The Treatment of Antibiotic Excess Sludge via Catalytic Wet Oxidation with Cu-Ce/γ-Al2O3 and the Production of a Carbon Source
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Cu-Ce/γ-Al2O3
2.2. Characterization
2.3. Catalytic Wet Oxidation Experiments
2.4. Experimental Setup of Oxidation Liquid as Organic Carbon Sources
3. Results and Discussion
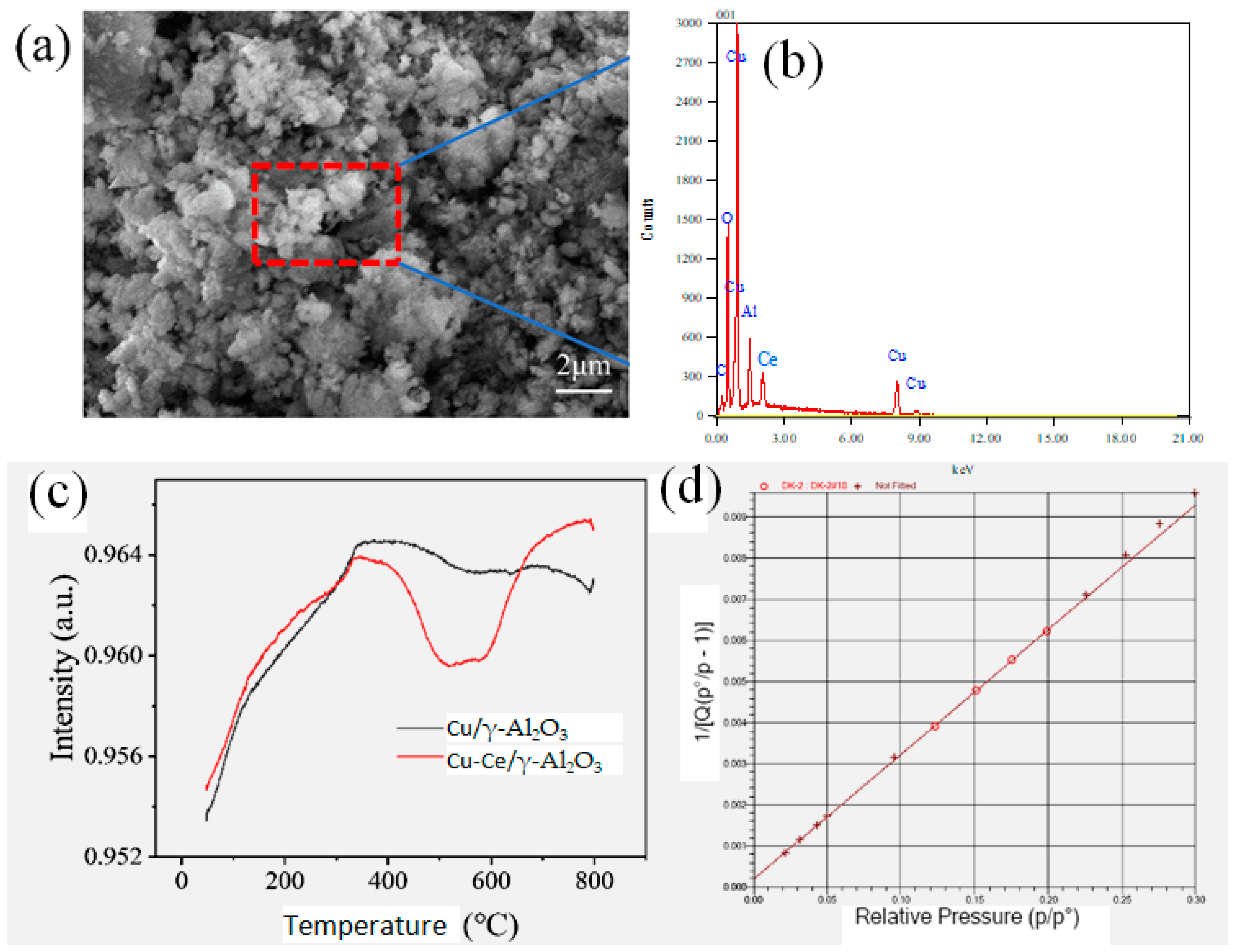
3.1. Physicochemical Properties of the Cu-Ce/γ-Al2O3
3.2. Influence of Experimental Parameters on Antibiotic/Sludge Degradation
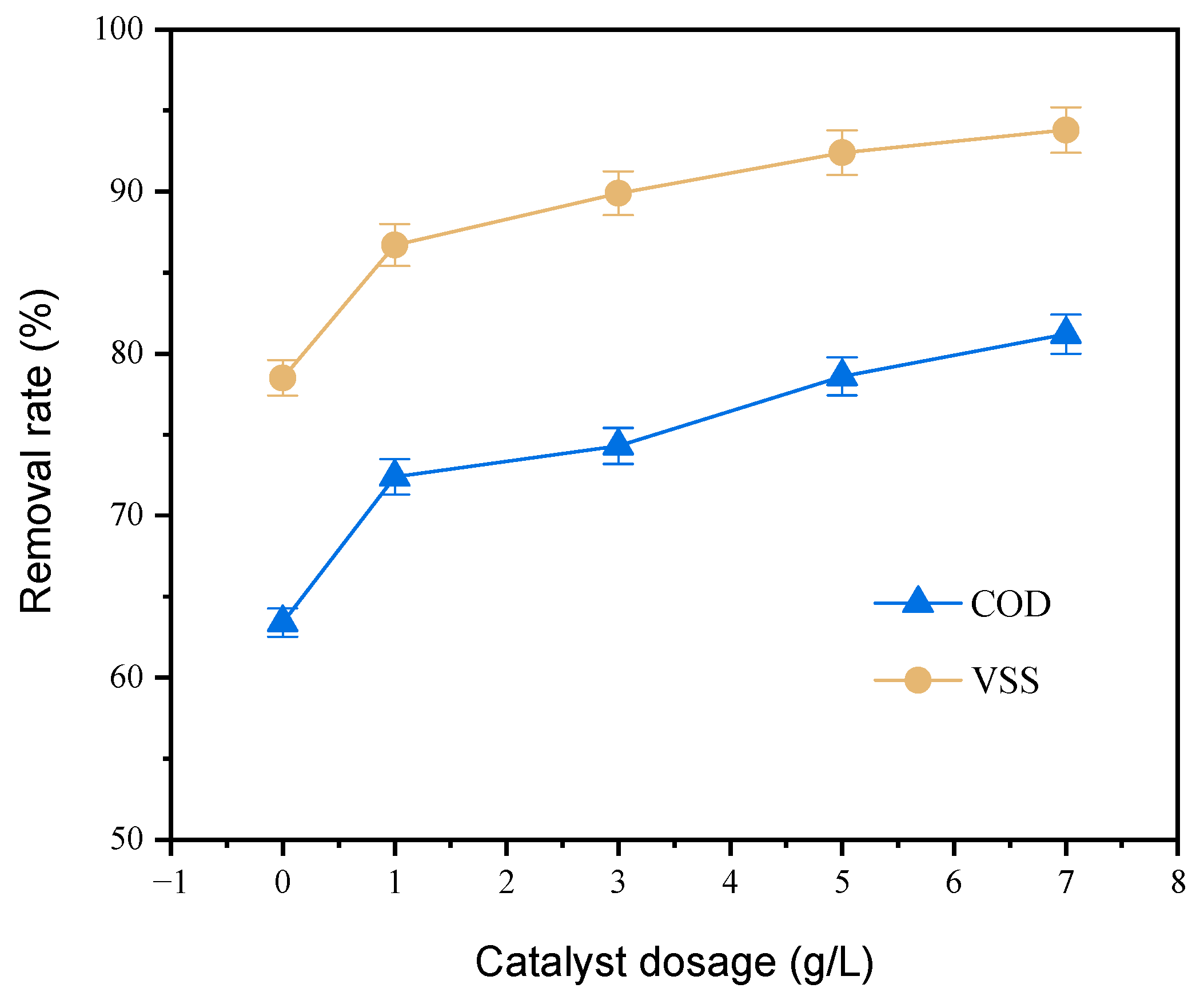
3.2.1. Cu-Ce/γ-Al2O3 Dosage
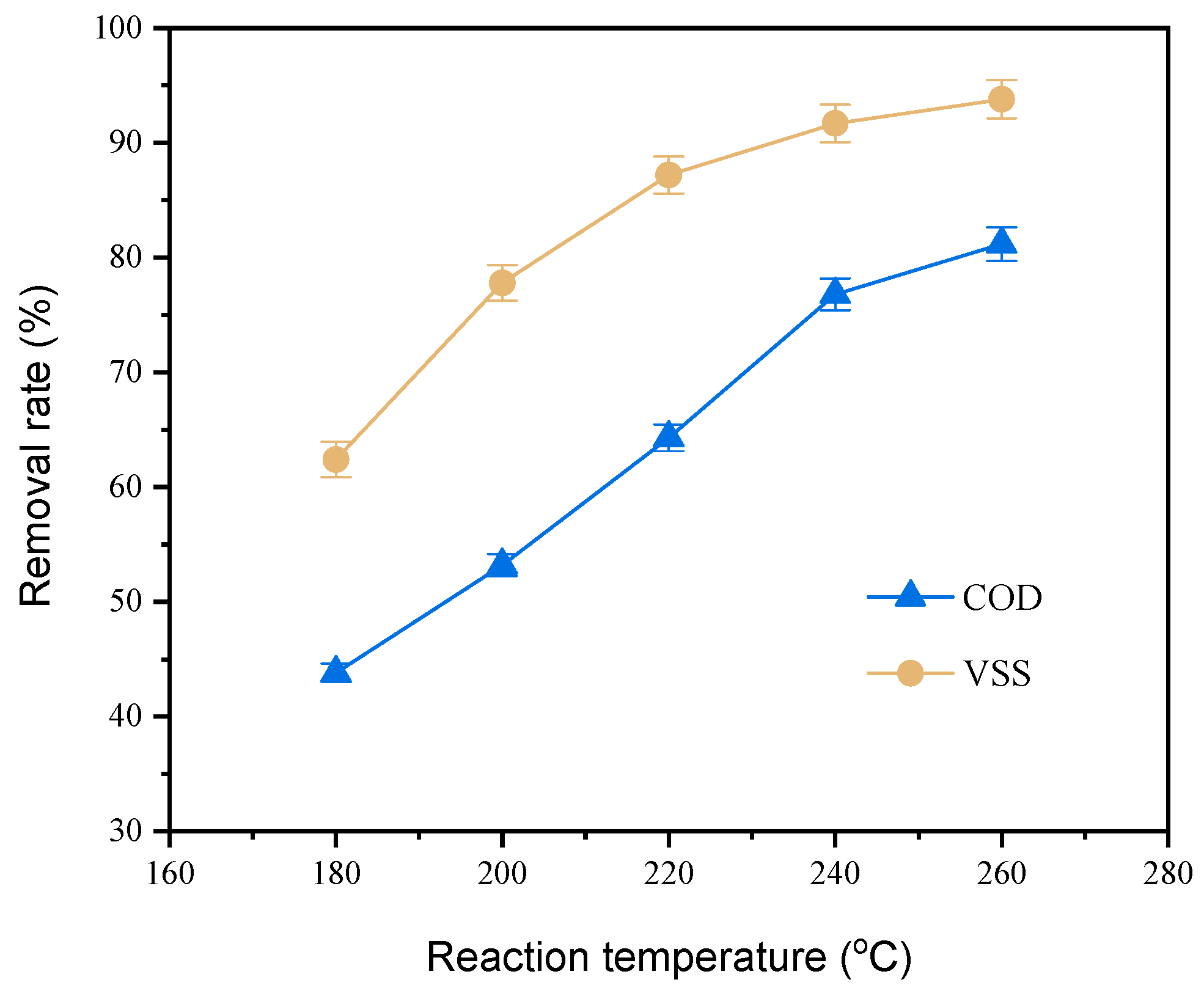
3.2.2. Reaction Temperature
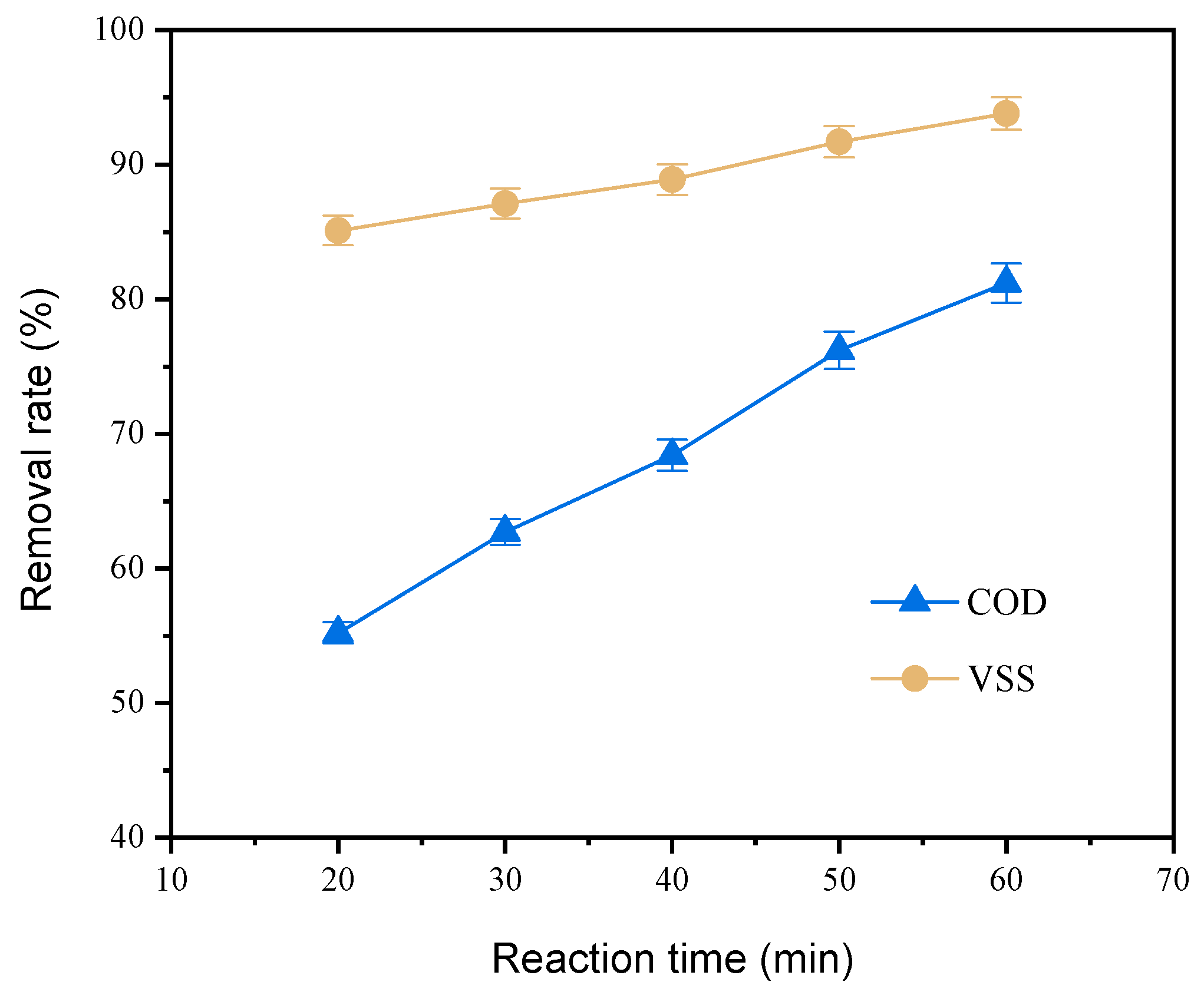
3.2.3. Reaction Time
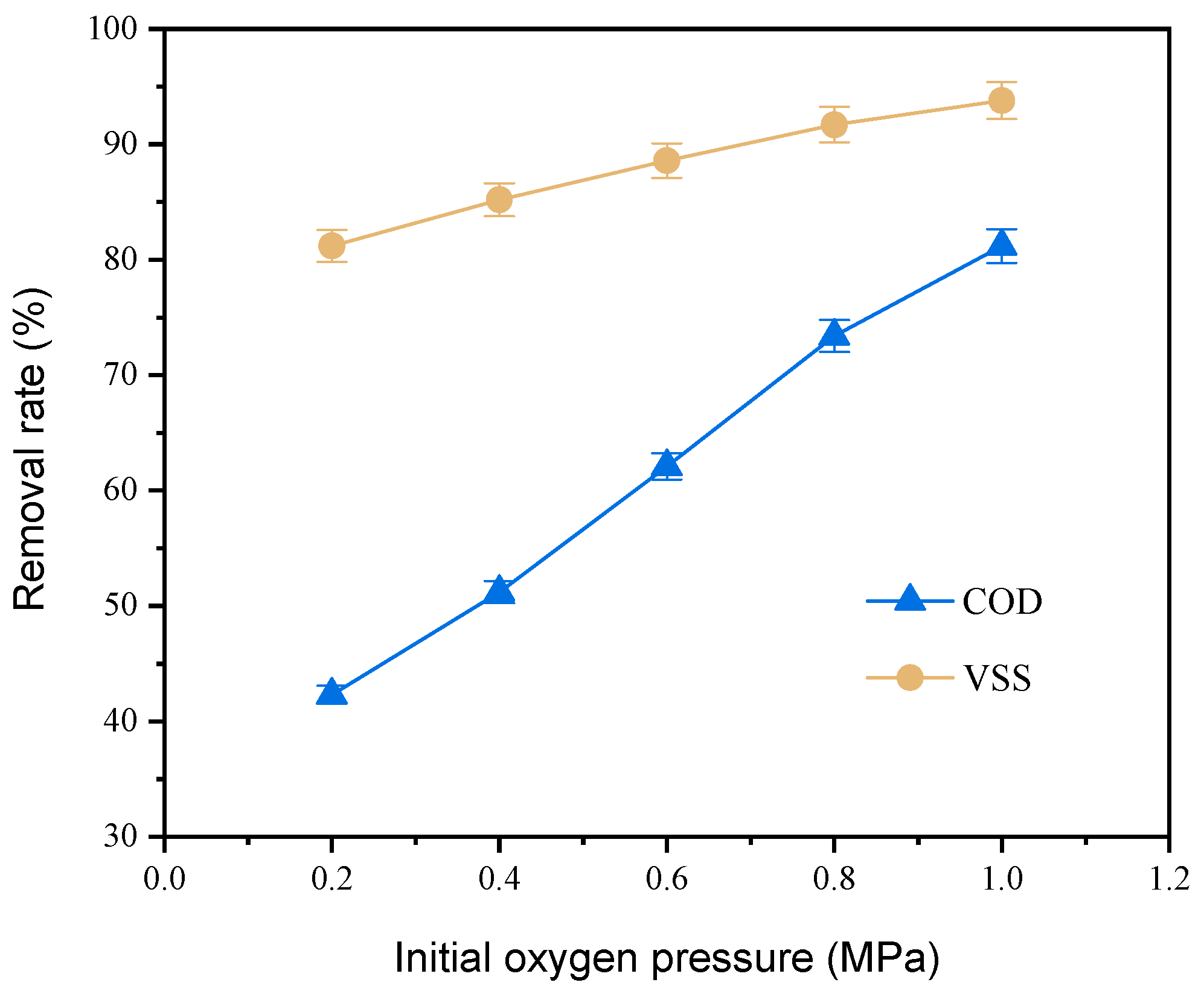
3.2.4. Supplement of Oxygen
3.3. Optimization of Reaction Parameters
3.4. Production of Carboxylic Acids
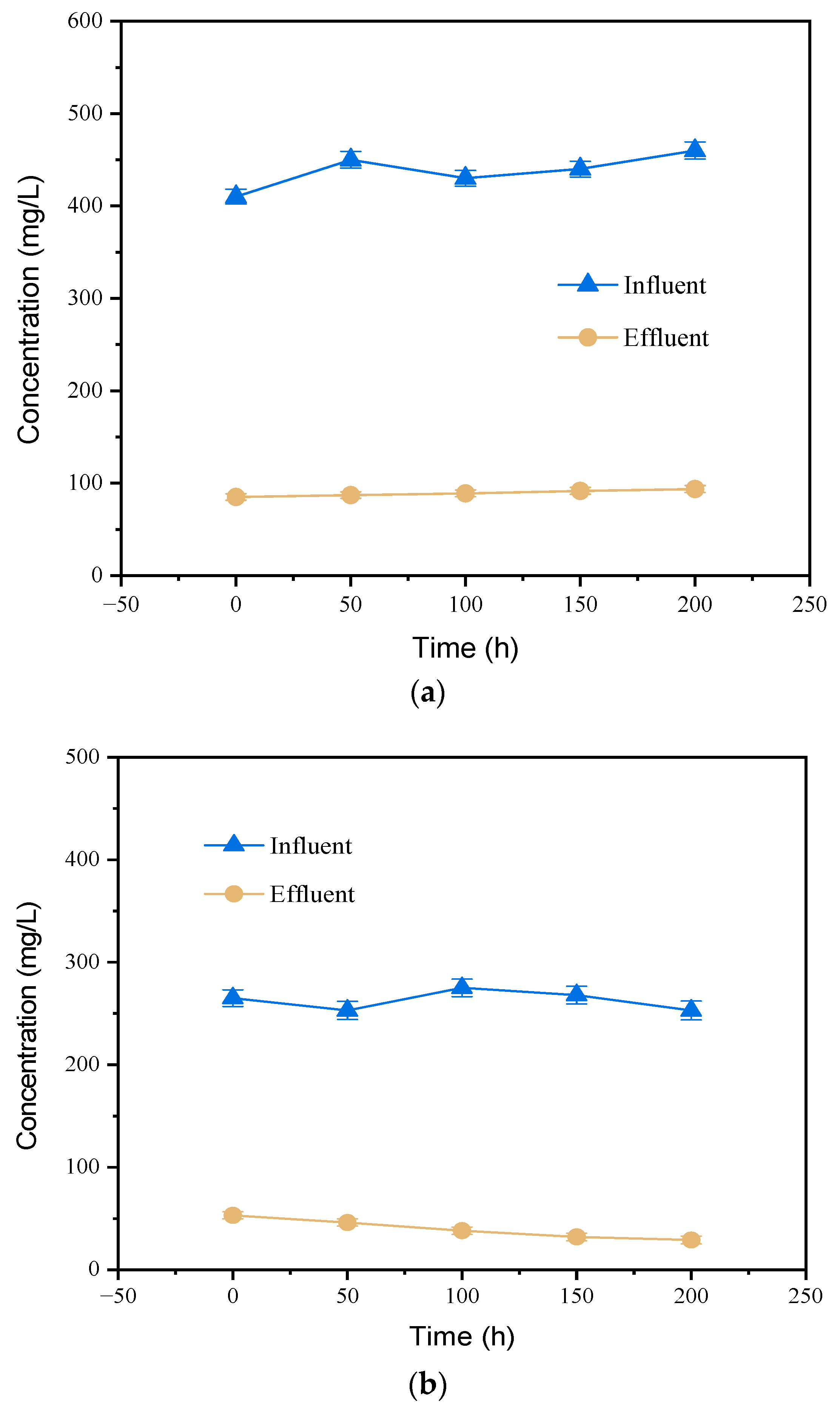
3.5. Oxidation Solution as an Organic Carbon Source for Sewage Treatment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Liu, J.; Yu, H. Sources, fates and treatment strategies of typical viruses in urban sewage collection/treatment systems: A review. Desalination 2022, 534, 115798–115811. [Google Scholar] [CrossRef] [PubMed]
- Arun, S.; Xin, L.; Gaonkar, O.; Neppolian, B.; Zhang, G.; Chakraborty, P. Antibiotics in sewage treatment plants, receiving water bodies and groundwater of Chennai city and the suburb, South India: Occurrence, removal efficiencies, and risk assessment. Sci. Total Environ. 2022, 851, 158195–158204. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wang, Q.; Wang, N.; Yang, S.; Qi, H. High-risk antibiotics positively correlated with antibiotic resistance genes in five typical urban wastewater. J. Environ. Manag. 2023, 342, 118296–118307. [Google Scholar] [CrossRef] [PubMed]
- Hazra, M.; Joshi, H.; Williams, J.B.; Watts, J.E.M. Antibiotics and antibiotic resistant bacteria/genes in urban wastewater: A comparison of their fate in conventional treatment systems and constructed wetlands. Chemos 2022, 303, 135148–135156. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, S.; Zhao, K.; Song, G.; Zhao, S.; Liu, R. Risk control of antibiotics, antibiotic resistance genes (ARGs) and antibiotic resistant bacteria (ARB) during sewage sludge treatment and disposal: A review. Sci. Total Environ. 2023, 877, 162772–162787. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Chen, Q.; Loosdrecht, M.C.M.; Li, J.; Jiang, H. Sustainable disposal of excess sludge: Incineration without anaerobic digestion. Water Res. 2020, 170, 115298–115308. [Google Scholar] [CrossRef] [PubMed]
- De la Torre-Bayo, J.J.; Martín-Pascual, J.; Torres-Rojo, J.C.; Zamorano, M. Characterization of screenings from urban wastewater treatment plants: Alternative approaches to landfill disposal. J. Clean. Prod. 2022, 380, 134884–134896. [Google Scholar] [CrossRef]
- Kaya, Y.; Ersan, G.; Vergili, I. The treatment of pharmaceutical waste-water using in a submerged membrane bioreactor under different sludge retention times. J. Membr. Sci. 2013, 442, 72–78. [Google Scholar] [CrossRef]
- Wang, S.; Han, Y.; Lu, X.; Zhi, Z.; Zhang, R.; Cai, T.; Zhang, Z.; Qin, X.; Song, Y.; Zhen, G. Microbial mechanism underlying high methane pro duction of coupled alkali-microwave–H2O2–oxidation pretreated sewage sludge by in-situ bioelectrochemical regulation. J. Clean. Prod. 2021, 305, 127195–127206. [Google Scholar] [CrossRef]
- Zhu, Y.; Xiao, K.; Ou, B.; Liu, Y.; Yu, W.; Jian, S.; Hu, X.; Liu, H.; Lei, P.; Yang, J. Behavior of organic components and the migration of heavy metals during sludge dewatering by different advanced oxidation processes via optical spectroscopy and molecular fingerprint analysis. Water Res. 2023, 243, 120336–120343. [Google Scholar] [CrossRef]
- Liang, J.; Zhou, Y. Iron-based advanced oxidation processes for enhancing sludge dewaterability: State of the art, challenges, and sludge reuse. Water Res. 2022, 218, 118499–118506. [Google Scholar] [CrossRef]
- Javid, F.; Ang, T.N.; Hanning, S.; Svirskis, D.; Burrell, R.; Taylor, M.; Wright, L.J.; Baroutian, S. Hydrothermal deconstruction of two antibiotics (amoxicillin and metronidazole). J. Clean. Prod. 2021, 325, 129330–129342. [Google Scholar] [CrossRef]
- Boucher, V.; Beaudon, M.; Ramirez, P.; Lemoine, P.; Volk, K.; Yargeau, V.; Segura, P.A. Comprehensive evaluation of non-catalytic wet air oxidation as a pretreatment to remove pharmaceuticals from hospital effluents. Environ. Sci. Water Res. Technol. 2021, 7, 1301–1314. [Google Scholar] [CrossRef]
- Kong, L.M.; Zhou, X.; Yao, Y. Catalytic wet peroxide oxidation of aniline in wastewater using copper modified SBA-15 as catalyst. Environ. Technol. 2016, 37, 422–429. [Google Scholar] [CrossRef]
- Peralta, Y.M.; Sanabria, N.R.; Carriazo, J.G. Catalytic wet hydrogen peroxide oxidation of phenolic compounds in coffee wastewater using Al-Fe-pillared clay extrudates. Desal. Water Treat. 2015, 55, 647–654. [Google Scholar] [CrossRef]
- Luck, F. A review of industrial catalytic wet air oxidation processes. Catal. Today 1996, 27, 195–202. [Google Scholar] [CrossRef]
- Marco, B.; Didier, C.; Stephane, D. Performances of soluble metallic salts in the catalytic wet air oxidation of sewage sludge. Catal. Today 2010, 157, 420–424. [Google Scholar]
- Zeng, X.; Liu, J.; Zhao, J.F. Catalytic Wet Oxidation of Pharmaceutical Sludge by Molecular Sieve Loaded with Cu/Ce. Catalysts 2018, 8, 67. [Google Scholar] [CrossRef]
- Cuauhtémoc, I.; Del Angel, G.; Torres, G.; Bertin, V. Catalytic wet air oxidation of gasoline oxygenates using Rh/γ-Al2O3 and Rh/γ-Al2O3–CeO2 catalysts. Catal. Today 2008, 133, 135588–135593. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, S.; Zhang, Y.; Hu, X.; Li, F.; Chen, X.; Cai, H.; Wang, J.; Shi, L.; Chen, X. Synergistic effect over a remarkable durable and active polymetallic Ru-doped Fe-Co-Ce/γ-Al2O3 nanocatalyst: Interfacial Lewis acid-base pair dependent reaction mechanism for landfill leachate. Chem. Eng. J. 2020, 382, 122938–122947. [Google Scholar] [CrossRef]
- Song, A.; Lü, G. Study of catalytic activity and product selectivity of M/Al2O3-CeO2 (M = Pt-Ru, Ru, and Pt) in catalytic wet air oxidation of methylamine. Chin. J. Catal. 2014, 35, 1212–1223. [Google Scholar] [CrossRef]
- Li, Y.Z.; Fan, Z.Y.; Shi, J.W.; Liu, Z.Y.; Zhou, J.W.; Shangguan, W.F. Catalytic oxidation of low concentration formaldehyde with the assist of ozone over supported cobalt-manganese composite oxides. J. Mol. Catal. 2014, 1, 60–66. [Google Scholar]
- Chou, B.; Tsai, J.L.; Cheng, S. Cu-substituted molecular sieves as liquid phase oxidation catalysts. Microp. Mesop. Mater. 2001, 48, 309–317. [Google Scholar] [CrossRef]
- Taran, O.P.; Zagoruiko, A.N.; Ayusheev, A.B.; Yashnik, S.A.; Prihod’ko, R.V.; Ismagilov, Z.R. Cu and Fe-containing ZSM-5 zeolites as catalysts for wet peroxide oxidation of organic contaminants: Reaction kinetics. Res. Chem. Intermed. 2015, 41, 9521–9537. [Google Scholar] [CrossRef]
- Wang, J.B.; Zhu, W.P.; Yang, S.X.; Wang, W.; Zhou, Y. Catalytic wet air oxidation of phenol with pelletized ruthenium catalysts. Appl. Catal. B Environ. 2008, 78, 30–37. [Google Scholar] [CrossRef]
- Barbier, J.; Oliviero, L.; Renard, B.; Duprez, D. Role of ceria-supported noble metal catalysts (Ru, Pd, Pt) in wet air oxidation of nitrogen and oxygen containing compounds. Top. Catal. 2005, 33, 77–86. [Google Scholar] [CrossRef]
- Kang, K.; Quitain, A.T.; Daimon, H.; Noda, R.; Goto, N.; Hu, H.Y.; Fujie, K. Optimization of amino acids production from waste fish entrails by hydrolysis in sub- and supercritical water. Canad. J. Chem. Eng. 2001, 79, 65–70. [Google Scholar] [CrossRef]
- Gapes, D.J.; Stuthridge, T.R.; Strong, P.J.; Lei, R.J.; Aggrey, A.; James, S.P.; Raymond, S.T. Treatment of Biomass. Patent WO2013128390-A1, 2015. [Google Scholar]
- Chung, J.; Lee, M.; Ahn, J.; Bae, W.; Lee, Y.W.; Shim, H. Effects of operational conditions on sludge degradation and organic acids formation in low-critical wet air oxidation. J. Hazard. Mater. 2009, 162, 10–16. [Google Scholar] [CrossRef]
- Das, T.; Al-Waili, I.; Balasubramanian, V.; Kaparaju, P.; Parthasarathy, R.; Eshtiaghi, N. Process modelling and techno-economic analysis of anaerobic digestion of sewage sludge integrated with wet oxidation using a gravity pressure vessel and thermal hydrolysis. Sci. Total Environ. 2024, 912, 169024. [Google Scholar] [CrossRef]
- Li, B.; Liu, S.; Zhu, H.; Qian, W.; Wang, P.; Yang, R.; Ning, P. Enhanced NOx absorption in flue gas by wet oxidation of red mud and phosphorus sludge. J. Hazard. Mater. 2024, 465, 133075. [Google Scholar] [CrossRef]

| Level | Factors | |||
|---|---|---|---|---|
| (A) | (B) | (C) | (D) | |
| Catalyst Dosage (g) | Temperature (°C) | Time (min) | Pressure of Oxygen (MPa) | |
| 1 | 4.0 | 220 | 30 | 0.6 |
| 2 | 5.0 | 240 | 45 | 0.8 |
| 3 | 6.0 | 260 | 60 | 1.0 |
| Reaction Temperature (°C) | Acetic Acid (mg/L) | Propionic Acid (mg/L) | Isobutyric Acid (mg/L) | Isovaleric Acid (mg/L) |
|---|---|---|---|---|
| 200 | 1620 | 90 | 50 | 70 |
| 220 | 2680 | 140 | 80 | 90 |
| 240 | 3470 | 180 | 90 | 100 |
| 260 | 3620 | 150 | 60 | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, S.; Lin, H.; Zeng, X. The Treatment of Antibiotic Excess Sludge via Catalytic Wet Oxidation with Cu-Ce/γ-Al2O3 and the Production of a Carbon Source. Water 2024, 16, 1249. https://doi.org/10.3390/w16091249
Chu S, Lin H, Zeng X. The Treatment of Antibiotic Excess Sludge via Catalytic Wet Oxidation with Cu-Ce/γ-Al2O3 and the Production of a Carbon Source. Water. 2024; 16(9):1249. https://doi.org/10.3390/w16091249
Chicago/Turabian StyleChu, Shangye, Hai Lin, and Xu Zeng. 2024. "The Treatment of Antibiotic Excess Sludge via Catalytic Wet Oxidation with Cu-Ce/γ-Al2O3 and the Production of a Carbon Source" Water 16, no. 9: 1249. https://doi.org/10.3390/w16091249
APA StyleChu, S., Lin, H., & Zeng, X. (2024). The Treatment of Antibiotic Excess Sludge via Catalytic Wet Oxidation with Cu-Ce/γ-Al2O3 and the Production of a Carbon Source. Water, 16(9), 1249. https://doi.org/10.3390/w16091249





