Immobilization Technology of Aerobic Denitrifying Bacteria and Its Enhanced Biological Denitrification: A Review of Recent Advances
Abstract
1. Introduction
2. Current Research Status of Aerobic Denitrifying Bacteria
2.1. Isolation and Screening of Aerobic Denitrifying Bacteria
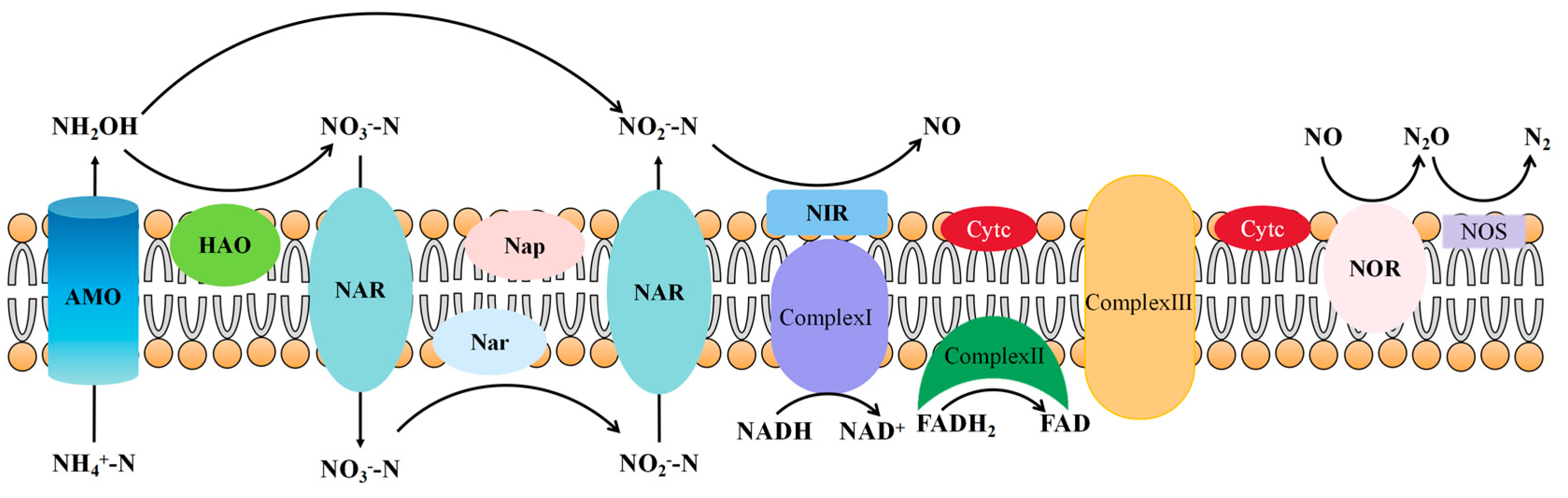
2.2. Key Enzymes and Genes of Aerobic Denitrifying Bacteria
2.3. Current Status of Application of Aerobic Denitrification Technology
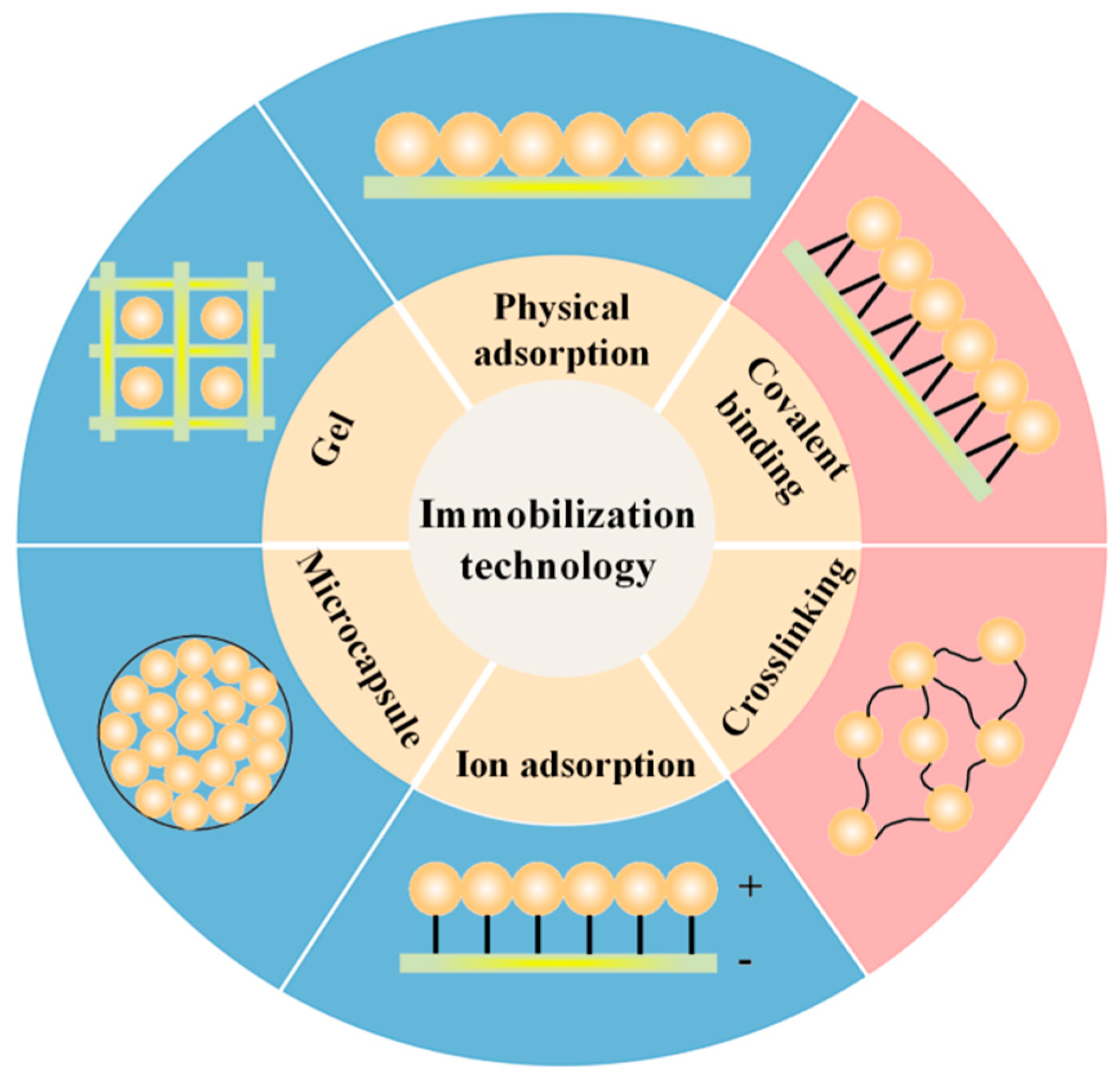
3. Current Status of Immobilized Aerobic Denitrification Technology
3.1. Traditional Immobilized Microbial Technology
3.1.1. Chemical Methods
3.1.2. Adsorption Method
3.1.3. Encapsulation Method
3.1.4. Composite Immobilization Method
3.2. Novel Microbial Immobilization Methods
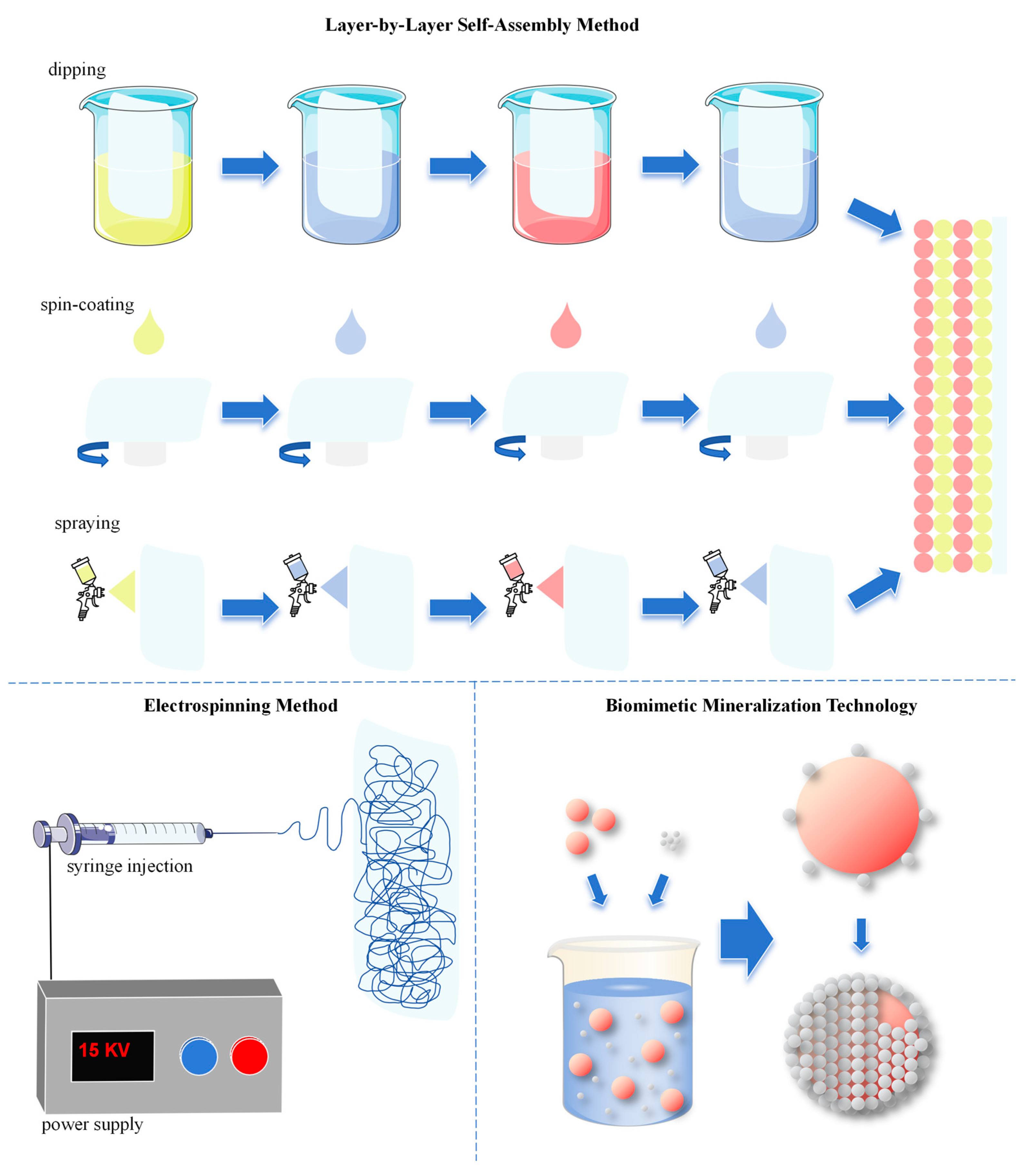
3.2.1. Layer-by-Layer Self-Assembly Method
3.2.2. Biomimetic Mineralization Technology
3.2.3. Electrospinning Method
3.3. Mechanism of Enhanced Biological Denitrification by Gel Entrapment Method
3.3.1. Providing Protection for Microorganisms
3.3.2. Accelerating Microbial Enrichment
3.3.3. Differences in Dissolved Oxygen Concentration Inside and Outside the Gel Beads
3.3.4. Providing Additional Functional Microorganisms and Nutrients
4. Current Status of Immobilized Aerobic Denitrifiers
5. Future Research
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, X.; Wang, X.; Zhang, H.; Wu, H. Enhanced Nitrogen Removal of Low C/N Domestic Wastewater Using a Biochar-Amended Aerated Vertical Flow Constructed Wetland. Bioresour. Technol. 2017, 241, 269–275. [Google Scholar] [CrossRef]
- Luo, G.; Xu, G.; Tan, H.; Gao, J.; Liu, W. Effect of Dissolved Oxygen On Denitrification Using Polycaprolactone as Both the Organic Carbon Source and the Biofilm Carrier. Int. Biodeterior. Biodegrad. 2016, 110, 155–162. [Google Scholar] [CrossRef]
- Rajta, A.; Bhatia, R.; Setia, H.; Pathania, P. Role of Heterotrophic AEROBIC denitrifying Bacteria in Nitrate Removal from Wastewater. J. Appl. Microbiol. 2020, 128, 1261–1278. [Google Scholar] [CrossRef]
- Ruan, Y.; Taherzadeh, M.J.; Kong, D.; Lu, H.; Zhao, H.; Xu, X.; Liu, Y.; Cai, L. Nitrogen Removal Performance and Metabolic Pathways Analysis of a Novel Aerobic Denitrifying Halotolerant Pseudomonas balearica Strain RAD-17. Microorganisms 2020, 8, 72. [Google Scholar] [CrossRef]
- Robertson, L.A.; Kuenen, J.G. Aerobic Denitrification—Old Wine in New Bottles? Antonie Van Leeuwenhoek 1984, 50, 525–544. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.; Sun, H.; Huang, W.; Zhao, Y.; Ma, Y.; Chen, W.; Sun, Y.; Chen, X.; Zhong, P.; Yang, H.; et al. Nitrogen Removal Characteristics and Potential Application of The Heterotrophic Nitrifying-Aerobic Denitrifying Bacteria Pseudomonas Mendocina S16 and Enterobacter Cloacae DS‘5 Isolated from Aquaculture Wastewater Ponds. Bioresour. Technol. 2022, 345, 126541. [Google Scholar] [CrossRef]
- Vinothkumar, R.; Dar, J.Y.; Bharti, V.S.; Singh, A.; Vennila, A.; Bhat, I.A.; Pandey, P.K. Heterotrophic Nitrifying and Aerobic Denitrifying Bacteria: Characterization and Comparison of Shrimp Pond and Effluent Discharge Channel in Aspects of Composition and Function. Aquaculture 2021, 539, 736659. [Google Scholar] [CrossRef]
- Hattori, T.; Furusaka, C. Chemical Activities of Escherichia coli Adsorbed on a Resin. Biochim. Et Biophys. Acta 1959, 31, 581–582. [Google Scholar] [CrossRef]
- Gao, D.; Chen, G.; Hou, Z.; Tao, H.; Du, X.; Liang, H. Effects of Modified Bentonites Immobilized Crude Enzymes on Soil Properties and Microbial Community of BaP Contaminated Soil. J. Environ. Chem. Eng. 2025, 13, 116574. [Google Scholar] [CrossRef]
- Hu, H.; Lu, W.; Li, S.; Zhou, X.; Zhu, C.; Wang, X.; Dai, H.; Geng, H. Hydrogel-Based Materials for Microbial/Enzyme Immobilization: Advanced Applications in Wastewater Treatment. Chem. Eng. J. 2025, 511, 161878. [Google Scholar] [CrossRef]
- Zeng, G.; Dai, J.; Jian, J.; Yan, C.; Peng, D.; Liu, H.; Xu, H. The Effect of Phosphate Solubilizing Bacteria on the Fate of Cadmium Immobilized by Microbial Induced Phosphate Precipitation. J. Environ. Manag. 2025, 380, 125125. [Google Scholar] [CrossRef]
- Hou, L.; Hu, K.; Huang, F.; Pan, Z.; Jia, X.; Liu, W.; Yao, X.; Yang, Z.; Tang, P.; Li, J. Advances in Immobilized Microbial Technology and Its Application to Wastewater Treatment: A Review. Bioresour. Technol. 2024, 413, 131518. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liang, J.; Sun, L.; Li, G.; Temmink, H.; Rijnaarts, H.H.M. Granule-Based Immobilization and Activity Enhancement of Anammox Biomass via PVA/CS and PVA/CS/Fe Gel Beads. Bioresour. Technol. 2020, 309, 123448. [Google Scholar] [CrossRef]
- Dong, H.; Wang, W.; Song, Z.; Dong, H.; Wang, J.; Sun, S.; Zhang, Z.; Ke, M.; Zhang, Z.; Wu, W.-M.; et al. A High-Efficiency Denitrification Bioreactor for the Treatment of Acrylonitrile Wastewater Using Waterborne Polyurethane Immobilized Activated Sludge. Bioresour. Technol. 2017, 239, 472–481. [Google Scholar] [CrossRef]
- Hou, L.-G.; Li, J.; Sun, F.-Y.; Zhang, X.-Y.; Liu, Y. High-Efficiency Denitrification for Steel Wastewater Treatment by Immobilized Bacteria. Desalination Water Treat. 2021, 211, 117–122. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, Z.; Hu, Y.; Song, C.; Li, F.; He, W.; Wang, X.; Li, Z.; Lin, H. Immobilization of Nitrifying Bacteria in Magnetic PVA–SA-Diatomite Carrier for Efficient Removal of NH4+-N from Effluents. Environ. Technol. Innov. 2021, 22, 101407. [Google Scholar] [CrossRef]
- Manonmani, U.; Joseph, K. Granulation of Anammox Microorganisms for Autotrophic Nitrogen Removal from Wastewater. Environ. Chem. Lett. 2018, 16, 881–901. [Google Scholar] [CrossRef]
- Song, Z.F.; An, J.; Fu, G.H.; Yang, X.L. Isolation and Characterization of an Aerobic Denitrifying Bacillus sp. YX-6 from Shrimp Culture ponds. Aquaculture 2011, 319, 188–193. [Google Scholar] [CrossRef]
- Zhang, Q.-L.; Liu, Y.; Ai, G.-M.; Miao, L.-L.; Zheng, H.-Y.; Liu, Z.-P. The Characteristics of a Novel Heterotrophic Nitrification–Aerobic Denitrification Bacterium, Bacillus Methylotrophicus Strain L7. Bioresour. Technol. 2012, 108, 35–44. [Google Scholar] [CrossRef]
- Chen, P.; Li, J.; Li, Q.X.; Wang, Y.; Li, S.; Ren, T.; Wang, L. Simultaneous Heterotrophic Nitrification and Aerobic Denitrification by Bacterium Rhodococcus sp. CPZ24. Bioresour. Technol. 2012, 116, 266–270. [Google Scholar] [CrossRef]
- Guo, Y.; Zhou, X.; Li, Y.; Li, K.; Wang, C.; Liu, J.; Yan, D.; Liu, Y.; Yang, D.; Xing, J. Heterotrophic Nitrification and Aerobic Denitrification by a Novel Halomonas Campisalis. Biotechnol. Lett. 2013, 35, 2045–2049. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhang, Y.; Zhou, J.; Chen, M.; Wang, X. Biological Removal of Nitrate and Ammonium under Aerobic Atmosphere by Paracoccus Versutus LYM. Bioresour. Technol. 2013, 148, 144–148. [Google Scholar] [CrossRef]
- Huang, X.; Li, W.; Zhang, D.; Qin, W. Ammonium Removal by a Novel Oligotrophic Acinetobacter sp. Y16 Capable of Heterotrophic Nitrification–Aerobic Denitrification at Low Temperature. Bioresour. Technol. 2013, 146, 44–50. [Google Scholar] [CrossRef]
- Kundu, P.; Pramanik, A.; Dasgupta, A.; Mukherjee, S.; Mukherjee, J. Simultaneous Heterotrophic Nitrification and Aerobic Denitrification by Chryseobacterium sp. R31 Isolated from Abattoir Wastewater. BioMed Res. Int. 2014, 2014, 436056. [Google Scholar] [CrossRef]
- Chen, M.; Wang, W.; Feng, Y.; Zhu, X.; Zhou, H.; Tan, Z.; Li, X. Impact Resistance of Different Factors on Ammonia Removal by Heterotrophic Nitrification–Aerobic Denitrification Bacterium Aeromonas sp. HN-02. Bioresour. Technol. 2014, 167, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Li, Y.; An, H.; Lv, Y. Nitrogen Removal Characteristics of Heterotrophic Nitrification-Aerobic Denitrification by Alcaligenes Faecalis C16. Chin. J. Chem. Eng. 2015, 23, 827–834. [Google Scholar] [CrossRef]
- Ge, Q.; Yue, X.; Wang, G. Simultaneous Heterotrophic Nitrification and Aerobic Denitrification at High Initial Phenol Concentration by Isolated Bacterium Diaphorobacter sp. PD-7. Chin. J. Chem. Eng. 2015, 23, 835–841. [Google Scholar] [CrossRef]
- He, D.; Zheng, M.; Ma, T.; Li, C.; Ni, J. Interaction of Cr(VI) Reduction and Denitrification by Strain Pseudomonas aeruginosa PCN-2 Under Aerobic Conditions. Bioresour. Technol. 2015, 185, 346–352. [Google Scholar] [CrossRef]
- Sun, Z.; Lv, Y.; Liu, Y.; Ren, R. Removal of Nitrogen by Heterotrophic Nitrification-Aerobic Denitrification of a Novel Metal Resistant Bacterium Cupriavidus sp. S1. Bioresour. Technol. 2016, 220, 142–150. [Google Scholar] [CrossRef]
- He, T.; Li, Z.; Sun, Q.; Xu, Y.; Ye, Q. Heterotrophic Nitrification and Aerobic Denitrification by Pseudomonas Tolaasii Y-11 without Nitrite Accumulation During Nitrogen Conversion. Bioresour. Technol. 2016, 200, 493–499. [Google Scholar] [CrossRef]
- Su, J.f.; Lian, T.t.; Huang, T.l.; Liang, D.h.; Ma, M.; Lu, J.s. Microcystis Aeruginosa Flour as Carbon and Nitrogen Source for Aerobic Denitrification and Algicidal Effect of Raoultella sp. R11. Ecol. Eng. 2017, 105, 162–169. [Google Scholar] [CrossRef]
- Su, J.f.; Shi, J.x.; Ma, F. Aerobic Denitrification and Biomineralization by a Novel Heterotrophic Bacterium, Acinetobacter sp. H36. Mar. Pollut. Bull. 2017, 116, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; He, T.; Li, Z.; Ye, Q.; Chen, Y.; Xie, E.; Zhang, X. Nitrogen Removal Characteristics of Pseudomonas putida Y-9 Capable of Heterotrophic Nitrification and Aerobic Denitrification at Low Temperature. BioMed Res. Int. 2017, 2017, 1429018. [Google Scholar] [CrossRef]
- Zhao, B.; Cheng, D.Y.; Tan, P.; An, Q.; Guo, J.S. Characterization of an Aerobic Denitrifier Pseudomonas Stutzeri Strain XL-2 to Achieve Efficient Nitrate Removal. Bioresour. Technol. 2018, 250, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, X.-H.; Cui, S.; Ren, Y.-X.; Yu, J.; Chen, N.; Xiao, Q.; Guo, L.-K.; Wang, R.-H. Simultaneous Removal of Nitrogen and Phosphorous by Heterotrophic Nitrification-Aerobic Denitrification of a Metal Resistant Bacterium Pseudomonas Putida Strain NP5. Bioresour. Technol. 2019, 285, 121360. [Google Scholar] [CrossRef]
- Wen, G.; Wang, T.; Li, K.; Wang, H.; Wang, J.; Huang, T. Aerobic Denitrification Performance of Strain Acinetobacter johnsonii WGX-9 Using Different Natural Organic Matter as Carbon Source: Effect of Molecular Weight. Water Res. 2019, 164, 114956. [Google Scholar] [CrossRef]
- Chen, S.; He, S.; Wu, C.; Du, D. Characteristics of Heterotrophic Nitrification and Aerobic Denitrification Bacterium Acinetobacter sp. T1 and Its Application for Pig Farm Wastewater Treatment. J. Biosci. Bioeng. 2019, 127, 201–205. [Google Scholar] [CrossRef]
- Lu, Z.; Gan, L.; Lin, J.; Chen, Z. Aerobic Denitrification by Paracoccus sp. YF1 in the Presence of Cu(II). Sci. Total Environ. 2019, 658, 80–86. [Google Scholar] [CrossRef]
- Yang, J.-R.; Wang, Y.; Chen, H.; Lyu, Y.K. Ammonium Removal Characteristics of an Acid-Resistant Bacterium Acinetobacter sp. Jr1 from Pharmaceutical Wastewater Capable of Heterotrophic Nitrification-Aerobic Denitrification. Bioresour. Technol. 2019, 274, 56–64. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Zhang, Y.; Sun, Z.; Zhang, J.; Chen, G.; Li, J. Simultaneous Nitrification and Denitrification by A Novel Isolated Pseudomonas sp. JQ-H3 Using Polycaprolactone as Carbon Source. Bioresour. Technol. 2019, 288, 121506. [Google Scholar] [CrossRef]
- Lang, X.; Li, Q.; Ji, M.; Yan, G.; Guo, S. Isolation and Niche Characteristics in Simultaneous Nitrification and Denitrification Application of an Aerobic Denitrifier, Acinetobacter sp. YS2. Bioresour. Technol. 2020, 302, 122799. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Liao, M.; Liang, Y.; Guo, J.; Zhang, Y.; Xie, X.; Fan, Q.; Zhu, Y. Biological Nitrogen Removal Capability and Pathways Analysis of a Novel Low C/N Ratio Heterotrophic Nitrifying and Aerobic Denitrifying Bacterium (Bacillus thuringiensis Strain WXN-23). Environ. Res. 2021, 195, 110797. [Google Scholar] [CrossRef]
- Wang, F.; Li, L.; Li, X.; Hu, X.; Zhang, B. Pulsed Electric Field Promotes the Growth Metabolism of Aerobic Denitrifying Bacteria Pseudomonas Putida W207-14 by Improving Cell Membrane Permeability. Environ. Technol. 2023, 44, 2327–2340. [Google Scholar] [CrossRef]
- Yang, X.; Wan, Q.; Wu, D.; Wang, J.; Abbas, T.; Zhang, Q. The Impact of Novel Azotobacter Bacillus Sp. T28 Combined Sea Buckthorn Pomace on Microbial Community Structure in Paddy Soil. Environ. Res. 2023, 224, 115548. [Google Scholar] [CrossRef]
- Chen, P.; Chen, D.; Yang, B.; Zhang, K.; Li, S. Enhancing the Sewage Treatment Effect, Reducing Membrane Fouling, and Increasing Microbial Community Diversity by a Novel Biofiller-Carrying Functional Bacteria Rhodococcus Sp. Cpz24 in a Moving Bed Biofilm Reactor. J. Water Process Eng. 2023, 56, 104576. [Google Scholar] [CrossRef]
- Chen, P.; Wang, J.; Lv, J.; Wang, Q.; Zhang, C.; Zhao, W.; Li, S. Nitrogen Removal by Rhodococcus Sp. Sy24 under Linear Alkylbenzene Sulphonate Stress: Carbon Source Metabolism Activity, Kinetics, and Optimum Culture Conditions. Bioresour. Technol. 2023, 368, 128348. [Google Scholar] [CrossRef] [PubMed]
- Huan, C.; Lyu, Q.; Wang, Z.; Tian, X.; Yan, Z.; Ji, G. Conversion Behavior of Heterotrophic Nitrification—Aerobic Denitrification Bacterium Paracoccus denitrificans Hy-1 in Nitrogen and Phosphorus Removal. J. Water Process Eng. 2024, 62, 105347. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Q.; Wang, Y.; Zhan, W.; Wu, Z.; Zhou, H.; Cheng, H.; Chen, Z. Effects of Cd(Ii) on Nitrogen Removal by a Heterotrophic Nitrification Aerobic Denitrification Bacterium Pseudomonas Sp. Xf-4. Ecotoxicol. Environ. Saf. 2024, 280, 116588. [Google Scholar] [CrossRef]
- Xiang, Z.; Xu, Y.; Dong, W.; Zhao, Y.; Chen, X. Effects of Sliver Nanoparticles on Nitrogen Removal by the Heterotrophic Nitrification-Aerobic Denitrification Bacteria Zobellella Sp. B307 and Their Toxicity Mechanisms. Mar. Pollut. Bull. 2024, 203, 116381. [Google Scholar] [CrossRef]
- Yan, W.; Wang, N.; Wang, Z.; Shi, J.; Tang, T.; Liu, L. Nitrogen Removal Characteristics and Mechanism of the Aerobic Denitrifying Bacterium Stutzerimonas Stutzeri Os3 Isolated from Shrimp Aquaculture Sediment. Mar. Pollut. Bull. 2025, 214, 117711. [Google Scholar] [CrossRef]
- Wei, X.; Li, S.; Li, C.; Liao, J.; Yang, Y.; He, Z.; Dong, K.; Lee, S.-S. Characterization and Genomic Insights into the Nitrogen Metabolism of Heterotrophic Nitrifying and Aerobic Denitrifying Bacterium Pseudomonas Aeruginosa Ws-03. J. Environ. Manag. 2025, 376, 124405. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Yao, T.; Liu, X.; Zhang, A.; Zhang, J.; Pang, L. Efficient Heterotrophic Nitrification—Aerobic Denitrification by a Novel Bacterium Ralstonia Pickettii J4: Isolation, Identification, and Application. Biochem. Eng. J. 2024, 210, 109417. [Google Scholar] [CrossRef]
- Chang, F.; Liu, W.; Fan, X.; Yu, C.; Liu, H.; Li, Q. Characteristics of Aerobic Denitrification and Ammonia Assimilation for Nitrogen Removal by Paracoccus binzhouensis Wg1. J. Environ. Chem. Eng. 2025, 13, 116529. [Google Scholar] [CrossRef]
- Wang, F.-R.; Feng, S.-Y.; Liang, S.; Du, W.-Y.; Wang, L.-Q.; Zhang, Y.-W.; Ren, J.-Y.; Gao, S.; Zhu, Y.-J.; Cong, Y.-T.; et al. Rapid and Efficient Nitrogen Removal by a Novel Heterotrophic Nitrification-Aerobic Denitrification Bacteria Marinobacterium Maritimum 5-Js in Aquaculture Wastewater: Performance and Potential Applications. Environ. Res. 2025, 276, 121500. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Li, K.; He, T.; Wang, Y.; Zhang, X.; Xie, E.; Ding, N.; Li, Z. Characteristics of Heterotrophic Nitrifying and Aerobic Denitrifying Arthrobacter Nicotianae D51 Strain in the Presence of Copper. Water 2019, 11, 434. [Google Scholar] [CrossRef]
- Ren, J.; Ma, H.; Liu, Y.; Ruan, Y.; Wei, C.; Song, J.; Wu, Y.; Han, R. Characterization of a Novel Marine Aerobic Denitrifier Vibrio Spp. Ad2 for Efficient Nitrate Reduction without Nitrite Accumulation. Environ. Sci. Pollut. Res. 2021, 28, 30807–30820. [Google Scholar] [CrossRef]
- Takeo, M.; Ohtaki, S.; Ishizawa, H. Complete Genome Sequence of Hydroquinonesulfonate-Assimilating Bacterium, Delftia Lacustris Hqs1. Microbiol. Resour. Announc. 2024, 14, e00371-24. [Google Scholar] [CrossRef]
- Yang, L.; Lu, H.; Wang, Y.; Liu, Y.; Tu, L.; Meng, H.; Ren, Y.; Lan, J. Nitrogen Removal Characteristics and Cr(Vi) Tolerance Mechanisms of Heterotrophic Nitrifying Bacterium Pseudomonas Putida Strain Lx1. J. Water Process Eng. 2024, 64, 105647. [Google Scholar] [CrossRef]
- Rusmana, I.; Nedwell, D.B. Use of Chlorate as a Selective Inhibitor to Distinguish Membrane-Bound Nitrate Reductase (Nar) and Periplasmic Nitrate Reductase (Nap) of Dissimilative Nitrate Reducing Bacteria in Sediment. FEMS Microbiol. Ecol. 2004, 48, 379–386. [Google Scholar] [CrossRef]
- Chen, Q.; Ni, J. Ammonium Removal by Agrobacterium Sp. Lad9 Capable of Heterotrophic Nitrification–Aerobic Denitrification. J. Biosci. Bioeng. 2012, 113, 619–623. [Google Scholar] [CrossRef]
- Duarte, A.G.; Cordas, C.M.; Moura, J.J.G.; Moura, I. Steady-State Kinetics with Nitric Oxide Reductase (nor): New Considerations on Substrate Inhibition Profile and Catalytic Mechanism. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2014, 1837, 375–384. [Google Scholar] [CrossRef]
- Zheng, M. Nitrogen Removal Characteristics of Aerobic Denitrifying Bacteria and Their Applications in Nitrogen Oxides Emission Mitigation; Springer: Singapore, 2018. [Google Scholar]
- AbdelGawwad, M.R.; Mahmutović, E.; Al Farraj, D.A.; Elshikh, M.S. In Silico Prediction of Silver Nitrate Nanoparticles and Nitrate Reductase a (Nar a) Interaction in the Treatment of Infectious Disease Causing Clinical Strains of E. coli. J. Infect. Public Health 2020, 13, 1580–1585. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wei, Y.; Lu, J.; Yin, D.; Liang, Y.; Li, J.; Xiao, J.; Mo, Z.; Yi, H.; Zhang, H.; et al. Heterologous Expression, Enzymatic Properties, Product Analysis and Molecular Docking of Assimilative Nitrite Reductase (Nir) in Bacillus Velezensis Gxmzu-B1 Derived from Mariculture. Int. J. Biol. Macromol. 2025, 291, 139047. [Google Scholar] [CrossRef] [PubMed]
- Carneiro Fidélis Silva, L.; Santiago Lima, H.; Antônio de Oliveira Mendes, T.; Sartoratto, A.; de Paula Sousa, M.; Suhett de Souza, R.; Oliveira de Paula, S.; Maia de Oliveira, V.; Canêdo da Silva, C. Heterotrophic Nitrifying/Aerobic Denitrifying Bacteria: Ammonium Removal under Different Physical-Chemical Conditions and Molecular Characterization. J. Environ. Manag. 2019, 248, 109294. [Google Scholar] [CrossRef]
- Nittami, T.; Magura, T.; Imai, Y.; Matsumoto, K. Influence of the Electron Acceptor on Nitrite Reductase Gene (Nir) Diversity in an Activated Sludge Community. J. Biosci. Bioeng. 2009, 108, 394–399. [Google Scholar] [CrossRef]
- Su, J.J.; Liu, B.Y.; Liu, C.Y. Comparison of Aerobic Denitrification under High Oxygen Atmosphere by Thiosphaera Pantotropha Atcc 35512 and Pseudomonas Stutzeri Su2 Newly Isolated from the Activated Sludge of a Piggery Wastewater Treatment System. J. Appl. Microbiol. 2001, 90, 457–462. [Google Scholar] [CrossRef]
- Fujiwara, T.; Fukumori, Y. Cytochrome cb-type nitric oxide reductase with cytochrome c oxidase activity from Paracoccus denitrificans ATCC 35512. J. Bacteriol. 1996, 178, 1866–1871. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Simon, J.; Einsle, O.; Kroneck, P.M.H.; Zumft, W.G. The Unprecedented Nos Gene Cluster of Wolinella Succinogenes Encodes a Novel Respiratory Electron Transfer Pathway to Cytochrome C Nitrous Oxide Reductase. FEBS Lett. 2004, 569, 7–12. [Google Scholar] [CrossRef]
- McGuirl, M.A.; Nelson, L.K.; Bollinger, J.A.; Chan, Y.-K.; Dooley, D.M. The Nos (Nitrous Oxide Reductase) Gene Cluster from the Soil Bacterium Achromobacter Cycloclastes: Cloning, Sequence Analysis, and Expression. J. Inorg. Biochem. 1998, 70, 155–169. [Google Scholar] [CrossRef]
- Chen, Q.; Ni, J. Heterotrophic Nitrification–Aerobic Denitrification by Novel Isolated Bacteria. J. Ind. Microbiol. Biotechnol. 2011, 38, 1305–1310. [Google Scholar] [CrossRef]
- Ferousi, C.; Schmitz, R.A.; Maalcke, W.J.; Lindhoud, S.; Versantvoort, W.; Jetten, M.S.M.; Reimann, J.; Kartal, B. Characterization of a Nitrite-Reducing Octaheme Hydroxylamine Oxidoreductase That Lacks the Tyrosine Cross-Link. J. Biol. Chem. 2021, 296, 100476. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.-W.; Liu, J.-F.; Gu, J.-D.; Mu, B.-Z. Nitrate-Reducing Community in Production Water of Three Oil Reservoirs and Their Responses to Different Carbon Sources Revealed by Nitrate-Reductase Encoding Gene (Napa). Int. Biodeterior. Biodegrad. 2011, 65, 1081–1086. [Google Scholar] [CrossRef]
- Klotz, M.G.; Norton, J.M. Multiple Copies of Ammonia Monooxygenase (Amo) Operons Have Evolved under Biased at/Gc Mutational Pressure in Ammonia-Oxidizing Autotrophic Bacteria. FEMS Microbiol. Lett. 1998, 168, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; He, D.; Xue, Z. Removal of Nitrogen and Phosphorus by Heterotrophic Nitrification-Aerobic Denitrification of a Denitrifying Phosphorus-Accumulating Bacterium Enterobacter Cloacae Hw-15. Ecol. Eng. 2017, 99, 199–208. [Google Scholar] [CrossRef]
- Deng, M.; Zhao, X.; Senbati, Y.; Song, K.; He, X. Nitrogen Removal by Heterotrophic Nitrifying and Aerobic Denitrifying Bacterium Pseudomonas Sp. Dm02: Removal Performance, Mechanism and Immobilized Application for Real Aquaculture Wastewater Treatment. Bioresour. Technol. 2021, 322, 124555. [Google Scholar] [CrossRef]
- Chen, Q.; Ni, J.; Ma, T.; Liu, T.; Zheng, M. Bioaugmentation Treatment of Municipal Wastewater with Heterotrophic-Aerobic Nitrogen Removal Bacteria in a Pilot-Scale Sbr. Bioresour. Technol. 2015, 183, 25–32. [Google Scholar] [CrossRef]
- Ma, B.; Zhang, H.; Ma, M.; Huang, T.; Guo, H.; Yang, W.; Huang, Y.; Liu, X.; Li, H. Nitrogen Removal by Two Strains of Aerobic Denitrification Actinomycetes: Denitrification Capacity, Carbon Source Metabolic Ability, and Raw Water Treatment. Bioresour. Technol. 2022, 344, 126176. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Y.; Han, Y.; Zhang, Y.; Jia, X.; Li, W.; Li, D.; Jing, L. Nitrate Removal from Low C/N Wastewater at Low Temperature by Immobilized Pseudomonas Sp. Y39-6 with Versatile Nitrate Metabolism Pathways. Bioresour. Technol. 2021, 326, 124794. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Wei, M.; Chen, J.; Liu, H.; Kou, M. Comparative Study of the Adsorption/Immobilization of Cu by Turmeric Residues after Microbial and Chemical Extraction. Sci. Total Environ. 2019, 691, 1082–1088. [Google Scholar] [CrossRef]
- Ahmad, M.; Ok, Y.S.; Rajapaksha, A.U.; Lim, J.E.; Kim, B.-Y.; Ahn, J.-H.; Lee, Y.H.; Al-Wabel, M.I.; Lee, S.-E.; Lee, S.S. Lead and Copper Immobilization in a Shooting Range Soil Using Soybean Stover- and Pine Needle-Derived Biochars: Chemical, Microbial and Spectroscopic Assessments. J. Hazard. Mater. 2016, 301, 179–186. [Google Scholar] [CrossRef]
- Alamsyah, G.; Albels, V.A.; Sahlan, M.; Hermansyah, H. Effect of Chitosan’s Amino Group in Adsorption-Crosslinking Immobilization of Lipase Enzyme on Resin to Catalyze Biodiesel Synthesis. Energy Procedia 2017, 136, 47–52. [Google Scholar] [CrossRef]
- Rong, Q.; Lu, D.; Zhong, K.; Yang, S.; Li, Z.; Zhang, C. Mechanism of Antimony Oxidation and Adsorption Using Immobilized Klebsiella Aerogenes Hc10 in Soil. Sci. Total Environ. 2024, 956, 177404. [Google Scholar] [CrossRef]
- An, Q.; Jin, N.; Deng, S.; Zhao, B.; Liu, M.; Ran, B.; Zhang, L. Ni(Ii), Cr(Vi), Cu(Ii) and Nitrate Removal by the Co-System of Pseudomonas Hibiscicola Strain L1 Immobilized on Peanut Shell Biochar. Sci. Total Environ. 2022, 814, 152635. [Google Scholar] [CrossRef] [PubMed]
- Zvulunov, Y.; Ben-Barak-Zelas, Z.; Fishman, A.; Radian, A. A Self-Regenerating Clay-Polymer-Bacteria Composite for Formaldehyde Removal from Water. Chem. Eng. J. 2019, 374, 1275–1285. [Google Scholar] [CrossRef]
- Baigorria, E.; Cano, L.A.; Sanchez, L.M.; Alvarez, V.A.; Ollier, R.P. Bentonite-Composite Polyvinyl Alcohol/Alginate Hydrogel Beads: Preparation, Characterization and Their Use as Arsenic Removal Devices. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100364. [Google Scholar] [CrossRef]
- Wen, Y.; Xu, X.; Wang, B.; He, Z.; Bai, J.; Chen, X.; Cui, J.; Xu, X. Pahs Biodegradation in Soil Washing Effluent by Native Mixed Bacteria Embedded in Polyvinyl Alcohol-Sodium Alginate-Nano Alumina Gel Beads. J. Environ. Manag. 2021, 297, 113415. [Google Scholar] [CrossRef]
- Sun, Y.; Ali, A.; Zheng, Z.; Su, J.; Zhang, S.; Min, Y.; Liu, Y. Denitrifying Bacteria Immobilized Magnetic Mycelium Pellets Bioreactor: A New Technology for Efficient Removal of Nitrate at a Low Carbon-to-Nitrogen Ratio. Bioresour. Technol. 2022, 347, 126369. [Google Scholar] [CrossRef]
- Ahmed, H.A.M.; Mohmmed, R. Micro/Nano Encapsulation Methods of Bioactive Materials Controlled Release Using Chitosan for Functionalization of Textiles Substrates: Review. Carbohydr. Polym. Technol. Appl. 2025, 100704. [Google Scholar] [CrossRef]
- Muthulakshmi, L.; Mohan, S.; Kanthimathi, G.; Rajaram, R. Immobilization of Eps-Modified Sodium Alginate Microcapsule by Co-Polymerization for Methylene Blue Dye Adsorption and Kinetics. Total Environ. Adv. 2024, 11, 200109. [Google Scholar] [CrossRef]
- Cui, J.; Li, T.; Zhao, Y.; Yuan, C.; Dong, D.; Li, J.; Zhao, M.; Cui, B. Enhancement in Stability and Reusability: Immobilization and Characterization of Inulin Fructotransferase from Paenarthrobacter Aurescens Ql1.001 in Calcium Alginate Microcapsules. Food Biosci. 2025, 64, 105815. [Google Scholar] [CrossRef]
- Mollaei, M.; Abdollahpour, S.; Atashgahi, S.; Abbasi, H.; Masoomi, F.; Rad, I.; Lotfi, A.S.; Zahiri, H.S.; Vali, H.; Noghabi, K.A. Enhanced Phenol Degradation by Pseudomonas Sp. Sa01: Gaining Insight into the Novel Single and Hybrid Immobilizations. J. Hazard. Mater. 2010, 175, 284–292. [Google Scholar] [CrossRef]
- Liu, C.; Yu, D.; Wang, Y.; Chen, G.; Tang, P.; Huang, S. A Novel Control Strategy for the Partial Nitrification and Anammox Process (Pn/a) of Immobilized Particles: Using Salinity as a Factor. Bioresour. Technol. 2020, 302, 122864. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.O.; Park, J.K.; Kim, J.H.; Jin, S.G.; Yong, C.S.; Li, D.X.; Choi, J.Y.; Woo, J.S.; Yoo, B.K.; Lyoo, W.S.; et al. Development of Polyvinyl Alcohol–Sodium Alginate Gel-Matrix-Based Wound Dressing System Containing Nitrofurazone. Int. J. Pharm. 2008, 359, 79–86. [Google Scholar] [CrossRef]
- Xie, S.; Wei, H.; Xu, L.; Pu, K.; Li, X.; Su, J. Sludge Reduction Using Polyvinyl Alcohol-Sodium Alginate (Pva/Sa)-Immobilized Refined Iron Ore and Microorganisms: Optimization and Mechanism. Chem. Eng. J. 2025, 510, 161864. [Google Scholar] [CrossRef]
- Wang, W.; Ding, Y.; Wang, Y.; Song, X.; Ambrose, R.F.; Ullman, J.L.; Winfrey, B.K.; Wang, J.; Gong, J. Treatment of Rich Ammonia Nitrogen Wastewater with Polyvinyl Alcohol Immobilized Nitrifier Biofortified Constructed Wetlands. Ecol. Eng. 2016, 94, 7–11. [Google Scholar] [CrossRef]
- Xu, X.; Jin, Z.; Wang, B.; Lv, C.; Hu, B.; Shi, D. Treatment of High-Strength Ammonium Wastewater by Polyvinyl Alcohol–Sodium Alginate Immobilization of Activated Sludge. Process Biochem. 2017, 63, 214–220. [Google Scholar] [CrossRef]
- Zheng, Z.; Ali, A.; Su, J.; Huang, T.; Wang, Y.; Zhang, S. Fungal Pellets Immobilized Bacterial Bioreactor for Efficient Nitrate Removal at Low C/N Wastewater. Bioresour. Technol. 2021, 332, 125113. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Su, J.; Huang, T.; Li, G.; Ali, A.; Shi, J. Simultaneous Removal of Nitrate and Diethyl Phthalate Using a Novel Sponge–Based Biocarrier Combined Modified Walnut Shell Biochar with Fe3o4 in the Immobilized Bioreactor. J. Hazard. Mater. 2021, 414, 125578. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhang, Y.; Tu, B. Immobilization of Ammonia-Oxidizing Bacteria by Polyvinyl Alcohol and Sodium Alginate. Braz. J. Microbiol. 2017, 48, 515–521. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, J.; Zhang, H.; Zheng, C.; Wei, R.; Gao, Y.; Yang, L. Efficient Nitrate Removal by Pseudomonas Mendocina Gl6 Immobilized on Biochar. Bioresour. Technol. 2021, 320, 124324. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, Y.; Chen, J.; Zhang, L.; Wu, W.; Li, J.; Liu, Y.; Gai, T.; Yu, J.; Zhang, H. Important Roles of Immobilization in Improving Low-Temperature Nitrogen Removal of Cold-Shock Anammox Sludge and Related Mechanism. Environ. Technol. Innov. 2025, 38, 104170. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, D.; Xiong, W.; Wu, Z.; Xiao, G.; Wang, S.; Su, H. Enhancing Nitrogen Removal Performance Using Immobilized Aerobic Denitrifying Bacteria by Modified Polyvinyl Alcohol/Sodium Alginate (Pva/Sa). Chemosphere 2024, 357, 141954. [Google Scholar] [CrossRef]
- Shen, J.-n.; Li, D.-d.; Jiang, F.-y.; Qiu, J.-h.; Gao, C.-j. Purification and Concentration of Collagen by Charged Ultrafiltration Membrane of Hydrophilic Polyacrylonitrile Blend. Sep. Purif. Technol. 2009, 66, 257–262. [Google Scholar] [CrossRef]
- Liu, H.; Tian, F.; Lei, L.; Zhang, C.; Bai, Y.; Zhao, Y.; Dong, L. Metal-Incorporated Covalent Organic Framework Membranes Via Layer-by-Layer Self-Assembly for Efficient Antibiotic Desalination. Desalination 2025, 600, 118537. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.-Q.; Huang, F.; Tu, C.; Cui, l. Layer by Layer Self-Assembly Mos2/Zif-8 Composites on Carboxyl Cotton Fabric for Enhanced Visible Light Photocatalysis and Recyclability. Appl. Surf. Sci. 2021, 565, 150458. [Google Scholar] [CrossRef]
- Li, S.; Sun, J.; Yan, J.; Zhang, S.; Shi, C.; McClements, D.J.; Liu, X.; Liu, F. Development of Antibacterial Nanoemulsions Incorporating Thyme Oil: Layer-by-Layer Self-Assembly of Whey Protein Isolate and Chitosan Hydrochloride. Food Chem. 2021, 339, 128016. [Google Scholar] [CrossRef]
- Yao, Z.; Xia, A.; Zhang, K.; Yu, M.; Wang, D.; Xiao, W.; Liu, W.; Wang, J. Layer by Layer Self-Assembly of Metal-Phenolic Networks on Carbon Fibers for Enhancement in Interfacial Adhesion of Epoxy Composites. Compos. Commun. 2025, 53, 102237. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, G.; Tang, G.; Zhao, Z.; Li, P. Preparation of High-Performance Pervaporation Membranes for Ethanol Dehydration Using a Layer-by-Layer Self-Assembly Method. Adv. Membr. 2025, 5, 100132. [Google Scholar] [CrossRef]
- Chen, H.; Lan, X.; Zhang, S.; Zhang, Q.; Zhang, X.; Chi, H.; Meng, Q.; Fan, F.; Tang, J. Properties of Gelatin-Zein Films Prepared by Blending Method and Layer-by-Layer Self-Assembly Method. Int. J. Biol. Macromol. 2025, 292, 139172. [Google Scholar] [CrossRef]
- Gongsun, K.; Gao, X.; Feng, K.; Yuan, B.; Qiu, J.; Chen, C.; Sang, C.; Wang, C.; Ma, H. Multifunctional Corrosion Inhibition of Brass by Interface Engineering Based on Ternary Layer-by-Layer Self-Assembly with Trivalent Cerium Captured. Corros. Sci. 2024, 237, 112310. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, C.; Zou, Y.; Li, Y.; Zhang, H. Immobilization of Lysozyme on Layer-by-Layer Self-Assembled Electrospun Nanofibers Treated by Post-Covalent Crosslinking. Food Hydrocoll. 2021, 121, 106999. [Google Scholar] [CrossRef]
- Gao, K.; Yang, Y.; Graham, N.J.D.; Zhang, Y.; Jiang, Y.; Duan, C.; Li, A.; Zhang, Q.; An, X.; Hou, L.-a. Self-Assembled Membrane with Excellent Antifouling Performance for Enhanced Treatment of Petrochemical Wastewater. Chem. Eng. J. 2024, 496, 153931. [Google Scholar] [CrossRef]
- Guo, Z.; Huang, A.; Gu, Z.; Guo, Z.; Yuan, L.; Gao, R.; Xin, Y.; Zhang, L. Ultrasound-Assisted Biomimetic Mineralization Immobilization Improves the Stability and Catalytic Performance of Laccases Derived from Bacillus Licheniformis. Mol. Catal. 2025, 574, 114869. [Google Scholar] [CrossRef]
- Liu, G.; Yuan, H.; Chen, Y.; Mao, L.; Yang, C.; Zhang, R.; Zhang, G. Magnetic Silica-Coated Cutinase Immobilized Via Elps Biomimetic Mineralization for Efficient Nano-Pet Degradation. Int. J. Biol. Macromol. 2024, 279, 135414. [Google Scholar] [CrossRef]
- Lu, Y.; Sui, L.; Dai, C.; Zheng, W.; Zhao, Y.; Li, Q.; Liang, X.; Li, Q.; Zhang, Z. Immobilization of Bacillus Thuringiensis Cry1ac in Metal-Organic Frameworks through Biomimetic Mineralization for Sustainable Pest Management. Int. J. Biol. Macromol. 2024, 274, 133388. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Feng, T.; Han, L.; Zhang, M.; Jiang, T. Fabricating Biopolymer-Inorganic Hybrid Microspheres for Enzyme Immobilization: Connect Membrane Emulsification with Biomimetic Mineralization. Particuology 2022, 64, 171–177. [Google Scholar] [CrossRef]
- Lei, T.; Zhang, T.; Fang, T.; Han, J.; Gu, C.; Liao, Y.; Fei, Y.; Luo, J.; Liu, H.; Wu, Y.; et al. Engineering a Stem Cell-Embedded Bilayer Hydrogel with Biomimetic Collagen Mineralization for Tendon-Bone Interface Healing. Bioact. Mater. 2025, 49, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Han, S.Y.; Lee, H.; Nguyen, D.T.; Yun, G.; Kim, S.; Park, J.H.; Choi, I.S. Single-Cell Nanoencapsulation of Saccharomyces Cerevisiae by Cytocompatible Layer-by-Layer Assembly of Eggshell Membrane Hydrolysate and Tannic Acid. Adv. NanoBiomed Res. 2021, 1, 2170013. [Google Scholar] [CrossRef]
- Naidja, A.; Liu, C.; Huang, P.M. Formation of Protein–Birnessite Complex: Xrd, Ftir, and Afm Analysis. J. Colloid Interface Sci. 2002, 251, 46–56. [Google Scholar] [CrossRef]
- Hwang, E.T.; Tatavarty, R.; Chung, J.; Gu, M.B. New Functional Amorphous Calcium Phosphate Nanocomposites by Enzyme-Assisted Biomineralization. ACS Appl. Mater. Interfaces 2013, 5, 532–537. [Google Scholar] [CrossRef]
- Xue, S.; Guo, H.; Li, Y.; Dong, N.; Dai, Y.; Ji, C.; Zhu, B.; Zhang, S. Immobilized Rhodotorula Mucilaginosa Dl-Xsy01 with Electrospinning for Ethyl Carbamate Degradation and Flavor Preservation in Chinese Baijiu. Food Biosci. 2025, 64, 105858. [Google Scholar] [CrossRef]
- Tang, P.; Hou, L.; Yin, M.; Huang, F.; Pan, Z.; Shi, T.; Li, J.; Zhu, Y.; Zhang, X.; Gao, P. Towards Robust Partial Nitrification in Low-Ammonia Wastewater: Electrospinning Nanofiber Composite-Enhanced Hydrogel Beads Immobilized Comammox Nitrospira. Bioresour. Technol. 2025, 429, 132541. [Google Scholar] [CrossRef] [PubMed]
- Abid, M.B.; Wahab, R.A.; Salam, M.A.; Moujdin, I.A.; Gzara, L. Desalination Technologies, Membrane Distillation, and Electrospinning, an Overview. Heliyon 2023, 9, e12810. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Yang, S.; Wu, S.; Yin, M.; Kuang, H. A Functional Pva Aerogel-Based Membrane Obtaining Sutureability through Modified Electrospinning Technology and Achieving Promising Anti-Adhesion Effect after Cardiac Surgery. Bioact. Mater. 2022, 10, 355–366. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L. Research Progress of Carbon Nanofiber-Based Precious-Metal-Free Oxygen Reaction Catalysts Synthesized by Electrospinning for Zn-Air Batteries. J. Power Sources 2021, 507, 230280. [Google Scholar] [CrossRef]
- Song, J.; Deng, Q.; Huang, M.; Kong, Z. Carbon Nanotube Enhanced Membrane Distillation for Salty and Dyeing Wastewater Treatment by Electrospinning Technology. Environ. Res. 2022, 204, 111892. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Khumsupan, D.; Patel, A.K.; Kee, P.E.; Ng, H.-S.; Hsu, H.-Y.; Lin, S.-P.; Cheng, K.-C. Immobilization of Kluyveromyces Marxianus K21 Via Coaxial Electrospinning of Pva and Sugarcane Bagasse Composite for Bioethanol Production. Appl. Energy 2024, 356, 122405. [Google Scholar] [CrossRef]
- Jayani, T.; Sanjeev, B.; Marimuthu, S.; Uthandi, S. Bacterial Cellulose Nano Fiber (Bcnf) as Carrier Support for the Immobilization of Probiotic, Lactobacillus Acidophilus 016. Carbohydr. Polym. 2020, 250, 116965. [Google Scholar] [CrossRef]
- Sarioglu, O.F.; San Keskin, N.O.; Celebioglu, A.; Tekinay, T.; Uyar, T. Bacteria Immobilized Electrospun Polycaprolactone and Polylactic Acid Fibrous Webs for Remediation of Textile Dyes in Water. Chemosphere 2017, 184, 393–399. [Google Scholar] [CrossRef]
- Fan, Y.; Tian, X.; Zheng, L.; Jin, X.; Zhang, Q.; Xu, S.; Liu, P.; Yang, N.; Bai, H.; Wang, H. Yeast Encapsulation in Nanofiber Via Electrospinning: Shape Transformation, Cell Activity and Immobilized Efficiency. Mater. Sci. Eng. C 2021, 120, 111747. [Google Scholar] [CrossRef]
- Li, J.; Wan, X.; Wang, H.; Zhang, Y.; Ma, Z.; Yang, W.; Hu, Y. Electrospun Nanofibers Electrostatically Adsorb Heterotrophic Nitrifying and Aerobic Denitrifying Bacteria to Degrade Nitrogen in Wastewater. J. Environ. Manag. 2024, 353, 120199. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hussain, A.; Liu, Y.; Yang, Z.; Zhao, T.; Bamanu, B.; Su, D. Electrospinning Micro-Nanofibers Immobilized Aerobic Denitrifying Bacteria for Efficient Nitrogen Removal in Wastewater. J. Environ. Manag. 2023, 343, 118230. [Google Scholar] [CrossRef]
- Yu, X.; Shi, J.; Khan, A.; Yun, H.; Zhang, P.; Zhang, P.; Kakade, A.; Tian, Y.; Pei, Y.; Jiang, Y.; et al. Immobilized-Microbial Bioaugmentation Protects Aerobic Denitrification from Heavy Metal Shock in an Activated-Sludge Reactor. Bioresour. Technol. 2020, 307, 123185. [Google Scholar] [CrossRef]
- Zerva, I.; Remmas, N.; Melidis, P.; Ntougias, S. Biotreatment Efficiency, Hydrolytic Potential and Bacterial Community Dynamics in an Immobilized Cell Bioreactor Treating Caper Processing Wastewater under Highly Saline Conditions. Bioresour. Technol. 2021, 325, 124694. [Google Scholar] [CrossRef] [PubMed]
- Bouabidi, Z.B.; El-Naas, M.H.; Zhang, Z. Immobilization of Microbial Cells for the Biotreatment of Wastewater: A Review. Environ. Chem. Lett. 2019, 17, 241–257. [Google Scholar] [CrossRef]
- Wang, B.-B.; Liu, X.-T.; Chen, J.-M.; Peng, D.-C.; He, F. Composition and Functional Group Characterization of Extracellular Polymeric Substances (Eps) in Activated Sludge: The Impacts of Polymerization Degree of Proteinaceous Substrates. Water Res. 2018, 129, 133–142. [Google Scholar] [CrossRef]
- Li, L.; Pagilla, K.R. Biomass Density-Function Relationships in Suspended Growth Biological Processes—A Critical Review. Water Res. 2017, 111, 274–287. [Google Scholar] [CrossRef]
- Wang, X.; Yang, H.; Liu, X.; Su, Y. Effects of Biomass and Environmental Factors on Nitrogen Removal Performance and Community Structure of an Anammox Immobilized Filler. Sci. Total Environ. 2020, 710, 135258. [Google Scholar] [CrossRef]
- Tan, X.; Yang, Y.-L.; Li, X.; Gao, Y.-X.; Fan, X.-Y. Multi-Metabolism Regulation Insights into Nutrients Removal Performance with Adding Heterotrophic Nitrification-Aerobic Denitrification Bacteria in Tidal Flow Constructed Wetlands. Sci. Total Environ. 2021, 796, 149023. [Google Scholar] [CrossRef]
- Wittorf, L.; Jones, C.M.; Bonilla-Rosso, G.; Hallin, S. Expression of Nirk and Nirs Genes in Two Strains of Pseudomonas Stutzeri Harbouring Both Types of No-Forming Nitrite Reductases. Res. Microbiol. 2018, 169, 343–347. [Google Scholar] [CrossRef]
- Ma, F.; Sun, Y.; Li, A.; Zhang, X.; Yang, J. Activation of Accumulated Nitrite Reduction by Immobilized Pseudomonas Stutzeri T13 during Aerobic Denitrification. Bioresour. Technol. 2015, 187, 30–36. [Google Scholar] [CrossRef]
- Wen, H.; Zhu, H.; Yan, B.; Xu, Y.; Shutes, B. Treatment of Typical Antibiotics in Constructed Wetlands Integrated with Microbial Fuel Cells: Roles of Plant and Circuit Operation Mode. Chemosphere 2020, 250, 126252. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Jing, Z.; Hu, J.; Yuan, P.; Liu, Y.; Cao, S. Degradation of Nitrobenzene-Containing Wastewater by a Microbial-Fuel-Cell-Coupled Constructed Wetland. Ecol. Eng. 2018, 112, 65–71. [Google Scholar] [CrossRef]
- Zhang, S.; Xiao, R.; Liu, F.; Zhou, J.; Li, H.; Wu, J. Effect of Vegetation on Nitrogen Removal and Ammonia Volatilization from Wetland Microcosms. Ecol. Eng. 2016, 97, 363–369. [Google Scholar] [CrossRef]
- Wang, W.; Ding, Y.; Wang, Y.; Song, X.; Ambrose, R.F.; Ullman, J.L. Intensified Nitrogen Removal in Immobilized Nitrifier Enhanced Constructed Wetlands with External Carbon Addition. Bioresour. Technol. 2016, 218, 1261–1265. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Peng, H.; Fu, Y.; Yan, X.; Du, C.; Chen, H. Enhanced Nitrogen Removal of Low C/N Wastewater in Constructed Wetlands with Co-Immobilizing Solid Carbon Source and Denitrifying Bacteria. Bioresour. Technol. 2019, 280, 337–344. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, H.; Yan, B.; Shutes, B.; Bañuelos, G.; Wen, H.; Cheng, R. Improving Denitrification Efficiency in Constructed Wetlands Integrated with Immobilized Bacteria under High Saline Conditions. Environ. Pollut. 2021, 287, 117592. [Google Scholar] [CrossRef]
- Zhao, L.; Fu, G.; Pang, W.; Tang, J.; Guo, Z.; Hu, Z. Biochar Immobilized Bacteria Enhances Nitrogen Removal Capability of Tidal Flow Constructed Wetlands. Sci. Total Environ. 2022, 836, 155728. [Google Scholar] [CrossRef]
- Zhang, X.; You, S.; Ma, L.; Chen, C.; Li, C. The Application of Immobilized Microorganism Technology in Wastewater Treatment. In Proceedings of the 2015 2nd International Conference on Machinery, Materials Engineering, Chemical Engineering and Biotechnology, Chongqing, China, 28–29 November 2015; Atlantis Press: Dordrecht, The Netherlands, 2016. [Google Scholar]
- Ahmad, H.A.; Ni, S.-Q.; Ahmad, S.; Zhang, J.; Ali, M.; Ngo, H.H.; Guo, W.; Tan, Z.; Wang, Q. Gel Immobilization: A Strategy to Improve the Performance of Anaerobic Ammonium Oxidation (Anammox) Bacteria for Nitrogen-Rich Wastewater Treatment. Bioresour. Technol. 2020, 313, 123642. [Google Scholar] [CrossRef]
- Tang, M.; Jiang, J.; Lv, Q.; Yang, B.; Zheng, M.; Gao, X.; Han, J.; Zhang, Y.; Yang, Y. Denitrification Performance of Pseudomonas Fluorescens Z03 Immobilized by Graphene Oxide-Modified Polyvinyl-Alcohol and Sodium Alginate Gel Beads at Low Temperature. R. Soc. Open Sci. 2020, 7, 191542. [Google Scholar] [CrossRef]
- Dayu, Y.; Bo, G.; Mengyu, Y.; Wenchao, L.; Nan, Q. The Treatment of Heterotrophic Nitrification-Aerobic Denitrifier Bacteria Loaded with Bacterial Cellulose Membrane to Nitrogenous Wastewater. Fresenius Environ. Bull. 2011, 20, 1208–1215. [Google Scholar]
- Zhao, Y.; Lu, W.; Liu, Y.; Wang, J.; Zhou, S.; Mao, Y.; Li, G.; Deng, Y. Efficient Total Nitrogen Removal from Wastewater by Paracoccus denitrificans Dytn-1. Lett. Appl. Microbiol. 2020, 70, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Ma, J.; Du, Y.; Wang, W.; Wang, J.; Gao, M.; Jin, C.; Zhao, Y.; Zhang, Z.; Ji, J. Effect of Hydraulic Residence Time on Nitrogen Removal from a Synthetic Mariculture Wastewater Using a Bench-Scale Recirculating Bioreactor Embedded with Aerobic Denitrifying Bacteria Marinobacter Alkaliphilus Strain Jy28. J. Environ. Manag. 2025, 381, 125289. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Ma, H.; Su, X.; Song, J.; Liu, P.; Wu, Y.; Liu, Y. Improved Nitrate Removal by Polyvinyl Alcohol/Sodium Alginate Hydrogel Beads Entrapping Salt-Tolerant Composite Bioagent Ahm M3. Desalination 2024, 591, 118009. [Google Scholar] [CrossRef]
- Tian, Z.; Zhou, N.; You, W.; He, D.; Chang, F.; Zheng, M. Mitigating NO and N2O Emissions from a Pilot-Scale Oxidation Ditch Using Bioaugmentation of Immobilized Aerobic Denitrifying Bacteria. Bioresour. Technol. 2021, 340, 125704. [Google Scholar] [CrossRef]
| Genus | Species | Source | Year |
|---|---|---|---|
| Bacillus sp. | YX-6 | Fishery pond | 2011 [18] |
| Bacillus methylotrophicus | L7 | Wastewater sample | 2012 [19] |
| Rhodococcus sp. | CPZ24 | Swine wastewater | 2012 [20] |
| Halomonas campisalis | ha3 | Saline–alkali lake | 2013 [21] |
| Paracoccus versutus | LYM | Seabed sludge | 2013 [22] |
| Acinetobacter sp. | Y16 | Drinking water source | 2013 [23] |
| Chryseobacterium sp. | R31 | Slaughterhouse wastewater | 2014 [24] |
| Aeromonas sp. | HN-02 | CASS reactor | 2014 [25] |
| Alcaligenes faecalis | C16 | Aeration tank | 2015 [26] |
| Diaphorobacter sp. | PD-7 | Coking-plant wastewater ponds | 2015 [27] |
| Pseudomonas aeruginosa | PCN-2 | Landfill leachate treating reactor | 2015 [28] |
| Cupriavidus sp. | S1 | Coking wastewater | 2016 [29] |
| Pseudomonas tolaasii | Y-11 | Long-term flooded paddy soil | 2016 [30] |
| Raoultella sp. | R11 | Eutrophic lake | 2017 [31] |
| Acinetobacter sp. | H36 | Sediment | 2017 [32] |
| Pseudomonas putida | Y-9 | Long-term flooded paddy soil | 2017 [33] |
| Pseudomonas stutzeri | XL-2 | Secondary sedimentation tank | 2018 [34] |
| Pseudomonas putida | NP5 | Activated sludge | 2019 [35] |
| Acinetobacter johnsonii | WGX-9 | Sediment of a drinking-water reservoir | 2019 [36] |
| Acinetobacter sp. | T-1 | Membrane bioreactor | 2019 [37] |
| Paracoccus sp. | YF1 | Activated sludge | 2019 [38] |
| Acinetobacter sp. | JR1 | Pharmaceutical raw water | 2019 [39] |
| Pseudomonas sp. | JQ-H3 | Packed-bed reactor | 2020 [40] |
| Acinetobacter sp. | YS2 | Aerobic pond | 2020 [41] |
| Bacillus thuringiensis | WXN-23 | Piggery bran feed filtrate | 2021 [42] |
| Pseudomonas putida | W207-14 | Landfill leachate | 2022 [43] |
| Bacillus sp. | T28 | Paddy soil | 2023 [44] |
| Rhodococcus sp. | CPZ24 | Biofilm reactor | 2023 [45] |
| Rhodococcus sp. | SY24 | Soil | 2023 [46] |
| Paracoccus sp. | HY-1 | Landfill leachate treatment plant | 2024 [47] |
| Pseudomonas | XF-4 | Activated sludge | 2024 [48] |
| Zobellella | B307 | Jiaozhou Bay sediment | 2024 [49] |
| Stutzerimonas sp. | os3 | Shrimp aquaculture sediment | 2025 [50] |
| Pseudomonas sp. | WS-03 | Sludge of an actual wastewater treatment plant | 2025 [51] |
| Ralstonia sp. | J4 | Piggery wastewater | 2025 [52] |
| Paracoccus binzhouensis | wg1 | Propylene oxide saponification-activated sludge | 2025 [53] |
| Marinobacterium maritimum | 5-JS | Sea cucumber aquaculture pond | 2025 [54] |
| Material Composition | Immobilization Method | Strain Name | Target Pollutant | Removal Efficiency (%) | Reference |
|---|---|---|---|---|---|
| PVA-SA | Gel method | Mixed Nitrifying Bacteria | High-concentration ammoniacal nitrogen | 46 | [96] |
| PVA-SA | Gel method | Mixed Nitrifying Bacteria | Different concentrations of ammoniacal nitrogen | 48.3–100 | [97] |
| Mycelium balls | Adsorption method | Pseudomonas GF3 | Nitrate nitrogen | 95.91 | [98] |
| Sponge–walnut shell Carbon–Magnetite | Adsorption method | Zoogloea L2 | Nitrate nitrogen, diethyl phthalate | 83.97, 67.87 | [99] |
| Mycelium balls–magnetite | Adsorption method | Pseudomonas GF2 | Nitrate nitrogen | 98.14 | [88] |
| PVA-SA | Gel method | Nitrosomonas GH22 | Ammoniacal nitrogen | 90.3 | [100] |
| Biochar adsorption | Adsorption method | Pseudomonas mendocina GL6 | Nitrate nitrogen | 95.8 | [101] |
| Polyethylene suspended balls | Adsorption method | Pseudomonas Y39-6 | Nitrate nitrogen under low temperature and low carbon–nitrogen ratio | 24.83 | [79] |
| PVA-SA | Gel method | Anammox granular sludge | Nitrogen removal of different low temperatures | 52–72 | [102] |
| PVA-SA | Gel method | Stutzerimonas stutzeri W-2 | Nitrogen-containing wastewater | 99.06 | [103] |
| Immobilization Method | Principle | Advantages | Disadvantages | Cost |
|---|---|---|---|---|
| Chemical methods | Microorganisms are connected to each other or to the carrier through chemical bonds | Strong binding force, and microorganisms are highly concentrated and not easily detached from the carrier | Chemical reagents are toxic to microorganisms, leading to reduced microbial activity | High |
| Adsorption method | Microorganisms are connected to the carrier through weak interactions, such as van der Waals forces and ionic bonds | Simple operation, non-toxic to microorganisms, and the carrier can be regenerated | The binding force is weak, and microorganisms are easily detached from the carrier | Middle |
| Encapsulation method | Microorganisms are trapped in water-insoluble gel polymers | Simple operation, can immobilize specific microorganisms, and has wide applicability | The mass transfer resistance is large, and microorganisms are prone to leakage from the gel polymer after long-term operation | Low |
| Layer-by-layer self-assembly | Specific materials are alternately deposited on microorganisms, layer by layer, through electrostatic forces | The assembly process is controllable, the conditions are mild, and the properties of the materials can be imparted to the microorganisms | Poor stability, long production cycle, and less application in biological nitrogen removal | High |
| Electrospinning | Nanofibers are formed from a mixture of microorganisms and polymer solution under a high-voltage electric field | Simple operation, and the nanofibers produced have a large specific surface area and high porosity | Low strength of nanofibers, low yield, and less application in biological nitrogen removal | High |
| Biomimetic mineralization | Mineralization-related molecules are introduced onto cells that lack biomineralization ability, forming a protective shell for microorganisms | Environmentally friendly, has good biocompatibility, and is highly controllable | The synthesis process requires a lot of time, making it difficult to meet the needs of large-scale production, and it is less applied in biological nitrogen removal | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Li, J.; Mu, H.; Xie, H.; Zhao, W. Immobilization Technology of Aerobic Denitrifying Bacteria and Its Enhanced Biological Denitrification: A Review of Recent Advances. Water 2025, 17, 1433. https://doi.org/10.3390/w17101433
Li J, Li J, Mu H, Xie H, Zhao W. Immobilization Technology of Aerobic Denitrifying Bacteria and Its Enhanced Biological Denitrification: A Review of Recent Advances. Water. 2025; 17(10):1433. https://doi.org/10.3390/w17101433
Chicago/Turabian StyleLi, Jing, Jie Li, Hao Mu, Huina Xie, and Wei Zhao. 2025. "Immobilization Technology of Aerobic Denitrifying Bacteria and Its Enhanced Biological Denitrification: A Review of Recent Advances" Water 17, no. 10: 1433. https://doi.org/10.3390/w17101433
APA StyleLi, J., Li, J., Mu, H., Xie, H., & Zhao, W. (2025). Immobilization Technology of Aerobic Denitrifying Bacteria and Its Enhanced Biological Denitrification: A Review of Recent Advances. Water, 17(10), 1433. https://doi.org/10.3390/w17101433






