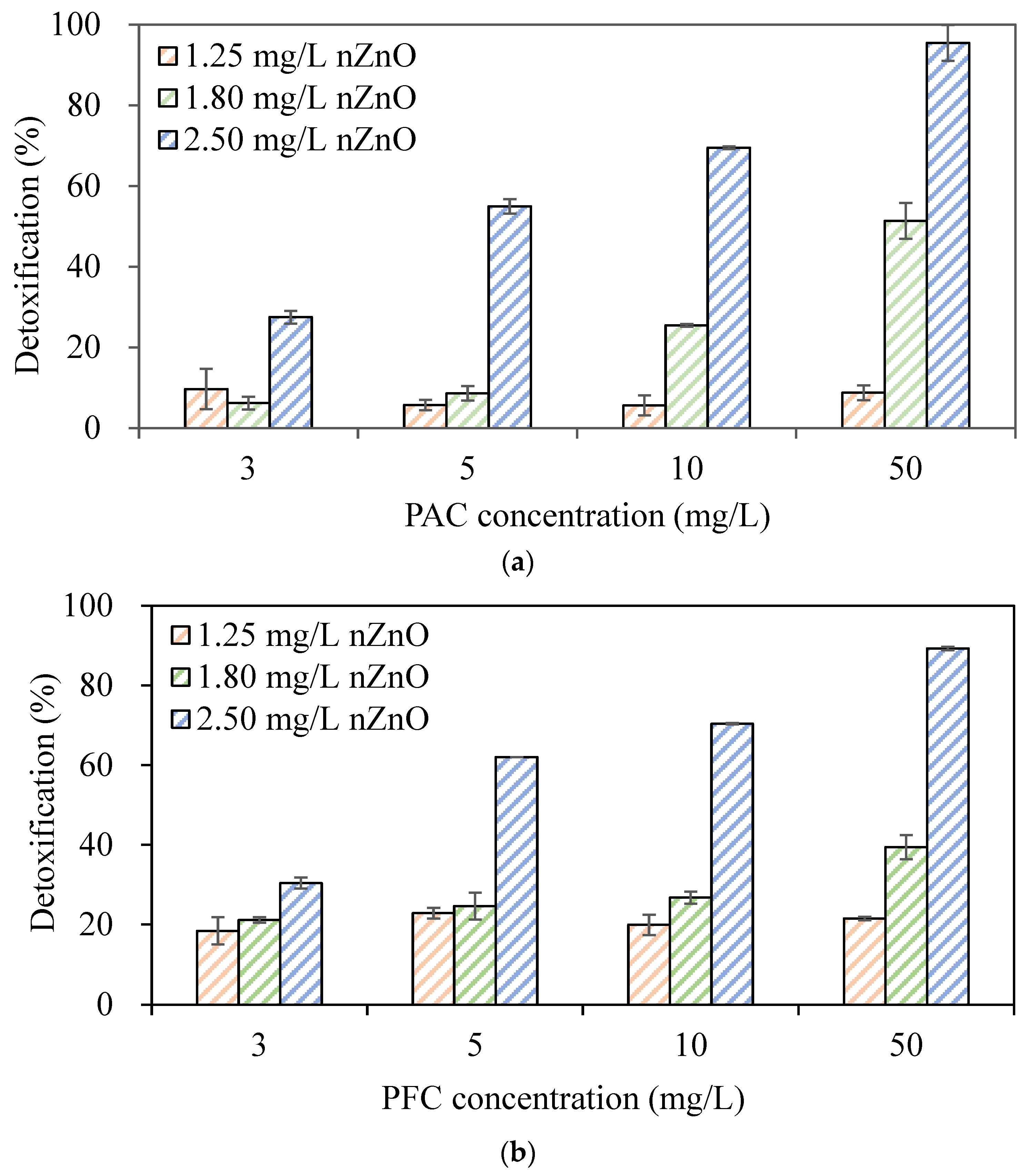
3.2. Effects of Coagulants on Inhibition of PO43− Removal by PAOs Due to nZnO Toxicity
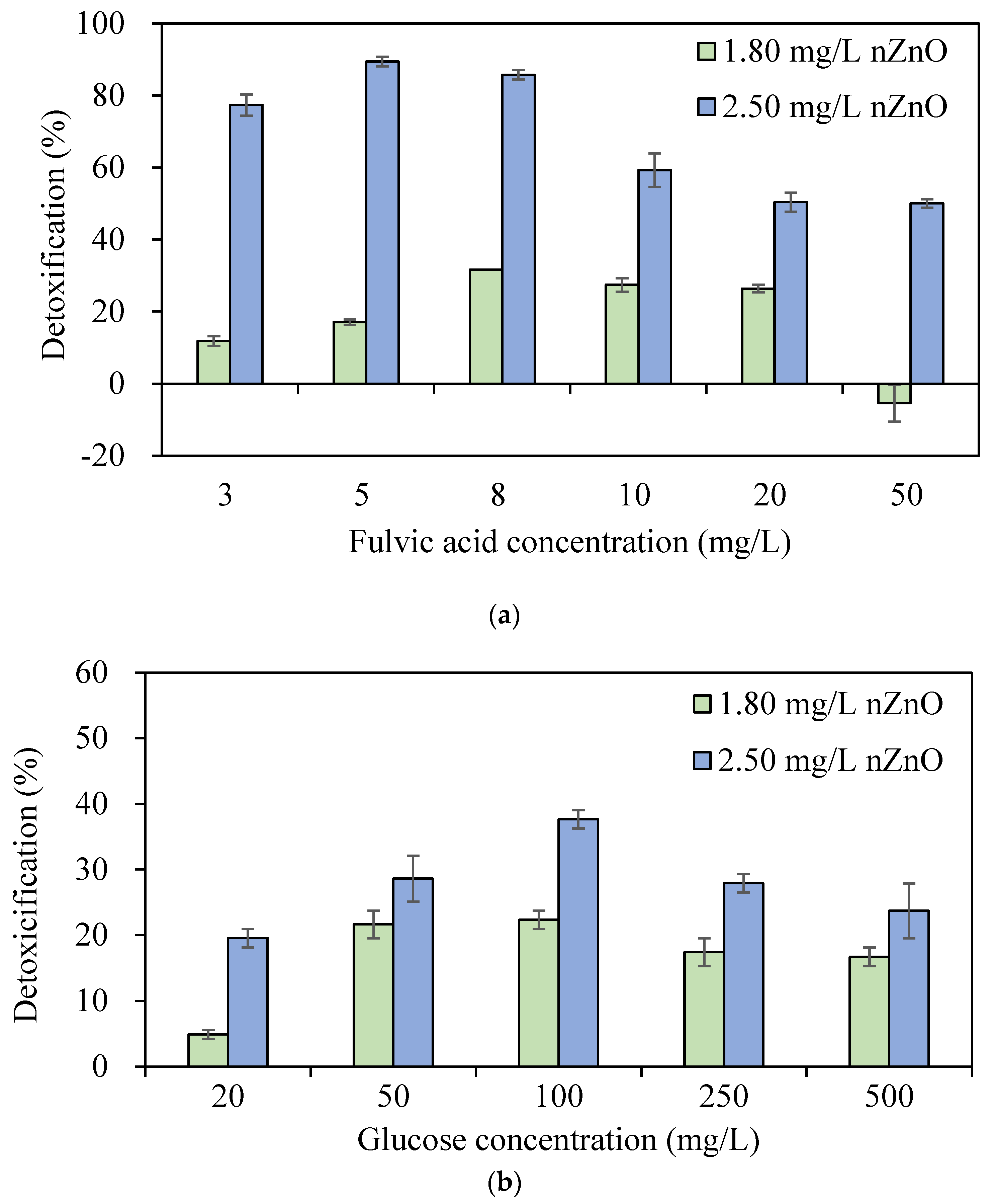
3.3. Effects of DOM on Inhibition of PO43− Removal by PAOs Due to nZnO Toxicity
3.4. Complex Toxicity Mechanism
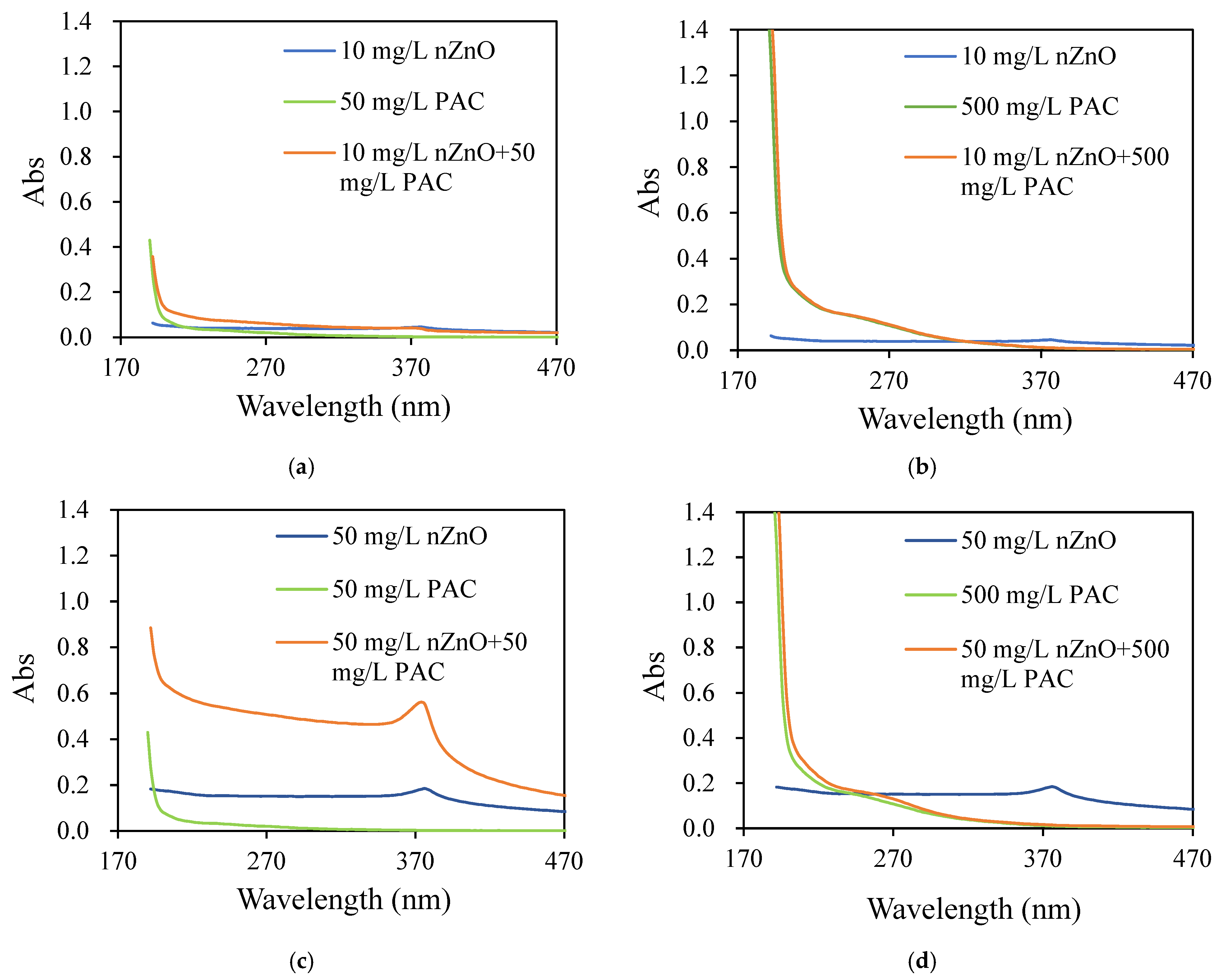
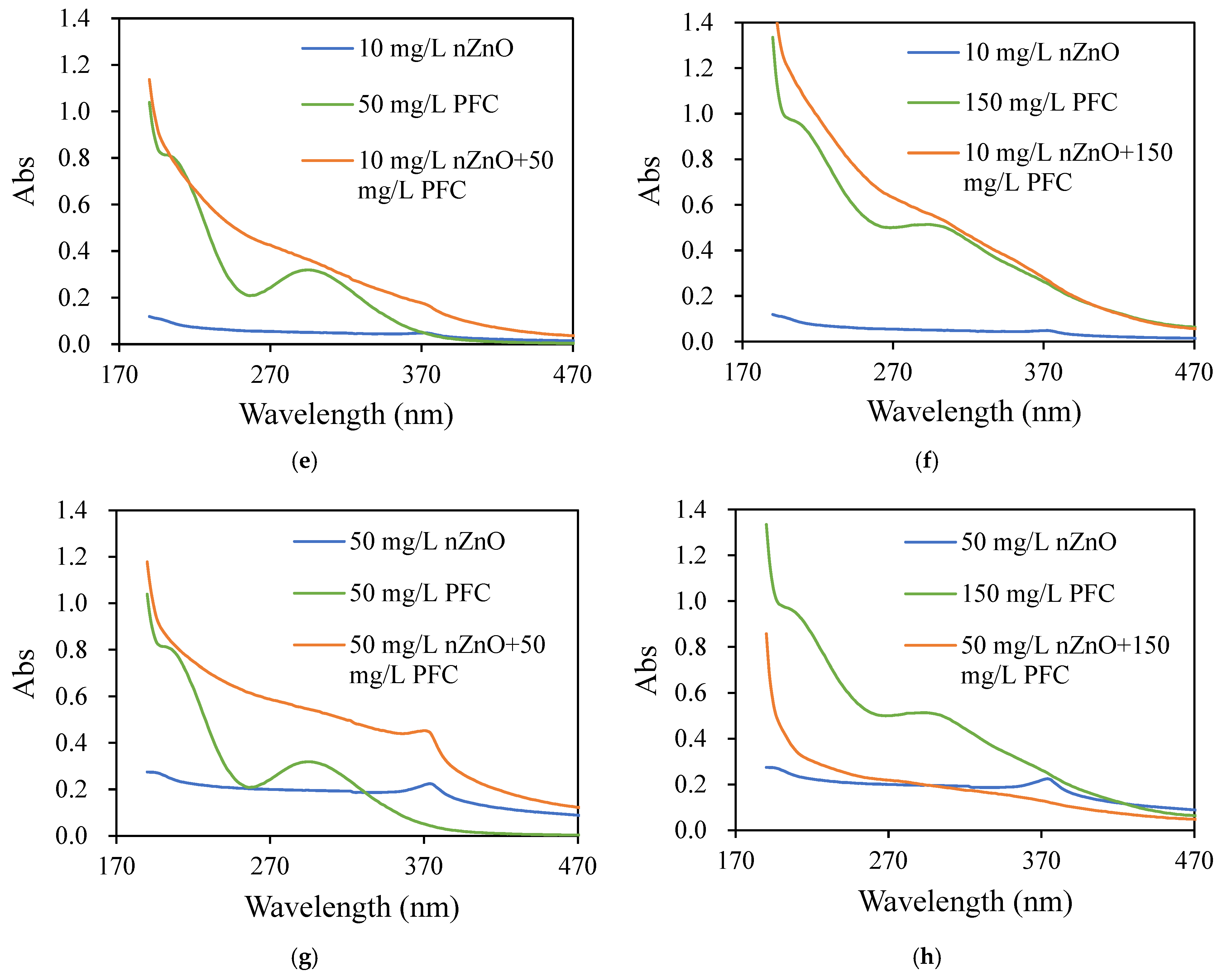
3.4.1. Compound Reaction Identification
- (1)
- PAC and PFC
- (2)
- Fulvic acid, glucose, and aspartic acid
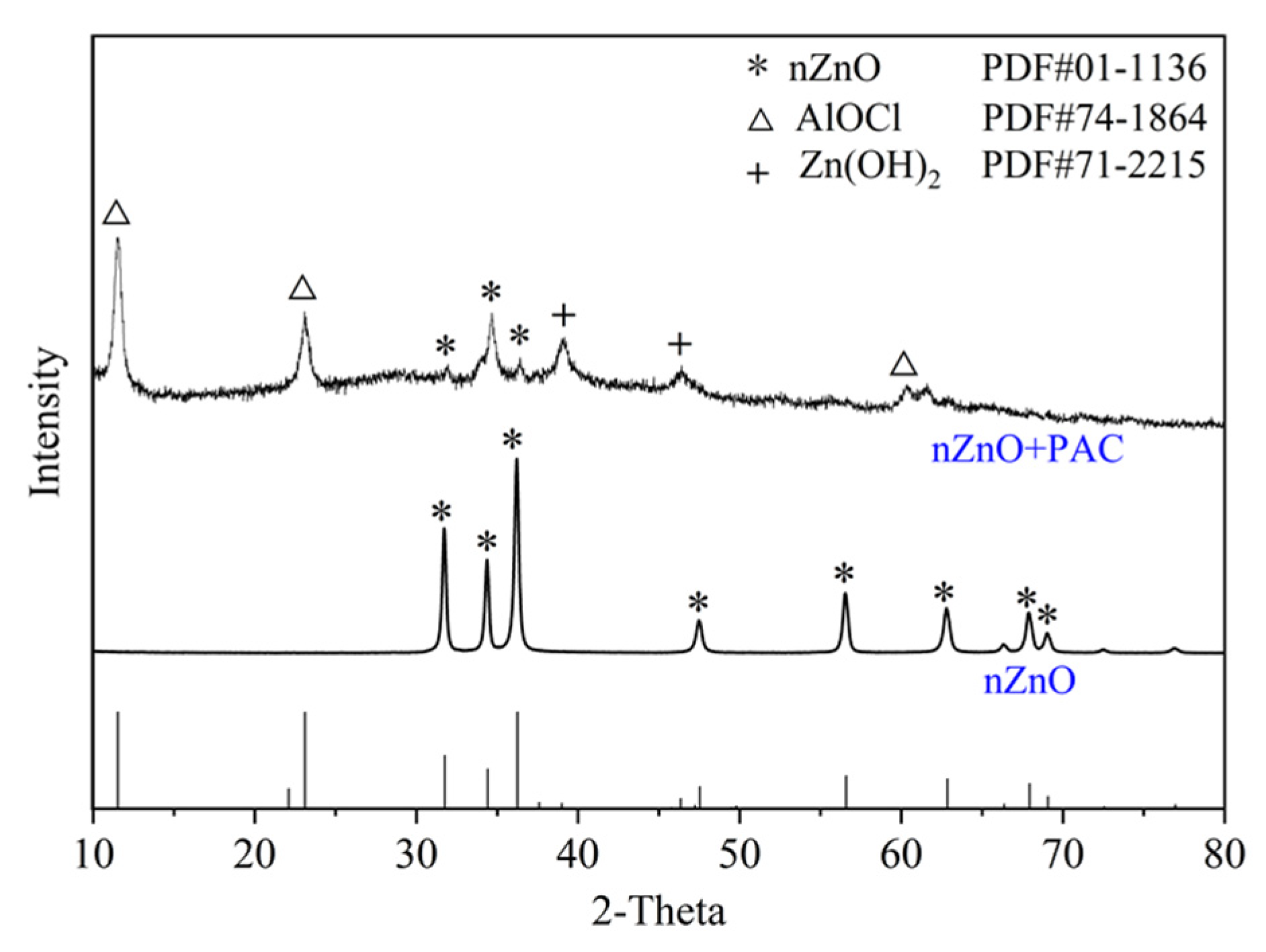
3.4.2. Crystal Structure Analysis
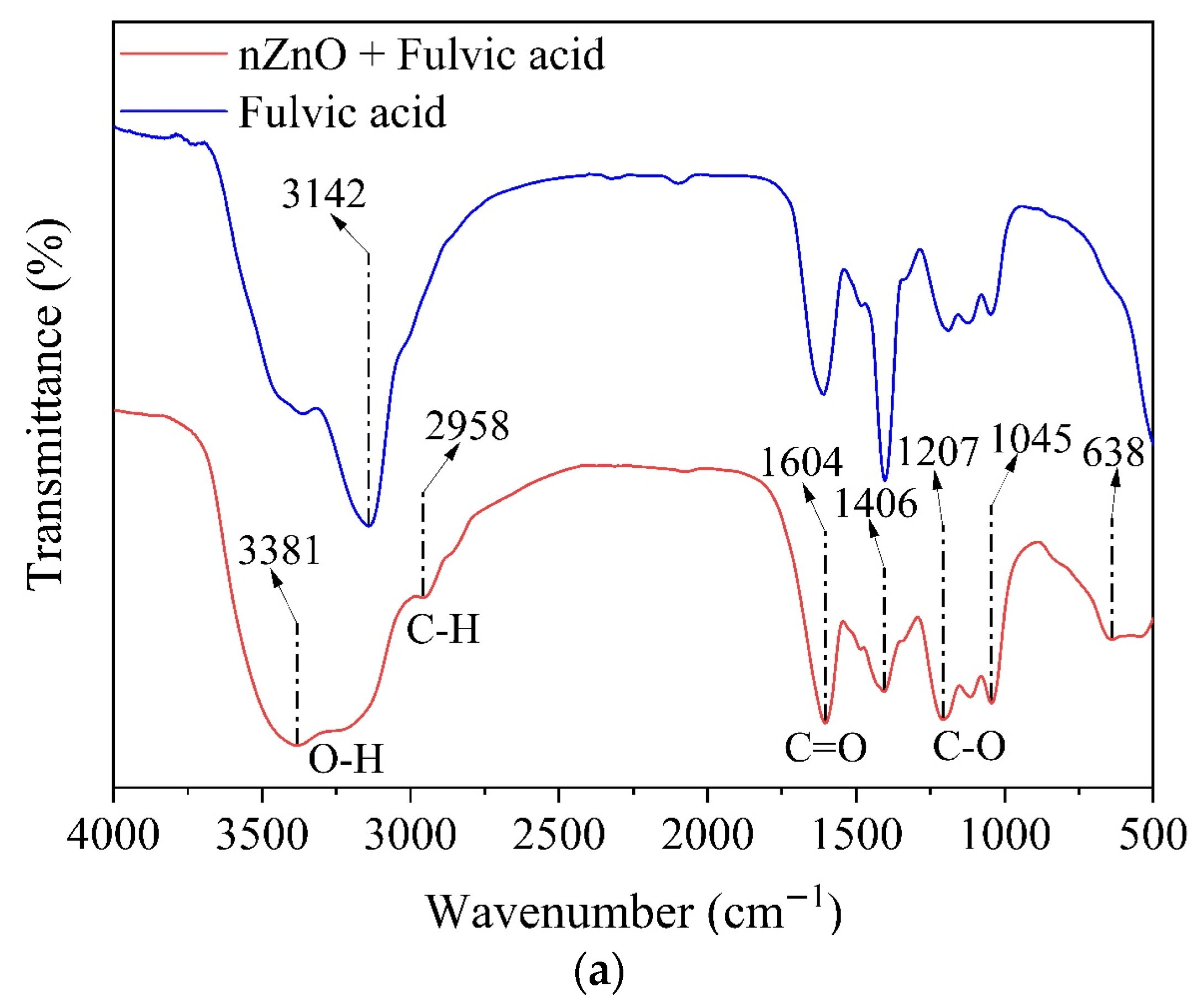
3.4.3. Functional Group Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dinesh, R.; Sreena, C.P.; Sheeja, T.E.; Kumar, I.P.V.; Praveena, R.; Charles, S.; Srinivasan, V.; Jayarajan, K.; Sajith, V.; Subila, K.P.; et al. Soil polluted with nano ZnO reveals unstable bacterial communities and decoupling of taxonomic and functional diversities. Sci. Total Environ. 2020, 889, 164285. [Google Scholar] [CrossRef]
- Gomez-Gonzalez, M.A.; Rehkämper, M.; Han, Z.; Ryan, M.P.; Laycock, A.; Porter, A.E. ZnO Nanomaterials and Ionic Zn Partition within Wastewater Sludge Investigated by Isotopic Labeling. Glob. Chall. 2022, 6, 2100091. [Google Scholar] [CrossRef]
- Zhang, B.; Tang, X.; Fan, C.; Hao, W.; Zhao, Y.; Zeng, Y. Cationic polyacrylamide alleviated the inhibitory impact of ZnO nanoparticles on anaerobic digestion of waste activated sludge through reducing reactive oxygen species induced. Water Res. 2021, 205, 117651. [Google Scholar] [CrossRef]
- Al-Mur, B.A. Green Zinc Oxide (ZnO) Nanoparticle Synthesis Using Mangrove Leaf Extract from Avicenna marina: Properties and Application for the Removal of Toxic Metal Ions (Cd2+ and Pb2+). Water 2023, 15, 455. [Google Scholar] [CrossRef]
- Soltani, S.; Gacem, A.; Choudhary, N.; Yadav, V.K.; Alsaeedi, H.; Modi, S.; Patel, A.; Khan, S.H.; Cabral-Pinto, M.M.S.; Yadav, K.K.; et al. Scallion Peel Mediated Synthesis of Zinc Oxide Nanoparticles and Their Applications as Nano fertilizer and Photocatalyst for Removal of Organic Pollutants from Wastewater. Water 2023, 15, 1672. [Google Scholar] [CrossRef]
- Ahmed, T.; Noman, M.; Manzoor, N.; Ali, S.; Rizwan, M.; Ijaz, M.; Allemailem, K.S.; BinShaya, A.S.; Alhumaydhi, F.A.; Li, B. Recent advances in nanoparticles associated ecological harms and their biodegradation: Global environmental safety from nano-invaders. J. Environ. Cheml. Eng. 2021, 9, 106093. [Google Scholar] [CrossRef]
- Jan, N.; Majeed, N.; Ahmad, M.; Ahmad Lone, W.; John, R. Nano-pollution: Why it should worry us. Chemosphere 2022, 302, 134746. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.F.; Zhang, Z.Z.; Li, G.F.; Zhu, B.Q.; Zhang, Q.; Liu, Y.Y.; Zhu, W.Q.; Fan, N.S.; Jin, R.C. Effects of ZnO nanoparticles on high-rate denitrifying granular sludge and the role of phosphate in toxicity attenuation. Environ. Pollut. 2019, 251, 166–174. [Google Scholar] [CrossRef]
- Mu, H.; Su, Y.; Zhang, Y.; Qiu, Q.; Zhu, L.; Zhao, C. Reduction of ZnO nanoparticles toxicity to methanogenic wastewater treatment by the presence of Fe3O4 nanoparticles: Focusing on nanomaterial interaction and direct interspecies electron transfer. Biochem. Eng. J. 2022, 180, 108365. [Google Scholar] [CrossRef]
- USEPA. Targeted National Sewage Sludge Survey Sampling and Analysis Technical Report; USEPA: Washington, DC, USA, 2009.
- Ma, X.-L.; He, E.-J.; Cao, F.-T.; Fan, Y.-Y.; Zhou, X.-T.; Xiao, X. Re-evaluation of the environmental hazards of nZnO to denitrification: Performance and mechanism. Chemosphere 2022, 291 Pt 2, 132824. [Google Scholar] [CrossRef]
- Bordin, E.R.; Ramsdorf, W.A.; Lotti Domingos, L.M.; de Souza Miranda, L.P.; Mattoso Filho, N.P.; Cestari, M.M. Ecotoxicological effects of zinc oxide nanoparticles (ZnO-NPs) on aquatic organisms: Current research and emerging trends. J. Environ. Manag. 2024, 349, 119396. [Google Scholar] [CrossRef]
- Du, J.; Qv, W.; Niu, Y.; Yuan, S.; Zhang, L.; Yang, H.; Zhang, Y. Co-exposures of acid rain and ZnO nanoparticles accelerate decomposition of aquatic leaf litter. J. Hazard. Mater. 2022, 426, 128141. [Google Scholar] [CrossRef]
- Bulacio Fischer, P.T.; Di Trapani, D.; Laudicina, V.A.; Muscarella, S.M.; Mannina, G. Nutrient Recovery from Zeolite and Biochar Columns: The Case Study of Marineo (Italy) Wastewater Treatment Plant. Water 2025, 17, 848. [Google Scholar] [CrossRef]
- Li, G.; Srinivasan, V.; Tooker, N.B.; Wang, D.; Yan, Y.; Onnis-Hayden, A.; Gu, A.Z. Distinct microdiversity of phosphate accumulating organisms (PAOs) between side-stream and conventional enhanced biological phosphorus removal (EBPR) systems with performance implications. Water Res. 2024, 266, 122280. [Google Scholar] [CrossRef]
- Liu, H.; Zeng, W.; Meng, Q.; Fan, Z.; Peng, Y. Phosphorus removal performance, intracellular metabolites and clade-level community structure of Tetrasphaera-dominated polyphosphate accumulating organisms at different temperatures. Sci. Total Environ. 2022, 842, 156913. [Google Scholar] [CrossRef]
- Akbari, A.; Wang, Z.; He, P.; Wang, D.; Lee, J.; Han, I.L.; Li, G.; Gu, A.Z. Unrevealed roles of polyphosphate-accumulating microorganisms. Microb. Biotechnol. 2021, 14, 82–87. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, X.; Wang, Z.-W.; Li, X.; Ma, J. In-depth characterization of secondary effluent from a municipal wastewater treatment plant located in Northern China for advanced treatment. Water Sci. Technol. 2014, 69, 1482–1488. [Google Scholar] [CrossRef]
- Rippner, D.A.; Green, P.G.; Young, T.M.; Parikh, S.J. Dissolved organic matter reduces CuO nanoparticle toxicity to duckweed in simulated natural systems. Environ. Pollut. 2018, 234, 692–698. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Z.; Xu, J.; Zhang, C.; Gao, P.; Zhu, L. Effects of dissolved organic matter on the environmental behavior and toxicity of metal nanomaterials: A review. Chemosphere 2024, 358, 142208. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, H.; Lu, K.; Gao, H.; Duan, L.; Yu, H.; Li, Q. New insights into pollution source analysis using receptor models: Effects of interaction between heavy metals and DOM on source identification and apportionment in rivers across industrial city. J. Hazard. Mater. 2025, 484, 136792. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Ge, Z.; Ou, C.; Wei, J.; Liu, H.; Wei, Y. The binding effects and mechanisms of dissolved organic matter (DOM) on the fate of mercury in sludge anaerobic digestion combined with thermal hydrolysis. Water Res. 2024, 259, 121845. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhou, Z. Spectroscopic Indices Reveal Spatiotemporal Variations of Dissolved Organic Matter in Subtropical Karst Cave Drip Water. Water 2024, 16, 2438. [Google Scholar] [CrossRef]
- Hochella, M.F.; Mogk, D.W.; Ranville, J.; Allen, I.C.; Luther, G.W.; Marr, L.C.; McGrail, B.P.; Murayama, M.; Qafoku, N.P.; Rosso, K.M.; et al. Natural, incidental, and engineered nanomaterials and their impacts on the Earth system. Science 2019, 363, eaau8299. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Sun, T.; Han, T.; Guo, Z.; Wang, X.; Chen, Y. Aggregation behavior of zinc oxide nanoparticles and their biotoxicity to Daphnia magna: Influence of humic acid and sodium alginate. Environ. Res. 2020, 191, 110086. [Google Scholar] [CrossRef]
- Yang, Y.; Xue, T.; Xiang, F.; Zhang, S.; Hanamoto, S.; Sun, P.; Zhao, L. Toxicity and combined effects of antibiotics and nano ZnO on a phosphorus-removing Shewanella strain in wastewater treatment. J. Hazard. Mater. 2021, 416, 125532. [Google Scholar] [CrossRef]
- Zheng, X.; Wu, R.; Chen, Y. Effects of ZnO Nanoparticles on Wastewater Biological Nitrogen and Phosphorus Removal. Environ. Sci. Technol. 2011, 45, 2826–2832. [Google Scholar] [CrossRef]
- Cao, F.T.; Ma, X.L.; Zhou, X.T.; Han, J.C.; Xiao, X. Performance and mechanisms exploration of nano zinc oxide (nZnO) on anaerobic decolorization by Shewanella oneidensis MR-1. Chemosphere 2022, 305, 135510. [Google Scholar] [CrossRef]
- Xiao, X.; He, E.J.; Lu, X.R.; Wu, L.J.; Fan, Y.Y.; Yu, H.Q. Evaluation of antibacterial activities of silver nanoparticles on culturability and cell viability of Escherichia coli. Sci. Total Environ. 2021, 794, 148765. [Google Scholar] [CrossRef]
- Wang, S.; Gao, M.; Ma, B.; Xi, M.; Kong, F. Size-dependent effects of ZnO nanoparticles on performance, microbial enzymatic activity and extracellular polymeric substances in sequencing batch reactor. Environ. Pollut. 2020, 257, 113596. [Google Scholar] [CrossRef]
- Mu, H.; Zheng, X.; Chen, Y.; Chen, H.; Liu, K. Response of anaerobic granular sludge to a shock load of zinc oxide nanoparticles during biological wastewater treatment. Environ. Sci. Technol. 2012, 46, 5997–6003. [Google Scholar] [CrossRef]
- Sarvajith, M.; Nancharaiah, Y.V. De novo granulation of sewage-borne microorganisms: A proof of concept on cultivating aerobic granular sludge without activated sludge and effective enhanced biological phosphorus removal. Environ. Res. 2023, 224, 115500. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Wan, L.; Liang, Z.; Yang, D.; Han, H.; Yi, J.; Dai, X. Enhanced biological phosphorus removal by high concentration powder carrier bio-fluidized bed (HPB): Phosphorus distribution, cyclone separation, and metagenomics. Chemosphere 2023, 337, 139353. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yang, H.; Wang, X.; Liu, W. Experimental study on the establishment of independent enhanced biological phosphorus removal system based on immobilized fillers for wastewater nitrogen removal. J. Water Process Eng. 2025, 70, 106917. [Google Scholar] [CrossRef]
- Sguanci, S.; Lubello, C.; Caffaz, S.; Lotti, T. Long-term stability of aerobic granular sludge for the treatment of very low-strength real domestic wastewater. J. Clean. Prod. 2019, 222, 882–890. [Google Scholar] [CrossRef]
- Wang, B.; Ni, M.; Zeng, Y.; Yang, B.; Shang, L.; Bin, L.; Chen, W.; Li, P.; Chen, X.; Wen, S.; et al. Transforming behavior and removal mechanism of phosphorus from municipal wastewater in a continuous flow aerobic granular sludge-membrane bioreactor: Unveiling the functional genes associated with phosphorus metabolism. Chem. Eng. J. 2025, 507, 160578. [Google Scholar] [CrossRef]
- Luo, H.; Yan, B.; Xing, C.; Guo, W. Integrating enhanced biological phosphorus removal in adsorption-stage to treat real domestic sewage. Bioresour. Technol. 2024, 411, 131334. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, C.; Chen, Z.; Yang, Y.; Lin, Z.; Deng, Z.; Wang, X. Fe(II)-driven spatiotemporal assembly of heterotrophic and anammox bacteria enhances simultaneous nitrogen and phosphorus removal for low-strength municipal wastewater. Bioresour. Technol. 2024, 401, 130713. [Google Scholar] [CrossRef]
- Gu, C.; Li, X.; Zhang, S.; Li, J.; Gao, X.; Chen, G.; Wang, Z.; Peng, Y. Advanced nitrogen and phosphorus removal in pilot-scale anaerobic/aerobic/anoxic system for municipal wastewater in Northern China. Bioresour. Technol. 2024, 399, 130616. [Google Scholar] [CrossRef]
- Wang, D.; Han, I.; McCullough, K.; Klaus, S.; Lee, J.; Srinivasan, V.; Li, G.; Wang, Z.L.; Bott, C.B.; McQuarrie, J.; et al. Side-Stream Enhanced Biological Phosphorus Removal (S2EBPR) enables effective phosphorus removal in a pilot-scale A-B stage shortcut nitrogen removal system for mainstream municipal wastewater treatment. Water Res. 2024, 251, 121050. [Google Scholar] [CrossRef]
- Fu, J.; Lin, Z.; Zhao, P.; Wang, Y.; He, L.; Zhou, J. Establishment and efficiency analysis of a single-stage denitrifying phosphorus removal system treating secondary effluent. Bioresour. Technol. 2019, 288, 121520. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Xue, N.; Yu, C.; Meng, Y.; Gao, B.; Li, Q. The effect of DOM on floc formation and membrane fouling in coagulation/ultrafiltration process for treating TiO2 nanoparticles in various aquatic media. Chem. Eng. J. 2017, 316, 429–437. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, H.; Li, D.; Sun, W.; Zheng, H. Copper oxide nanoparticles removal by coagulation and optimization by matter–element analysis model. J. Environ. Chem. Eng. 2022, 10, 107096. [Google Scholar] [CrossRef]
- Tan, J.; Huang, Y.; Chi, B.; Xiong, Z.; Zhou, W.; Yang, Z.; Zhou, K.; Ruan, X.; Duan, X.; Wang, M.; et al. Comparative study of iron and aluminium coagulants in conditioning sludge: Sludge dewatering performance, physicochemical properties, and risk of heavy metal migration. J. Environ. Chem. Eng. 2024, 12, 113168. [Google Scholar] [CrossRef]
- Thomas, M.; Hybská, H.; Masten, S.J.; Šuránek, M.; Melichová, Z.; Samešová, D. Removal of heavy metals and organic compounds from wastewater printing circuit board manufacturers via a hybrid ferrate(VI)-trithiocarbonate process: An optimization study and toxicity assessment. Chem. Eng. J. 2025, 503, 158330. [Google Scholar] [CrossRef]
- Yang, Z.; Ma, X.; Zhou, H.; Ma, J.; Ding, Y.; He, D. Chelated heavy metals removal by in-situ formed Fe(II) and Fe(III) iron (oxy)hydroxides: Mechanism and performance. J. Environ. Chem. Eng. 2023, 11, 110531. [Google Scholar] [CrossRef]
- Khan, R.; Inam, M.A.; Lee, K.H. Removal of Tannic Acid Stabilizes CuO Nanoparticles from Aqueous Media by PAFC: Effect of Process Conditions and Water Chemistry. Molecules 2021, 26, 5615. [Google Scholar] [CrossRef]
- Sun, Q.; Li, Y.; Tang, T.; Yuan, Z.; Yu, C.-P. Removal of silver nanoparticles by coagulation processes. J. Hazard. Mater. 2013, 261, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wang, H.; Keller, A.A.; Wang, T.; Li, F. The effect of humic acid on the aggregation of titanium dioxide nanoparticles under different pH and ionic strengths. Sci. Total Environ. 2014, 487, 375–380. [Google Scholar] [CrossRef]
- Li, M.; Pokhrel, S.; Jin, X.; Mädler, L.; Damoiseaux, R.; Hoek, E.M.V. Stability, Bioavailability, and Bacterial Toxicity of ZnO and Iron-Doped ZnO Nanoparticles in Aquatic Media. Environ. Sci. Technol. 2011, 45, 755–761. [Google Scholar] [CrossRef]
- He, X.; McAlliser, D.; Aker, W.G.; Hwang, H.-m. Assessing the effect of different natural dissolved organic matters on the cytotoxicity of titanium dioxide nanoparticles with bacteria. J. Environ. Sci. 2016, 48, 230–236. [Google Scholar] [CrossRef]
- Wang, S.; Wu, Y.; An, J.; Liang, D.; Tian, L.; Zhou, L.; Wang, X.; Li, N. Geobacter Autogenically Secretes Fulvic Acid to Facilitate the Dissimilated Iron Reduction and Vivianite Recovery. Environ. Sci. Technol. 2020, 54, 10850–10858. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, J.; Liang, L.; Yao, L.; Fang, T. Dual impact of dissolved organic matter on cytotoxicity of PVP-Ag NPs to Escherichia coli: Mitigation and intensification. Chemosphere 2019, 214, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Lu, Q.; Zhou, H.; Shen, F.; Liu, Z.; Zhu, W.; Huang, H. Tuning oxygenated functional groups on biochar for water pollution control: A critical review. J. Hazard. Mater. 2021, 420, 126547. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Tang, Y.; Sun, J.; Dong, B.; Zuxin, X. Tracking of the conversion and transformation pathways of dissolved organic matter in sludge hydrothermal liquids during Cr(VI) reduction using FT-ICR MS. J. Hazard. Mater. 2024, 466, 133566. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, Q.; Chen, L.; Zhong, Y.; Hu, F.; Wu, K.; Yin, X.; Hamza, M.F.; Wei, Y.; Ning, S. Phosphination of amino-modified mesoporous silica for the selective separation of strontium. J. Hazard. Mater. 2024, 467, 133741. [Google Scholar] [CrossRef]
- Artifon, V.; Zanardi-Lamardo, E.; Fillmann, G. Aquatic organic matter: Classification and interaction with organic microcontaminants. Sci. Total Environ. 2019, 649, 1620–1635. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, R.; Qin, B.; Gan, J.; Peng, X. Energy-efficient monosaccharides electrooxidation coupled with green hydrogen production by bifunctional CO9S8/Ni3S2 electrode. Chem. Eng. J. 2022, 446, 136950. [Google Scholar] [CrossRef]
- Zhang, Q.; Wan, Z.; Yu, I.K.M.; Tsang, D.C.W. Sustainable production of high-value gluconic acid and glucaric acid through oxidation of biomass-derived glucose: A critical review. J. Clean. Prod. 2021, 312, 127745. [Google Scholar] [CrossRef]
- Ma, J.; Guan, Z.; Shen, G.; Zhang, K.; Zhang, X.; Zhao, L.; Zhou, Y.; Yu, Z. Substrate preference of Tetrasphaera in dual PAOs synergistic EBPR system and its phosphorus removal performance. J. Water Process Eng. 2024, 67, 106269. [Google Scholar] [CrossRef]
- Qiu, G.; Liu, X.; Saw, N.M.M.T.; Law, Y.; Zuniga-Montanez, R.; Thi, S.S.; Ngoc Nguyen, T.Q.; Nielsen, P.H.; Williams, R.B.; Wuertz, S. Metabolic Traits of Candidatus Accumulibacter clade IIF Strain SCELSE-1 Using Amino Acids As Carbon Sources for Enhanced Biological Phosphorus Removal. Environ. Sci. Technol. 2020, 54, 2448–2458. [Google Scholar] [CrossRef]
- Chen, L.; Wang, C.; Li, Y.; Xie, X.; Deng, X.; Chen, H.; Ji, S.; Yuan, J.; Wang, K.; Zhang, Y.; et al. Metabolisms of Microlunatus phosphovorus NM-1 Using Glucose, Glutamate, and Aspartate as Carbon Sources for Enhanced Biological Phosphorus Removal. ACS ES&T Water 2024, 4, 4150–4164. [Google Scholar] [CrossRef]
- Ganda, L.; Zhou, Y.; Lim, C.-P.; Liu, Y.; Ng, W.J. Free nitrous acid inhibition on carbon storage microorganisms: Accumulated inhibitory effects and recoverability. Chem. Eng. J. 2016, 287, 285–291. [Google Scholar] [CrossRef]
- Li, Y.; Dong, R.; Guo, J.; Wang, L.; Zhao, J. Effects of Mn2+ and humic acid on microbial community structures, functional genes for nitrogen and phosphorus removal, and heavy metal resistance genes in wastewater treatment. J. Environ. Manag. 2022, 313, 115028. [Google Scholar] [CrossRef]
- Liu, B.; Gao, Y.; Pan, J.; Feng, Q.; Yue, Q.; Guo, K.; Gao, B. Coagulation behavior of polyaluminum-titanium chloride composite coagulant with humic acid: A mechanism analysis. Water Res. 2022, 220, 118633. [Google Scholar] [CrossRef]
- Sousa, V.S.; Ribau Teixeira, M. Removal of a mixture of metal nanoparticles from natural surface waters using traditional coagulation process. J. Water Process Eng. 2020, 36, 101285. [Google Scholar] [CrossRef]
- Serrão Sousa, V.; Corniciuc, C.; Ribau Teixeira, M. The effect of TiO2 nanoparticles removal on drinking water quality produced by conventional treatment C/F/S. Water Res. 2017, 109, 1–12. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, Y.; Liang, Y.; Sun, W.; Shah, K.J.; Zheng, H. Flocculation performance of PCFA composite coagulant for removing nanoparticles. Colloids Surf. A 2023, 673, 131828. [Google Scholar] [CrossRef]
- Devarajan, Y. Nanomaterials-Based Wastewater Treatment: Addressing Challenges and Advancing Sustainable Solutions. BioNanoScience 2025, 15, 149. [Google Scholar] [CrossRef]
- Gao, J.; Yu, F.; Zhang, P.; Wu, Z.; Liu, H.; Li, W. Distribution characteristics of TiO2 NPs in Daihai lake and their stability regulated by abiotic factors. Environ. Res. 2024, 263, 120203. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Gong, G.; Liu, W.; Chu, M.; Zhou, J.; Zarebska, K.; Kuttybaevna, K.M.; Toleukhanuly, Y.B. Kinetics and molecular structure of the binding process between coal-based fulvic acid and zinc ions. J. Environ. Chem. Eng. 2024, 12, 113340. [Google Scholar] [CrossRef]
- Guo, J.; Fu, Q.; Tang, M.; Bai, J.; Liu, R.; Zhang, H.; Siddique, K.H.M.; Mao, H. Fulvic acid modified ZnO nanoparticles improve nanoparticle stability, mung bean growth, grain zinc content, and soil biodiversity. Sci. Total Environ. 2024, 913, 169840. [Google Scholar] [CrossRef] [PubMed]
- Mohd Omar, F.; Abdul Aziz, H.; Stoll, S. Aggregation and disaggregation of ZnO nanoparticles: Influence of pH and adsorption of Suwannee River humic acid. Sci. Total Environ. 2014, 468–469, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Zhang, Y.; Wei, W. Effects of macromolecular humic/fulvic acid on Cd(II) adsorption onto reed-derived biochar as compared with tannic acid. Int. J. Biol. Macromol. 2019, 134, 43–55. [Google Scholar] [CrossRef]
- Xue, S.; Yi, X.; Peng, J.; Bak, F.; Zhang, L.; Duan, G.; Liesack, W.; Zhu, Y. Fulvic Acid Enhances Nitrogen Fixation and Retention in Paddy Soils through Microbial-Coupled Carbon and Nitrogen Cycling. Environ. Sci. Technol. 2024, 58, 18777–18787. [Google Scholar] [CrossRef]
- López-Maldonado, E.A.; Oropeza-Guzmán, M.T. Nejayote biopolyelectrolytes multifunctionality (glucurono ferulauted arabinoxylans) in the separation of hazardous metal ions from industrial wastewater. Chem. Eng. J. 2021, 423, 130210. [Google Scholar] [CrossRef]
- Wang, D.-C.; Yang, X.; Yu, H.-Y.; Gu, J.; Qi, D.; Yao, J.; Ni, Q. Smart nonwoven fabric with reversibly dual-stimuli responsive wettability for intelligent oil-water separation and pollutants removal. J. Hazard. Mater. 2020, 383, 121123. [Google Scholar] [CrossRef]
- Liang, H.; Lu, Q.; Liu, M.; Ou, R.; Wang, Q.; Quirino, R.L.; Luo, Y.; Zhang, C. UV absorption, anticorrosion, and long-term antibacterial performance of vegetable oil based cationic waterborne polyurethanes enabled by amino acids. Chem. Eng. J. 2021, 421, 127774. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Yao, Y.; Liu, J.-Y.; Gao, Y.; Hua, Q.; Jiao, Q.; Liu, J.; Jin, Y.; Zhang, H.; et al. A mild one-pot self-assembly approach to encapsulating enzymes into metal-organic framework with Asp-boosted enzymatic performance for clean production. J. Clean. Prod. 2023, 401, 136710. [Google Scholar] [CrossRef]
- Dong, J.; Fang, L.; Li, J.; Gao, X.; Li, D.; Wang, S. Synthesis of aspartic acid-derived chiral carbon dots and their effect on bovine serum albumin amyloid fibrillation by multispectral and molecular interaction studies. J. Mol. Struct. 2023, 1291, 136045. [Google Scholar] [CrossRef]
- Syeda, H.I.; Muthukumaran, S.; Baskaran, K. Polyglutamic acid and its derivatives as multi-functional biopolymers for the removal of heavy metals from water: A review. J. Water Process Eng. 2023, 56, 104367. [Google Scholar] [CrossRef]



| Sewage Treatment Process | Wastewater Type | Phosphorus Concentration in the Influent (mg/L) | Mixed Liquor Suspended Solid (g/L) | Sludge Volume Index (mL/g) | Phosphorus Removal (%) | Reference |
|---|---|---|---|---|---|---|
| Aerobic granular sludge | Practical | 1.6–6.5 | 8.0 | <30.0 | 67 | [32] |
| Aerobic granular sludge | Practical | 1.6–6.5 | 8.0 | <30.0 | 94 | [32] |
| Anaerobic–anoxic–oxic | Practical | 4.0 | - | - | 85 | [33] |
| High concentration powder carrier bio-fluidized bed | Practical | 4.0 | - | - | 88 | [33] |
| Enhanced biological phosphorus removal | Practical | 1.8–6.1 | 2.8 | 63.6 | 83 | [34] |
| Aerobic granular sludge | Practical | - | 3.5 | <50.0 | 30–80 | [35] |
| Aerobic granular sludge | Practical | - | 8.0 | - | 30–70 | [36] |
| Enhanced biological phosphorus removal | Practical | 1.8–5.7 | - | - | 93 | [37] |
| Partial denitrification coupled anammox | Artificial | 2.4 | - | - | 91 | [38] |
| Anaerobic–anoxic–oxic | Practical | 2.3–4.3 | 3.5 | - | 96 | [39] |
| Enhanced biological phosphorus removal | Practical | 1.7–3.6 | 4.1 | - | 78–96 | [40] |
| Denitrifying phosphorus removal | Artificial | 1.0 | - | - | 61 | [41] |
| Enhanced biological phosphorus removal | Artificial | 10.0 | - | - | 63 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, S.; Zhao, W.; Wang, Y.; Zhang, Y.; Liu, J.; Yang, Y. Effect of Inorganic Coagulant and Dissolved Organic Matter on the Toxicity of Nano-Zinc Oxide to Phosphorus-Accumulating Organisms in Wastewater. Water 2025, 17, 1563. https://doi.org/10.3390/w17111563
Qu S, Zhao W, Wang Y, Zhang Y, Liu J, Yang Y. Effect of Inorganic Coagulant and Dissolved Organic Matter on the Toxicity of Nano-Zinc Oxide to Phosphorus-Accumulating Organisms in Wastewater. Water. 2025; 17(11):1563. https://doi.org/10.3390/w17111563
Chicago/Turabian StyleQu, Sen, Wen Zhao, Yushu Wang, Yuan Zhang, Jinyi Liu, and Yongkui Yang. 2025. "Effect of Inorganic Coagulant and Dissolved Organic Matter on the Toxicity of Nano-Zinc Oxide to Phosphorus-Accumulating Organisms in Wastewater" Water 17, no. 11: 1563. https://doi.org/10.3390/w17111563
APA StyleQu, S., Zhao, W., Wang, Y., Zhang, Y., Liu, J., & Yang, Y. (2025). Effect of Inorganic Coagulant and Dissolved Organic Matter on the Toxicity of Nano-Zinc Oxide to Phosphorus-Accumulating Organisms in Wastewater. Water, 17(11), 1563. https://doi.org/10.3390/w17111563






