Efficacy and Adaptation Mechanisms of Algal-Bacterial Granular Sludge Treatment for Phenolic Wastewater
Abstract
1. Introduction
2. Materials and Methods
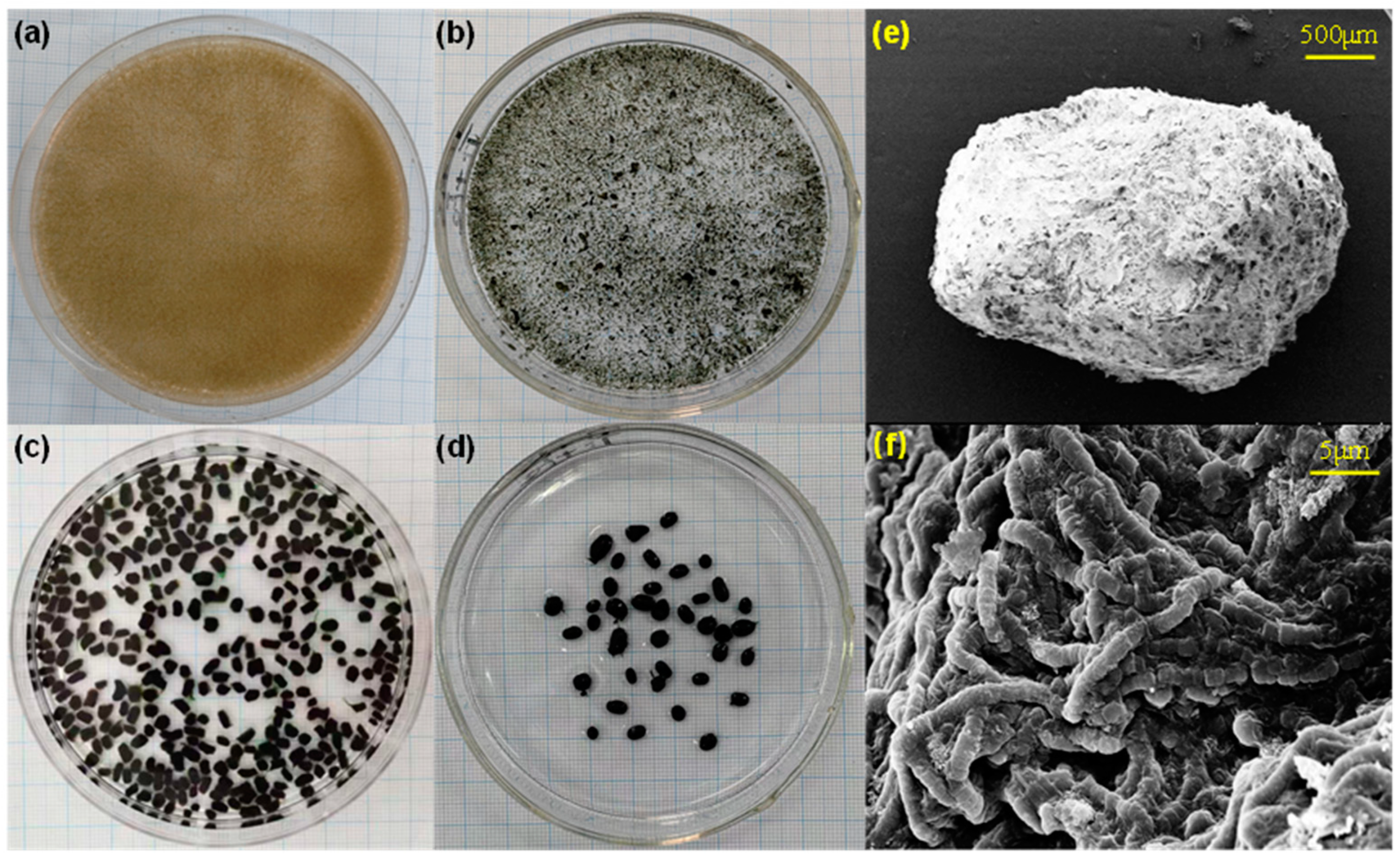
2.1. Synthetic Wastewater and Algal-Bacterial Granular Sludge
2.2. Experimental Design
2.3. Analysis Methods
3. Results and Discussion
3.1. Performance of Algal-Bacterial Granular Sludge on Phenolic Wastewater
3.2. Microbiological Analysis and Photosynthetic Pigment Analysis
3.3. Defensive Responses of Algal-Bacterial Granular Sludge Under Phenols Stress
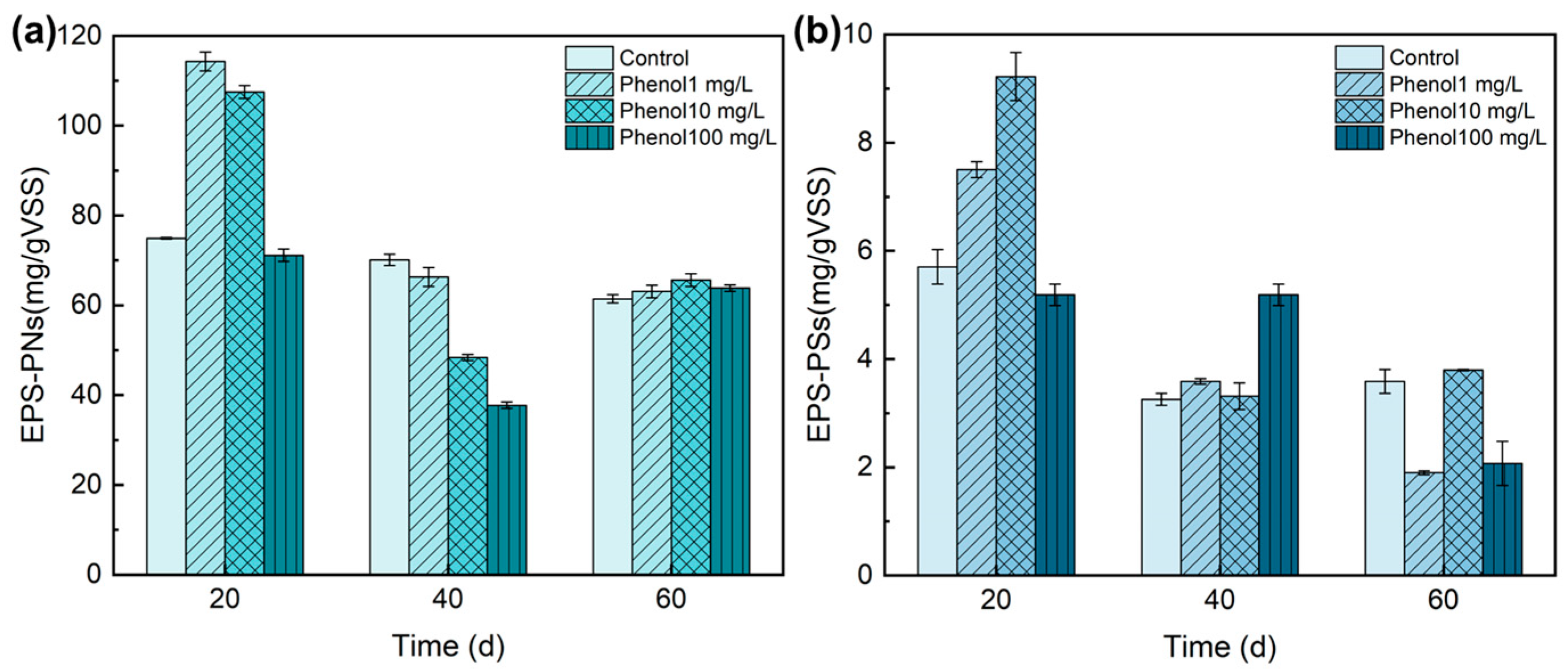
3.3.1. EPS Variations
3.3.2. Antioxidant Enzyme Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ji, B.; Liu, Y. Assessment of Microalgal-Bacterial Granular Sludge Process for Environmentally Sustainable Municipal Wastewater Treatment. ACS ES&T Water 2021, 1, 2459–2469. [Google Scholar] [CrossRef]
- Ji, B.; Zhang, M.; Gu, J.; Ma, Y.; Liu, Y. A self-sustaining synergetic microalgal-bacterial granular sludge process towards energy-efficient and environmentally sustainable municipal wastewater treatment. Water Res. 2020, 179, 115884. [Google Scholar] [CrossRef] [PubMed]
- Quijano, G.; Arcila, J.S.; Buitrón, G. Microalgal-bacterial aggregates: Applications and perspectives for wastewater treatment. Biotechnol. Adv. 2017, 35, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, R.; Kim, B.-H.; Cho, D.-H.; Oh, H.-M.; Kim, H.-S. Algae–bacteria interactions: Evolution, ecology and emerging applications. Biotechnol. Adv. 2016, 34, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Abouhend, A.S.; McNair, A.; Kuo-Dahab, W.C.; Watt, C.; Butler, C.S.; Milferstedt, K.; Hamelin, J.; Seo, J.; Gikonyo, G.J.; El-Moselhy, K.M.; et al. The Oxygenic Photogranule Process for Aeration-Free Wastewater Treatment. Environ. Sci. Technol. 2018, 52, 3503–3511. [Google Scholar] [CrossRef]
- Brockmann, D.; Gérand, Y.; Park, C.; Milferstedt, K.; Hélias, A.; Hamelin, J. Wastewater treatment using oxygenic photogranule-based process has lower environmental impact than conventional activated sludge process. Bioresour. Technol. 2021, 319, 124204. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, B.; Lens, P.N.L.; Shi, W. Zero-Valent Iron Enhances Nutrient Removal and Long-Term Stability of Algal–Bacterial Granular Sludge under Low Carbon Conditions. ACS ES&T Water 2024, 4, 3568–3578. [Google Scholar] [CrossRef]
- Cardeña, R.; Moreno, G.; Bakonyi, P.; Buitrón, G. Enhancement of methane production from various microalgae cultures via novel ozonation pretreatment. Chem. Eng. J. 2017, 307, 948–954. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Liu, J.; Chen, X.; Lei, Z.; Yuan, T.; Shimizu, K.; Zhang, Z.; Lee, D.-J.; Lin, Y.; et al. Highly efficient carbon assimilation and nitrogen/phosphorus removal facilitated by photosynthetic O2 from algal-bacterial aerobic granular sludge under controlled DO/pH operation. Water Res. 2023, 238, 120025. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Liu, C.; Li, A.; Ji, B. Using carbon dioxide-added microalgal-bacterial granular sludge for carbon-neutral municipal wastewater treatment under outdoor conditions: Performance, granule characteristics and environmental sustainability. Sci. Total Environ. 2022, 848, 157657. [Google Scholar] [CrossRef]
- You, X.; Xu, N.; Yang, X.; Sun, W. Pollutants affect algae-bacteria interactions: A critical review. Environ. Pollut. 2021, 276, 116723. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Y.; Ge, H.; Hou, H.; Zhang, H.; Pi, K. Cultivation of algal-bacterial granular sludge and degradation characteristics of tetracycline. Water Environ. Res. 2023, 95, e10846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, S.; Pi, K.; Gerson, A.R. New Insight into the Performance and Self-Defensive Responses of the Algal–Bacterial Granular Sludge Process under Cr(VI)-Induced Stress. Sustainability 2023, 15, 16754. [Google Scholar] [CrossRef]
- Duan, W.; Meng, F.; Cui, H.; Lin, Y.; Wang, G.; Wu, J. Ecotoxicity of phenol and cresols to aquatic organisms: A review. Ecotoxicol. Environ. Saf. 2018, 157, 441–456. [Google Scholar] [CrossRef] [PubMed]
- Grace Pavithra, K.; Sundar Rajan, P.; Arun, J.; Brindhadevi, K.; Hoang Le, Q.; Pugazhendhi, A. A review on recent advancements in extraction, removal and recovery of phenols from phenolic wastewater: Challenges and future outlook. Environ. Res. 2023, 237, 117005. [Google Scholar] [CrossRef]
- Al-Khalid, T.; El-Naas, M.H. Aerobic Biodegradation of Phenols: A Comprehensive Review. Crit. Rev. Environ. Sci. Technol. 2012, 42, 1631–1690. [Google Scholar] [CrossRef]
- Li, H.; Meng, F.; Duan, W.; Lin, Y.; Zheng, Y. Biodegradation of phenol in saline or hypersaline environments by bacteria: A review. Ecotoxicol. Environ. Saf. 2019, 184, 109658. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Chen, X.; Cui, C.; Hou, Y.; Li, W.; You, H. Biodegradation of saline phenolic wastewater in a biological contact oxidation reactor with immobilized cells of Oceanimonas sp. Biotechnol. Lett. 2017, 39, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Papazi, A.; Karamanli, M.; Kotzabasis, K. Comparative biodegradation of all chlorinated phenols by the microalga Scenedesmus obliquus—The biodegradation strategy of microalgae. J. Biotechnol. 2019, 296, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Bucci, P.; García-Depraect, O.; Montero, E.J.M.; Zaritzky, N.; Caravelli, A.; Muñoz, R. Phenol and nitrogen removal in microalgal–bacterial granular sequential batch reactors. J. Chem. Technol. Biotechnol. 2023, 98, 2274–2282. [Google Scholar] [CrossRef]
- Shi, Y.; Xu, C.; Ji, B.; Li, A.; Zhang, X.; Liu, Y. Microalgal-bacterial granular sludge can remove complex organics from municipal wastewater with algae-bacteria interactions. Commun. Earth Environ. 2024, 5, 347. [Google Scholar] [CrossRef]
- Zhang, C. Degradation and Application of O-Cresol in Wastewater by Microalgae. Master’s Thesis, University of Jinan, Jinan, China, 2021. [Google Scholar]
- Hu, G.; Fan, S.; Wang, H.; Ji, B. Adaptation responses of microalgal-bacterial granular sludge to sulfamethoxazole. Bioresour. Technol. 2022, 364, 128090. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Cao, B.; Wang, D.; Ma, T.; Xia, H.; Yu, D. Influence of wastewater sludge treatment using combined peroxyacetic acid oxidation and inorganic coagulants re-flocculation on characteristics of extracellular polymeric substances (EPS). Water Res. 2016, 88, 728–739. [Google Scholar] [CrossRef] [PubMed]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Wang, S.; Ji, B.; Cui, B.; Ma, Y.; Guo, D.; Liu, Y. Cadmium-effect on performance and symbiotic relationship of microalgal-bacterial granules. J. Clean. Prod. 2021, 282, 125383. [Google Scholar] [CrossRef]
- GB 18918-2002; Discharge Standard of Pollutants for Municipal Wastewater Treatment Plants. State Quality Inspection Administration: Beijing, China, 2002.
- Wang, S.; Ji, B.; Zhang, M.; Gu, J.; Ma, Y.; Liu, Y. Tetracycline-induced decoupling of symbiosis in microalgal-bacterial granular sludge. Environ. Res. 2021, 197, 111095. [Google Scholar] [CrossRef] [PubMed]
- Sells, M.D.; Brown, N.; Shilton, A.N. Determining variables that influence the phosphorus content of waste stabilization pond algae. Water Res. 2018, 132, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, M.; El-Naeb, E.H.; Hifney, A.F.; Adam, M.S.; Fawzy, M.A. Hormesis effects of phenol on growth and cellular metabolites of Chlorella sp. under different nutritional conditions using response surface methodology. Environ. Sci. Pollut. Res. 2023, 30, 56904–56919. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, J.; Wang, C. Rapid cultivation of the aerobic granules for simultaneous phenol degradation and ammonium oxidation in a sequencing batch reactor. Bioresour. Technol. 2021, 325, 124414. [Google Scholar] [CrossRef]
- Council of European Communities. Directive Concerning Urban Wastewater Treatment (91/271/EEC); Council of European Communities: Washington, DC, USA, 1991. [Google Scholar]
- Liu, Y.; Zhang, B.; Shi, W. The stability mechanism of algal-bacterial granular sludge. China Environ. Sci. 2022, 42, 1696–1705. [Google Scholar] [CrossRef]
- Abouhend, A.S.; Milferstedt, K.; Hamelin, J.; Ansari, A.A.; Butler, C.; Carbajal-González, B.I.; Park, C. Growth Progression of Oxygenic Photogranules and Its Impact on Bioactivity for Aeration-Free Wastewater Treatment. Environ. Sci. Technol. 2020, 54, 486–496. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M.; Mishra, S.R.; Gadore, V.; Yadav, G.; Roy, S.; Bhattacharjee, B.; Bhuyan, A.; Hazarika, B.; Darabdhara, J.; Kumari, K. Phenolic compounds in water: From toxicity and source to sustainable solutions—An integrated review of removal methods, advanced technologies, cost analysis, and future prospects. J. Environ. Chem. Eng. 2024, 12, 112964. [Google Scholar] [CrossRef]
- Yu, H.-Q. Molecular Insights into Extracellular Polymeric Substances in Activated Sludge. Environ. Sci. Technol. 2020, 54, 7742–7750. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Gong, X.; Li, A.; Shao, S.; Ji, B. A typical acidic extracellular polysaccharide alludes to algae-bacteria-collaboration in microalgal-bacterial symbiosis. Sci. Total Environ. 2024, 929, 172545. [Google Scholar] [CrossRef]
- Hou, X.; Liu, S.; Zhang, Z. Role of extracellular polymeric substance in determining the high aggregation ability of anammox sludge. Water Res. 2015, 75, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Feng, X.; Zhu, N.; Chen, J. Role of extracellular protein in the formation and stability of aerobic granules. Enzym. Microb. Technol. 2007, 41, 551–557. [Google Scholar] [CrossRef]
- Kurade, M.B.; Kim, J.R.; Govindwar, S.P.; Jeon, B.-H. Insights into microalgae mediated biodegradation of diazinon by Chlorella vulgaris: Microalgal tolerance to xenobiotic pollutants and metabolism. Algal Res. 2016, 20, 126–134. [Google Scholar] [CrossRef]
- Chen, X.; Su, L.; Yin, X.; Pei, Y. Responses of Chlorella vulgaris exposed to boron: Mechanisms of toxicity assessed by multiple endpoints. Environ. Toxicol. Pharmacol. 2019, 70, 103208. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Pandey, L.K.; Kumar, D.; Yadav, A.; Rai, J.; Gaur, J.P. Morphological abnormalities in periphytic diatoms as a tool for biomonitoring of heavy metal pollution in a river. Ecol. Indic. 2014, 36, 272–279. [Google Scholar] [CrossRef]




| Samples | Observed_otus | Chao1 | Simpson |
|---|---|---|---|
| Control | 480 | 480.07 | 0.94 |
| Phenol 1-10-100 mg/L | 383 | 384.16 | 0.97 |
| Phenol 1 mg/L | 657 | 657.00 | 0.96 |
| Phenol 10 mg/L | 565 | 565.00 | 0.96 |
| Phenol 100 mg/L | 525 | 525.68 | 0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, A.; Ouyang, R.; Wang, S.; Ji, B.; Cai, L. Efficacy and Adaptation Mechanisms of Algal-Bacterial Granular Sludge Treatment for Phenolic Wastewater. Water 2025, 17, 127. https://doi.org/10.3390/w17010127
Yu A, Ouyang R, Wang S, Ji B, Cai L. Efficacy and Adaptation Mechanisms of Algal-Bacterial Granular Sludge Treatment for Phenolic Wastewater. Water. 2025; 17(1):127. https://doi.org/10.3390/w17010127
Chicago/Turabian StyleYu, Aoxue, Rui Ouyang, Shulian Wang, Bin Ji, and Lu Cai. 2025. "Efficacy and Adaptation Mechanisms of Algal-Bacterial Granular Sludge Treatment for Phenolic Wastewater" Water 17, no. 1: 127. https://doi.org/10.3390/w17010127
APA StyleYu, A., Ouyang, R., Wang, S., Ji, B., & Cai, L. (2025). Efficacy and Adaptation Mechanisms of Algal-Bacterial Granular Sludge Treatment for Phenolic Wastewater. Water, 17(1), 127. https://doi.org/10.3390/w17010127








