Abstract
Industrial munition facilities are increasingly manufacturing insensitive high explosives (IHEs) to improve safety. The explosive residues in wastewater from these facilities are treated to meet regulatory standards. However, the resulting effluent contains elevated levels of mineralized nitrogen species. This study evaluated the potential of vetiver grass (Chrysopogon zizanioides), a non-invasive perennial species, to remove high concentrations of nitrate, nitrite, and ammonium from munition plant wastewater. Vetiver was grown hydroponically in synthetic wastewater containing high levels of nitrogen compounds simulating munitions plant effluents. Vetiver plants were treated with one nitrogen species at a time, with concentrations ranging from 165 to 24,700 mg N/L of nitrate, 100 to 4000 mg N/L of nitrite, and 260 to 39,000 mg N/L of ammonium. Nitrogen concentrations in the media and plant responses were monitored over time. The results showed significant nitrogen removal at lower concentration ranges. When concentrations exceeded 3800 mg N/L of nitrate, 800 mg N/L of nitrite, and 2600 mg N/L of ammonium, the removal rates declined after 7 days. At higher nitrogen levels, vetiver exhibited stress symptoms such as chlorosis and elevated antioxidant enzyme activity. Our study demonstrates the potential of vetiver grass in treating nitrogen-rich wastewater from the munition industry and provides a baseline for future large-scale studies to optimize the technology.
1. Introduction
The development of energetic compounds (ECs) began with nitroglycerin in the 19th century, followed by 2,4,6-trinitrotoluene (TNT) and hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX), which were widely used in both World Wars. Due to their sensitivity and associated risks, the U.S. Department of Defense has worked in recent decades to develop insensitive high explosives (IHEs) to enhance safety. Wastewater effluents produced by munition manufacturing facilities contain a diverse array of ECs [1]. These waste streams must comply with regulations and undergo pretreatment before being discharged. In the upstream stage of wastewater treatment, munition manufacturing facilities commonly employ chemical, biochemical, and advanced pretreatment technologies—such as oxidation, advanced oxidation, and biological mineralization—to convert ECs into less reactive inorganic nitrogen species like nitrate, nitrite, and ammonium, thereby reducing toxicity and enabling safer downstream remediation [2].
If the mineralized nitrogen species in wastewater are not subjected to advanced treatment and released into natural water bodies, severe environmental impacts such as eutrophication, reduction in biodiversity, impairment of commercial and recreational use, and fish kills can occur [3]. Conventional treatment processes include a combination of physical, chemical, and biological methods to remove nitrogen species in wastewater [4,5]. Although these methods have certain advantages, they also come with drawbacks such as high costs, the potential for subsequent pollution during disposal, limitations in their practicality for large-scale implementation, and limited potential for resource recovery [6,7]. Hence, developing more sustainable treatment methods for nitrogen-rich wastewater is crucial. Phytoremediation could be a viable and cost-effective biological approach, which is sustainable and readily applicable with vetiver grass (Chrysopogon zizanioides), serving as a downstream polishing method specifically targeting the removal of inorganic nitrogen species [6].
Vetiver grass, a fast-growing perennial grass with a large root system capable of deep penetration (up to 3–4 m), possesses distinct qualities that enable it to withstand high concentrations of nitrogen, phosphorus, heavy metals, etc. [7,8,9], making it a suitable choice for phytoremediation [6]. Vetiver grass has demonstrated its effectiveness in remediating explosive compounds and various pollutants found in the environment [10]. Worku et al. found that when employing a vetiver hydroponic system, the ammonium–nitrogen removal rate reached 35% at an initial nitrogen concentration of 4 mg/L. At a higher initial concentration of 11 mg/L, the removal rate significantly increased to 58% [11]. Akbarzadeh et al. found that a vetiver-planted floating wetland achieved 85% nitrogen removal efficiency in secondary treated domestic wastewater, with initial nitrate, nitrite, and ammonium levels of 46.8 mg/L, 0.98 mg/L, and 0.53 mg/L, respectively [12]. Davamani et al. demonstrated that a vetiver grass hydroponic system led to a reduction of 59.24% in nitrogen content from paperboard mill wastewater. Goren et al. investigated vetiver’s ability to remove nitrogen from olive mill wastewater at different ranges of nitrogen concentration up to 0.5 g N/L. Vetiver achieved a significant nitrogen removal rate of approximately 38.9% [13]. Junio et al. found that vetiver achieved removal efficiencies of 87.72% at a nitrogen concentration of 338.71 mg N/L [14]. Panja et al. exposed vetiver to very high NO3− concentrations in wastewater from munition plants and found that vetiver showed diminished capacity for growth and nitrogen removal at a threshold as extreme as 352,734 mg N/L [1].
Understanding the nitrogen accumulation capacity of vetiver grass is a key parameter for the phytoremediation process. However, there is a lack of research on the suitability of a phytoremediation system to remove various nitrogen species from industrial wastewater with a high nitrogen content. To design a sustainable remediation strategy for munition plant wastewater, the threshold capacity for nitrogen uptake needs to be established. Our goal was to develop a vetiver-based remediation system for wastewater with high levels of nitrogen species. The accumulation thresholds of nitrate, nitrite, and ammonium in synthetic wastewater by vetiver grass were tested in a hydroponic setup. The impact of high nitrogen concentration on plant health was determined by measuring growth parameters, the chlorophyll content, and the antioxidant enzymatic response of vetiver grass during the study.
2. Materials and Methods
2.1. Experimental Design
Batch experiments were conducted in a greenhouse using synthetic wastewater prepared according to Kangwannarakul et al. [15] as shown in Table S1. The synthetic wastewater was prepared to mimic effluents from a munition manufacturing facility containing very high levels of nitrogen species. The synthetic wastewater was spiked with eight concentration levels (L1–L8) of nitrogen species (nitrate, nitrite, and ammonium) as outlined in Table S2, reflecting typical ranges reported in the literature for the wastewater composition of munition manufacturing facilities (Table S3). In specific, concentrations were designed as 165, 330, 930, 1850, 3800, 8000, 17,800, and 24,200 mg N/L for nitrate, 100, 200, 400, 800, 1200, 1600, 2000, and 4000 mg N/L for nitrite, and 260, 520, 1300, 2600, 5200, 13,800, 26,500, and 39,100 mg N/L for ammonium. Vetiver grass was trimmed and placed in 1 L high-density polyethylene (HDPE) bottles (4% w/v), each containing 800 mL of synthetic wastewater spiked with nitrogen species (Table S2). A control setup without nitrogen was also included. All treatments were performed in triplicate.
During the 30-day experiment, wastewater samples were collected at regular intervals (day 1, day 2, day 15, and day 30). Volume adjustments were made to compensate for water loss due to evapotranspiration. Additionally, vetiver shoot and root samples were collected and preserved at −80 °C for later analysis of the chlorophyll content, protein levels, and antioxidant enzyme activity.
2.2. Chlorophyll Content
The measurement of chlorophyll (Chl) in the plant leaves was conducted using the method outlined by [16]. Shoot tissue (100 mg) was homogenized in 5 mL of 80% (v/v) aqueous acetone, and the mixture was kept at 4 °C in the dark for 24 h. The chlorophyll content was subsequently determined by analyzing the absorbance at 663 nm and 645 nm, and the Chl content was calculated following the equations established by [17].
Chlorophyll a = 12.47 × A663 − 3.62 × A645,
Chlorophyll b = 25.06 × A645 − 6.5 × A663,
2.3. Protein Extraction and Quantification
The extraction of proteins from root and shoot was carried out using the Pierce Plant Total Protein Extraction Kit (Thermo Fisher Scientific, Waltham, MA, USA). Following the extraction process, the Pierce™ bicinchoninic acid (BCA) protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA) was used to determine the protein concentrations.
2.4. Antioxidant Enzyme Activity
Catalase (CAT), glutathione S-transferase (GST), superoxide dismutase (SOD), and glutathione peroxidase (GPX) were measured on the initial and final day of the experiment using standardized assay kits from Cayman Chemical Company (Ann Arbor, MI, USA).
2.5. Data Analysis
The sample data were subjected to statistical analysis using Microsoft Excel (version 2302, Microsoft, Redmond, WA, USA). To compare the phytoremediation performance of the hydroponic treatment units, a one-way analysis of variance (ANOVA) was employed. The mean values, along with one standard deviation, were reported with a confidence interval of 95%.
3. Results and Discussion
3.1. Removal of Nitrogen Species from Simulated Wastewater
Figure 1 shows the results of nitrogen uptake by vetiver grass. Nitrate, nitrite, and ammonium concentrations were measured in water samples collected at four different time points (day 1, day 2, day 15, and day 30) to assess the nutrient uptake capability of vetiver at eight concentration levels.
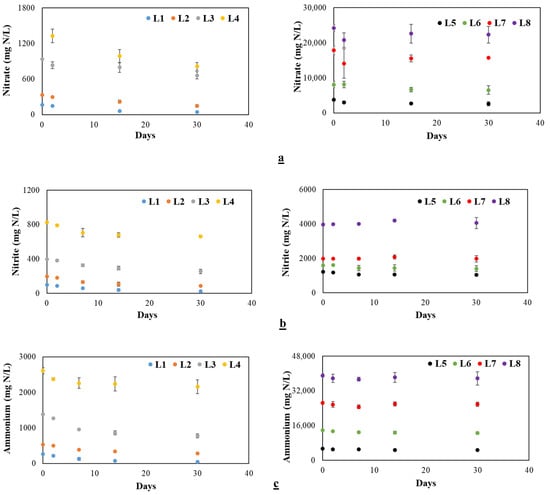
Figure 1.
Removal of nitrogen species from synthetic wastewater by vetiver grass at various initial concentrations for 30 days. (a) Treatment at different nitrate levels, (b) treatment at different nitrite levels, and (c) treatment at different ammonium levels. Note: the designations L1 to L8 correspond to specific initial concentrations of nitrate, nitrite, and ammonium, as detailed in Table S2 of the SI.
3.1.1. Nitrate
Vetiver lowered nitrate levels significantly (p < 0.05), demonstrating its potential as a sustainable and natural remediation method (Figure 1a). At the initial concentration level of L1 (Table S2), nitrate levels were reduced to 44.3 mg/L after one month, resulting in an impressive removal efficiency of 73%. At the concentration level (L2), a removal rate of 54.6% was observed, while at level L3, a reduction to 663.8 mg/L, corresponding to a removal rate of 29.2%, was observed. At the L4 treatment level, the second-highest removal efficiency of 55.6% was observed, following closely behind the first concentration level. At higher concentrations, nitrate uptake was reduced progressively (Figure 1a). Overall, removal percentages ranging from 7% to 73% were observed. Our findings are similar to the results reported by Zhang [18], where floating treatment wetlands were used, showing better nitrate removal efficiencies (73.45%) compared to those of free-water surface constructed wetlands (51.63%). Additionally, a nitrogen removal rate ranging from 75% to 85% was reported using floating treatment systems containing vetiver [12]. Also, a study conducted by Effendi et al. demonstrated the use of a vetiver treatment system to reduce nitrate concentrations in tilapia cultivation wastewater [19]. These investigations show that plant-based removal systems can achieve effective removal efficiencies in various types of wastewater [20].
3.1.2. Nitrite
Vetiver consistently and significantly reduced nitrite levels across treatments (p < 0.05) from L1 through L7 with decreasing removal as the nitrite concentration increased (Figure 1b). In L1, nitrite dropped to 24.95 mg N/L, achieving a 75% removal rate. L2, starting at 200 mg N/L, decreased to 87 mg N/L (55.4% removal). A higher concentration of L3 showed a reduction to 255.05 mg N/L (35.8% removal), while L4 to L6 had removal rates between 10% and 20%. Minimal removal occurred at L7, with L8 exhibiting a slight increase of 2%, possibly due to microbial activity [21]. Gholipour et al. observed the efficacy of vetiver grass in removing nitrite from influent and effluent samples of a sewage treatment plant. Initially, a rise in nitrite concentrations was noted, potentially attributed to microbial processes such as ammonium conversion and environmental factors, including the presence of nutrients and microbial nitrate conversion. Subsequently, nitrate-induced conversion initially increased nitrite levels, followed by a gradual decline, likely influenced by both vetiver grass absorption and microbial activity over the experimental duration [21]. Supporting these observations, Effendi et al. reported a significant reduction (27.51%) in nitrite concentration following vetiver treatment of wastewater from tilapia cultivation [19].
3.1.3. Ammonium
Vetiver consistently and significantly removed ammonium from wastewater at various concentration levels (p < 0.05) (Figure 1c). In L1, it achieved the highest removal rate at 83.6%. L2 and L3 both showed approximately 45% removal. However, as the concentrations increased, the removal rates declined. L4 showed a 17.21% reduction, while L5, L6, and L7 each had around 10% removal. At L7 and L8, removal dropped below 3%, indicating reduced effectiveness at higher concentrations. No detectable ammonium was found in the control experiments. The findings from Boonsong and Chansiri revealed average removal efficiency rates for ammonium ranging from 13% to 57% when utilizing vetiver floating platforms for the treatment of domestic wastewater, which corroborates the outcomes obtained in our study [22]. Similarly, Minh Tran et al. reported average removal efficiency values of 63.5% for nitrogen using vetiver grass [23]. Effendi et al. further demonstrated vetiver’s effectiveness by observing a 30.17% reduction in the ammonium concentration in wastewater from tilapia cultivation [19]. This aligns with Gholipour et al., who found that the ammonium uptake rate in vetiver increased with higher concentrations in wastewater. In their study, vetiver absorbed ammonium between days 4 and 18, despite the low effluent concentration of 53.18 mg/L [21]. Our study shows vetiver grass’s ability as a sustainable solution to remove nitrate, nitrite, and ammonium from synthetic wastewater, with removal efficiencies peaking at 73%, 75%, and 83.6%, respectively, and higher effectiveness at lower concentrations.
3.2. Plant Growth
Plant growth was evaluated across different experimental conditions, including a control group in synthetic wastewater without nitrogen. Throughout the experiment, vetiver shoots maintained a green appearance, and roots remained healthy. The study involved assessing nitrate uptake across eight experimental groups (L1 to L8), with initial plant weights ranging from 32 g to 34 g shown in Figure S1. The results indicated varying degrees of growth and nitrate uptake. Plants exposed to lower nitrogen concentrations (up to 1650 mg N/L for nitrate, 400 mg N/L for nitrite, and 1300 mg N/L for ammonium) showed slight increases in weight, while higher concentrations led to plant desiccation and weight loss. In the control group, the plant weight increased from 32 g to 39.4 g, and similar increments were observed in L1 (32.9 g to 42.1 g) and L2 (34 g to 39 g). Conversely, higher concentrations resulted in reduced plant weight, especially in L5 to L8. When exposed to nitrite, L1, L2, and L3 exhibited slight weight increases, indicating biomass accumulation and effective nitrite removal. Similarly, exposure to ammonium resulted in weight increases for L1, L2, and L3, suggesting some degree of growth or mass accumulation.
The effect of different nitrate concentrations (L1–L4) on the height of vetiver plants was evaluated (Figure S2). A significant increase in height was observed across the first four nitrate levels, with L1 showing growth from 61.38 cm to 71 cm. Similar trends were observed for the first three nitrite and ammonium concentrations, indicating a consistent response of vetiver to these nitrogen compounds. However, higher nitrogen concentrations elicited an inhibitory effect. The control group, with no external nitrogen, showed the greatest growth, increasing from 48.6 cm to 104.1 cm, surpassing the height achieved under any specific nitrate concentration. These findings contribute valuable insights into vetiver’s response to varying nitrogen levels, showcasing both stimulatory and inhibitory effects on plant height depending on the concentration thresholds. The data demonstrate that plants exposed to higher doses of nitrogen in various forms experienced stress symptoms akin to those documented by [19,24,25,26], including drying leaves, decreased biomass, and mild chlorosis (Figure S3).
3.3. Chlorophyll
Chlorophyll content is a key indicator of a plant’s photosynthetic and metabolic health. Photosynthesis depends on chlorophyll a, which captures light energy and releases high-energy electrons, and chlorophyll b, which aids by transferring light energy to chlorophyll a to enhance efficiency [17]. Chlorophyll a, chlorophyll b, and total chlorophyll in vetiver leaves were measured at two time points: day 0 and day 30. The total chlorophyll content from all nitrogen treatments and the control group is shown in Figure S4. Under the control conditions, the chlorophyll content decreased by 45% (p < 0.05) from an initial concentration of 31 μg/mL. This was likely due to the shock of transplanting the plants from soil to wastewater. For nitrate concentrations from L1 to L4, chlorophyll reduction ranged from 38% to 55%, while higher concentrations caused a significant decrease of 70% to 95% (p < 0.05). In nitrite treatments, L1, with an initial concentration of 28 μg/mL, showed a significant 75% reduction in chlorophyll content compared to all other concentrations. Ammonium treatments showed a lower impact in treatments L1 through L3 (52% to 74% reduction), but from L4 onwards, chlorophyll decline exceeded 90% (p < 0.05), with the highest reduction observed at L4, where the initial concentration of 37 μg/mL decreased to 3.5 μg/mL. Vetiver grew well and survived in lower nitrogen treatments, but a severe reduction in chlorophyll was seen at higher levels. These results suggest that photosynthesis is strongly influenced by nitrogen type and concentration, with significant differences (p < 0.05) between pre- and post-experimental chlorophyll levels. Several other studies have also documented notable reductions in chlorophyll content in different plant species as a consequence of toxicity from nitrogen species. While it is well known that the availability of nitrate increases plant growth, excessive nitrate uptake can be detrimental to plants. Plants have been reported to exhibit toxicity symptoms when exposed to high levels of nitrate, including decreased yield and biomass [27]. Elevated nitrite in plants also causes a reduction in growth, reduced biomass, and root discoloration [28]. Excessive ammonium is known to cause various symptoms in plants including reduced growth and yield, chlorosis, stunted root growth, and oxidative stress [29].
3.4. Analysis of Protein
Fluctuations in the concentration of proteins may be indicative of shifts in the metabolic processes of plants [30]. The protein concentration in vetiver shoots and roots for all nitrogen species treatment levels is depicted in Figure S5. Nitrate stress caused a pronounced impact on the protein concentration in vetiver grass shoots. Under the control conditions, the protein concentration rose by 7.9%, increasing from 4.67 mg/mL to 5.04 mg/mL in treatments L1 through L4. However, significant variations were observed at higher nitrate concentrations, particularly between the L5 and L8 levels, where the protein levels surged by 0.5 to 2.3 times compared to the control conditions (p < 0.05). The elevated protein content in vetiver shoots under nitrate stress is likely attributable to increased nitrogen assimilation and enhanced metabolic activity, and compared to roots, the shoot tissues act as primary sinks for assimilated nitrogen [31,32,33]. Nitrate serves not only as a nutrient but also as a signaling molecule that regulates gene expression related to protein synthesis. Under nitrate-rich conditions, plants often upregulate enzymes involved in nitrogen assimilation (e.g., nitrate reductase and glutamine synthetase), leading to increased amino acid and protein synthesis in shoots. In contrast, the protein concentration in the root of vetiver decreased, a trend consistent with that observed under the control conditions, unlike the initial four rates.
Under nitrite treatment, the protein concentration in the shoot exhibited a significant increase in all treatments, while the protein concentration in the root decreased significantly in these concentration levels (p < 0.05). Vetiver grass exhibited a similar trend under varying concentrations of ammonium, except at the L2 concentration level, where the protein content decreased in the shoot of vetiver and increased in the root (Figure S5). Notably, concentrations from L4 to L8 demonstrated a particularly pronounced and statistically significant increase in protein content in the shoots. Simultaneously, within this concentration range, there was a marked and statistically significant decrease in protein content in the roots of vetiver (p < 0.05).
In the current investigation, as the concentration of nitrogen species in wastewater increased, there was a significant elevation in protein levels observed in the shoot and across most root concentrations, excluding specific instances like high nitrate concentrations, particularly between L5 and L8, and L2 in ammonium. Previous reports have shown that increased nitrate levels significantly enhanced protein accumulation in poplar, with a higher protein increase observed in the shoots compared to the roots [30]. Exposure to ammonium resulted in a 5% increase in the total protein content in Salvinia molesta, rising from 0.2582 g/g biomass to 0.2703 g/g biomass [34].
3.5. Antioxidant Enzyme Response Due to Nitrogen Species Stress
Environmental stresses can trigger the enhanced generation of reactive oxygen species (ROS), which result in detrimental effects such as lipid peroxidation, enzyme activity inhibition, protein oxidation, and nucleic acid deterioration, resulting in cell damage and death [35]. The antioxidative defense system, which encompasses enzymatic antioxidants such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione S-transferase (GST), plays a significant role in scavenging ROS from cells. This system helps minimize stress in plants by efficiently neutralizing ROS [35]. The impact of nitrate, nitrite, and ammonium stress on vetiver roots and shoots was examined through the assessment of antioxidant enzyme activity over a month.
3.5.1. Superoxide Dismutase
The initial defense mechanism converting superoxide (O2−) and water to hydrogen peroxide (H2O2) and molecular oxygen (O2) is catalyzed by SOD [36]. As shown in Figure S6, there was minimal variation in SOD levels in both the shoots and roots of vetiver across all coverage rates. The concentration remained around 15.5 U/mL in the roots and 16 U/mL in the shoots, regardless of nitrogen species stress. Abiotic stresses, such as drought, floods, heat, heavy metal contamination, and deficiency of micronutrients, have been reported to increase SOD activity [37]. To our knowledge, no previous report on the effect of very high levels of nitrogen species on SOD activity has been reported. Our results are similar to those of Medici. et al., who reported glutathione reductase (GR) and CAT activities increased in barley, maize, and Arabidopsis roots grown at different nitrogen concentrations, but no significant change in SOD activity was reported [38].
3.5.2. Catalase
Catalase enzymes play a crucial role in transforming H2O2 into water (H2O) [39]. The activities of CAT enzymes under the influence of nitrate, nitrite, and ammonium stress on vetiver shoots and roots over one month are depicted in Figure 2. CAT activities in the shoot of vetiver exhibited a consistently significant decrease under nitrate and nitrite stress across all concentration levels and conditions, including the control group, with p-values less than 0.05. In contrast, under ammonium stress, the observed changes did not reach statistical significance, as the associated p-value exceeded the threshold of 0.05. Interestingly, under the control conditions, CAT activities in the root of vetiver showed a significant decrease by 15% (p < 0.05). However, in the presence of nitrate, nitrite, or ammonium, this figure exhibited an increase in all concentration levels, with CAT activities rising significantly under nitrate (22–77%), nitrite (40–155%), and ammonium stress (8–33%).
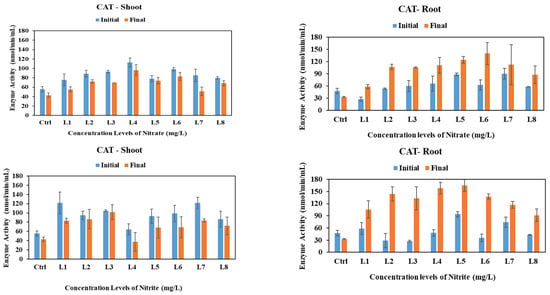
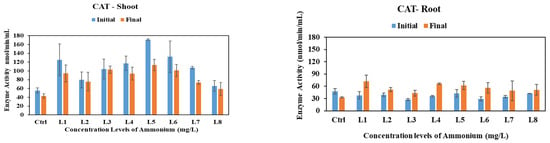
Figure 2.
CAT activities in shoots and roots of vetiver grass after exposure to various initial concentrations of nitrate, nitrite, and ammonium. Enzyme activities were analyzed at the initial (day 0) vs. final (day 30) stages of plant exposure to nitrogen.
3.5.3. Guaiacol Peroxidase
Similar to CAT, GPx is responsible for the breakdown of H2O2 [40]. As shown in Figure 3, GPx activity in vetiver exhibited distinct patterns in shoots and roots under different conditions. While not statistically significant, GPx levels increased in the shoots across all stressors, while in the roots, GPx decreased under the control conditions and at certain nitrite (L8) and ammonium concentrations (L4 to L8), but increased under other stress conditions, reflecting complex regulation. It is noteworthy that the level of CAT enzyme activity surpassed that of GPx, implying that CAT likely played a substantial role in the breakdown of H2O2.
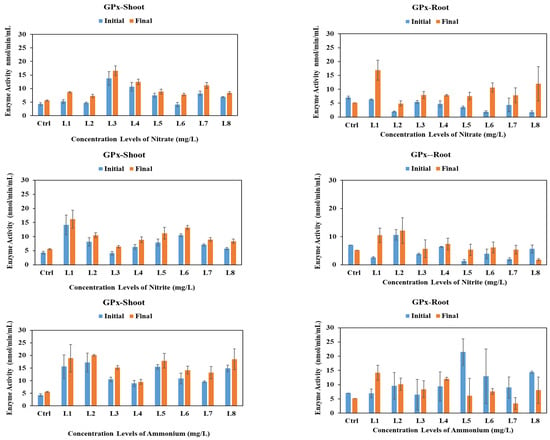
Figure 3.
GPx activities in vetiver shoots and roots after 30-day exposure to various initial concentrations of nitrate, nitrite, and ammonium. Enzyme activities were analyzed at the initial (day 0) vs. final (day 30) stages of plant exposure to nitrogen.
3.5.4. Glutathione S-Transferase
Glutathione S-transferases are a group of enzymes that combine reactive compounds with glutathione and neutralize them [41]. GST levels in vetiver shoots varied under different stress conditions (Figure 4). A significant increase was noted from concentration level L3 onward under nitrate stress, but not in the control, L1, or L2 (p < 0.05). Under nitrite stress, GST levels significantly decreased in the control and L5, while increasing at other concentrations (p < 0.05). For ammonium stress, GST levels in the shoots decreased in the control, remained stable in L1 and L2, rose between L3 and L4, and declined from L5 to L8, with no statistically significant changes (p > 0.05). In vetiver roots, GST levels declined under nitrate, nitrite, and ammonium stress, except for an increase observed between L5 and L8 under nitrite stress (p > 0.05). There are no previous studies on the effect of such high concentrations of nitrogen species on plants. However, a few studies have shown that high nitrogen stress can be countered by the activation of antioxidant enzymes. Lin et al. [42] studied the impact of high nitrate on feed crops alfalfa, ryegrass, and tall fescue. They reported that all three nitrogen species showed high antioxidant activity, including SOD and GPx. In addition, a few previous studies have reported ammonium-induced oxidative stress response in various plant species. In leaf tissues of the aquatic plant Myriophyllum mattogrossense, as well as in tobacco, increased ROS generation was observed [43,44]. Higher activities of SOD, glutathione reductase, and guaiacol peroxidase were reported in Arabidopsis roots exposed to high levels of ammonium [44]. In wheat leaves, antioxidant enzymes SOD, ascorbate peroxidase, glutathione reductase, and CAT were significantly higher in ammonia-treated plants compared to those of nitrate [45].
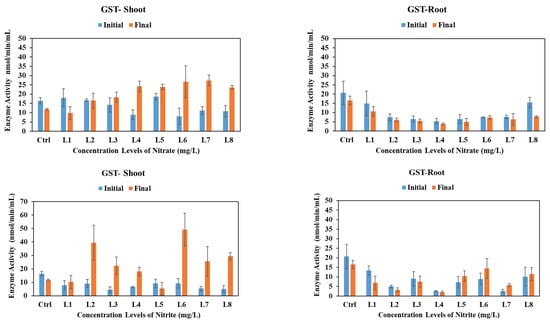
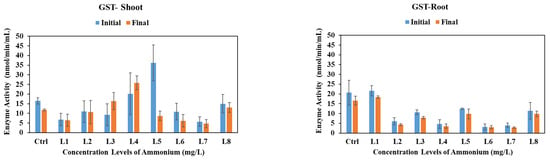
Figure 4.
GST activities in vetiver shoots and roots after 30-day exposure to various initial concentrations of nitrate, nitrite, and ammonium. Enzyme activities were analyzed at the initial (day 0) vs. final (day 30) stages of plant exposure to nitrogen.
To aid in cross-comparison and highlight overarching trends, a summary table consolidating key findings from all nitrogen treatments is provided below (Table 1).

Table 1.
Summary of vetiver grass responses to varying concentrations of nitrate, nitrite, and ammonium in synthetic wastewater.
4. Conclusions
On assessing the uptake of a range of concentration levels of nitrate, nitrite, and ammonia in munition plant wastewater by vetiver, we found that vetiver was effective in taking up all three nitrogen species. Eight concentration levels were tested, ranging from 165 to 24,700 mg N/L for nitrate, 100 to 4000 mg N/L for nitrite, and 260 to 39,000 mg N/L for ammonium. The results from this preliminary study showed that nitrogen concentrations decreased over time in all treatments, demonstrating the efficacy of vetiver in treating nitrogen in wastewater at lower concentrations. However, very high nitrogen caused oxidative stress, reduced growth, and chlorosis. Vetiver was able to tolerate nitrate concentrations up to 3800 mg N/L, nitrite concentrations up to 800 mg N/L, and ammonium concentrations up to 2600 mg N/L. Our findings highlight the role of antioxidant enzymes in the detoxification of nitrogen species. This study did not look into the conversion of nitrate species within the plant tissue. A larger-scale study on nitrogen uptake using a floating treatment system is necessary, to understand the effectiveness of a vetiver-based system to remediate munition plant wastewater to optimize plant coverage and scale this approach for practical applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w17101464/s1. Figure S1. Change in vetiver weight. (A) nitrate levels; (B) nitrite levels; (C) ammonium levels; Figure S2. Change in vetiver height. (A) nitrate levels; (B) nitrite levels; (C) ammonium levels; Figure S3. Visual comparison of vetiver grass response to nitrate, nitrite, and ammonium exposure at Day 0 and Day 30; Figure S4. Change in vetiver total chlorophyll under nitrate, nitrite, and ammonium stress; Figure S5. Protein concentrations in vetiver shoots (a) and roots (b) under nitrate, nitrite, ammonium stress; Figure S6. SOD activities in vetiver shoots and roots exposed to nitrate, nitrite, and ammonium. Initial (Day 0) vs. Final (Day 30) stages of plant exposure to nitrogen; Table S1. Chemical compositions of the synthetic wastewater; Table S2. Concentrations of nitrogen species (mg N/L) in the synthetic wastewater; Table S3. Summary of reported nitrogen species concentrations and pH in industrial wastewater. References [46,47,48,49,50,51,52] are citied in the Supplementary Materials.
Author Contributions
Conceptualization, D.S.; methodology, A.A. and Z.Z.; validation, A.A, Z.Z., C.C. and R.D.; formal analysis, A.A.; investigation, A.A.; data curation, A.A., Z.Z. and R.D.; writing—original draft preparation, A.A.; writing—review and editing, Z.Z., R.D., C.C. and D.S.; visualization, A.A.; resources, C.C. and D.S., supervision, D.S.; project administration, D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We sincerely thank Roley and Ina Nofkey of Hydromulch Ltd. for their generous provision of vetiver slips for this study. We also extend our gratitude to Vijay Persaud for his invaluable assistance with the experiments.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| IHEs | insensitive high explosives |
| ECs | energetic compounds |
| Chl | chlorophyll |
| ROS | reactive oxygen species |
| CAT | catalase |
| GST | glutathione S-transferase |
| SOD | superoxide dismutase |
| GPX | glutathione peroxidase |
References
- Panja, S.; Sarkar, D.; Datta, R. Vetiver grass (Chrysopogon zizanioides) is capable of removing insensitive high explosives from munition industry wastewater. Chemosphere 2018, 209, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Zoh, K.-D.; Stenstrom, M.K. Fenton oxidation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) and octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX). Water Res. 2002, 36, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Resende, J.D.; Nolasco, M.A.; Pacca, S.A. Life cycle assessment and costing of wastewater treatment systems coupled to constructed wetlands. Resour. Conserv. Recycl. 2019, 148, 170–177. [Google Scholar] [CrossRef]
- Karimi, H.; Mohammadi, F.; Rajabi, S.; Mahvi, A.H.; Ghanizadeh, G. Biological 2,4,6-trinitrotoluene removal by extended aeration activated sludge: Optimization using artificial neural network. Sci. Rep. 2023, 13, 9053. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Chen, N.; Feng, Z.; Feng, C.; Deng, Y. Treatment of nitrate containing wastewater by adsorption process using polypyrrole-modified plastic-carbon: Characteristic and mechanism. Chemosphere 2022, 297, 134107. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef]
- Andra, S.S.; Sarkar, D.; Saminathan, S.K.M.; Datta, R.; Andra, S.S.; Saminathan, S.K.M.; Sarkar, D.; Datta, R. Exchangeable lead from prediction models relates to vetiver lead uptake in different soil types. Environ. Monit. Assess. 2011, 183, 571–579. [Google Scholar] [CrossRef]
- Ketaubon, P.; Ritthikasem, N.; Tanheng, P.; Prapagdee, B. Enhancing heavy metal phytoremediation in landfill soil by Chrysopogon zizanioides (L.) roberty through the application of bacterial-biochar pellets. Environ. Technol. Innov. 2024, 35, 103738. [Google Scholar] [CrossRef]
- RoyChowdhury, A.; Datta, R.; Sarkar, D. Heavy Metal Pollution and Remediation. Green Chem. Incl. Approach 2018, 359–373. [Google Scholar] [CrossRef]
- Corredor, D.; Duchicela, J.; Flores, F.J.; Maya, M.; Guerron, E. Review of Explosive Contamination and Bioremediation: Insights from Microbial and Bio-Omic Approaches. Toxics 2024, 12, 249. [Google Scholar] [CrossRef]
- Worku, A.; Tefera, N.; Kloos, H.; Benor, S. Bioremediation of brewery wastewater using hydroponics planted with vetiver grass in Addis Ababa, Ethiopia. Bioresour. Bioprocess. 2018, 5, 1–12. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Jamshidi, S.; Vakhshouri, M. Nutrient uptake rate and removal efficiency of Vetiveria zizanioides in contaminated waters. Pollution 2015, 1, 1–8. [Google Scholar]
- Goren, A.Y.; Yucel, A.; Sofuoglu, S.C.; Sofuoglu, A. Phytoremediation of olive mill wastewater with Vetiveria zizanioides (L.) Nash and Cyperus alternifolius L. Environ. Technol. Innov. 2021, 24, 102071. [Google Scholar] [CrossRef]
- Junio, C.J.; Ontar, P.A.; Rutaquio, V.J.; Manlapaz, J.M.; Braga, E.B.; Tugade, C.; Pescos, C.J. Horizontal-flow constructed wetlands by phytoremediation using vetiver grass, common reed, and canna lily as tertiary wastewater treatment for the reduction of pollutant concentrations of ammonia, phosphates, and nitrates. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2024; Volume 1372, p. 12045. [Google Scholar]
- Kangwannarakul, N.; Wantawin, C.; Noophan, P. Anammox bacteria with attached-growth media for nitrogen removal in wastewater. Clean Technol. Environ. Policy 2018, 20, 219–226. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Liu, C.; Wang, Z.; Dong, J.; Zhu, G.; Huang, X. Potential effect and accumulation of veterinary antibiotics in Phragmites australis under hydroponic conditions. Ecol. Eng. 2013, 53, 138–143. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Q.; Jinadasa, K.; Gersberg, R.M.; Liu, Y.; Ng, W.J.; Tan, S.K. Application of constructed wetlands for wastewater treatment in developing countries—A review of recent developments (2000–2013). J. Environ. Manag. 2014, 141, 116–131. [Google Scholar] [CrossRef]
- Effendi, H.; Utomo, B.A.; Pratiwi, N.T.M. Ammonia and orthophosphate removal of tilapia cultivation wastewater with Vetiveria zizanioides. J. King Saud Univ. 2020, 32, 207–212. [Google Scholar] [CrossRef]
- Norton, S. Removal Mechanisms in Constructed Wastewater Wetlands. 2014. Available online: https://home.engineering.iastate.edu/tge/ce421-521/stephen.pdf (accessed on 5 May 2025).
- Gholipour, M.; Mehrabanjoubani, P.; Abdolzadeh, A.; Raghimi, M.; Seyedkhademi, S.; Karimi, E.; Sadeghipour, H.R. Facilitated decrease of anions and cations in influent and effluent of sewage treatment plant by vetiver grass (Chrysopogon zizanioides): The uptake of nitrate, nitrite, ammonium, and phosphate. Environ. Sci. Pollut. Res. 2020, 27, 21506–21516. [Google Scholar] [CrossRef]
- Boonsong, K.; Chansiri, M. Domestic wastewater treatment using vetiver grass cultivated with floating platform technique. AU J. Technol. 2008, 12, 73–80. [Google Scholar]
- Panja, S.; Sarkar, D.; Li, K.; Datta, R. Uptake and transformation of ciprofloxacin by vetiver grass (Chrysopogon zizanioides). Int. Biodeterior. Biodegrad. 2019, 142, 200–210. [Google Scholar] [CrossRef]
- Panja, S.; Sarkar, D.; Zhang, Z.; Datta, R. Removal of antibiotics and nutrients by vetiver grass (Chrysopogon zizanioides) from a plug flow reactor based constructed wetland model. Toxics 2021, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- RoyChowdhury, A.; Mukherjee, P.; Panja, S.; Datta, R.; Christodoulatos, C.; Sarkar, D. Evidence for Phytoremediation and Phytoexcretion of NTO from Industrial Wastewater by Vetiver Grass. Molecules 2020, 26, 74. [Google Scholar] [CrossRef]
- Ahmed, M.; Rauf, M.; Akhtar, M.; Mukhtar, Z.; Saeed, N.A. Hazards of nitrogen fertilizers and ways to reduce nitrate accumulation in crop plants. Environ. Sci. Pollut. Res. 2020, 27, 17661–17670. [Google Scholar] [CrossRef]
- Hoque, M.A.; Okuma, E.; Banu, M.N.A.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. Exogenous proline mitigates the detrimental effects of salt stress more than exogenous betaine by increasing antioxidant enzyme activities. J. Plant Physiol. 2007, 164, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Shilpha, J.; Song, J.; Jeong, B.R. Ammonium phytotoxicity and tolerance: An insight into ammonium nutrition to improve crop productivity. Agronomy 2023, 13, 1487. [Google Scholar] [CrossRef]
- Singh, P.K.; Tewari, R.K. Cadmium toxicity induced changes in plant water relations and oxidative metabolism of Brassica juncea L. plants. J. Environ. Biol. 2003, 24, 107–112. [Google Scholar] [PubMed]
- Wang, R.; Guegler, K.; LaBrie, S.T.; Crawford, N.M. Genomic analysis of a nutrient response in Arabidopsis reveals diverse expression patterns and novel metabolic and potential regulatory genes induced by nitrate. Plant Cell 2000, 12, 1491–1509. [Google Scholar] [CrossRef]
- Stitt, M. Nitrate regulation of metabolism and growth. Curr. Opin. Plant Biol. 1999, 2, 178–186. [Google Scholar] [CrossRef]
- Krapp, A.; David, L.C.; Chardin, C.; Girin, T.; Marmagne, A.; Leprince, A.-S.; Chaillou, S.; Ferrario-Méry, S.; Meyer, C.; Daniel-Vedele, F. Nitrate transport and signalling in Arabidopsis. J. Exp. Bot. 2014, 65, 789–798. [Google Scholar] [CrossRef]
- Ng, Y.S.; Chan, D.J.C. Wastewater phytoremediation by Salvinia molesta. J. Water Process Eng. 2017, 15, 107–115. [Google Scholar] [CrossRef]
- Kapoor, D.; Singh, S.; Kumar, V.; Romero, R.; Prasad, R.; Singh, J. Antioxidant enzymes regulation in plants in reference to reactive oxygen species (ROS) and reactive nitrogen species (RNS). Plant Gene 2019, 19, 100182. [Google Scholar] [CrossRef]
- Navabpour, S.; Yamchi, A.; Bagherikia, S.; Kafi, H. Lead-induced oxidative stress and role of antioxidant defense in wheat (Triticum aestivum L.). Physiol. Mol. Biol. Plants 2020, 26, 793–802. [Google Scholar] [CrossRef]
- Stephenie, S.; Chang, Y.P.; Gnanasekaran, A.; Esa, N.M.; Gnanaraj, C. An insight on superoxide dismutase (SOD) from plants for mammalian health enhancement. J. Funct. Foods 2020, 68, 103917. [Google Scholar] [CrossRef]
- Medici, L.O.; Azevedo, R.A.; Smith, R.J.; Lea, P.J. The influence of nitrogen supply on antioxidant enzymes in plant roots. Funct. Plant Biol. 2004, 31, 1–9. [Google Scholar] [CrossRef]
- Alfonso-Prieto, M.; Biarnés, X.; Vidossich, P.; Rovira, C. The molecular mechanism of the catalase reaction. J. Am. Chem. Soc. 2009, 131, 11751–11761. [Google Scholar] [CrossRef] [PubMed]
- Rocha, S.; Gomes, D.; Lima, M.; Bronze-da-Rocha, E.; Santos-Silva, A. Peroxiredoxin 2, glutathione peroxidase, and catalase in the cytosol and membrane of erythrocytes under H2O2-induced oxidative stress. Free Radic. Res. 2015, 49, 990–1003. [Google Scholar] [CrossRef]
- Kumar, S.; Trivedi, P.K. Glutathione S-transferases: Role in combating abiotic stresses including arsenic detoxification in plants. Front. Plant Sci. 2018, 9, 751. [Google Scholar] [CrossRef]
- Lin, D.; Huang, Y.; Zhao, J.; Wu, Z.; Liu, S.; Qin, W.; Wu, D.; Chen, H.; Zhang, Q. Evaluation of seed nitrate assimilation and stimulation of phenolic-linked antioxidant on pentose phosphate pathway and nitrate reduction in three feed-plant species. BMC Plant Biol. 2020, 20, 1–12. [Google Scholar] [CrossRef]
- Nimptsch, J.; Pflugmacher, S. Ammonia triggers the promotion of oxidative stress in the aquatic macrophyte Myriophyllum mattogrossense. Chemosphere 2007, 66, 708–714. [Google Scholar] [CrossRef]
- Skopelitis, D.S.; Paranychianakis, N.V.; Paschalidis, K.A.; Pliakonis, E.D.; Delis, I.D.; Yakoumakis, D.I.; Kouvarakis, A.; Papadakis, A.K.; Stephanou, E.G.; Roubelakis-Angelakis, K.A. Abiotic stress generates ROS that signal expression of anionic glutamate dehydrogenases to form glutamate for proline synthesis in tobacco and grapevine. Plant Cell 2006, 18, 2767–2781. [Google Scholar] [CrossRef] [PubMed]
- Motamedi-Tehrani, J.; Peyghan, R.; Shahriari, A.; Razijalali, M.; Ebrahimi, E. The influence of ammonia-N and salinity levels on oxidative stress markers, hepatic enzymes, and acid phosphatase activity in Nile tilapia (Oreochromis niloticus). Sci. Rep. 2025, 15, 559. [Google Scholar] [CrossRef]
- Polesskaya, O.G.; Kashirina, E.I.; Alekhina, N.D. Changes in the activity of antioxidant enzymes in wheat leaves and roots as a function of nitrogen source and supply. Russ. J. Plant Physiol. 2004, 51, 615–620. [Google Scholar] [CrossRef]
- Abraham, J.; Lin, Y.; RoyChowdhury, A.; Christodoulatos, C.; Conway, M.; Smolinski, B.; Braida, W. Algae toxicological assessment and valorization of energetic-laden wastewater streams using Scenedesmus obliquus. J. Clean. Prod. 2018, 202, 838–845. [Google Scholar] [CrossRef]
- Brenner, A.; Ronen, Z.; Harel, Y.; Abeliovich, A. Use of Hexahydro-1,3,5-trinitro-1,3,5-triazine as a Nitrogen Source in Biological Treatment of Munitions Wastes. Water Environ. Res. 2000, 72, 469–475. [Google Scholar] [CrossRef]
- Huibregtse, K.R.; Fulk, R.; Zanoni, A.E.; Zenker, D. Feasibility Study Regarding Landfill of Nitrocellulose Lime Sludge and Oxidation of Nitroglycerin Wastewater Stream; Envirex, Inc.: Milwaukee, WI, USA, 1978. [Google Scholar]
- Oh, S.-Y.; Cha, D.K.; Chiu, P.-C.; Kim, B.J. Zero-valent iron treatment of RDX-containing and perchlorate-containing wastewaters from an ammunition-manufacturing plant at elevated temperatures. Water Sci. Technol. 2006, 54, 47–53. [Google Scholar] [CrossRef]
- Ronen, Z.; Brenner, A.; Abeliovich, A. Biodegradation of RDX-contaminated wastes in a nitrogen-deficient environment. Water Sci. Technol. 1998, 38, 219–224. [Google Scholar] [CrossRef]
- Terracciano, A.; Christodoulatos, C.; Koutsospyros, A.; Zheng, Z.; Su, T.-L.; Smolinski, B.; Arienti, P.; Meng, X. Degradation of 3-nitro-1,2,4-trizole-5-one (NTO) in wastewater with UV/H2O2 oxidation. Chem. Eng. J. 2018, 354, 81–491. [Google Scholar] [CrossRef]
- Terracciano, A.; Koutsospyros, A.; Christodoulatos, C.; Mai, A.; Meng, X.; Su, T.-L.; Smolinski, B. Oxidative degradation of nitroguanidine (NQ) by UV-C and oxidants: Hydrogen peroxide, persulfate and peroxymonosulfate. Chemosphere 2022, 292, 133357. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).