Comparative Studies of Regeneration and Single Batch Design for the Properties of Basic Blue-41 Removal Using Porous Clay and Porous Acid-Activated Heterostructures
Abstract
1. Introduction
2. Materials and Characterization
2.1. Materials and Chemicals
2.2. Acid Activation of Montmorillonite
2.3. Preparation of Porous Acid-Activated Clay Heterostructures
2.4. Batch Removal of BB-41
2.5. Regeneration Procedure
2.6. Characterization Techniques
3. Results and Discussion
3.1. XRF Data Analysis
3.2. XRD Data Analysis
3.3. TGA Data Analysis
3.4. 29Si NMR Data Analysis
3.5. The Textural Properties
3.6. Acidity Measurements
3.7. Influence of BB-41 Removal Parameters
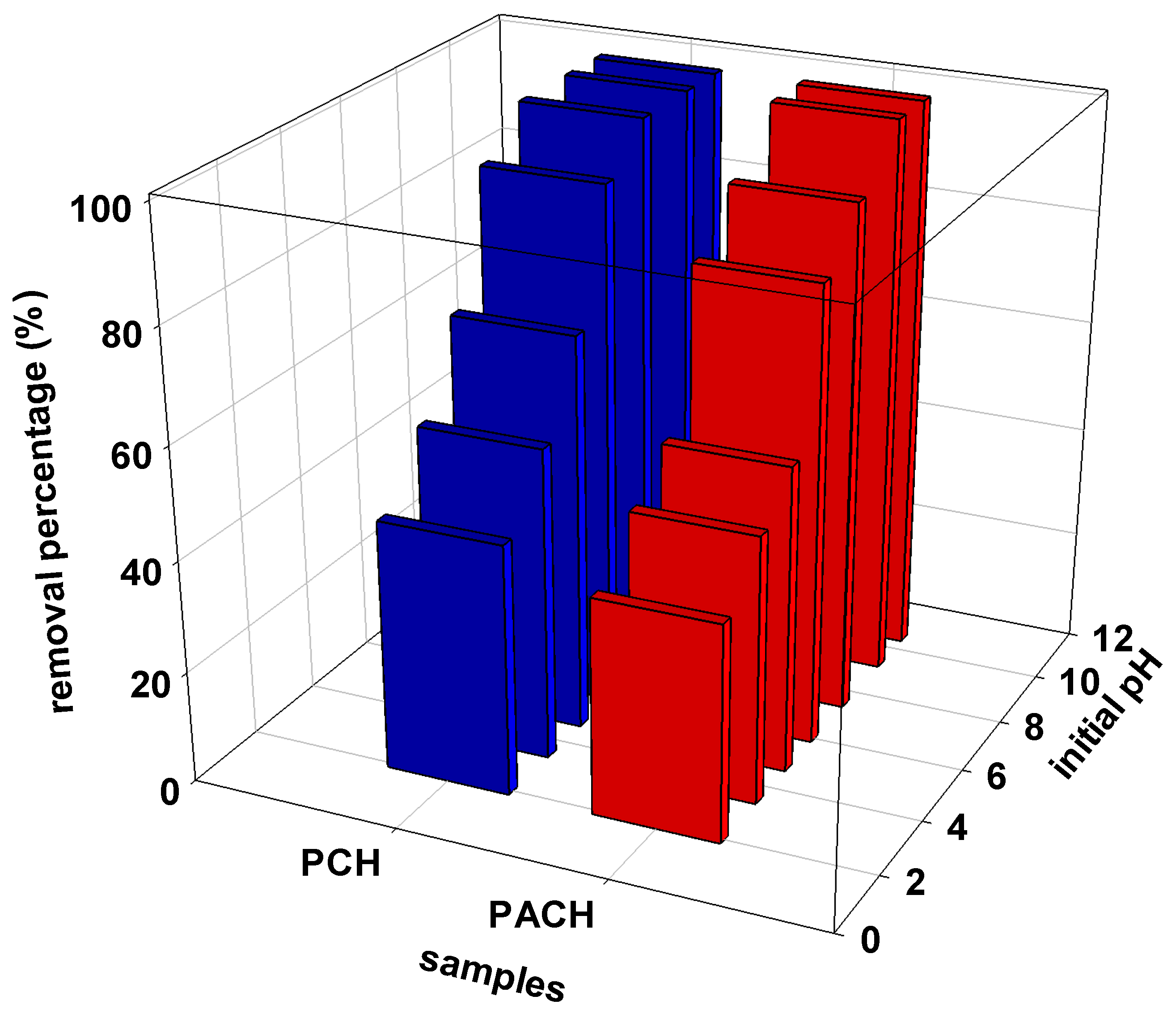
3.7.1. Effect of pH
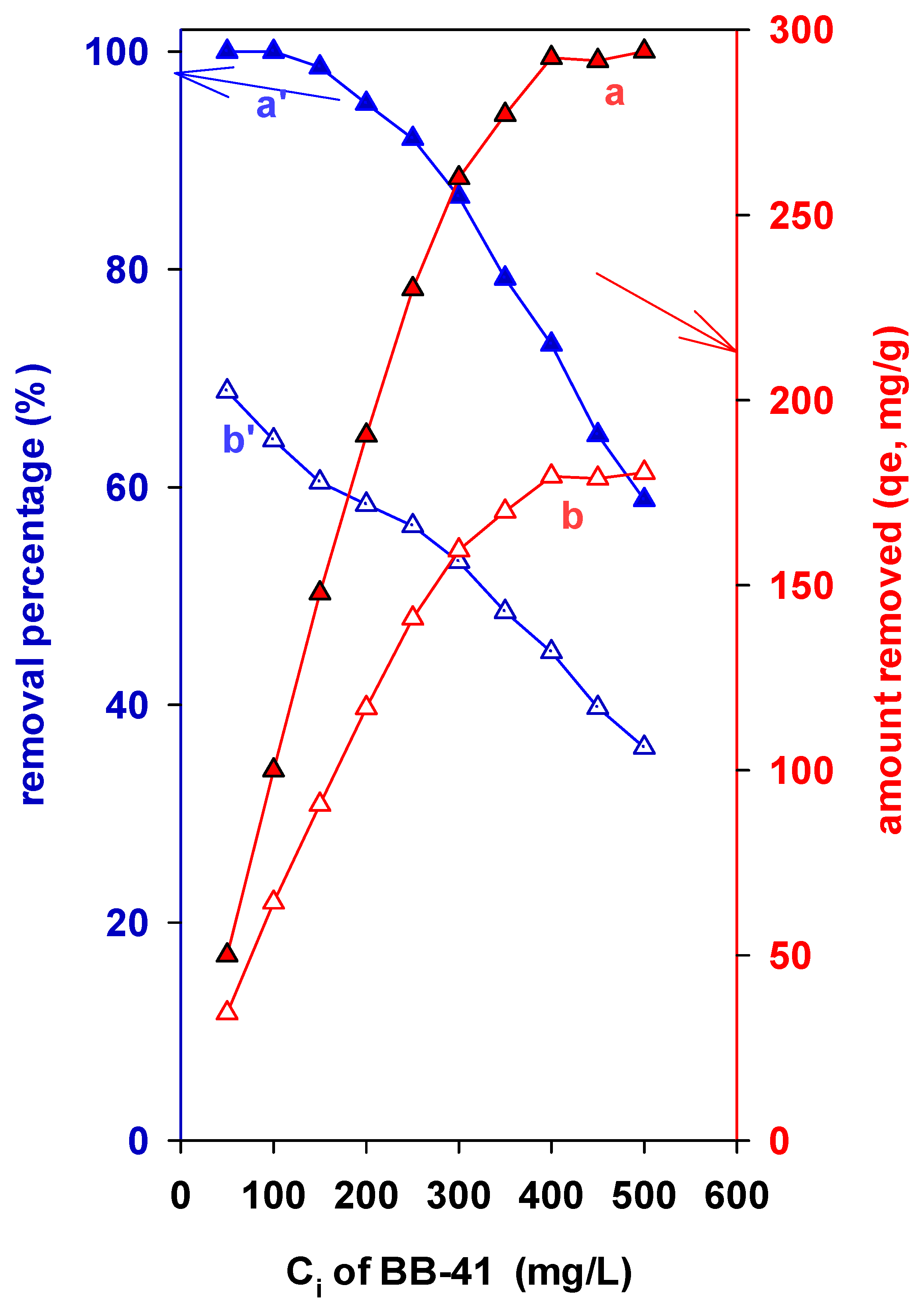
3.7.2. Effect of the Initial Concentration
3.7.3. Effect of the Dose
3.7.4. Effect of Acid Activation
3.7.5. Adsorption Model
3.7.6. Regeneration Studies
3.7.7. Single-Batch Design
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mekonnen, M.M.; Hoekstra, A.Y. Four billion people facing severe water scarcity. Sci. Adv. 2016, 2, e1500323. [Google Scholar] [CrossRef] [PubMed]
- World Economic Forum. Global Risks Report. 2019. Available online: https://www.weforum.org/reports/the-global-risks-report-2019 (accessed on 25 March 2019).
- Angelakis, A.; Voudouris, K.; Tchobanoglous, G. Evolution of water supplies in the Hellenic world focusing on water treatment and modern parallels. Water Supply 2020, 20, 773–786. [Google Scholar] [CrossRef]
- Morote, Á.-F.; Olcina, J.; Hernández, M. The use of non-conventional water resources as a means of adaptation to drought and climate change in Semi-Arid Regions: South-Eastern Spain. Water 2019, 11, 93. [Google Scholar] [CrossRef]
- Yannopoulos, S.; Giannopoulou, I.; Kaiafa-Saropoulou, M. Investigation of the current situation and prospects for the development of rainwater harvesting as a tool to confront water scarcity worldwide. Water 2019, 11, 2168. [Google Scholar] [CrossRef]
- Al-Madanat, O.; AlSalka, Y.; Ramadan, W.; Bahnemann, D.W. TiO2 Photocatalysis for the Transformation of Aromatic Water Pollutants into Fuels. Catalysts 2021, 11, 317. [Google Scholar] [CrossRef]
- Shen, K.-W.; Li, L.; Wang, J.-Q. Circular economy model for recycling waste resources under government participation: A case study in industrial wastewater circulation in China. Technol. Econ. Dev. Econ. 2020, 26, 21–47. [Google Scholar] [CrossRef]
- Lu, Y.; Song, S.; Wang, R.; Liu, Z.; Meng, J.; Sweetman, A.J.; Jenkins, A.; Ferrier, R.C.; Li, H.; Luo, W.; et al. Impacts of soil and water pollution on food safety and health risks in China. Environ. Int. 2015, 77, 5–15. [Google Scholar] [CrossRef]
- Jiries, A.; Al-Nasir, F.; Hijazin, T.J.; Al-Alawi, M.; El Fels, L.; Mayyas, A.; Al-Dmour, R.; Al-Madanat, O.Y. Polycyclic aromatic hydrocarbons in citrus fruit irrigated with fresh water under arid conditions: Concentrations, sources, and risk assessment. Arab. J. Chem. 2022, 15, 104027. [Google Scholar] [CrossRef]
- Al-Nasir, F.; Hijazin, T.J.; Al-Alawi, M.M.; Jiries, A.; Al-Madanat, O.Y.; Mayyas, A.; Al-Dalain, S.A.; Al-Dmour, R.; Alahmad, A.; Batarseh, M.I. Accumulation, Source Identification, and Cancer Risk Assessment of Polycyclic Aromatic Hydrocarbons (PAHs) in Different Jordanian Vegetables. Toxics 2022, 10, 643. [Google Scholar] [CrossRef]
- Panhwar, A.; Jatoi, A.S.; Mazari, S.A.; Kandhro, A.; Rashid, U.; Qaisar, S. Water resources contamination and health hazards by textile industry effluent and glance at treatment techniques: A review. Waste Manag. Bull. 2024, 1, 158–163. [Google Scholar] [CrossRef]
- Samsami, S.; Mohamadi, M.; Sarrafzadeh, M.H.; Rene, E.R.; Firoozbahr, M. Recent advances in the treatment of dye-containing wastewater from textile industries: Overview and perspectives. Process Saf. Environ. Prot. 2020, 143, 138–163. [Google Scholar] [CrossRef]
- Ombaka, L.M.; McGettrick, J.D.; Oseghe, E.O.; Al-Madanat, O.; Rieck genannt Best, F.; Msagati, T.A.M.; Davies, M.L.; Bredow, T.; Bahnemann, D.W. Photocatalytic H2 production and degradation of aqueous 2-chlorophenol over B/N-graphene-coated Cu0/TiO2: A DFT, experimental and mechanistic investigation. J. Environ. Manag. 2022, 311, 114822. [Google Scholar] [CrossRef] [PubMed]
- Younas, F.; Mustafa, A.; Farooqi, Z.U.R.; Wang, X.; Younas, S.; Mohy-Ud-Din, W.; Ashir Hameed, M.; Mohsin Abrar, M.; Maitlo, A.A.; Noreen, S.; et al. Current and Emerging Adsorbent Technologies for Wastewater Treatment: Trends, Limitations, and Environmental Implications. Water 2021, 13, 215. [Google Scholar] [CrossRef]
- Kausar, A.; Iqbal, M.; Javed, A.; Aftab, K.; Bhatti, H.N.; Nouren, S. Dyes adsorption using clay and modified clay: A review. J. Mol. Liq. 2018, 256, 395–407. [Google Scholar] [CrossRef]
- Ochirkhuyag, A.; Temuujin, J. The Catalytic Potential of Modified Clays: A review. Minerals 2024, 14, 629. [Google Scholar] [CrossRef]
- Baloyi, J.; Ntho, T.; Moma, J. Synthesis and application of pillared clay heterogeneous catalysts for wastewater treatment: A review. RSC Adv. 2018, 8, 5197. [Google Scholar] [CrossRef]
- Abdalqadir, M.; Gomari, S.R.; Pak, T.; Hughes, D.; Shwan, D. A comparative study of acid-activated non-expandable kaolinite and expandable montmorillonite for their CO2 sequestration capacity. React. Kinet. Mech. Catal. 2024, 137, 375–398. [Google Scholar] [CrossRef]
- Pardo, L.; Cecilia, J.A.; Moreno, C.L.; Hernández, V.; Pozo, M.; Bentabol, M.J.; Franco, F. Influence of the Structure and Experimental Surfaces Modifications of 2:1 Clay Minerals on the Adsorption Properties of Methylene Blue. Minerals 2018, 8, 359. [Google Scholar] [CrossRef]
- Dim, P.E.; Mustapha, L.S.; Termtanun, M. Isotherms, kinetics and thermodynamic studies of adsorption of Cd and Ni from textile effluent with acid modified clay. J. Chem. Technol. Metal. 2021, 56, 1016–1029. [Google Scholar]
- Khan, A.; Hassan, S.; Naqvi, J.; Kazmi, A.M.; Ashraf, Z. Surface activation of fuller’s earth (bentonite clay) using organic acids. Sci. Int. 2015, 27, 329–332. [Google Scholar]
- Nagendrappa, G.; Chowreddy, R.R. Organic reactions using clay and clay-supported catalysts: A survey of recent literature. Catal. Surv. Asia 2021, 25, 231–278. [Google Scholar] [CrossRef]
- España, V.A.A.; Sarkar, B.; Biswas, B.; Rusmin, R.; Naidu, R. Environmental applications of thermally modified and acid activated clay minerals: Current status of the art. Environ. Technol. Innov. 2019, 13, 383–397. [Google Scholar] [CrossRef]
- Yang, L.; Chen, X.; Ni, K.; Li, Y.; Wu, J.; Chen, W.; Ji, Y.; Feng, L.; Li, F.; Chen, D. Proton-exchanged montmorillonite-mediated reactions of hetero-benzyl acetates: Application to the synthesis of Zafirlukast. Tetrahedron Lett. 2020, 61, 152123. [Google Scholar] [CrossRef]
- Mokaya, R.; Jones, W. Pillared clays and pillared acid-activated clays: A comparative-study of physical, acidic, and catalytic properties. J. Catal. 1995, 153, 76–85. [Google Scholar] [CrossRef]
- Kooli, F.; Jones, W. Al and Zr pillared acid-activated saponite clays: Characterization and properties. J. Mater. Chem. 1998, 8, 2119–2124. [Google Scholar] [CrossRef]
- Kooli, F. The effects of acid activation on the thermal properties of polyvinylpyrrolidone and organoclay composites. J. Chem. 2015, 2015, 919636. [Google Scholar] [CrossRef][Green Version]
- Kooli, F. Thermal stability investigation of organo-acid-activated clays by TG-MS and in situ XRD techniques. Thermochim. Acta 2016, 486, 71–76. [Google Scholar] [CrossRef]
- Kooli, F.; Rakass, S.; Liu, Y.; Abboudi, M.; Oudghiri Hassani, H.; Ibrahim, S.M.; Al Wadaani, F.; Al-Faze., R. Eosin Removal by cetyl trimethylammonium-cloisites: Influence of the surfactant solution type and regeneration properties. Molecules 2019, 24, 3015–3044. [Google Scholar] [CrossRef]
- Kooli, F.; Khimyak, Y.Z.; Alshahateet, S.F.; Chen, F. Effect of the acid activation levels of montmorillonite clay on the cetyltrimethylammonium cations adsorption. Langmuir 2005, 21, 8717–8723. [Google Scholar] [CrossRef]
- Kooli, F.; Yan, L. Chemical and thermal properties of organoclays derived from highly stable bentonite in sulfuric acid. Appl. Clay Sci. 2013, 83–84, 349–356. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.W.; Olson, D.H.; Sheppard, E.W.; Mccullen, S.B.; et al. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Galarneau, A.; Barodawalla, A.; Pinnavaia, T. Porous clay heterostructures formed by gallery-templated synthesis. Nature 1995, 374, 529–531. [Google Scholar] [CrossRef]
- Garea, S.A.; Mihal, A.; Vasile, E.; Voicu, G. Synthesis and characterization of porous clay heterostructures. Rev. Chim. 2014, 65, 649–656. [Google Scholar]
- Garea, S.A.; Mihai, A.I.; Ghebaur, A.; Nistor, C.; Sarbu, A. Porous clay heterostructures: A new inorganic host for 5-fluorouracil encapsulation. Int. J. Pharm. 2015, 491, 299–309. [Google Scholar] [CrossRef]
- Sorina, A.; Gârea, S.A.; Ghebaur, A.; Nistor, C.L.; Andrei Sârbu, A.; Vasile, E.; Mitran, R.; Iovu, H. New nanocarriers based on Porous Clay Heterostructures (PCH) designed for methotrexate delivery. Microporous Mesoporous Mater. 2021, 328, 111434. [Google Scholar]
- Cecilia, J.A.; García-Sancho, C.; Vilarrasa-García, E.; Jiménez-Jiménez, J.; Rodriguez-Castellón, E. Synthesis, characterization, uses and applications of porous clays heterostructures: A review. Chem. Rec. 2018, 18, 1085–1104. [Google Scholar] [CrossRef]
- Popoola, S.A.; Al Dmour, H.; Al-Faze, R.; Alam, M.G.; Rakass, S.; Oudghiri Hassani, H.; Kooli, F. Regeneration and Single Stage Batch Adsorber Design for Efficient Basic Blue-41 Dye Removal by Porous Clay Heterostructures Prepared from Al13 Montmorillonite and Pillared Derivatives. Materials 2024, 17, 4948. [Google Scholar] [CrossRef]
- Chmielarz, L.; Kowalczyk, A.; Skoczek, M.; Rutkowska, M.; Gil, B.; Natkański, P.; Radko, M.; Motak, M.; Dębek, R.; Ryczkowski, J. Porous clay heterostructures intercalated with multicomponent pillars as catalysts for dehydration of alcohols. Appl. Clay Sci. 2018, 160, 116–125. [Google Scholar] [CrossRef]
- Yuan, M.; Deng, W.; Dong, S.; Li, Q.; Zhao, B.; Su, Y. Montmorillonite based porous clay heterostructures modified with Fe as catalysts for selective catalytic reduction of NO with propylene. Chem. Eng. J. 2018, 353, 839–848. [Google Scholar] [CrossRef]
- Kooli, F.; Jones, W. Characterization and catalytic properties of a saponite clay modified by acid activation. Clay Miner. 1997, 32, 633–643. [Google Scholar] [CrossRef]
- Kooli, F.; Liu, Y.; Al-Faze, R.; Al Suhaimi, A. Effect of acid activation of Saudi local clay mineral on removal properties of basic blue 41 from an aqueous solution. Appl. Clay Sci. 2015, 1165, 23–30. [Google Scholar] [CrossRef]
- Espantaleón, A.G.; Nieto, J.A.; Fernández, M.; Marsal, A. Use of activated clays in the removal of dyes and surfactants from tannery waste waters. Appl. Clay Sci. 2003, 24, 105–110. [Google Scholar] [CrossRef]
- Alanazi, A.M.; Jefri, O.A.; Gulfam Alam, M.; Al-Faze, R.; Kooli, F. Organo acid-activated clays for water treatment as removal agent of Eosin-Y: Properties, regeneration, and single batch design absorber. Heliyon 2024, 10, e30530. [Google Scholar] [CrossRef] [PubMed]
- Pichowicza, M.; Mokaya, R. Porous clay heterostructures with enhanced acidity obtained from acid-activated clays. Chem. Commun. 2001, 2001, 2100–2101. [Google Scholar] [CrossRef]
- Kooli, F.; Hian, P.C.; Weirong, Q.; Alshahateet, S.F.; Chen, F. Effect of the acid-activated clays on the properties of porous clay heterostructures. J. Porous Mater. 2006, 13, 319–324. [Google Scholar] [CrossRef]
- Prashantha Kumar, T.K.M.; Mandlimath, T.R.; Sangeetha, P.; Sakthivel, P.; Revathi, S.K.; Ashok Kumar, S.K.; Sahoo, S.K. Highly efficient performance of activated carbon impregnated with Ag, ZnO and Ag/ZnO nanoparticles as antimicrobial materials. RSC Adv. 2015, 5, 108034. [Google Scholar] [CrossRef]
- Kooli, F.; Liu, Y.; Alshahateet, S.F.; Messali, M.; Bergaya, F. Reaction of acid activated montmorillonites with hexadecyl trimethylammonium bromide solution. Appl. Clay Sci. 2009, 43, 357–363. [Google Scholar] [CrossRef]
- Tomić, Z.P.; Antić Mladenović, S.B.; Babić, B.M.; Poharc Logar, V.A.; Dorđević, A.R.; Cupać, S.B. Modification of smectite structure by sulphuric acid and characteristics of the modified smectite. J. Agric. Sci. 2011, 56, 25–35. [Google Scholar]
- Arellano-Cárdenas, S.; Gallardo-Velázquez, T.; Osorio-Revilla, G.; López-Cortez, M.d.S. Preparation of a porous clay heterostructure and study of its adsorption capacity of phenol and chlorinated phenols from aqueous solutions. Water Environ. Res. 2008, 80, 60–67. [Google Scholar] [CrossRef]
- Kooli, F. Porous clay heterostructures (PCHs) from Al13-intercalated and Al13-pillared montmorillonites: Properties and heptane hydro-isomerization catalytic activity. Microporous Mesoporous Mater. 2014, 184, 184–192. [Google Scholar] [CrossRef]
- Morodome, S.; Kawamura, K. Swelling behavior of na- and ca-montmorillonite up to 150ºC by in situ x-ray diffraction experiments. Clays Clay Miner. 2009, 57, 150–160. [Google Scholar] [CrossRef]
- Krupskaya, V.V.; Zakusin, S.V.; Tyupina, E.A.; Dorzhieva, O.V.; Zhukhlistov, A.P.; Belousov, P.E.; Timofeeva, M.N. Experimental Study of Montmorillonite Structure and Transformation of Its Properties under Treatment with Inorganic Acid Solutions. Minerals 2017, 7, 49. [Google Scholar] [CrossRef]
- Bahranowski, K.; Klimek, A.; Gaweł, A.; Olejniczak, Z.; Serwicka, E.M. Rehydration Driven Acid Impregnation of Thermally Pretreated Ca-Bentonite-Evolution of the Clay Structure. Materials 2022, 15, 2067. [Google Scholar] [CrossRef]
- Ferrage, E.; Tournassat, C.; Rinnert, E.; Lanson, B. Influence of pH on the interlayer cationic composition and hydration state of Ca-montmorillonite: Analytical chemistry, chemical modelling and XRD profile modelling study. Geochim. Cosmochim. Acta 2005, 69, 2797–2812. [Google Scholar] [CrossRef][Green Version]
- Gavin, P.; Chevrier, V. Thermal alteration of nontronite and montmorillonite: Implications for the martian surface. Icarus 2010, 208, 721–734. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, P.; Wen, K.; Su, X.; Zhu, J.; He, H. A new insight into the compositional and structural control of porous clay heterostructures from the perspective of NMR and TEM. Microporous Mesoporous Mater. 2016, 224, 285–293. [Google Scholar] [CrossRef]
- Chmielarz, L.; Kustrowski, P.; Piwowarska, Z.; Dudek, B.; Gil, B.; Michalik, M. Montmorillonite, vermiculite and saponite based porous clay heterostructures modified with transition metals as catalysts for the DeNOx process. Appl. Catal. B Environ. 2009, 88, 331–340. [Google Scholar] [CrossRef]
- Alam, N.; Mokaya, R. Crystalline mesoporous silicates from layered precursors. J. Mater. Chem. 2008, 18, 1383–1391. [Google Scholar] [CrossRef]
- Garea, S.A.; Mihai, A.I.; Vasile, E.; Nistor, C.; Sarbu, A.; Mitran, R. Synthesis of new porous clay heterostructures: The influence of co-surfactant type. Mater. Chem. Phys. 2016, 179, 17–26. [Google Scholar] [CrossRef]
- Kashif, M.; Chaeyeon Kang, C.; Siddhartha, T.R.; Che, C.A.; Su, Y.; Heynderickx, P.M. Investigating the effects of calcination temperature on porous clay heterostructure characteristics. Vacuum 2024, 227, 113145. [Google Scholar] [CrossRef]
- Popoola, S.A.; Al Dmour, H.; Rakass, S.; Fatimah, I.; Liu, Y.; Mohmoud, A.; Kooli, F. Enhancement Properties of Zr Modified Porous Clay Heterostructures for Adsorption of Basic-Blue 41 Dye: Equilibrium, Regeneration, and Single Batch Design Adsorber. Materials 2022, 15, 5567. [Google Scholar] [CrossRef] [PubMed]
- Tkac, I.; Komadel, P.; Muller, D. Acid-treated montmoril-lonites: A study by 29Si and 27Al MAS NMR. Clay Miner. 1994, 29, 11–19. [Google Scholar] [CrossRef]
- Pálková, H.; Madejová, J.; Zimowska, M.; Bielanska, E.; Zbigniew Olejniczak, Z.; Lityńska-Dobrzynska, L.; Serwicka, E.M. Enotiadis,-derived porous clay heterostructures: I. Synthesis and physicochemical characterization. Microporous Mesoporous Mater. 2009, 127, 228–236. [Google Scholar] [CrossRef]
- Jozefaciuk, G.; Bowanko, G. Effect of acid and alkali treatments on surface areas and adsorption energies of selected minerals. Clays Clay Miner. 2002, 50, 771–778. [Google Scholar] [CrossRef]
- Amari, A.; Gannouni, H.; Khan, M.I.; Almesfer, M.K.; Abubakr, M.; Elkhaleefa, A.M.; Gannouni, A. Effect of Structure and Chemical Activation on the Adsorption Properties of Green Clay Minerals for the Removal of Cationic Dye. Appl. Sci. 2018, 8, 2302. [Google Scholar] [CrossRef]
- Yang, P.; Song, M.; Kim, D.; Jung, S.P.; Hwang, Y. Synthesis conditions of porous clay heterostructure (PCH) optimized for volatile organic compounds (VOC) adsorption. Korean J. Chem. Eng. 2019, 36, 1806–1813. [Google Scholar] [CrossRef]
- Enotiadis, A.; Tsokaridou, M.; Chalmpes, N.; Sakavitsi, V.; Spyrou, K.; Gournis, D. Synthesis and characterization of porous clay-organic heterostructures. J. Sol-Gel Sci. Techn. 2019, 91, 295–301. [Google Scholar] [CrossRef]
- Son, Y.; Kim, T.H.; Kim, D.; Hwang, Y. Porous clay heterostructure with alginate encapsulation for toluene removal. Nano-materials 2021, 11, 388. [Google Scholar] [CrossRef]
- Srithammaraj, K.; Magaraphan, R.; Manuspiya, H. Surfactant-Templated Synthesis of Modified Porous Clay Heterostructure (PCH). Adv. Mater. Res. 2008, 55–57, 317–320. [Google Scholar] [CrossRef]
- Besghaier, S.; Cecilia, J.A.; Chouikhi, N.; Vilarrasa-García, E.; Rodríguez-Castellón, E.; Chlendi, M.; Bagane, M. Glyphosate adsorption onto porous clay heterostructure (PCH): Kinetic and thermodynamic studies. Braz. J. Chem. Eng. 2022, 39, 903–917. [Google Scholar] [CrossRef]
- Perdigon, A.C.; Li, D.; Pesquera, C.; Gonzalez, F.; Ortiz, B.; Aguado, F.; Blanco, C. Synthesis of porous clay heterostructures from high charge mica-type aluminosilicates. J. Mater. Chem. A 2013, 1, 1213–1219. [Google Scholar] [CrossRef]
- Arab, C.; El Kurdi, R.; Patra, D. Effect of pH on the removal of anionic and cationic dyes using zinc curcumin oxide nanoparticles as adsorbent. Mater. Chem. Phys. 2022, 277, 125504. [Google Scholar] [CrossRef]
- Ta, M.; An, Y.; Yang, H.; Bai, C.; Wang, T.; Zhang, T.; Cai, H.; Zheng, H. Attraction behavior induces the aggregation and precipitation of organic dyes: Improving efficiency through co-treatment strategy. J. Mol. Liq. 2024, 415, 126331. [Google Scholar] [CrossRef]
- Mills, A.; Hazafy, D.; Parkinson, J.; Tuttle, T.; Hutchings, M.G. Effect of alkali on methylene blue (C.I. Basic Blue 9) and other thiazine dyes. Dyes Pigm. 2011, 88, 149–155. [Google Scholar] [CrossRef]
- Dodoo, D.; Fynn, G.E.; Yawson, E.S.C.; Appiah, G.; Suleiman, N.; Yaya, A. Eco-efficient treatment of hazardous bauxite liquid-residue using acid-activated clays. Clean. Chem. Eng. 2022, 3, 100040. [Google Scholar] [CrossRef]
- Nwosu, F.O.; Ajala, O.J.; Owoyemi, R.M.; Raheem, B.G. Preparation and characterization of adsorbents derived from bentonite and kaolin clays. Appl. Water Sci. 2018, 8, 195. [Google Scholar] [CrossRef]
- Dehmani, Y.; Franco, D.S.P.; Georgin, J.; Lamhasni, T.; Brahmi, Y.; Oukhrib, R.; Mustapha, B.; Moussout, H.; Ouallal, H.; Sadik, A. Comparison of phenol adsorption property and mechanism onto different moroccan clays. Water 2023, 15, 1881. [Google Scholar] [CrossRef]
- Aghazadeh, V.; Barakan, S.; Bidari, E. Determination of surface protonation-deprotonation behavior, surface charge, and total surface site concentration for natural, pillared and porous nano bentonite heterostructure. J. Mol. Struct. 2020, 1204, 127570. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; Elgarahy, A.M.; Elshoubaky, G.A.; Mohammad, S.H. Microwave assist sorption of crystal violet and Congo Red dyes onto amphoteric sorbent based on upcycled sepia shells. J. Environ. Health Sci. Eng. 2020, 18, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Rápó, E.; Tonk, S. Factors Affecting Synthetic Dye Adsorption; Desorption Studies: A Review of Results from the Last Five Years (2017–2021). Molecules 2021, 26, 5419. [Google Scholar] [CrossRef] [PubMed]
- Soltani, A.; Faramarzi, M.; Mousavi Parsa, S.A. A review on adsorbent parameters for removal of dye products from industrial wastewater. Water Qual. Res. J. 2021, 56, 181–193. [Google Scholar] [CrossRef]
- Agarwala, R.; Mulky, L. Adsorption of Dyes from Wastewater: A Comprehensive Review. Chem. Bio. Eng. Rev. 2023, 10, 326–335. [Google Scholar] [CrossRef]
- Mahdieh Mozaffari Majd, Vahid Kordzadeh-Kermani, Vahab Ghalandari, Anis Askari, Mika Sillanpää, Adsorption isotherm models: A comprehensive and systematic review (2010−2020). Sci. Total Environ. 2022, 812, 151334. [CrossRef]
- Saha, T.K.; Bishwas, R.K.; Karmaker, S.; Islam, Z. Adsorption Characteristics of Allura Red AC onto Sawdust and Hexa-decylpyridinium Bromide-Treated Sawdust in Aqueous Solution. ACS Omega 2020, 5, 13358–13374. [Google Scholar] [CrossRef]
- Humelnicu, I.; Baiceanu, A.; Ignat, M.; Dulman, V. The removal of Basic Blue 41 textile dye from aqueous solution by ad-sorption onto natural zeolitic tuff: Kinetics and thermodynamics. Process Saf. Environ. Prot. 2017, 105, 274–287. [Google Scholar] [CrossRef]
- Alanazi, A.M.; Al Dmour, H.; Popoola, S.A.; Oudghiri Hassani, H.; Rakass, S.; Al-Faze, R.; Kooli, F. Parameters Synthesis of Na-Magadiite Materials for Water Treatment and Removal of Basic Blue-41: Properties and Single-Batch Design Adsorber. Inorganics 2023, 11, 423. [Google Scholar] [CrossRef]
- Al-Madanat, O.Y.; Popoola, S.A.; Al Dmour, H.; Al-Faze, R.; Kooli, F. Na-Kenyaite as Efficient Basic Blue-41 Dye Removal: Synthesis and Regeneration Studies. Water 2024, 16, 2056. [Google Scholar] [CrossRef]
- Roulia, M.; Alexandros, V. Interactions between C.I. Basic Blue 41 and aluminosilicate sorbents. J. Colloid Interface Sci. 2005, 291, 37–44. [Google Scholar] [CrossRef]
- Zhu, R.; Zhu, J.; Ge, F.; Yuan, P. Regeneration of spent organoclays after the sorption of organic pollutants: A review. J. Environ. Manag. 2009, 90, 3212–3216. [Google Scholar] [CrossRef] [PubMed]
- El Messaoudi, N.; El Khomri, M.; El Mouden, A.; Bouich, A.; Jada, A.; Lacherai, A.; Iqbal, H.M.; Mulla, S.I.; Kumar, V.; Américo-Pinheiro, J.H. Regeneration and reusability of non-conventional low-cost adsorbents to remove dyes from wastewaters in multiple consecutive adsorption–desorption cycles: A review. Biomass Conv. Biorefin. 2024, 14, 11739–11756. [Google Scholar] [CrossRef]
- Khan, S.; Ajmal, S.; Hussain, T.; Ur Rahman, M. Clay-based materials for enhanced water treatment: Adsorption mechanisms, challenges, and future directions. J. Umm Al-Qura Univ. Appl. Sci. 2023, 1–16. [Google Scholar] [CrossRef]
- Ozacar, M.; Sengil, I.A. Equilibrium data and process design for adsorption of disperse dyes onto Alunite. Environ. Geol. 2004, 45, 762–768. [Google Scholar] [CrossRef]
- Popoola, S.A.; Al Dmour, H.; Messaoudi, B.; Fatimah, I.; Rakass, S.; Liu, Y.; Kooli, F. Organophilic clays for efficient removal of eosin Y dye properties. J. Saudi Chem. Soc. 2023, 27, 101723. [Google Scholar] [CrossRef]






| Samples | Mnt | A-Mnt | PCH | PACH |
|---|---|---|---|---|
| SiO2 | 56 | 63.9 | 79.9 | 84.2 |
| Al2O3 | 22.4 | 16.3 | 5.54 | 3.21 |
| MgO | 3.00 | 1.59 | 0.66 | 0.54 |
| CaO | 1.77 | 0.055 | 0.014 | 0.033 |
| Fe2O3 | 0.884 | 0.628 | 0.139 | 0.467 |
| TiO2 | 0.236 | 0.164 | 0.03 | 0.055 |
| K2O | 0.108 | 0.084 | 0.03 | 0.055 |
| CEC (meq/g) | 0.92 | 0.81 | --- | --- |
| Samples | SBET (m2 g−1) | T.P.V. (mL g−1) | A.P.D. (nm) | Acidity * |
|---|---|---|---|---|
| Mnt | 95 | 0.07 | 10.9 | 0.51 |
| A-Mnt | 150 | 0.11 | 6.4 | 0.61 |
| PCH-pre | 197 | 0.246 | 4.98 | --- |
| PACH-pre | 271 | 0.269 | 3.97 | --- |
| PCH-cal | 740 | 0.49 | 2.64 | 0.58 |
| PACH-cal | 890 | 0.57 | 2.56 | 0.56 |
| Samples | qmax (mg/g) | KL (L/g) | R2 | PCC * | Γmax, mg/m2 |
|---|---|---|---|---|---|
| Mnt | 57 | 0.0289 | 0.9854 | 0.9993 | 0.633 |
| A-Mnt | 73 | 0.0518 | 0.9996 | 0.9996 | 0.4866 |
| PCH-cal | 243 | 0.0804 | 0.9997 | 0.9993 | 0.3283 |
| PACH-cal | 298 | 0.2673 | 0.9998 | 0.9996 | 0.3348 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Madanat, O.Y.; Popoola, S.A.; Altarawneh, R.M.; Alraddadi, T.S.; Alam, M.G.; Al Dmour, H.; Kooli, F.; Said, M.A. Comparative Studies of Regeneration and Single Batch Design for the Properties of Basic Blue-41 Removal Using Porous Clay and Porous Acid-Activated Heterostructures. Water 2025, 17, 2. https://doi.org/10.3390/w17010002
Al-Madanat OY, Popoola SA, Altarawneh RM, Alraddadi TS, Alam MG, Al Dmour H, Kooli F, Said MA. Comparative Studies of Regeneration and Single Batch Design for the Properties of Basic Blue-41 Removal Using Porous Clay and Porous Acid-Activated Heterostructures. Water. 2025; 17(1):2. https://doi.org/10.3390/w17010002
Chicago/Turabian StyleAl-Madanat, Osama Y., Saheed A. Popoola, Rakan M. Altarawneh, Thamer S. Alraddadi, Mohd Gulfam Alam, Hmoud Al Dmour, Fethi Kooli, and Musa A. Said. 2025. "Comparative Studies of Regeneration and Single Batch Design for the Properties of Basic Blue-41 Removal Using Porous Clay and Porous Acid-Activated Heterostructures" Water 17, no. 1: 2. https://doi.org/10.3390/w17010002
APA StyleAl-Madanat, O. Y., Popoola, S. A., Altarawneh, R. M., Alraddadi, T. S., Alam, M. G., Al Dmour, H., Kooli, F., & Said, M. A. (2025). Comparative Studies of Regeneration and Single Batch Design for the Properties of Basic Blue-41 Removal Using Porous Clay and Porous Acid-Activated Heterostructures. Water, 17(1), 2. https://doi.org/10.3390/w17010002







