Study on Photocatalytic Performance of Bi2O3-TiO2/Powdered Activated Carbon Composite Catalyst for Malachite Green Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Bi2O3-TiO2/PAC Composite Catalysts
2.3. Characterization
2.4. Photocatalytic Experiments
3. Results and Discussion
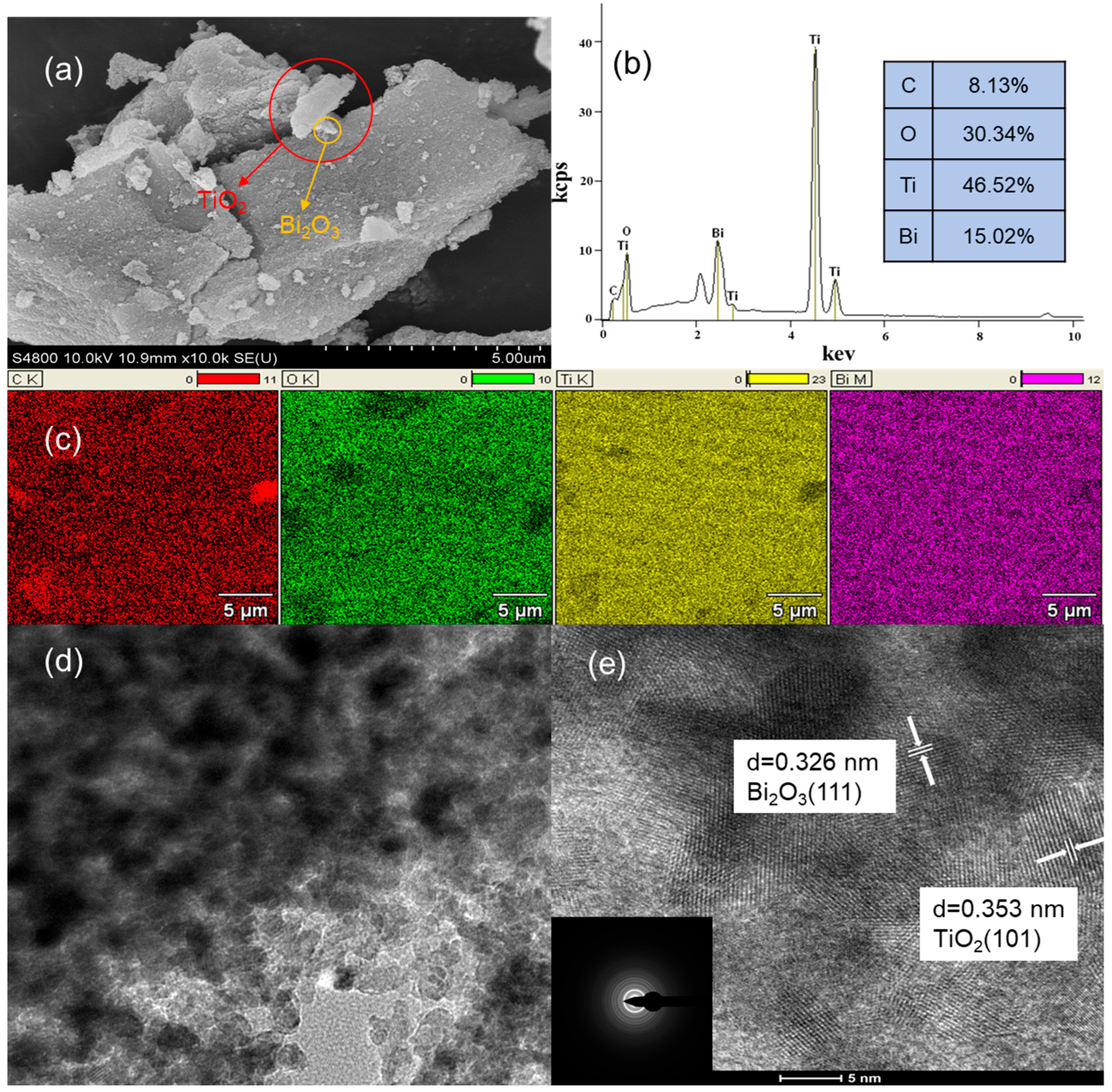
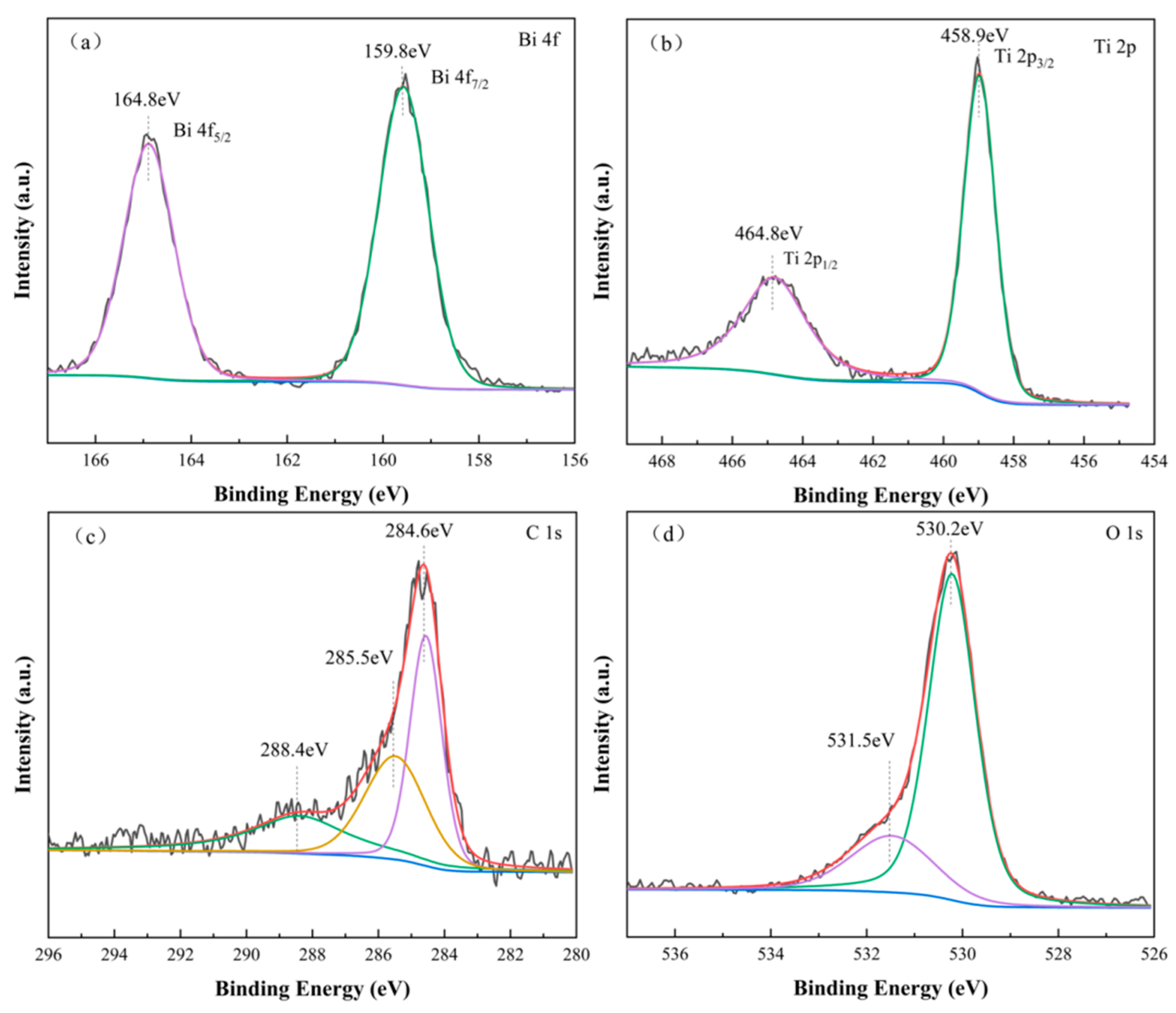
3.1. Characterization of Bi2O3-TiO2/PAC Composites
3.2. Photocatalytic Degradation Performances
3.2.1. Effect of Bi2O3-TiO2/PAC Dosage
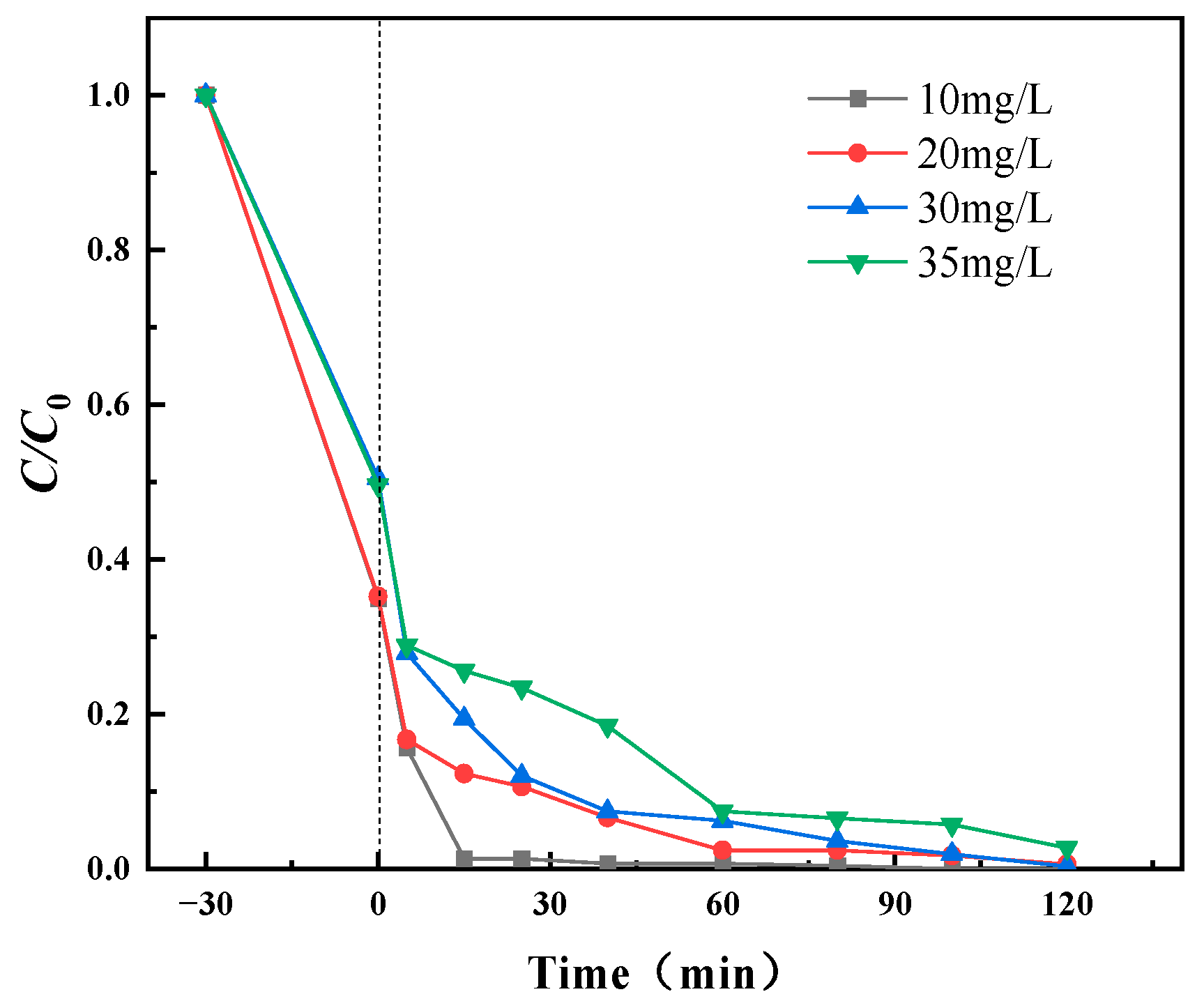
3.2.2. Effect of Initial MG Concentration
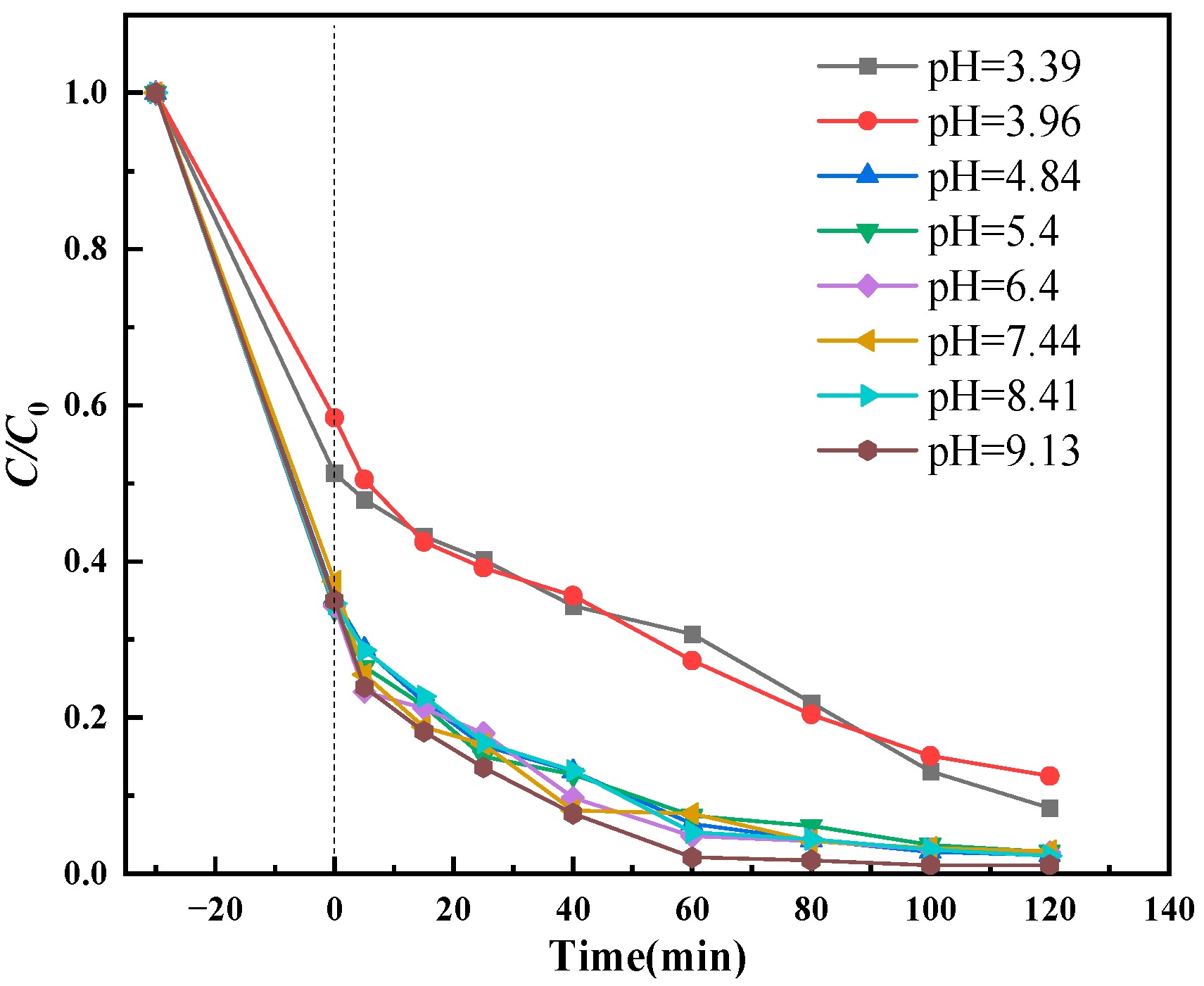
3.2.3. Effect of Solution pH
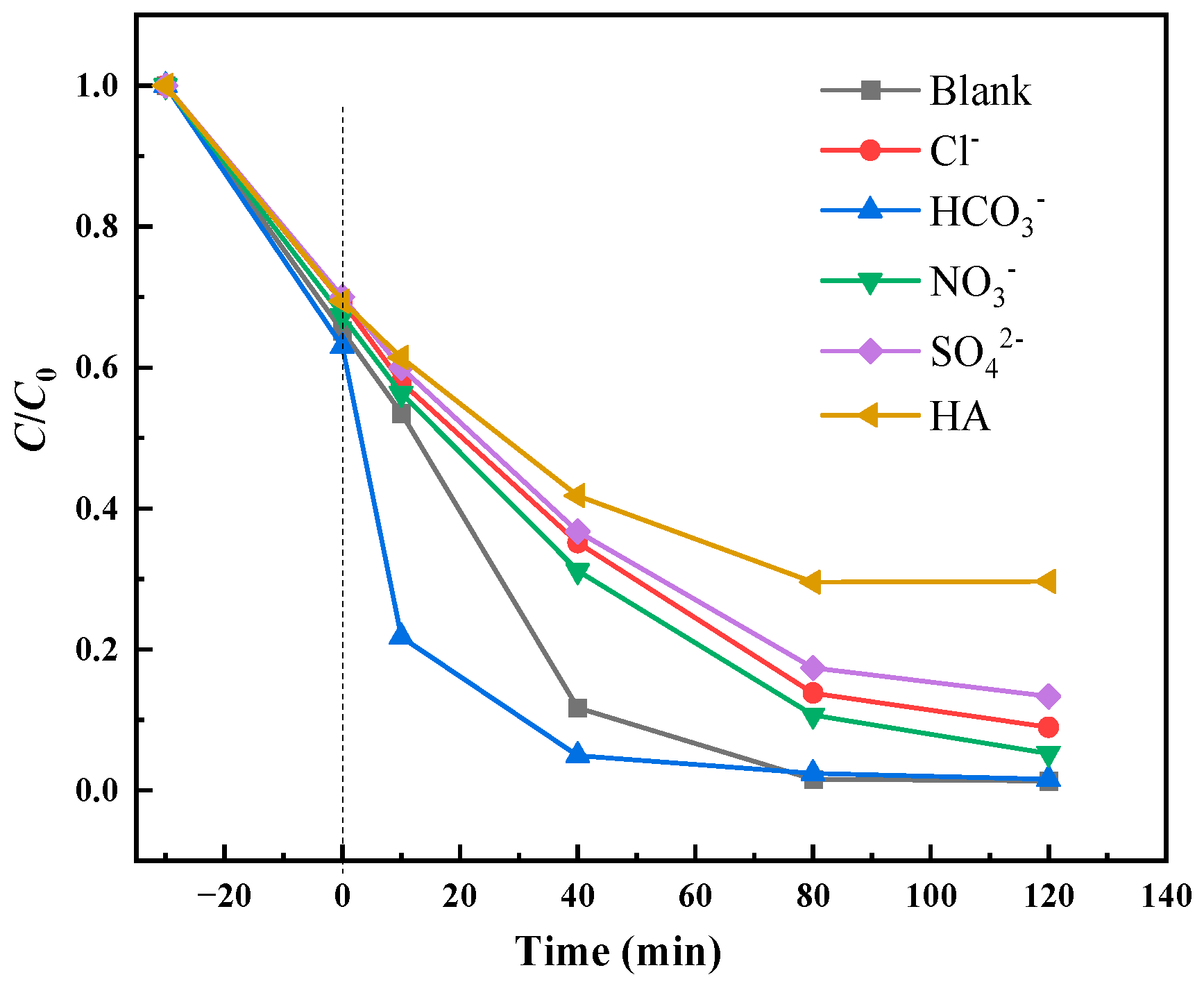
3.2.4. Effect of Coexisting Anions
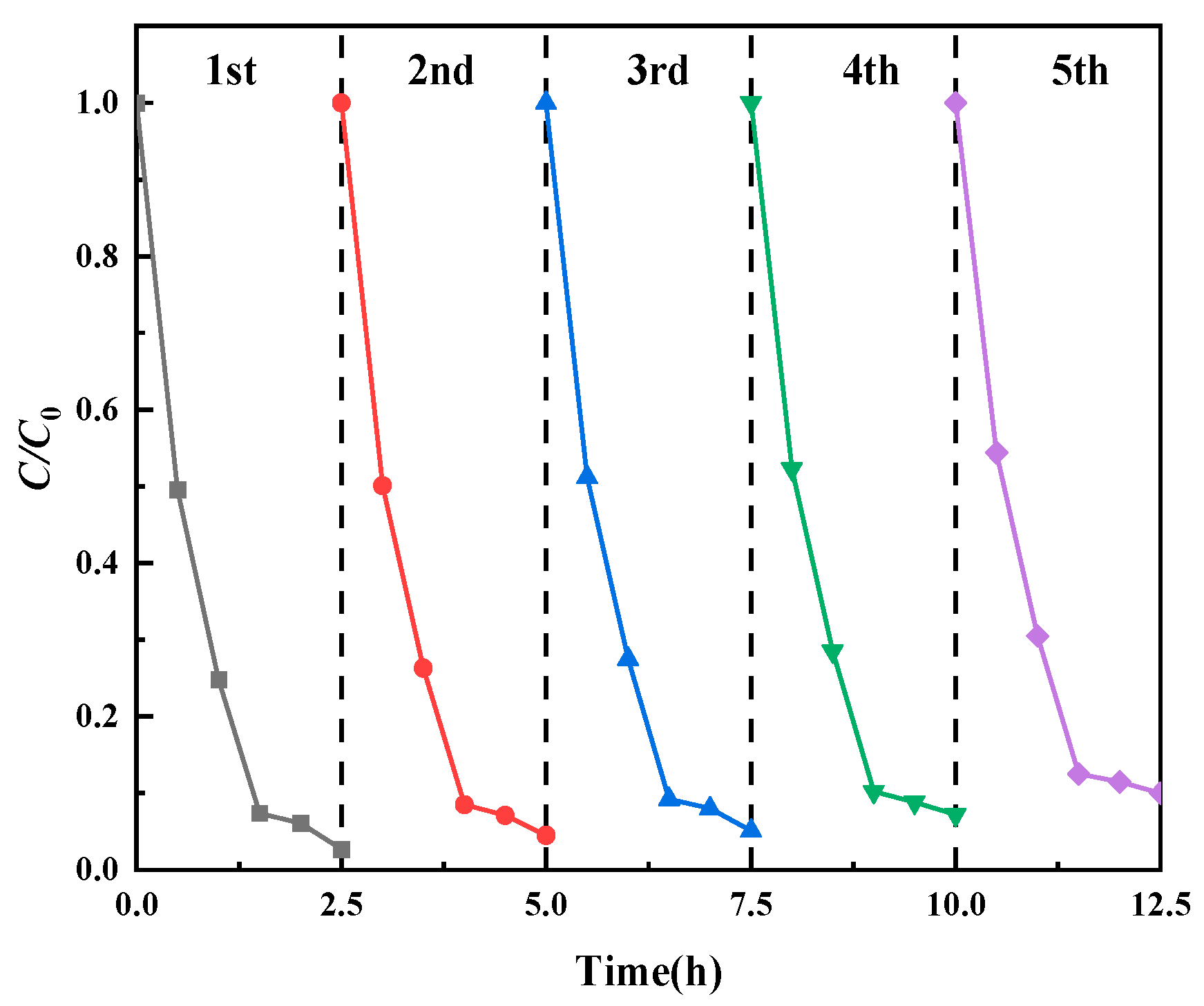
3.2.5. Stability and Reusability of Prepared Catalysts
3.3. Insight into the Photocatalytic Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Munyai, S.; Tetana, Z.N.; Mathipa, M.M.; Ntsendwana, B.; Hintsho-Mbita, N.C. Green synthesis of Cadmium Sulphide nanoparticles for the photodegradation of Malachite green dye, Sulfisoxazole and removal of bacteria. Optik 2021, 247, 167851. [Google Scholar] [CrossRef]
- Elavarasan, M.; Uma, K.; Yang, T.C.K. Nanocubes phase adaptation of In2O3/TiO2 heterojunction photocatalysts for the dye degradation and tracing of adsorbed species during photo-oxidation of ethanol. J. Taiwan Inst. Chem. Eng. 2021, 120, 169–177. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, S.; Wang, K.; Huang, H.; Zhang, F.; Kang, L. Optimization of photocatalytic degradation of malachite green using nano-scaled V/CeO2-functionalized steel-slag-based catalyst by response surface design approach. J. Mater. Res. Technol. 2023, 25, 6061–6073. [Google Scholar] [CrossRef]
- Ruiz-Santoyo, V.; García-Carvajal, S.; Arenas-Arrocena, M.C. Photocatalytic removal of synthetic dyes using Bi2O3–TiO2 nanocomposites obtained by simple hydrothermal route. J. Nanopart. Res. 2025, 27, 23. [Google Scholar] [CrossRef]
- Gong, L.; Wang, J.; Jiang, C.; Xiao, T.; Shen, K.; Lei, M.; Tang, Y. Study on magnetic porous carbon microspheres as a novel adsorbent for malachite green. ChemistrySelect 2021, 6, 3174–3182. [Google Scholar] [CrossRef]
- Zabolotnaya, E.; Men’Shova, I.I. A Membrane System for the Treatment of Waste Water Containing Organic Compounds. Fibre Chem. 2019, 4, 272–274. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Lopez, F.A. Organic Dyes versus Adsorption Processing. Molecules 2021, 26, 5440. [Google Scholar] [CrossRef]
- Hosny, M.; Eltaweil, A.S.; Mostafa, M.; El-Badry, Y.A.; Hussein, E.E.; Omer, A.M.; Fawzy, M. Facile Synthesis of Gold Nanoparticles for Anticancer, Antioxidant Applications, and Photocatalytic Degradation of Toxic Organic Pollutants. ACS Omega 2022, 7, 3121–3133. [Google Scholar] [CrossRef]
- Saha, S.; Wang, J.M.; Pal, A. Nano silver impregnation on commercial TiO2 and a comparative photocatalytic account to degrade malachite green. Sep. Purif. Technol. 2012, 89, 147–159. [Google Scholar] [CrossRef]
- Silva, G.N.; Martins, T.A.; Nogueira, I.C.; Santos, R.k.; Li, M.S.; Longo, E.; Botelho, G. Synthesis of Ag3PO4/SnO2 composite photocatalyst for improvements in photocatalytic activity under visible light. Mater. Sci. Semicond. Process. 2021, 135, 106064. [Google Scholar] [CrossRef]
- Bertagna Silva, D.; Buttiglieri, G.; Babic, S. State-of-the-art and current challenges for TiO2/UV-LED photocatalytic degradation of emerging organic micropollutants. Environ. Sci. Pollut. Res. 2021, 28, 103–120. [Google Scholar] [CrossRef]
- Eskandarian, M.R.; Choi, H.; Fazli, M.; Rasoulifard, M.H. Effect of UV-LED wavelengths on direct photolytic and TiO2 photocatalytic degradation of emerging contaminants in water. Chem. Eng. J. 2016, 300, 414–422. [Google Scholar] [CrossRef]
- Kelly, J.; Morrison, G.; Skillen, N.; Manesiotis, P.; Robertson, P.K. An investigation of the role of pH in the rapid photocatalytic degradation of MCPA and its primary intermediate by low-power UV LED irradiation. Chem. Eng. J. 2019, 359, 112–118. [Google Scholar] [CrossRef]
- Devika, S.; Tayade, R.J. Low temperature energy- efficient synthesis methods for bismuth-based nanostructured photocatalysts for environmental remediation application: A review. Chemosphere 2022, 304, 135300. [Google Scholar]
- Wang, Y.; Zhao, S.; Yang, Y.; Rodriguez, R.D.; Lipovka, A.; Lu, Y.; Huang, H.; Chen, J. Ag nanoparticle-decorated Bi2O3-TiO2 heterogeneous nanotubular photocatalysts for enhanced degradation of organic contaminants. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129233. [Google Scholar] [CrossRef]
- Dias, L.P.; Correia, F.C.; Ribeiro, J.M.; Tavares, C.J. Photocatalytic Bi2O3/TiO2:N Thin Films with Enhanced Surface Area and Visible Light Activity. Coatings 2020, 10, 445. [Google Scholar] [CrossRef]
- Jiang, Y.; Mofarah, S.S.; Koshy, P.; Chen, W.-F.; Fang, X.; Zheng, X.; Wang, D.; Sorrell, C.C. Na0.5Bi0.5TiO3 phase relations: Thermodynamics and phase equilibria in the systems Bi2O3–TiO2, Na2O–TiO2, and Na2O–Bi2O3–TiO2. J. Eur. Ceram. Soc. 2021, 41, 7005–7013. [Google Scholar] [CrossRef]
- He, R.; Liu, H.; Liu, H.; Xu, D.; Zhang, L. S-scheme photocatalyst Bi2O3/TiO2 nanofiber with improved photocatalytic performance. J. Mater. Sci. Technol. 2020, 52, 145–151. [Google Scholar] [CrossRef]
- Chen, J.; Tang, T.; Feng, W.; Liu, X.; Yin, Z.; Zhang, X.; Chen, J.; Cao, S. Large-Scale Synthesis of p–n Heterojunction Bi2O3/TiO2 Nanostructures as Photocatalysts for Removal of Antibiotics under Visible Light. ACS Appl. Nano Mater. 2021, 5, 1296–1307. [Google Scholar] [CrossRef]
- Li, J.-Q.; Zhou, Z.-W.; Li, X.; Yang, Y.-L.; Gao, J.-F.; Yu, R.; Wang, H.-P.; Wang, N. Synergistically boosting sulfamerazine degradation via activation of peroxydisulfate by photocatalysis of Bi2O3-TiO2/PAC under visible light irradiation. Chem. Eng. J. 2022, 428, 132613. [Google Scholar] [CrossRef]
- Qian, Y.; Shi, J.; Yang, X.; Yuan, Y.; Liu, L.; Zhou, G.; Yi, J.; Wang, X.; Wang, S. Integration of biochar into Ag3PO4/alpha-Fe2O3 heterojunction for enhanced reactive oxygen species generation towards organic pollutants removal. Environ. Pollut. 2022, 303, 119131. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Li, X.; Yang, Y.; Zhou, Z.; Shang, Y.; Zhuang, X.; Zhang, T. Two-stage calcination composite of Bi2O3-TiO2 supported on powdered activated carbon for enhanced degradation of sulfamethazine under solar irradiation. J. Water Process Eng. 2020, 35, 101220. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, H.; Li, S.; Wang, L.; Huang, F.; Guan, R.; Li, J.; Jiao, Y.; Sun, J. Rapidly degradation of di-(2-ethylhexyl) phthalate by Z-scheme Bi2O3/TiO2@reduced graphene oxide driven by simulated solar radiation. Chemosphere 2021, 272, 129631. [Google Scholar] [CrossRef]
- Wang, N.; Li, X.; Yang, Y.; Zhang, T.; Zhuang, X.; Ji, S.; Zhang, T.; Shang, Y.; Zhou, Z. Enhanced photocatalytic degradation of sulfamethazine by Bi-doped TiO2 nano-composites supported by powdered activated carbon under visible light irradiation. Sep. Purif. Technol. 2019, 211, 673–683. [Google Scholar] [CrossRef]
- Hu, Y.; Cao, Y.; Wang, P.; Li, D.; Chen, W.; He, Y.; Fu, X.; Shao, Y.; Zheng, Y. A new perspective for effect of Bi on the photocatalytic activity of Bi-doped TiO2. Appl. Catal. B Environ. 2012, 125, 294–303. [Google Scholar] [CrossRef]
- Liu, Y.; Xin, F.; Wang, F.; Luo, S.; Yin, X. Synthesis, characterization, and activities of visible light-driven Bi2O3–TiO2 composite photocatalysts. J. Alloys Compd. 2010, 498, 179–184. [Google Scholar] [CrossRef]
- Venkatalaxmi, A.; Padmavathi, B.S.; Amaranath, T. A general solution of unsteady Stokes equations. Fluid Dyn. Res. 2004, 35, 229–236. [Google Scholar] [CrossRef]
- Du, X.; Bai, X.; Xu, L.; Yang, L.; Jin, P. Visible-light activation of persulfate by TiO2/g-C3N4 photocatalyst toward efficient degradation of micropollutants. Chem. Eng. J. 2020, 384, 123245. [Google Scholar] [CrossRef]
- Wang, S.; Lei, C.; Liu, S.; Shen, R.; Feng, S.; Jin, Q. Selective photodegradation of malachite green in water by Bi2O3-doped TiO2 supported on molecularly imprinted powdered activated carbon. Mater. Lett. 2021, 304, 130692. [Google Scholar] [CrossRef]
- Lu, C.; Wu, Y.; Mai, F.; Chung, W.; Wu, C.; Lin, W.; Chen, C. Degradation efficiencies and mechanisms of the ZnO-mediated photocatalytic degradation of Basic Blue 11 under visible light irradiation. J. Mol. Catal. A Chem. 2009, 310, 159–165. [Google Scholar] [CrossRef]
- Evgenidou, E.; Chatzisalata, Z.; Tsevis, A.; Bourikas, K.; Torounidou, P.; Sergelidis, D.; Koltsakidou, A.; Lambropoulou, D.A. Photocatalytic degradation of a mixture of eight antibiotics using Cu-modified TiO2 photocatalysts: Kinetics, mineralization, antimicrobial activity elimination and disinfection. J. Environ. Chem. Eng. 2021, 9, 105295. [Google Scholar] [CrossRef]
- Irfan, M.; Nawaz, R.; Khan, J.A.; Ullah, H.; Haneef, T.; Legutko, S.; Rahman, S.; Józwik, J.; Alsaiari, M.A.; Khan, M.K.A.; et al. Synthesis and Characterization of Manganese-Modified Black TiO2 Nanoparticles and Their Performance Evaluation for the Photodegradation of Phenolic Compounds from Wastewater. Materials 2021, 14, 7422. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Hao, H.; Sheng, W.; Lang, X. Dye-TiO2/SiO2 assembly photocatalysis for blue light-initiated selective aerobic oxidation of organic sulfides. Chem. Eng. J. 2021, 423, 129419. [Google Scholar] [CrossRef]
- Yang, J.; Huang, Q.; Sun, Y.; An, G.; Li, X.; Mao, J.; Wei, C.; Yang, B.; Li, D.; Tao, T.; et al. Photocatalytic oxidation of formaldehyde under visible light using BiVO4-TiO2 synthesized via ultrasonic blending. Environ. Sci. Pollut. Res. 2024, 31, 30085–30098. [Google Scholar] [CrossRef]
- Paula, L.F.; Hofer, M.; Lacerda, V.P.B.; Bahnemann, D.W.; Patrocinio, A.O.T. Unraveling the photocatalytic properties of TiO2/WO3 mixed oxides. Photochem. Photobiol. Sci. 2020, 18, 2469–2483. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Su, Y.; Cheng, B.; Zhou, M. Effects of pH on the microstructures and photocatalytic activity of mesoporous nanocrystalline titania powders prepared via hydrothermal method. J. Mol. Catal. A Chem. 2006, 258, 104–112. [Google Scholar] [CrossRef]
- Lu, N.; Lu, Y.; Liu, F.; Zhao, K.; Yuan, X.; Zhao, Y.; Li, Y.; Qin, H.; Zhu, J. H3PW12O40/TiO2 catalyst-induced photodegradation of bisphenol A (BPA): Kinetics, toxicity and degradation pathways. Chemosphere 2013, 91, 1266–1272. [Google Scholar] [CrossRef]
- Li, K.; Dong, C.; Zhang, Y.; Wei, H.; Zhao, F.; Wang, Q. Ag–AgBr/CaWO4 composite microsphere as an efficient photocatalyst for degradation of Acid Red 18 under visible light irradiation: Affecting factors, kinetics and mechanism. J. Mol. Catal. A Chem. 2014, 394, 105–113. [Google Scholar] [CrossRef]
- Tang, T.; Yin, Z.; Chen, J.; Zhang, S.; Sheng, W.; Wei, W.; Xiao, Y.; Shi, Q.; Cao, S. Novel p-n heterojunction Bi2O3/Ti3+-TiO2 photocatalyst enables the complete removal of tetracyclines under visible light. Chem. Eng. J. 2021, 417, 128058. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, Y.; Sun, D.; Zhang, S.; Tang, T.; Zhang, X.; Cao, S. Bi2O3-Sensitized TiO2 Hollow Photocatalyst Drives the Efficient Removal of Tetracyclines under Visible Light. Inorg. Chem. 2020, 59, 18131–18140. [Google Scholar] [CrossRef]
- Barkouch, H.; Bessbousse, H.; Amar, M.; Bouzzine, S.M.; Hamidi, M.; El Mhammedi, M.A.; Alaoui, O.T. Bismuth-doped TiO2 enable solar photocatalytic water treatment. Opt. Mater. 2023, 146, 114507. [Google Scholar] [CrossRef]
- Bazmeh, A.; Fatehizadeh, A.; Bina, B.; Shoshtari-Yeganeh, B. Mechanism of oxidative decomposition of direct red 89 by Bi2O3/TiO2 composite under visible light irradiation: Effect of co-existing cations and anions and artificial neural network modeling of key factor. Desalination Water Treat. 2021, 212, 333–346. [Google Scholar] [CrossRef]
- Zeshan, M.; Bhatti, I.A.; Mohsin, M.; Iqbal, M.; Amjed, N.; Nisar, J.; AlMasoud, N.; Alomar, T.S. Remediation of pesticides using TiO2 based photocatalytic strategies: A review. Chemosphere 2022, 300, 134525. [Google Scholar] [CrossRef]
- Wang, M.; Li, C.; Liu, B.; Qin, W.; Xie, Y. Facile Synthesis of Nano-Flower beta-Bi2O3/TiO2 Heterojunction as Photocatalyst for Degradation RhB. Molecules 2023, 28, 882. [Google Scholar] [CrossRef] [PubMed]

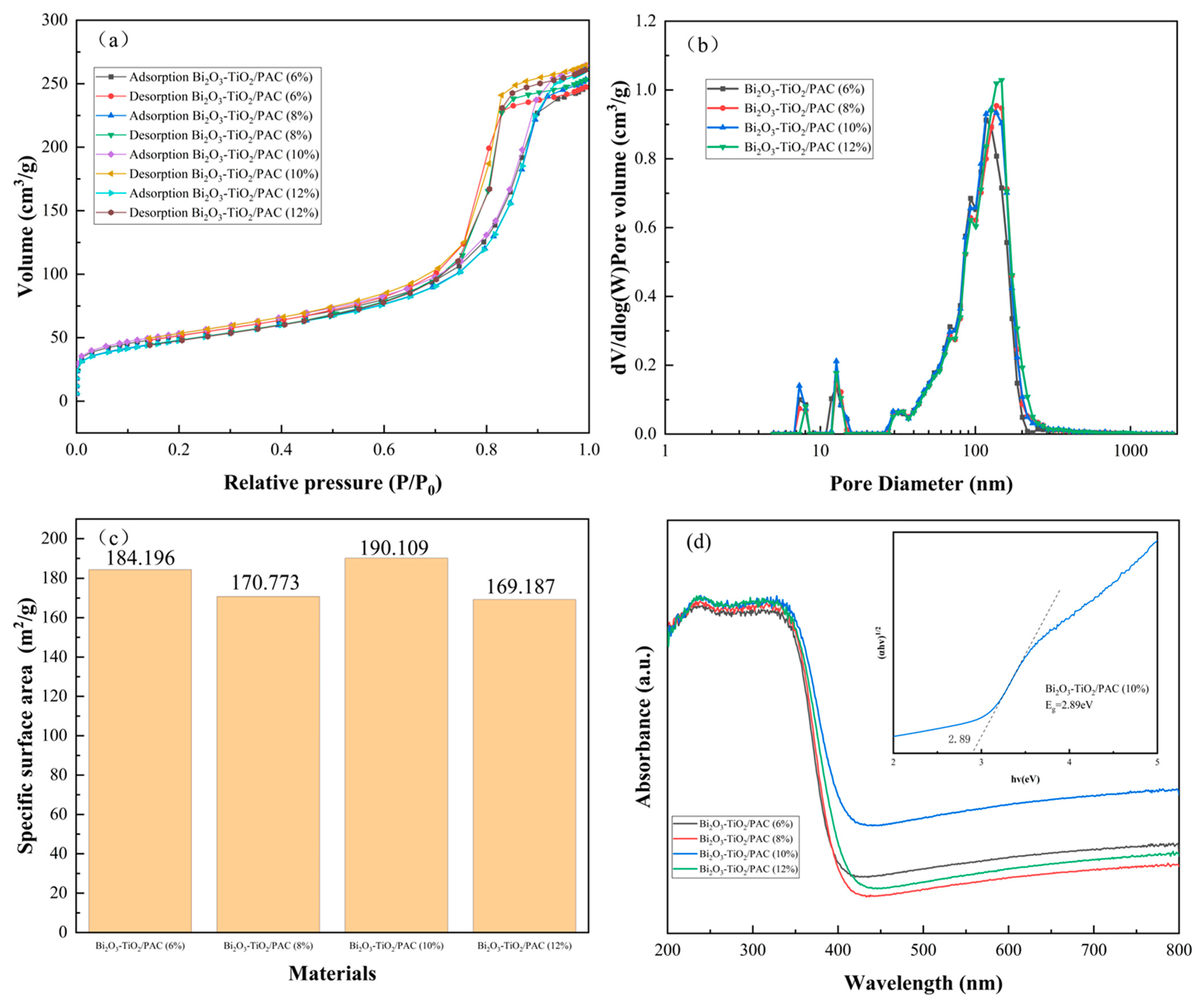



| Materials | Structure | Surface Area (Before–After/m2/g) | Bandgap Energy (Before–After/eV) | Pollutant | Degradation Efficiency |
|---|---|---|---|---|---|
| Bi2O3-TiO2/PAC | Mesopore | 84.56–190.1 | 3.2–2.89 | MG | 99% |
| Cu-TiO2 | Micropores | 43–46 | 3.08–2.78 | OC | 75% [31] |
| Mn-TiO2 | Mesopore | 3.20–2.21 | 3.20–2.21 | TPOME | 60.2% [32] |
| ZnO-TiO2 | Mesopore | 50.05–107.98 | 3.26–2.76 | TC | 92% [32] |
| TiO2-SiO2 | Mesopore | 217–256 | 3.22–3.22 | TC | 96% [33] |
| BiVO4-TiO2 | Mesopore | 60.6–95.3 | 3.2–3.03 | FA | 97% [34] |
| TiO2-WO3 | Mesopore | 95–117 | 3.0–2.6 | MB | 90% [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Cai, M.; Li, J.; Zhang, W. Study on Photocatalytic Performance of Bi2O3-TiO2/Powdered Activated Carbon Composite Catalyst for Malachite Green Degradation. Water 2025, 17, 1452. https://doi.org/10.3390/w17101452
Chen Y, Cai M, Li J, Zhang W. Study on Photocatalytic Performance of Bi2O3-TiO2/Powdered Activated Carbon Composite Catalyst for Malachite Green Degradation. Water. 2025; 17(10):1452. https://doi.org/10.3390/w17101452
Chicago/Turabian StyleChen, Yajun, Man Cai, Junfeng Li, and Wenshuo Zhang. 2025. "Study on Photocatalytic Performance of Bi2O3-TiO2/Powdered Activated Carbon Composite Catalyst for Malachite Green Degradation" Water 17, no. 10: 1452. https://doi.org/10.3390/w17101452
APA StyleChen, Y., Cai, M., Li, J., & Zhang, W. (2025). Study on Photocatalytic Performance of Bi2O3-TiO2/Powdered Activated Carbon Composite Catalyst for Malachite Green Degradation. Water, 17(10), 1452. https://doi.org/10.3390/w17101452






