The AMR assessment of the waste water microbiome of the selected urban WWTP was based on analyses of three culture-dependent parameters—number of resistant HPC bacteria and antibiotic susceptibility of Enterobacteriaceae or Enterococcus spp. isolates, as well as PCR analyses of ARGs encoding antibiotic resistance in Enterococcus spp. and ESBL production in Enterobacteriaceae. The population study assessed the overall AMR of the HPC bacteria involved in WW treatment, while the AMR analyses of faecal indicators in the WWTP influents revealed the contribution of the respective urban population to the AMR prevalence. The AMR assessment of the WWTP effluents took into account the influence and efficiency of WW treatment.
Comparing between the AMR level of UWW and TWW microbiomes on the base of the studied parameters reveals an inefficiency of the conducted WW treatment in the selected WWTP for complete removal of resistant HPC or faecal indicator bacteria and demonstrates a positive selective effect of increasing AMR to some ABs (most often CIP, COT, and TE).
4.1. Prevalence of AMR Among HPC Bacteria
Our investigations on the AMR of the waste water microbiome target ABs widely used to treat infections, such as ampicillin, tetracycline, ciprofloxacin, and sulfamethoxazole. These pharmaceuticals are categorized by the World Health Organization as high-priority ‘critically important antimicrobial agents’ (e.g., ampicillin), as ‘highly important antimicrobials’ (e.g., sulfamethoxazole and tetracycline), or as the highest-priority critically important antimicrobials in human medicine (e.g., fluoroquinolones) [
40]. Because AB substances are not completely utilized in the human body, their residues or metabolites leave the organism and end up in sewage or water intakes [
7,
41]. The amount of excreted beta-lactams, quinolones, tetracyclines, phenicols, and trimethoprim can exceed half of the administered dose [
41] and vary from nanograms to micrograms per litre of WW depending on usage intensity, chemical stability, WW treatment effectiveness for ABs removal, etc. [
16,
42]. AB residues can act as a driving force for selection of ARB and acquisition of AMR.
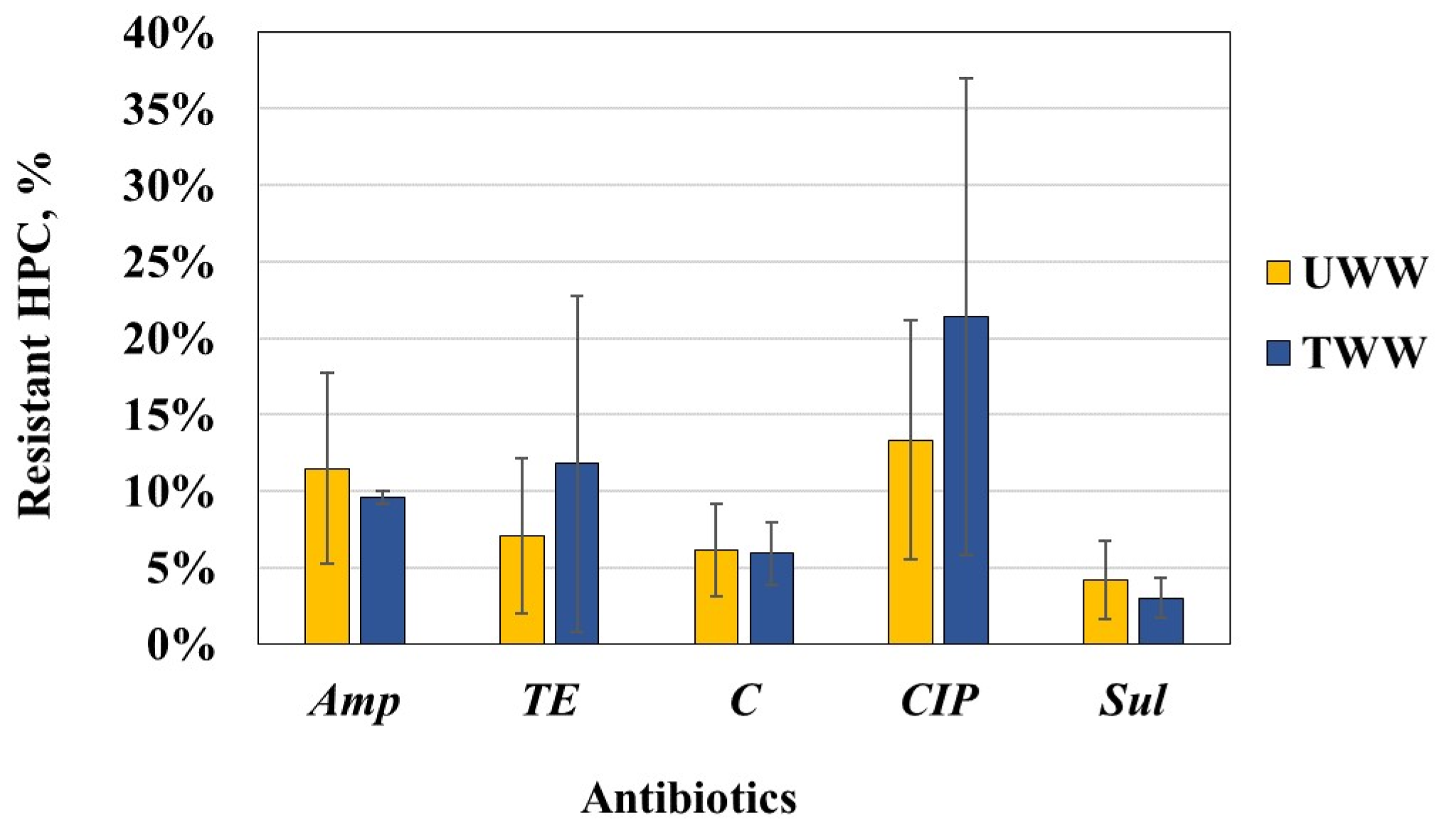
The data obtained show occurrence of bacteria resistant to the tested ABs in the raw and biologically treated waste water but with different abundances depending on WW type and AB substance. In both WW types, the level of resistance to Sul was the lowest, followed by resistance to C. However, the HPC bacteria in UWW were most commonly resistant to Amp or CIP, while those in TWW demonstrated the most common resistance to TE and CIP. These populational changes in the antibiotic resistance of HPC bacteria are illustrated by the shifts in the ascending order of AMR (Sul < C < TE < CIP/Amp (for UWW)) to the following one: Sul < C < Amp/TE < CIP (for TWW). Despite the increase in the relative proportion of HPC bacteria resistant to CIP or TE in TWW, the trend was not statistically confirmed.
Our data on the increased proportion of HPC bacteria resistant to various ABs in TWW are consistent with the trends established by other researchers [
15,
22,
43]. A significantly high level of heterotrophic bacteria resistant to penicillin, ampicillin, cephalothin, chloramphenicol, tetracycline, and rifampicin have been reported for the secondary effluents of a WWTP of Beijing, China, with relative proportions of 63%, 47%, 55%, 69%, 2.6%, and 11%, respectively [
22]. It has been also reported that the increased percentage share of ARB in the total number of bacteria after the WW treatment depended on the technology used or its modifications, with the highest effectiveness of ARB reduction being obtained in WWTPs with A
2O systems and the lowest in WWTPs with mechanical-biological treatment systems [
15].
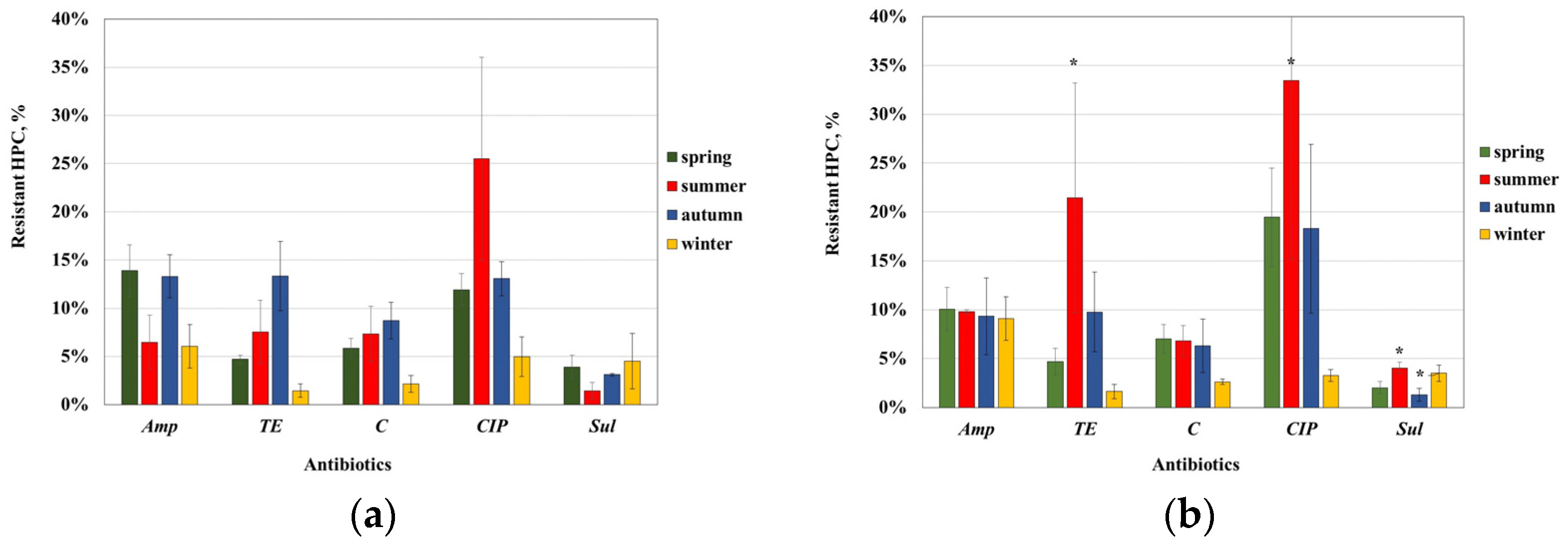
We observed a higher variability in the AMR rates in UWW than TWW, which lets us to suppose that this was related to fluctuations in the ABs content in UWW depending on sampling time. Moreover, the ABs content might occasionally reach concentrations capable of selecting for ARB. Unfortunately, we do not have data available on the AB content in wastewater. In UWW, ciprofloxacin resistance of HPC bacteria appeared to vary the most, with an exceptionally high rate of resistance (26%) detected in summer and the lowest value of 5% in winter; tetracycline resistance was in the range 2–13%, also with an increased value in summer; the proportion of Sul-resistant bacteria increased in summer but decreased in autumn. In TWW, CIP resistance varied in the broadest range (3–33%), followed by TE resistance (2–21%), while the resistance rate to Amp and C was relatively unchanged.
There is a scarcity of data regarding the seasonal changes in the abundance of resistant HPC bacteria in waste water. A study of tetracycline-resistant bacteria in a Polish WWTP has found fluctuation in the HPC population share, with the highest values in April regardless of the type of waste water, while the percentage share of oxytertacycline-resistant bacteria in UWW had the highest values in February and April, but in TWW, this was in April and October [
43]. The population proportion of
TE-resistant bacteria found in our study (7.0% and 11.8% for UWW and TWW, respectively) was higher than the values of 2.6% or 1.6% determined in the previous studies [
22,
43]. It should be noted that these studies [
22,
43] were conducted at higher concentrations of TE (16 μg/mL or 32 μg/mL), which may explain, to some extent, the difference from our data obtained at 8 μg/mL
TE. Despite the limited data, it can be assumed that the observed fluctuations are mainly related to the selective pressure of ABs, whose residues probably vary over time as a result of their use, as well as to the different treatment efficiency in the WWTPs, related to ambient temperature, amount of precipitation entering the sewer, etc.
A comparison of the AMR data of HPC bacteria in WW with those from our previous AMR study of the water of the Yantra River (recipient of the effluents from the studied WWTP) reveals significant differences in the levels of resistance to individual AB. In contrast to WW, the population proportion of
TE-resistant bacteria in the river water was the lowest, followed by
CIP-resistant bacteria, and the highest values were that of
Amp and
C-resistant bacteria [
44]. The quantity of HPC bacteria resistant to
CIP,
TE, and
Amp was greater in the WWTP effluents compared to Yantra River water, which may suggest involvement of the discharged effluents in spreading of resistance to these ABs in the receiving waters.
Our comparative data on the different types of ARB in the two types of WW clearly show the changes that occur in the levels of AMR in wastewater treatment processes, as well as the fact that, despite the rather high level of reduction in the number of ARB, the wastewater discharged from the WWTP significantly loads the aquatic environment with various ARB, thus contributing to the spread of AMR.
4.2. Prevalence of AMR Among Enterobacteriaceae Isolates from Waste Water of the Studied WWTP
E. coli and coliforms are commensal bacteria that enter sewage system with faeces. Some species are pathogenic. Due to their common habitats with other pathogens or ARB in living organisms or during WW treatment, they can acquire resistance by horizontal gene transfer. The increased frequency of resistant Enterobacteriaceae in discharged waste water suggests the possibility of increased prevalence of AMR in the receiving water body.
The AMR assessment of
Enterobacteriaceae isolates found the highest resistance rate to aminopenicillins (
Amp—up to 46%), ureidopenicillins (
PI—20%), and
TE (up to 28%) and the lowest to
GEN (2%). Resistance to cephalosporins of the 3rd and 4th generations was 9% or 8%, respectively, and a carbapenem resistance of 3% was found only among UWW isolates. The AMR rate of
Enterobacteriaceae found in our study was lower or within the range of some previous studies [
8,
15,
18,
45]. The data reported for 10 countries from different European regions (3 from western, 4 from northern, and 3 from southern Europe) on the resistant rates to aminopenicillines, fluoroquinolones, 3rd generation cephalosporins, and amynoglycosides found predominantly lower AMR rates than those determined in our study, excepting a higher resistance to
Amp [
34]. The AMR rates reported for the urban effluents of a large Romanian WWTP were higher in terms of
Amp (61.9%),
CTX and
CTR (42.86%), and
COT (76.19%) but similar for
CIP,
C, and
TE [
45]. A high occurrence of antibiotic-resistant faecal coliforms has been reported in two urban WWTPs in Ireland: more than 90% of isolates were resistant to
Amp; to
TE—up to 39.82%; to
CIP—up to 31.42%; and to
IMI—up to 15.93% [
18]. Meanwhile, a much lower level of AMR prevalence was found in another Irish WWTP [
14].
Waste water treatment in the studied WWTP reduced the proportion of
Enterobacteriaceae resistant to
Amp and
IMI but increased that of those resistant to cephalosporins,
CIP,
C, and
COT. However, the populations of
E. coli and coliforms underwent different changes during the WW treatment: the incidence of resistant
E. coli increased, especially those resistant to cephalosporins,
CIP,
C, and
COT, while the abundance of resistant coliforms decreased, especially those resistant to
Pi and the cephalosporins
CTR and
CPM, but the reduction in piperacillin resistance was most significant (
p < 0.05). Despite the AMR changes experienced during WW treatment, statistically insignificant differences in resistance determinants were found between UWW and TWW, except for resistance to
CIP and
COT (
p < 0.05). However, the treatment process exerted selective pressure, especially on
E. coli, increasing resistance to cephalosporins,
CIP,
COT, and
C. The obtained data on the increased resistance to cephalosporins,
CIP, and
COT in TWW are consistent with previous studies [
8,
16].
The greatest fractions of resistant
E. coli in UWW were to
Amp (44%) and
TE (30%), and the lowest were to
GEN (1%) and
IMI (1%). Resistance rates to
CTX and
CIP were 2% and 3%, respectively. Comparing
E. coli resistance phenotypes with WHO/ECDC data on clinical isolates from Bulgaria, a much lower (from 0.9 to 3.3 times) level of AMR among waterborne
E. coli was found [
46]. According to a WHO/ECDC report, in Bulgaria, the invasive
E. coli had a population-weighted mean value of 54.6% for ampicillin resistance, 14.9% for third-generation cephalosporin resistance, 23.8% to fluoroquinolones, 10.2% to aminoglycosides, and 0.2% to carbapenems [
46]. Moreover,
E. coli isolates from UWW samples showed a resistance rate strongly correlated with the reported data on clinical isolates (
r2 = 0.94). These data support the findings of Huijbers et al. (2020) that compared resistance prevalence in
E. coli isolates from sewage water to that in clinical
E. coli for 10 EU countries (based on data of the European Antibiotic Resistance Surveillance Network) and found a significant relationship (
r2 = 0.94). Based on these data, the possibility of using wastewater monitoring for the prediction of clinical resistance levels and provision of clinically relevant AMR data has been assumed [
34]. This assumption has been supported by comparative AMR data of
E. coli from a regional, primary care centre with urban wastewater data, which showed a significant relationship regarding the prevalence of resistance to eleven antibiotics (
r2 = 0.82) [
36], as the AMR rates in municipal sewage isolates were about half of those measured in primary care isolates.
The comparison between the AMR level of
Enterobacteriaceae in the WWTP effluents and in the receiving water of the Yantra River showed a slightly higher or similar AMR values in TWW. Moreover, the most common resistance among
Enterobacteriaceae isolates from TWW and the river water was towards the ‘old’ ABs—
Amp,
TE, and
COT—with the resistance rates in riverine bacteria being 36%, 25%, and 16%, respectively [
44], vs. 41%, 30%, and 16% in TWW. In both types of water, the lowest resistance to
GEN (2%) and susceptibility to imipenem was found. However, the river water contained a lower number of
Enterobacteriaceae resistant to
Cx,
AUG,
C,
CIP,
S, and
TE, suggesting a possible transitory negative effect of the TWW discharge.
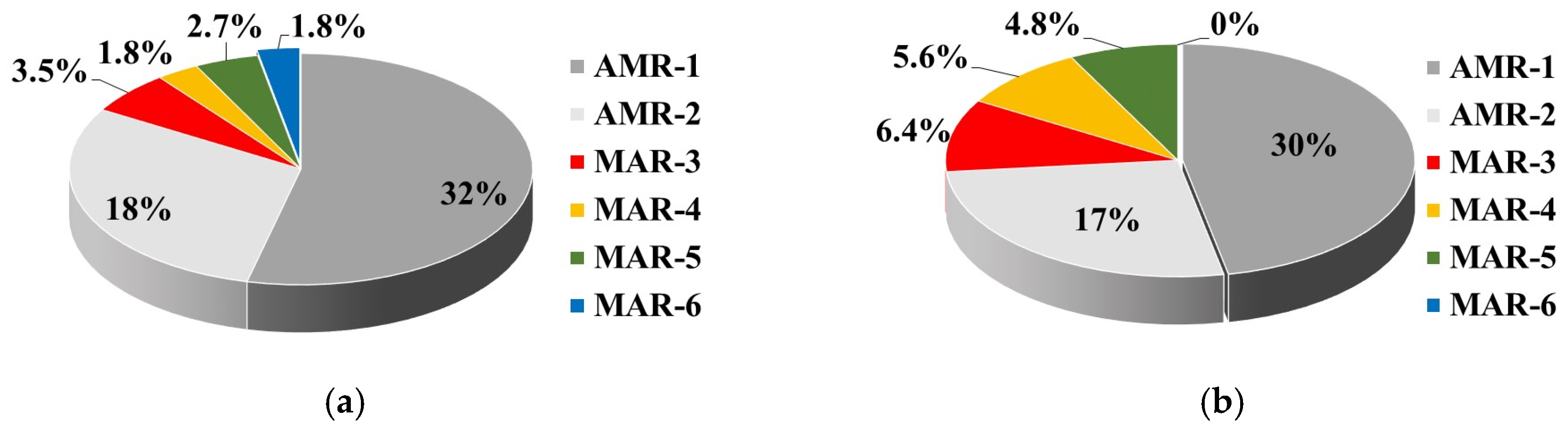
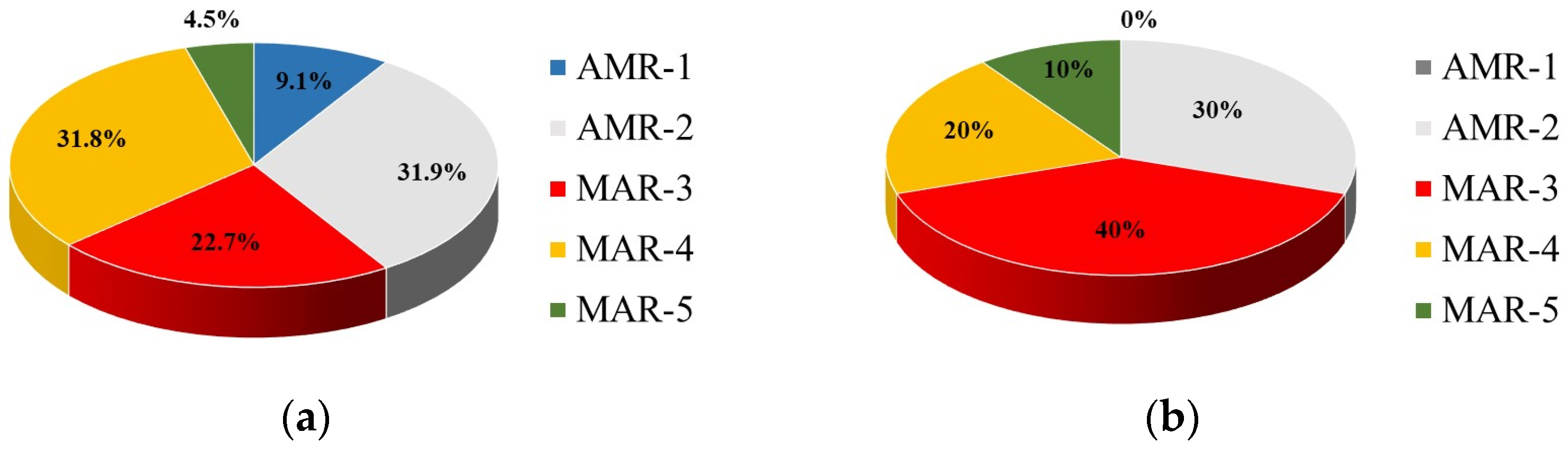
The WW treatment provided a higher proportion of multiple resistant isolates in TWW (16.8%) than in UWW (9.7%). A similar trend of a significant increase in the MAR level of the WWTP effluent compared to the influent water was reported [
45], but with many-times-higher MAR values. Besides their higher abundance, the MAR strains from TWW show a greater diversity of resistant phenotypes than UWW, exhibiting 14 MAR phenotypes vs. 6 resistant patterns of UWW isolates.
It is also important to note that 12 MAR strains (3 isolated from UWW and 9 from TWW) expressed the ESBL phenotype. The increased incidence of ESBL producers in TWW was associated with an increased co-resistance to non-beta-lactam ABs, most often
CIP,
COT, or
TE. The occurrence of ESBL-producing
Enterobacteriaceae isolates in TWW was 8.8%, which is consistent with the findings of 6% [
14] or 11.8% [
18] for urban effluents but is lower compared with the reported values of 18% [
25] or 19.8% [
28]. Data of a much higher prevalence of ESBL-producing
Enterobacteriaceae in WWTP effluents have also been reported, including rates of 56% [
47], 56.3% [
32], 56.3% [
48], and about 73% [
27], which is probably due to ineffective or insufficient WW treatment, including absence of biological treatment and/or disinfection.
4.3. AMR of ESBL-Producing Enterobacteriaceae Strains
To enrich the assessment of the prevalence of cephalosporins resistance, an additional study was carried out among 32
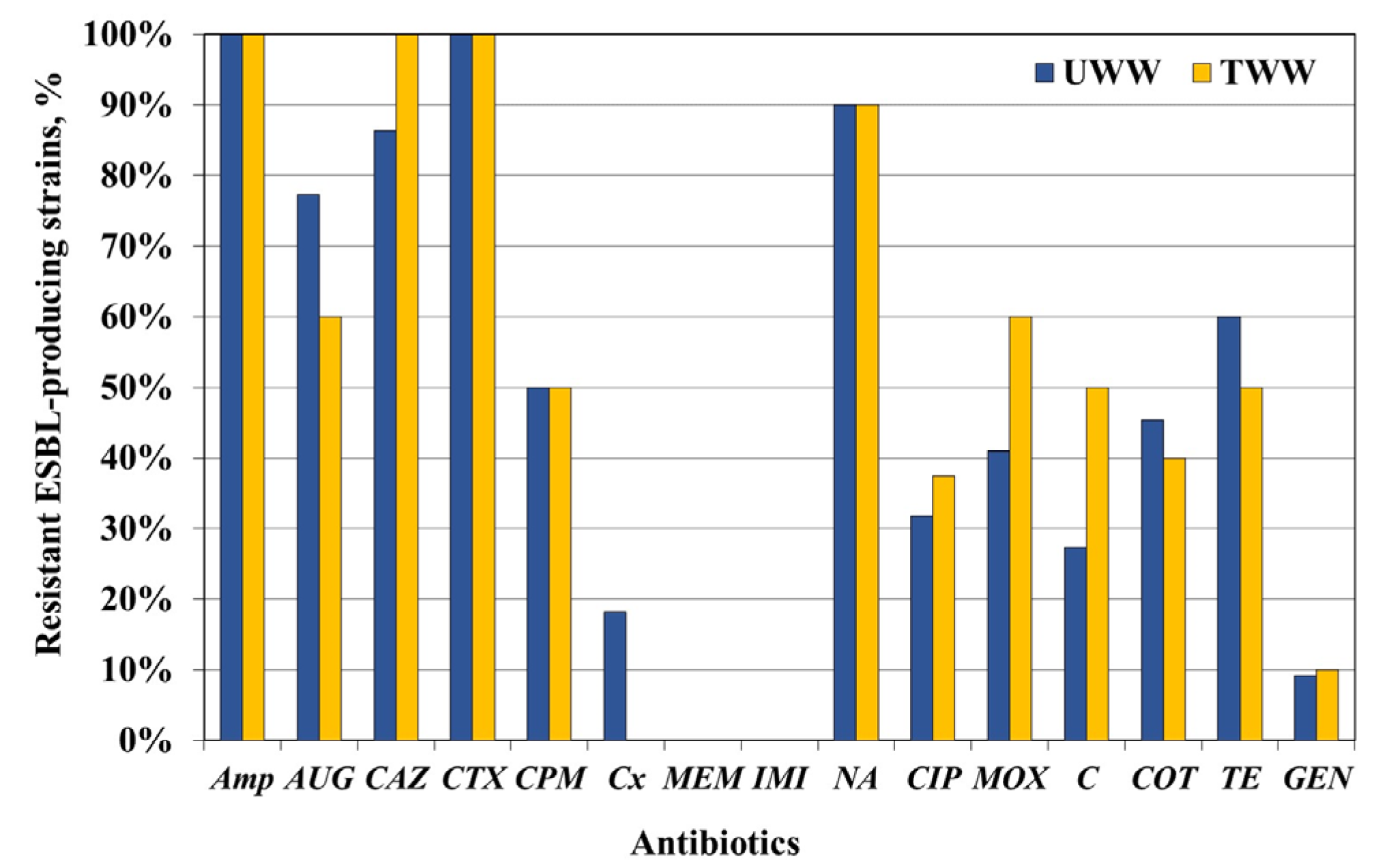
Enterobacteriaceae strains screened and confirmed as ESBL producers and tested for AB susceptibility and genes encoding ESBL production. As might be expected, ESBL-producing
Enterobacteriaceae isolates exhibited high resistance rates to beta-lactams—
Amp (100%),
CTX (100%),
CAZ (94%), and
CPM (50%). The ESBL phenotype was accompanied by a high resistance rate to other classes of ABs:
TE—up to 60%; fluoroquinolones—
CIP (up to 38%) or
MOX (up to 60%); and
COT—up to 45%. The resistance rate to non-beta-lactams was much higher than that of the total
Enterobacteriaceae isolates, which was associated with the high level of MAR among the ESBL producers (59% of UWW isolates and 70% of TWW ones). Similar high MAR values of ESBL isolates have been reported [
8,
18]. Furthermore, it is important to note that an increase in resistance to
CIP and
MOX among ESBL isolates from TWW was registered. Moreover, a study based on in-depth statistical analysis of the probability of detecting ARB or ARGs at the simultaneous presence of AB residues indicated
CIP in waste water as a good indicator of the presence of ESBL-producing
Enterobacteriaceae [
49].
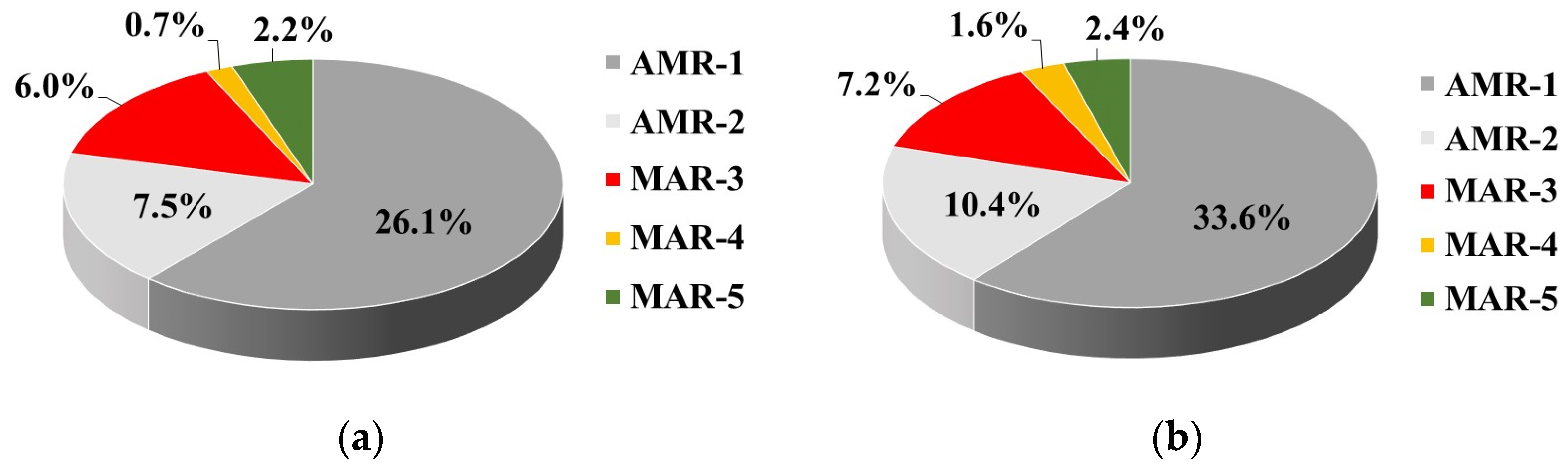
Among ESBL-producing
Enterobacteriaceae, six different genotypes encoded by the
bla-genes
TEM,
SHV, or
CTX-M, alone or in association, were determined. The
blaTEM gene occurred more often alone (22.7%) than the
blaCTX-M gene (13.6%), and the most common ESBL genotype was associated with co-carriage of
blaTEM and
blaCTX-M genes (found in 50% of isolates). A similar trend for high prevalence of the
blaCTX-M gene has been reported for several other WWTPs [
27,
28,
50], while in other studies,
blaTEM has been detected as the most prevalent gene encoding the ESBL phenotype [
18,
47]. The
blaSHV gene occurred rarely only among TWW isolates. Only one TWW isolate harboured all
bla-genes encoding ESBL production. However, most importantly, multidrug-resistant ESBL-producing
E. coli strains are present in the WWTP effluents, which may contribute to their spread in the receiving water and pose an increasing health risk in water intended use.
4.4. Prevalence of AMR Among Enterococcus spp. Isolates from Waste Water
Enterococci are ubiquitous Gram-positive bacteria inhabiting various ecological niches—humans, animals, food, water, and soil. As commensal bacteria in the gastrointestinal tracts of mammals, birds, fishes, and invertebrates, they enter waste water, environmental water, and soil through faeces [
51,
52]. They can cause urinary tract infections and are associated with nosocomial pathogenicity worldwide, particularly in immune-compromised peoples. Therefore,
Enterococcus spp., along with other important pathogens, are the focus of AMR research in the One Health humans–animals–environment continuum [
53,
54].
In our study, all
Enterococcus spp. isolates were analysed to determine their AMR phenotype and some of them were analysed for ARGs encoding resistance. The predominant species found in the raw and treated WW tested were
E. faecalis and
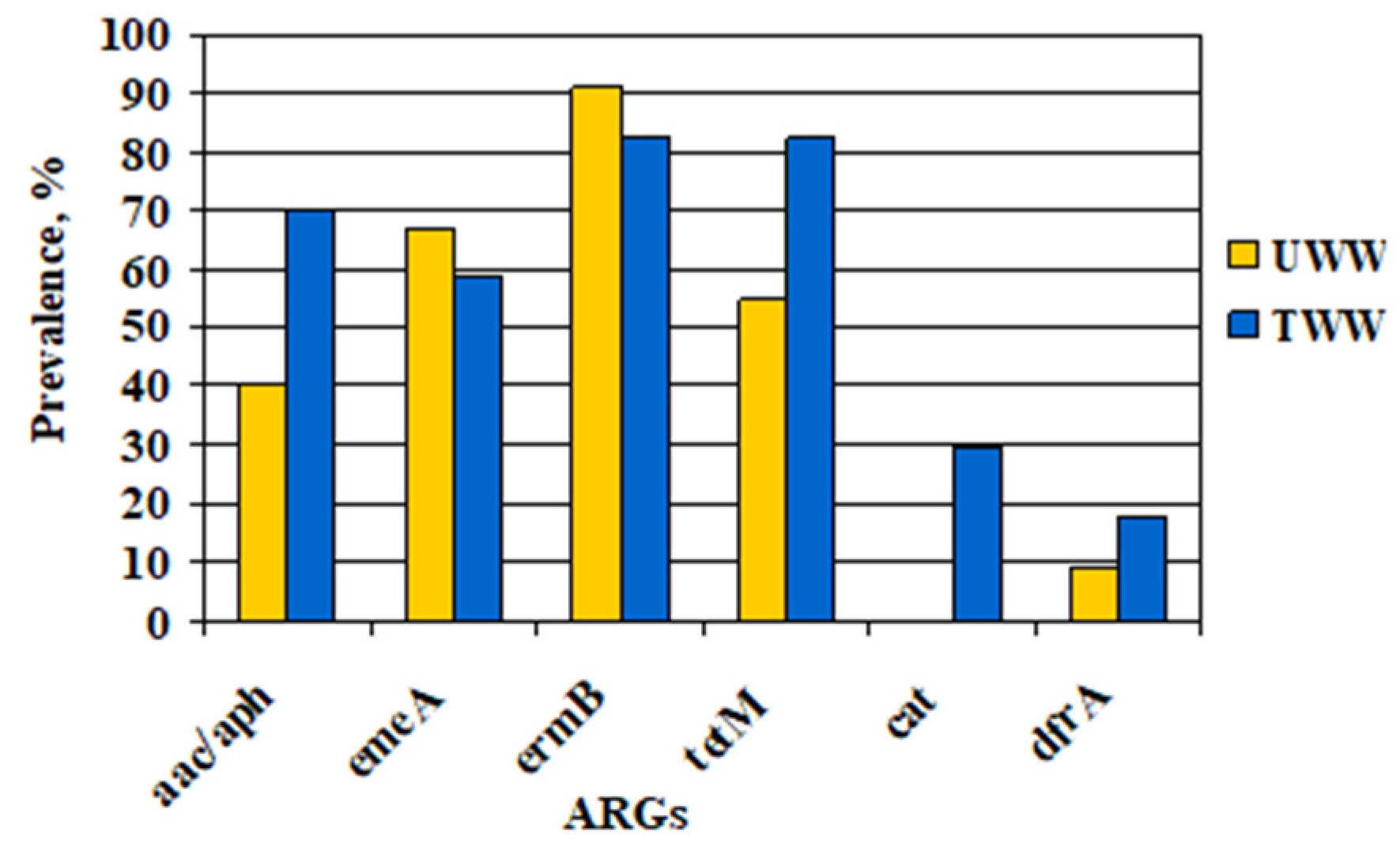
E. faecium. Among them, the most common resistance was to erythromycin (up to 39%) and tetracycline (up to 46%), followed by imipenem (up to 15%). The least common was resistance to chloramphenicol (up to 4%) and co-trimoxazole (up to 3%). The high levels of
E and
TE resistance found are consistent with the findings for a Portuguese WWTP [
8], but a lower resistance to
CIP was detected in our study (8% vs. 33%). Prevalence of resistance mostly to
E,
TE, and
CIP has been reported for several WWTPs, with
E. faecium exhibiting the highest AMR rate [
55]. A large-scale study on AMR of
Enterococcus spp. in the scope of a One Health continuum comprising 8430 isolates from sectors such as humans, agriculture, food, natural streams, and waste water found prevalent phenotypic resistance to tetracyclines and macrolides encoded by
tetM and
ermB, respectively [
53,
54]. It has been also clarified that urban waste water and human clinical isolates have a greater diversity of resistance phenotypes and exhibit higher levels of MAR compared to other sectors.
The prevalence of tetracycline and erythromycin resistance in the studied WWTP confirms the data reported for many other WWTPs [
8,
53,
55], but the lower level of
CIP resistance and higher beta-lactam resistance identified in our study are probably related to differences in the type and amount of ABs used by local communities. In the present study, all enterococcal isolates were susceptible to newer generations of ABs, such as glycopeptides, glycylcycline, and oxazilidinones. The susceptibility to vancomycin and teicoplanin of the tested strains is in contrast with the data for occurrence of vancomycin-resistant enterococci (VRE) in the effluents of some other WWTPs [
9,
56,
57]. VRE strains of
E. faecium have been isolated from a Czech WWTP, and all but one were of the
vanA phenotype [
56]. Some VRE
E. faecium isolates from WWTPs located in the North Spain–South France region were resistant to vancomycin and teicoplanin, expressing the
vanB phenotype [
9].
VanA-harbouring
E. faecium, as well as
vanC1/
vanC2-containing enterococcal strains, have been detected in a Portuguese WWTP [
57].
A comparison between the AMR phenotypes of waste water isolates obtained in our study and clinical isolates from Bulgarian hospitals [
58] found various resistance patterns. Among WW isolates, the
E-TE-HLG phenotype predominated, found in 50% of enterococcal strains from UWW and in 14.2% of TWW isolates, while the
Amp-CIP-HLG phenotype was the most common (79%) among the clinical isolates. Similarly, the incidence of MAR isolates in UWW and TWW of the studied WWTP amounted to 9.0% and 11.2%, respectively, while a higher MAR (39%) was reported for the clinical isolates [
58].
The AMR rate of
Enterococcus spp. in waste water was higher than that found for food and environmental enterococci. A variable but low level of AMR was found among enterococcal strains isolated from Bulgarian food and environmental habitats [
52]. Among them, the
ermB gene also was most frequently found but at a lower level (15.2%). In contrast with our data, the prevalence of the
aac(6′)-
aph(2″) gene was lower (1.4% vs. 57%), and the
vanA gene was detected as well [
52].
According to our data on gentamicin resistance, 58% of the tested enterococcal strains contained the
aac(6′)/
aph(2″) gene, but only 3% exhibited a high-level gentamicin-resistant phenotype. Such a discrepancy was also established for clinical isolates, 98% of whom harboured the
aac(6′)/
aph(2″) gene but just 58% of whom exhibited resistance to HLG by the disc diffusion method [
58]. However, the resistance rate to HLG was reduced in the studied WWTP, and the reduction effect was statistically significant.
In our study, the efflux pump gene emeA was detected in all ciprofloxacin-resistant enterococcal strains, suggesting that the drug efflux is the main mechanism involved in resistance to fluoroquinolones. Despite the reduced incidence of ermB and emeA genes and an increased prevalence of the rest of the tested ARGs, ermB and tetM remained the most common ARGs among the Enterococcus spp. in TWW. Therefore, all data obtained reveal that the WW treatment did not completely limit the incidence of resistant Enterococcus spp. in the WWTP effluents and could contribute to AMR spread in water environments.
The results of present study demonstrate a limited efficiency of the mechanical-biological treatment processes in the monitored WWTP with regard to HPC bacteria,
Enterobacteriaceae, and
Enterococcus spp. resistant to the studied antibiotics. The various effectiveness of removal of ARB and ARGs in WWTPs depending on the type of pollutants and operational characteristics of the treatment facilities [
1,
24] indicates a need for information on each different pollutant and particular waste water treatment facility. Usage of advanced WW treatment methods, including WWTP effluents disinfection with chlorine, ozone, or UV, has been explored as an approach to reduce AMR emissions into the environment [
11,
21,
59,
60]. Advanced WW treatment is a tool for removal of not only the target pollutants but also a range of additional ones, in this way providing collateral benefits. Therefore, when choosing measures to limit transmission of ARB in the environment, it is important to assess the risk level and the amount of costs to achieve certain benefits. Our study is a first step in assessing a regional situation of AMR spread, which reveals the need to assess AMR not only in urban WWTPs but also in other critical points of selection and transmission of AMR, such as urban sewage and hospital wastewater facilities. Considering the limitations of our study (a single WWTP studied, analysis of replicate samples, not pooled daily samples) we will enrich our research by directing it to other WWTPs, which differ in degree of treatment and type of treatment facilities, and by improving the sampling method as well. Continuing our investigations in this line will allow us to clarify the risks of selection of ARB in certain compartments of the urban water cycle and to prioritize measures to limit the spread of AMR in the receiving waters.