Comparative Analysis of Volumetric Mass Transfer Coefficients for Oxygen Uptake and Desorption with Nanobubbles
Abstract
1. Introduction
2. Materials and Methods
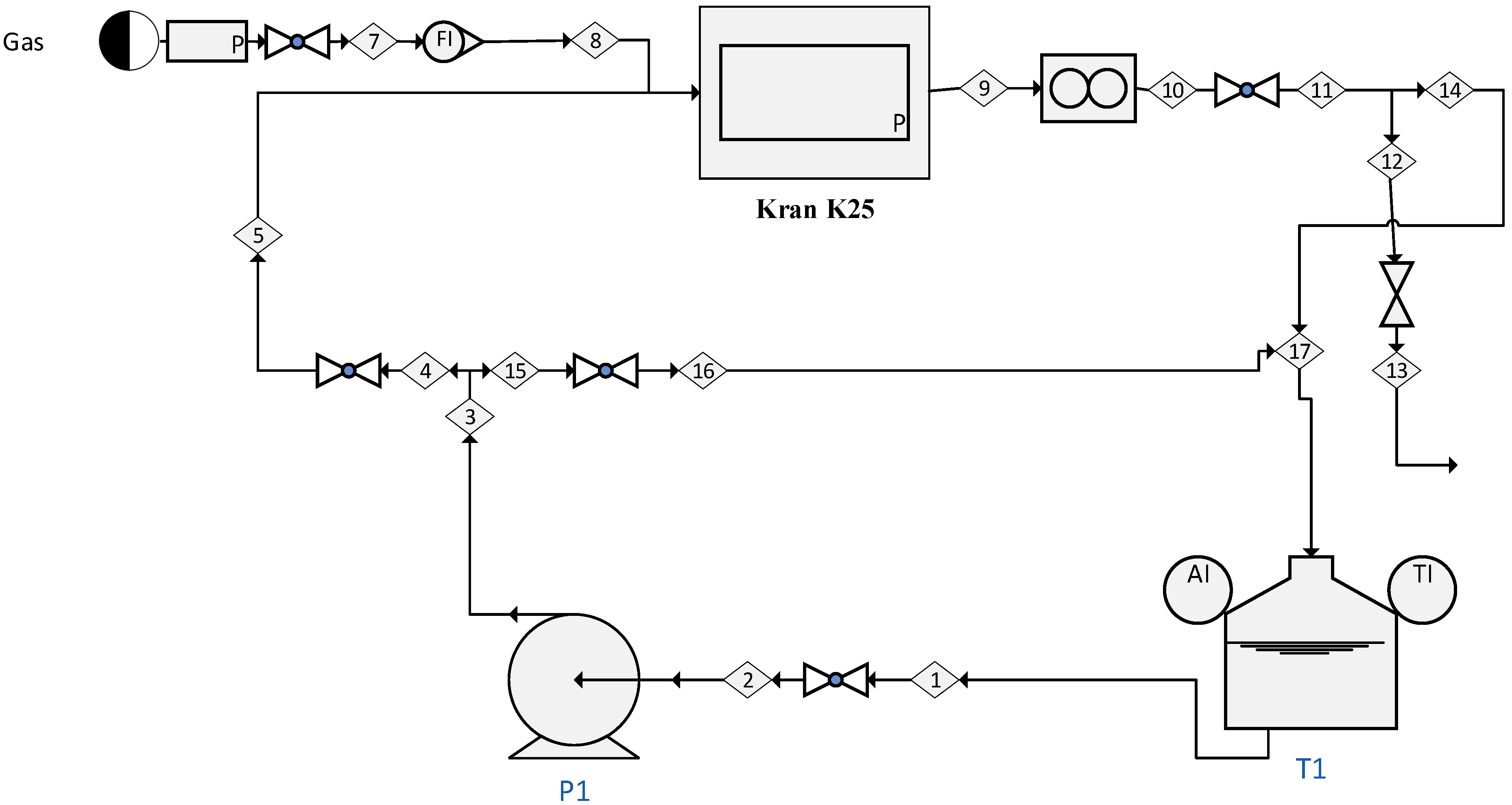
2.1. Experimental System
2.2. KLa Determination
2.3. Data Analysis
3. Results
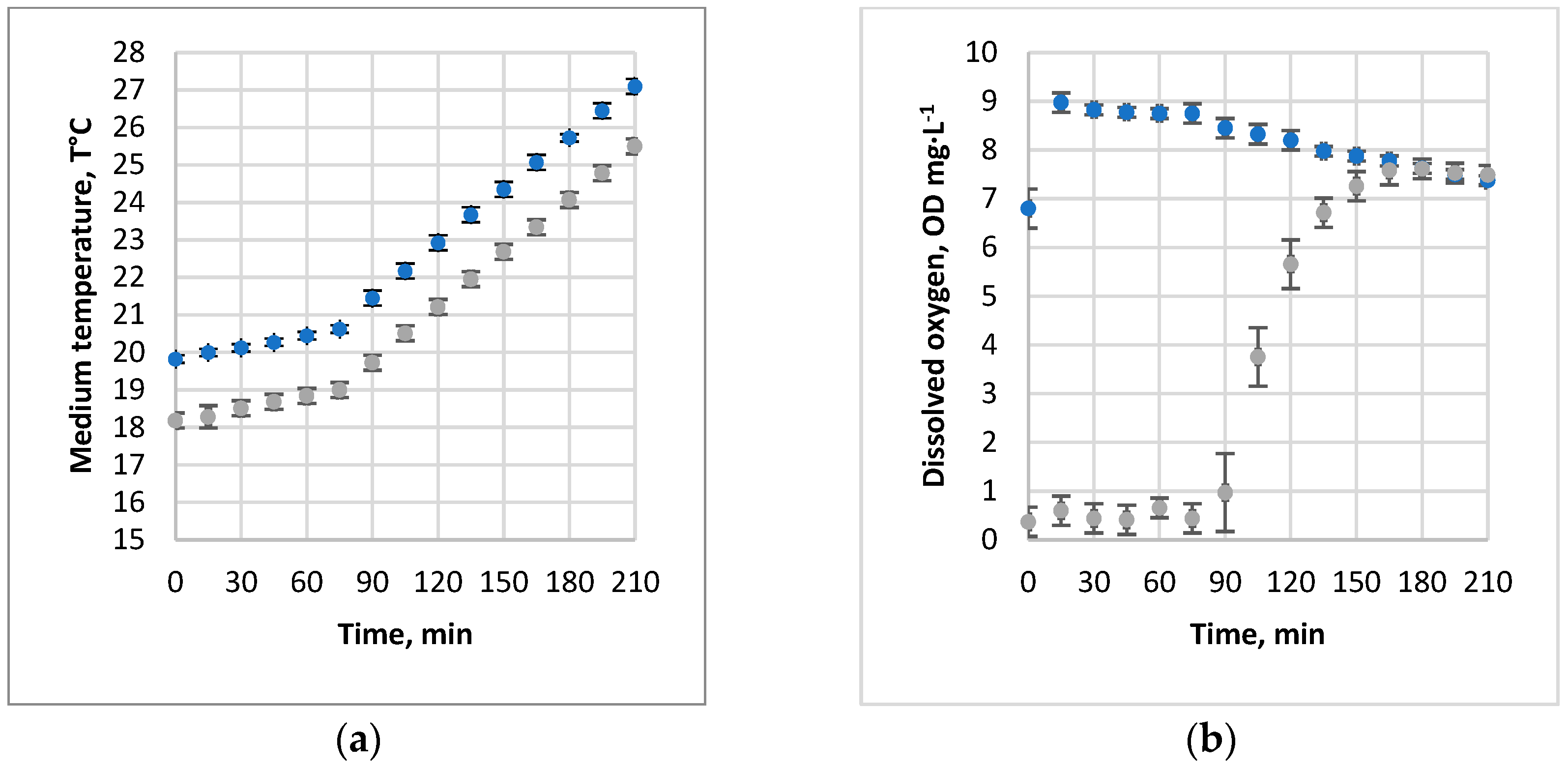
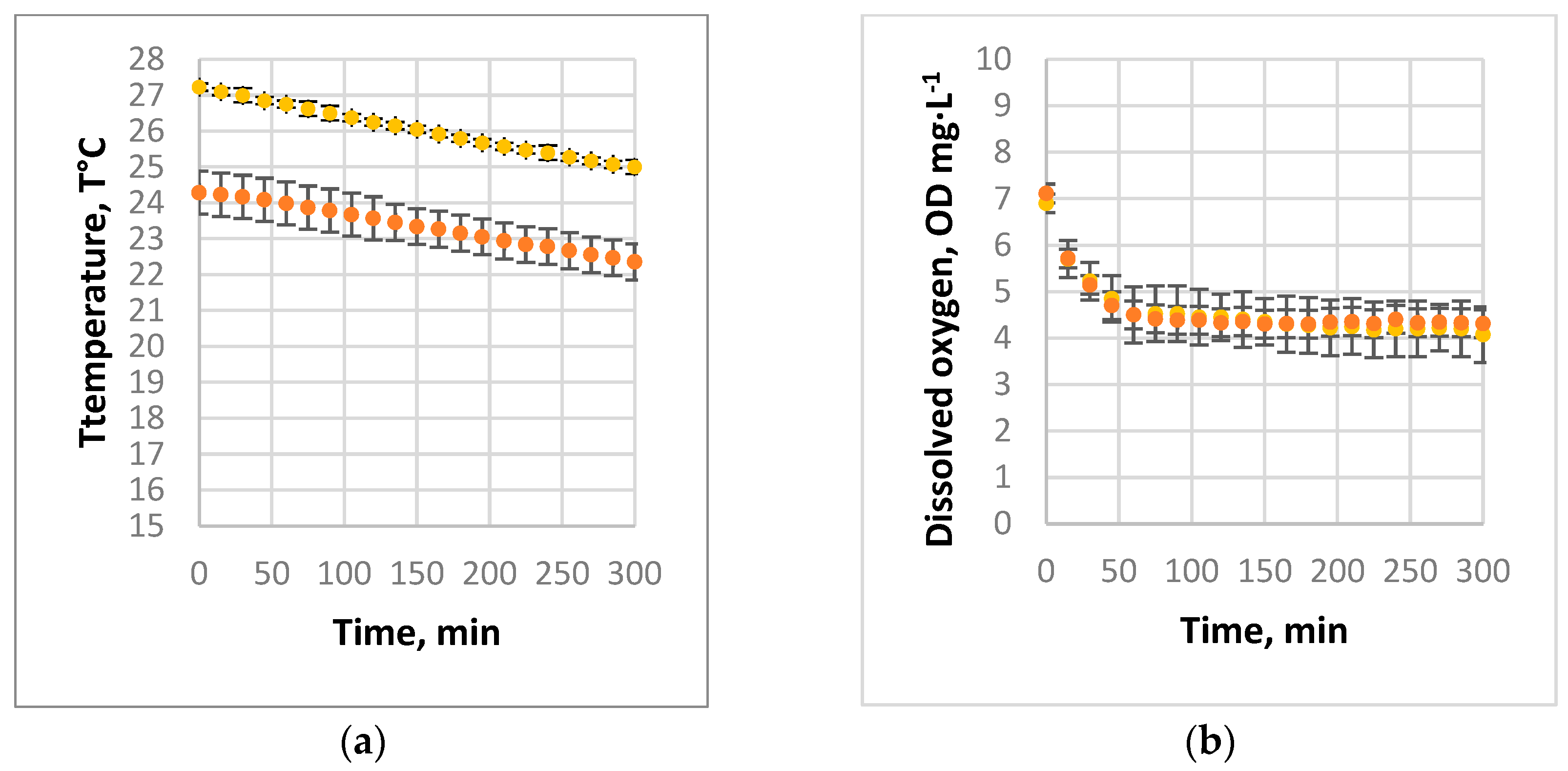
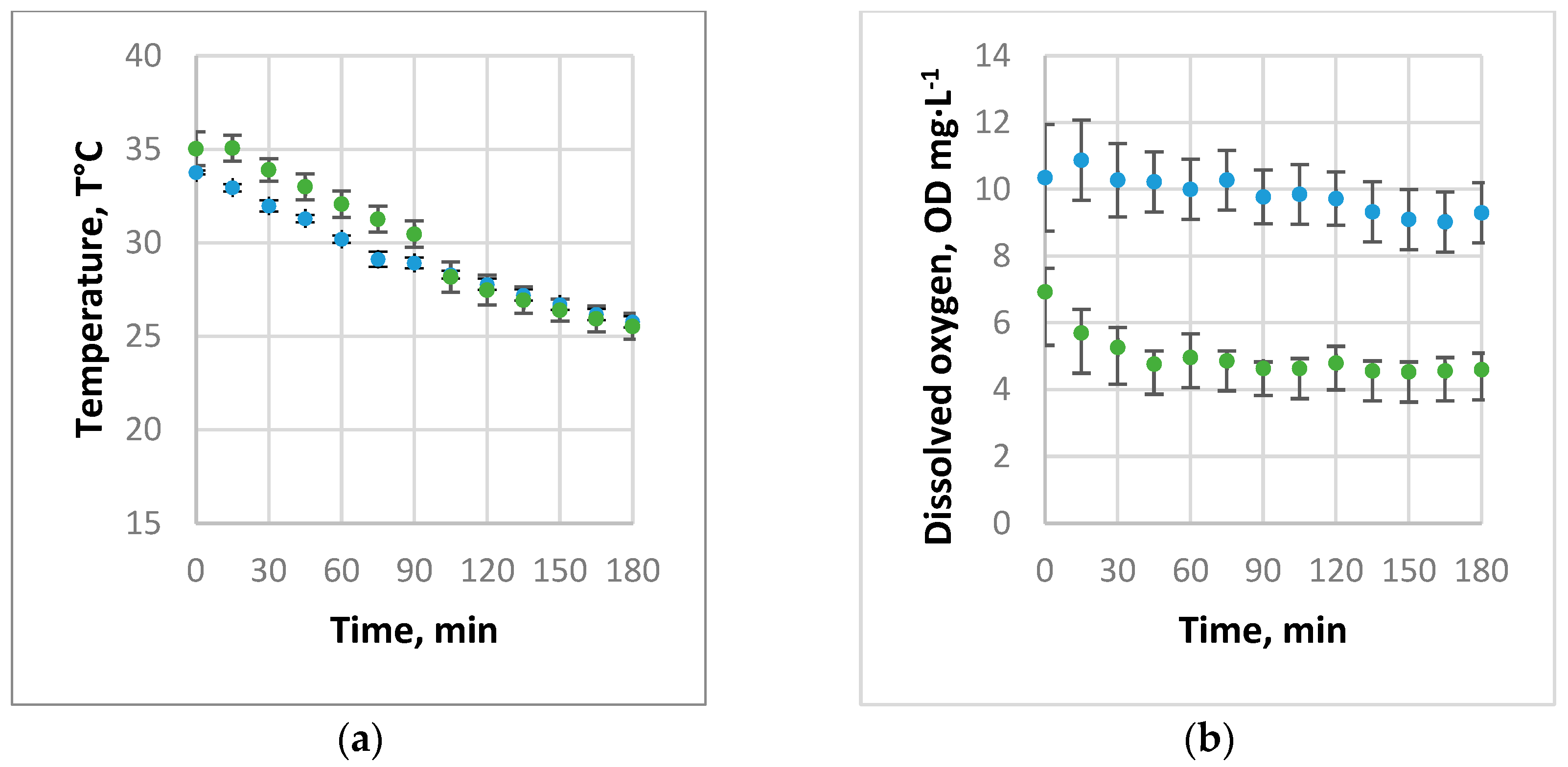
3.1. Absorption of Air and Oxygen by NBs
3.2. Oxygen Desorption Dynamics
3.3. Evaluation of kLa
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Thom, P.T.; Hai, L.D.; Khoi, N.T. Application of Nano Bubble Aeration Technology in Domestic Wastewater Treatment: Optimization of Gas Flow and Reaction Time. J. Glob. Ecol. Environ. 2024, 20, 146–154. [Google Scholar] [CrossRef]
- Jia, M.; Farid, M.U.; Ho, Y.-W.; Ma, X.; Wong, P.W.; Nah, T.; He, Y.; Boey, M.W.; Lu, G.; Fang, J.K.-H.; et al. Advanced Nanobubble Flotation for Enhanced Removal of Sub-10 Μm Microplastics from Wastewater. Nat. Commun. 2024, 15, 9079. [Google Scholar] [CrossRef]
- Guerra, J.; Lechón, J.; Mosquera, L.; Ferie, J.; Ormeño-Mejía, E.; Lechón, V. Nanobubbles Generation System for Bacteria Removal in Water through Hydrodynamic Cavitation. In Proceedings of the 2024 IEEE Eighth Ecuador Technical Chapters Meeting (ETCM), Cuenca, Ecuador, 15–18 October 2024; IEEE: Piscataway, NJ, USA, 2024; pp. 1–5. [Google Scholar]
- Radadiya, N.L.; Kumar, A.; Kalla, S. Micro-nanobubbles Assisted Fouling Reduction in Membrane Distillation for Desalination. Can. J. Chem. Eng. 2024. early review. [Google Scholar] [CrossRef]
- Zhu, N.; Li, M.; Shibata, K. The Development of a High-Concentration Oxygenated Water Generator Based on Nanobubbles and Its Application. Eng. Proc. 2023, 55, 23. [Google Scholar] [CrossRef]
- Yaparatne, S.; Morón-López, J.; Bouchard, D.; Garcia-Segura, S.; Apul, O.G. Nanobubble Applications in Aquaculture Industry for Improving Harvest Yield, Wastewater Treatment, and Disease Control. Sci. Total Environ. 2024, 931, 172687. [Google Scholar] [CrossRef] [PubMed]
- Foudas, A.W.; Kosheleva, R.I.; Favvas, E.P.; Kostoglou, M.; Mitropoulos, A.C.; Kyzas, G.Z. Fundamentals and Applications of Nanobubbles: A Review. Chem. Eng. Res. Des. 2023, 189, 64–86. [Google Scholar] [CrossRef]
- Patel, A.K.; Singhania, R.R.; Chen, C.-W.; Tseng, Y.-S.; Kuo, C.-H.; Wu, C.-H.; Dong, C. Di Advances in Micro- and Nano Bubbles Technology for Application in Biochemical Processes. Env. Technol. Innov. 2021, 23, 101729. [Google Scholar] [CrossRef]
- Thangadurai, D.; Shettar, A.K.; Sangeetha, J.; Adetunji, C.O.; Islam, S.; Al-Tawaha, A.R.M.S. Nanobubble Technology for Remediation of Metal-Contaminated Soil. In Nanomaterials for Soil Remediation; Elsevier: Amsterdam, The Netherlands, 2021; pp. 427–441. [Google Scholar]
- Zhang, Y.; Song, Z.; Sugita, K.; Xue, S.; Zhang, W. Impacts of Nanobubbles in Pore Water on Heavy Metal Pollutant Release from Contaminated Soil Columns. Nanomaterials 2023, 13, 1671. [Google Scholar] [CrossRef] [PubMed]
- DeBoer, E.J.; Richardson, M.D.; Gentimis, T.; McCalla, J.H. Analysis of Nanobubble-Oxygenated Water for Horticultural Applications. Horttechnology 2024, 34, 769–773. [Google Scholar] [CrossRef]
- Arablousabet, Y.; Povilaitis, A. The Impact of Nanobubble Gases in Enhancing Soil Moisture, Nutrient Uptake Efficiency and Plant Growth: A Review. Water 2024, 16, 3074. [Google Scholar] [CrossRef]
- Lee, Y.-G.; Han, J.-G.; Choi, J.-H.; Kim, D.-C.; You, S.-K.; Hong, K. Effect of Nano-Bubble on Removal of Complex Heavy Metals. J. Korean Geosynth. Soc. 2015, 14, 139–146. [Google Scholar] [CrossRef][Green Version]
- Lee, J.H.; Lee, S.H.; Suh, D.H. Using Nanobubblized Carbon Dioxide for Effective Microextraction of Heavy Metals. J. CO2 Util. 2020, 39, 101163. [Google Scholar] [CrossRef]
- Alkan, P.E.; Güneş, M.E.; Sabanci, A.Ü. Can Nanobubble Ozone Liposomes Be a New Agent in the Fight Against Foodborne Infections? Recent. Pat. Nanotechnol. 2024, 18, 17–21. [Google Scholar] [CrossRef]
- Shiroodi, S.; Schwarz, M.H.; Nitin, N.; Ovissipour, R. Efficacy of Nanobubbles Alone or in Combination with Neutral Electrolyzed Water in Removing Escherichia Coli O157:H7, Vibrio Parahaemolyticus, and Listeria Innocua Biofilms. Food Bioprocess Technol. 2021, 14, 287–297. [Google Scholar] [CrossRef]
- Magdaleno, A.L.; Cerrón-Calle, G.A.; dos Santos, A.J.; Lanza, M.R.V.; Apul, O.G.; Garcia-Segura, S. Unlocking the Potential of Nanobubbles: Achieving Exceptional Gas Efficiency in Electrogeneration of Hydrogen Peroxide (Small 3/2024). Small 2024, 20, 2470021. [Google Scholar] [CrossRef]
- Ho, D.; Kim, K.; Earmme, T.; Kim, C. Enhancing Gas–Liquid Volumetric Mass Transfer Coefficient. J. Ind. Eng. Chem. 2020, 87, 1–17. [Google Scholar] [CrossRef]
- Mast, Y.; Wild, M.; Takors, R. Optimizing Mass Transfer in Multiphase Fermentation: The Role of Drag Models and Physical Conditions. Processes 2023, 12, 45. [Google Scholar] [CrossRef]
- Sharma, H.; Nirmalkar, N. Enhanced Gas-Liquid Mass Transfer Coefficient by Bulk Nanobubbles in Water. Mater. Today Proc. 2022, 57, 1838–1841. [Google Scholar] [CrossRef]
- Wang, T.; Yang, C.; Sun, P.; Wang, M.; Lin, F.; Fiallos, M.; Khu, S.-T. Generation Mechanism of Hydroxyl Free Radicals in Micro–Nanobubbles Water and Its Prospect in Drinking Water. Processes 2024, 12, 683. [Google Scholar] [CrossRef]
- Nguyen, H.-H.T.; Jeong, Y.-H.; Choi, Y.-H.; Kwak, D.-H. Effect of Bubble Sizes on Oxygen Transfer Efficiency of Nano- and Micro-Sized Bubble Clouds for Improving Aquatic Environments. Int. J. Environ. Sci. Technol. 2024. [Google Scholar] [CrossRef]
- Kadier, A.; Akkaya, G.K.; Singh, R.; Niza, N.M.; Parkash, A.; Achagri, G.; Bhagawati, P.B.; Asaithambi, P.; Al-Qodah, Z.; Almanaseer, N.; et al. Micro and Nano-Sized Bubbles for Sanitation and Water Reuse: From Fundamentals to Application. Front. Env. Sci. Eng. 2024, 18, 147. [Google Scholar] [CrossRef]
- Sharma, H.; Nirmalkar, N.; Zhang, W. Nanobubbles Produced by Nanopores to Probe Gas-Liquid Mass Transfer Characteristics. J. Colloid. Interface Sci. 2024, 665, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Temesgen, T.; Han, M. Advancing Aerobic Digestion Efficiency Using Ultrafine Bubbles in Wastewater Treatment. J. Water Process Eng. 2023, 55, 104072. [Google Scholar] [CrossRef]
- Bai, M.; Liu, Z.; Liu, Z.; He, C.; Fan, Z.; Yuan, M. Effect of Surfactant Frequently Used in Soil Flushing on Oxygen Mass Transfer in Micro-Nano-Bubble Aeration System. Chin. J. Chem. Eng. 2024, 67, 174–181. [Google Scholar] [CrossRef]
- Koshoridze, S.I. On factors affecting the stability of nanobubbles. Nanosci. Technol. Int. J. 2025, 16, 79–85. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, S.; Zhang, L.; Hu, J. Generation and Stability of Bulk Nanobubbles: A Review and Perspective. Curr. Opin. Colloid Interface Sci. 2021, 53, 101439. [Google Scholar] [CrossRef]
- Lasek, L.; Krzywanski, J.; Skrobek, D.; Zylka, A.; Nowak, W. Review of Micro- and Nanobubble Technologies: Advancements in Theory and Applications and Perspectives on Adsorption Cooling and Desalination Systems. Energies 2023, 16, 8078. [Google Scholar] [CrossRef]
- Yasuda, K. Characteristics of Ultrafine Bubbles (Bulk Nanobubbles) and Their Application to Particle-Related Technology. KONA Powder Part. J. 2024, 41, 2024004. [Google Scholar] [CrossRef]
- Montazeri, S.M.; Kalogerakis, N.; Kolliopoulos, G. Effect of Chemical Species and Temperature on the Stability of Air Nanobubbles. Sci. Rep. 2023, 13, 16716. [Google Scholar] [CrossRef] [PubMed]
- Farid, M.U.; Kharraz, J.A.; Lee, C.-H.; Fang, J.K.-H.; St-Hilaire, S.; An, A.K. Nanobubble-Assisted Scaling Inhibition in Membrane Distillation for the Treatment of High-Salinity Brine. Water Res. 2022, 209, 117954. [Google Scholar] [CrossRef]
- Jiang, P.; Stenstrom, M.K. Oxygen Transfer Parameter Estimation: Impact of Methodology. J. Environ. Eng. 2012, 138, 137–142. [Google Scholar] [CrossRef]
- Mines, R.O. Oxygen Transfer Parameters and Oxygen Uptake Rates Revisited. J. Environ. Sci. Health Part A 2020, 55, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Rochoux, M.; Guo, Y.; Schuurman, Y.; Farrusseng, D. Determination of Oxygen Adsorption–Desorption Rates and Diffusion Rate Coefficients in Perovskites at Different Oxygen Partial Pressures by a Microkinetic Approach. Phys. Chem. Chem. Phys. 2015, 17, 1469–1481. [Google Scholar] [CrossRef]
- Ding, S.; Xing, Y.; Zheng, X.; Zhang, Y.; Cao, Y.; Gui, X. New Insights into the Role of Surface Nanobubbles in Bubble-Particle Detachment. Langmuir 2020, 36, 4339–4346. [Google Scholar] [CrossRef] [PubMed]
- Michailidi, E.D.; Bomis, G.; Varoutoglou, A.; Kyzas, G.Z.; Mitrikas, G.; Mitropoulos, A.C.; Efthimiadou, E.K.; Favvas, E.P. Bulk Nanobubbles: Production and Investigation of Their Formation/Stability Mechanism. J. Colloid Interface Sci. 2020, 564, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Linek, V.; Vacek, V. Chemical engineering use of catalyzed sulfite oxidation kinetics for the determination of mass transfer characteristics of gas—Liquid contactor. Chem. Eng. Sci. 1981, 36, 1747–1768. [Google Scholar] [CrossRef]
- Hoffstadt, K.; Cheenakula, D.; Nikolausz, M.; Krafft, S.; Harms, H.; Kuperjans, I. Design and Construction of a New Reactor for Flexible Biomethanation of Hydrogen. Fermentation 2023, 9, 774. [Google Scholar] [CrossRef]
- Garcia-Ochoa, F.; Gomez, E. Bioreactor Scale-up and Oxygen Transfer Rate in Microbial Processes: An Overview. Biotechnol. Adv. 2009, 27, 153–176. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Yin, M.; Lv, Q.; Hu, Y.; Liu, C.; Zhang, J. Oxygen Solubility, Diffusion Coefficient, and Solution Viscosity. In Rotating Electrode Methods and Oxygen Reduction Electrocatalysts; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–31. [Google Scholar]
- Li, T.; Cui, Z.; Sun, J.; Jiang, C.; Li, G. Generation of Bulk Nanobubbles by Self-Developed Venturi-Type Circulation Hydrodynamic Cavitation Device. Langmuir 2021, 37, 12952–12960. [Google Scholar] [CrossRef]
- Achour, S.H.; Sheng, K.; Lawal, T.; Okuno, R. Thermodynamic Modeling of Aqueous Nanobubble Dispersion. In Proceedings of the SPE Annual Technical Conference and Exhibition, The Woodlands, TX, USA, 31 January–2 February 2023; SPE: Kuala Lumpur, Malaysia, 2023. [Google Scholar]
- Li, M.; Gao, Y.; Ma, X.; Chen, C.; Wang, B.; Sun, C. How Bulk Nanobubbles Respond to Elevated External Pressures. Phys. Fluids 2024, 36, 092003. [Google Scholar] [CrossRef]
- Akshit, F.N.U.; Mao, T.; Mohan, M.S. Future Perspective of Nanobubble Technology in Dairy Processing Applications. Trends Food Sci. Technol. 2024, 147, 104420. [Google Scholar] [CrossRef]
- Sjogreen, C.A.; Landínez Téllez, D.A.; Rosas Pérez, J.E.; Plazas Hurtado, P.C.; Roa-Rojas, J. Experimental Study of Nanobubbles in Salt Solutions. Rev. Acad. Colomb. Cienc. Exactas Fis. Nat. 2018, 42, 41. [Google Scholar] [CrossRef]
- Prakash, R.; Lee, J.; Moon, Y.; Pradhan, D.; Kim, S.-H.; Lee, H.-Y.; Lee, J. Experimental Investigation of Cavitation Bulk Nanobubbles Characteristics: Effects of PH and Surface-Active Agents. Langmuir 2023, 39, 1968–1986. [Google Scholar] [CrossRef] [PubMed]


| Industry | Application | Key Benefits | Properties Involved | References |
|---|---|---|---|---|
| Water treatment | Elimination of organic matter, removal of microplastics, and disinfection of bacteria. | Increase in COD removal by up to 85%, elimination of microplastics (<10 µm), and total disinfection of E. coli. | High stability, increased DO, antimicrobial. | [1,2,3] |
| Aquaculture | Oxygenation in tanks and ponds. | Improves dissolved oxygen levels, optimizing fish health and growth. | High surface area-volume ratio, long-term stability. | [5,6] |
| Biotechnology | Improvement in bioreactors and fermentation. | Increase in kLa, optimizing the production processes of biomolecules and secondary metabolites. | Increased DO, resistance to coalescence. | [7,8] |
| Environmental remediation | Detoxification of contaminated soils. | Improving the efficiency of soil washing processes, reducing heavy metal contamination. | Electrostatic interactions, ph-dependent charge. | [9,10] |
| Membrane distillation | Reduction in membrane fouling. | Decreased fouling, reduced drop flow by 63%, and increased operating time. | Negative surface charge, induced turbulence. | [4] |
| Agriculture | Increasing irrigation efficiency. | Improves the oxygenation of irrigation water, promoting the absorption of nutrients by plants. | Prolonged suspension, unique interfacial properties. | [11,12] |
| Industrial wastewater treatment | Heavy metal removal. | Increase in the adsorption of heavy metals, improvement in flotation processes. | High surface area, adsorption on activated carbon. | [13,14] |
| Food industry | Washing and disinfection of fruits and vegetables. | Elimination of microorganisms and pesticides, preserving the quality of the final product. | Antimicrobial properties, long-term stability. | [15,16] |
| Chemical production | H2O2 electrogeneration. | Increased gas transfer efficiency, improving H2O2 yield by up to 84%. | Higher kLa, high gas transfer efficiency. | [17] |
| Injected Gas | Vessel Volume, L | Curve Type | Reagent Presence | Time Period, min | kLa, min−1 | Determination Coefficient, R2 | p-Value | kLa20, min−1 |
|---|---|---|---|---|---|---|---|---|
| Air | 100 | Absorption | No | ND | ND | ND | ND | ND |
| Air | 100 | Absorption | Yes | 90–135 | 0.0401 | 99.35% | 6.34·10−9 | 0.0393 |
| Oxygen | 100 | Absorption | No | 60–120 | 0.0322 | 95.34% | 8.47·10−7 | 0.0311 |
| Oxygen | 100 | Absorption | Yes | 75–120 | 0.0547 | 97.70% | 7.68·10−7 | 0.0546 |
| Air | 100 | Desorption | No | 0–60 | 0.0312 | 92.40% | 4.37·10−12 | 0.0265 |
| Air | 100 | Desorption | Yes | 0–60 | 0.0436 | 99.73% | 6.11·10−26 | 0.0395 |
| Oxygen | 100 | Desorption | No | 0–120 | 0.0325 | 95.45% | 3.32·10−14 | 0.0276 |
| Oxygen | 100 | Desorption | Yes | 0–120 | 0.0362 | 98.02% | 1.20·10−17 | 0.0330 |
| Air | 30 | Desorption | No | 0–45 | 0.0424 | 95.94% | 5.32·10−9 | 0.0333 |
| Oxygen | 30 | Desorption | No | 15–60 | 0.0067 | 75.14% | 2.89·10−5 | 0.0055 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arias-Torres, L.; Silva, J.; Ortiz, R.; Carlesi, C.; Aroca, G. Comparative Analysis of Volumetric Mass Transfer Coefficients for Oxygen Uptake and Desorption with Nanobubbles. Water 2025, 17, 130. https://doi.org/10.3390/w17010130
Arias-Torres L, Silva J, Ortiz R, Carlesi C, Aroca G. Comparative Analysis of Volumetric Mass Transfer Coefficients for Oxygen Uptake and Desorption with Nanobubbles. Water. 2025; 17(1):130. https://doi.org/10.3390/w17010130
Chicago/Turabian StyleArias-Torres, Laura, Javier Silva, Rodrigo Ortiz, Carlos Carlesi, and Germán Aroca. 2025. "Comparative Analysis of Volumetric Mass Transfer Coefficients for Oxygen Uptake and Desorption with Nanobubbles" Water 17, no. 1: 130. https://doi.org/10.3390/w17010130
APA StyleArias-Torres, L., Silva, J., Ortiz, R., Carlesi, C., & Aroca, G. (2025). Comparative Analysis of Volumetric Mass Transfer Coefficients for Oxygen Uptake and Desorption with Nanobubbles. Water, 17(1), 130. https://doi.org/10.3390/w17010130








