Safety of Tap Water in Terms of Changes in Physical, Chemical, and Biological Stability
Abstract
1. Introduction
2. Materials and Methods
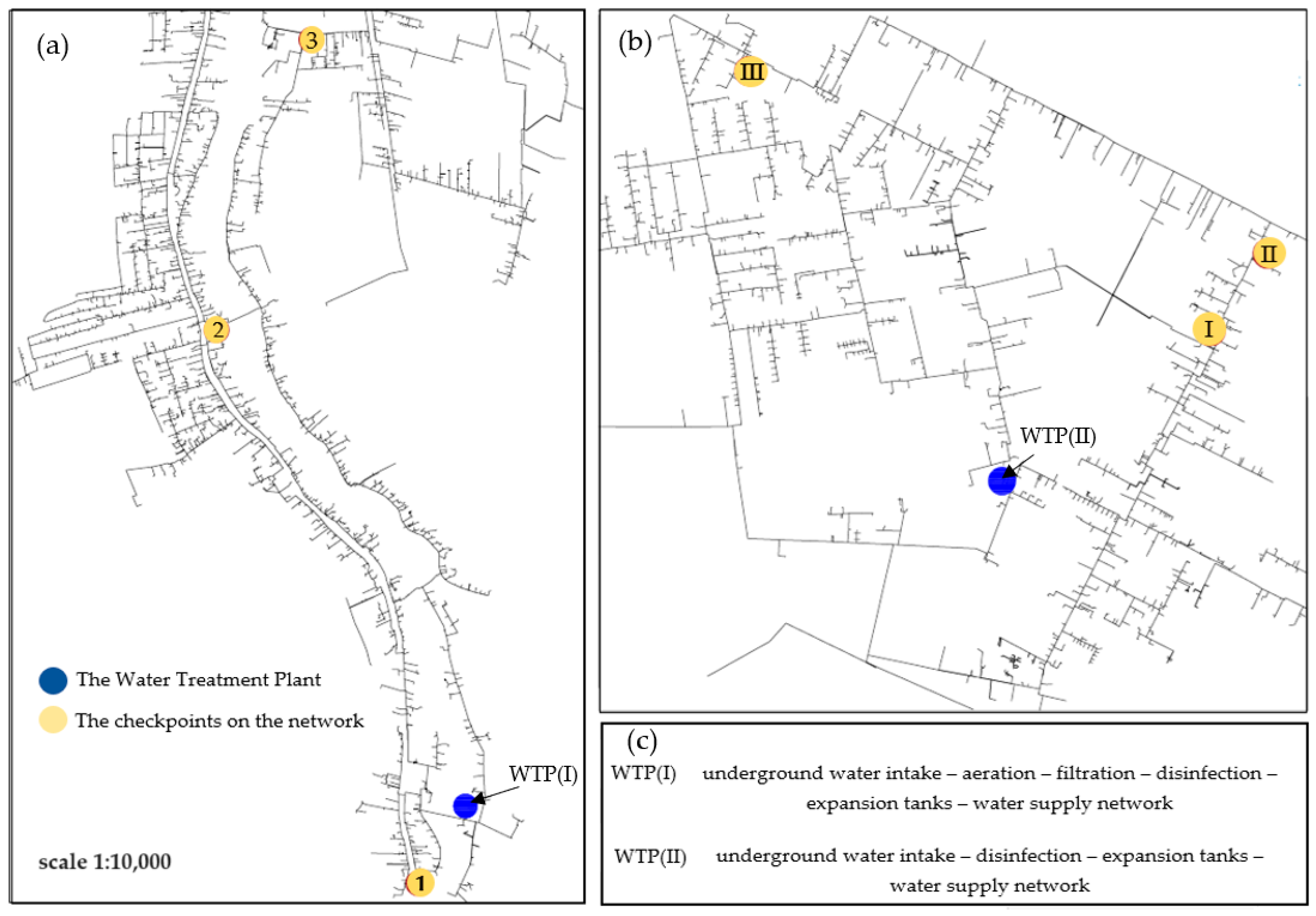
2.1. Subject of Study
2.2. Taking Samples for Analysis
2.3. Analysis of Physicochemical Water Quality
2.4. Water Stability Index
2.4.1. Biological Stability of Water
2.4.2. Physical Stability of Water
2.4.3. Chemical Stability of Water
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bichai, F.; Smeets, P.W.M.H. Using QMRA-Based Regulation as a Water Quality Management Tool in the Water Security Challenge: Experience from the Netherlands and Australia. Water Res. 2013, 47, 7315–7326. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tao, S.; Sun, Z.; Chen, Y.; Xu, J. Determination of Heavy Metals and Health Risk Assessment in Tap Water from Wuhan, China, a City with Multiple Drinking Water Sources. Water 2023, 15, 3709. [Google Scholar] [CrossRef]
- Olatunde, K.; Kane Patton, S.; Cameron, L.; Stankus, T.; James Milaham, P. Factors Affecting the Quality of Drinking Water in the United States of America: A Ten-Year Systematic Review. AJWR 2022, 10, 24–34. [Google Scholar] [CrossRef]
- Perveen, S. Amar-Ul-Haque Drinking Water Quality Monitoring, Assessment and Management in Pakistan: A Review. Heliyon 2023, 9, e13872. [Google Scholar] [CrossRef] [PubMed]
- Price, J.I.; Heberling, M.T. The Effects of Source Water Quality on Drinking Water Treatment Costs: A Review and Synthesis of Empirical Literature. Ecol. Econ. 2018, 151, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Council Directive 98/83/EC of 3 November 1998 on the Quality of Water Intended for Human Consumption. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A31998L0083 (accessed on 15 February 2024).
- World Health Organization. A Global Overview of National Regulations and Standards for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Zhang, J.; Li, W.; Chen, J.; Wang, F.; Qi, W.; Li, Y. Impact of Disinfectant on Bacterial Antibiotic Resistance Transfer between Biofilm and Tap Water in a Simulated Distribution Network. Environ. Pollut. 2019, 246, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, Y.; Knibbe, W.-J.; Feng, C.; Liu, W.; Medema, G.; van der Meer, W. Potential Impacts of Changing Supply-Water Quality on Drinking Water Distribution: A Review. Water Res. 2017, 116, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ling, F.; Bakker, G.; Liu, W.-T.; Medema, G.; Van Der Meer, W.; Liu, G. Assessing the Transition Effects in a Drinking Water Distribution System Caused by Changing Supply Water Quality: An Indirect Approach by Characterizing Suspended Solids. Water Res. 2020, 168, 115159. [Google Scholar] [CrossRef] [PubMed]
- Jachimowski, A. Factors affecting water quality in a water supply network. J. Ecol. Eng. 2017, 18, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Świetlik, J.; Magnucka, M. Chemical and Microbiological Safety of Drinking Water in Distribution Networks Made of Plastic Pipes. WIREs Water 2023, 11, e1704. [Google Scholar] [CrossRef]
- Act of 7 June 2001 on collective water supply and collective sewage disposal. J. Laws 2001, 72, 747. (In Polish)
- Lee, D.; Calendo, G.; Kopec, K.; Henry, R.; Coutts, S.; McCarthy, D.; Murphy, H.M. The Impact of Pipe Material on the Diversity of Microbial Communities in Drinking Water Distribution Systems. Front. Microbiol. 2021, 12, 779016. [Google Scholar] [CrossRef]
- Learbuch, K.L.G.; Smidt, H.; van der Wielen, P.W.J.J. Influence of Pipe Materials on the Microbial Community in Unchlorinated Drinking Water and Biofilm. Water Res. 2021, 194, 116922. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, S.; Lopez-Roldan, R.; Cortina, J.-L. Presence of Metals in Drinking Water Distribution Networks Due to Pipe Material Leaching: A Review. Toxicol. Environ. Chem. 2013, 95, 870–889. [Google Scholar] [CrossRef]
- Rożej, A.; Cydzik-Kwiatkowska, A.; Kowalska, B.; Kowalski, D. Structure and Microbial Diversity of Biofilms on Different Pipe Materials of a Model Drinking Water Distribution Systems. World J. Microbiol. Biotechnol. 2015, 31, 37–47. [Google Scholar] [CrossRef]
- Besner, M.; Lavoie, J.; Morissette, C.; Payment, P.; Prévost, M. Effect of Water Main Repairs on Water Quality. J. AWWA 2008, 100, 95–109. [Google Scholar] [CrossRef]
- Gholizadeh, A.; Mokhtari, M.; Naimi, N.; Shiravand, B.; Ehrampoush, M.H.; Miri, M.; Ebrahimi, A. Assessment of Corrosion and Scaling Potential in Groundwater Resources; a Case Study of Yazd-Ardakan Plain, Iran. Groundw. Sustain. Dev. 2017, 5, 59–65. [Google Scholar] [CrossRef]
- García-Ávila, F.; Ramos-Fernández, L.; Zhindón-Arévalo, C. Estimation of Corrosive and Scaling Trend in Drinking Water Systems in the City of Azogues, Ecuador. Rev. Ambient. Agua 2018, 13, e2237. [Google Scholar] [CrossRef]
- Vreeburg, J.H.G.; Schippers, D.; Verberk, J.Q.J.C.; van Dijk, J.C. Impact of Particles on Sediment Accumulation in a Drinking Water Distribution System. Water Res. 2008, 42, 4233–4242. [Google Scholar] [CrossRef]
- Wolska, M. Removal of Nutrients in Water Purification Technology Intended for Human Consumption; Oficyna Wydawnicza Politechniki Wrocławskiej: Wroclaw, Poland, 2015. (In Polish) [Google Scholar]
- Pietrucha-Urbanik, K.; Tchórzewska-Cieślak, B.; Papciak, D.; Skrzypczak, I. Analysis of Chemical Stability of Tap Water in Terms of Required Level of Technological Safety. Arch. Environ. Prot. 2017, 43, 3–12. [Google Scholar] [CrossRef]
- Atekwana, E.; Atekwana, E.; Rowe, R.; Werkemajr, D.; Legall, F. The Relationship of Total Dissolved Solids Measurements to Bulk Electrical Conductivity in an Aquifer Contaminated with Hydrocarbon. J. Appl. Geophys. 2004, 56, 281–294. [Google Scholar] [CrossRef]
- Baloïtcha, G.M.P.; Mayabi, A.O.; Home, P.G. Evaluation of Water Quality and Potential Scaling of Corrosion in the Water Supply Using Water Quality and Stability Indices: A Case Study of Juja Water Distribution Network, Kenya. Heliyon 2022, 8, e09141. [Google Scholar] [CrossRef]
- Sengupta, P. Potential Health Impacts of Hard Water. Int. J. Prev. Med. 2013, 4, 866–875. [Google Scholar]
- Sappa, G.; Ergul, S.; Ferranti, F. Water Quality Assessment of Carbonate Aquifers in Southern Latium Region, Central Italy: A Case Study for Irrigation and Drinking Purposes. Appl. Water Sci. 2014, 4, 115–128. [Google Scholar] [CrossRef]
- Rubenowitz-Lundin, E.; Hiscock, K.M. Water Hardness and Health Effects. In Essentials of Medical Geology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 337–350. [Google Scholar]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A. Impact of Heavy Metals on the Environment and Human Health: Novel Therapeutic Insights to Counter the Toxicity. J. King Saud Univ. Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Bafe Dilebo, W.; Desta Anchiso, M.; Tereke Kidane, T.; Eskezia Ayalew, M. Assessment of Selected Heavy Metals Concentration Level of Drinking Water in Gazer Town and Selected Kebele, South Ari District, Southern Ethiopia. Int. J. Anal. Chem. 2023, 2023, 1524850. [Google Scholar] [CrossRef]
- Mohod, C.V.; Dhote, J. Review of Heavy Metals in Drinking Water and Their Effect on Human Health. Int. J. Innov. Res. Sci. Eng. Technol. 2013, 2, 2992–2996. [Google Scholar]
- Ehi-Eromosele, C.O.; Okiei, W.O. Heavy Metal Assessment of Ground, Surface and Tap Water Samples in Lagos Metropolis Using Anodic Stripping Voltammetry. Resour. Environ. 2012, 2, 82–86. [Google Scholar]
- Zhang, P.; Yang, M.; Lan, J.; Huang, Y.; Zhang, J.; Huang, S.; Yang, Y.; Ru, J. Water Quality Degradation Due to Heavy Metal Contamination: Health Impacts and Eco-Friendly Approaches for Heavy Metal Remediation. Toxics 2023, 11, 828. [Google Scholar] [CrossRef]
- Chowdhury, S.; Mazumder, M.A.J.; Al-Attas, O.; Husain, T. Heavy Metals in Drinking Water: Occurrences, Implications, and Future Needs in Developing Countries. Sci. Total Environ. 2016, 569–570, 476–488. [Google Scholar] [CrossRef]
- Górski, J.; Siepak, M. Assessment of Metal Concentrations in Tap-Water–from Source to the Tap: A Case Study from Szczecin, Poland. Geologos 2014, 20, 25–33. [Google Scholar] [CrossRef]
- Chen, W.; Chen, J. Formation and Prevention of Pipe Scale in Water Supply Pipelines with Anti-Corrosion Lining. Water Supply 2022, 22, 4006–4014. [Google Scholar] [CrossRef]
- Sun, H.; Shi, B.; Yang, F.; Wang, D. Effects of Sulfate on Heavy Metal Release from Iron Corrosion Scales in Drinking Water Distribution System. Water Res. 2017, 114, 69–77. [Google Scholar] [CrossRef]
- Dinelli, E.; Lima, A.; Albanese, S.; Birke, M.; Cicchella, D.; Giaccio, L.; Valera, P.; De Vivo, B. Major and Trace Elements in Tap Water from Italy. J. Geochem. Explor. 2012, 112, 54–75. [Google Scholar] [CrossRef]
- Kowalska, B.; Kowalski, D.; Rożej, A. Organic Compounds Migrating from Plastic Pipes into Water. J. Water Supply Res. Technol. Aqua. 2011, 60, 137–146. [Google Scholar] [CrossRef]
- Verma, A.; Sharma, A.; Kumar, R.; Sharma, P. Nitrate Contamination in Groundwater and Associated Health Risk Assessment for Indo-Gangetic Plain, India. Groundw. Sustain. Dev. 2023, 23, 100978. [Google Scholar] [CrossRef]
- Erdei-Tombor, P.; Kiskó, G.; Taczman-Brückner, A. Biofilm Formation in Water Distribution Systems. Processes 2024, 12, 280. [Google Scholar] [CrossRef]
- Hammes, F.; Berney, M.; Wang, Y.; Vital, M.; Köster, O.; Egli, T. Flow-Cytometric Total Bacterial Cell Counts as a Descriptive Microbiological Parameter for Drinking Water Treatment Processes. Water Res. 2008, 42, 269–277. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Percival, S.L.; Walker, J.T. Contamination Potential of Biofilms in Water Distribution Systems. Water Supply 2002, 2, 271–280. [Google Scholar] [CrossRef]
- Maeng, M.; Hyun, I.; Choi, S.; Dockko, S. Effects of Rainfall Characteristics on Corrosion Indices in Korean River Basins. Desalin. Water Treat. 2015, 54, 1233–1241. [Google Scholar] [CrossRef]
- Alvarez-Bastida, C.; Martínez-Miranda, V.; Vázquez-Mejía, G.; Solache-Ríos, M.; Fonseca-Montes De Oca, G.; Trujillo-Flores, E. The Corrosive Nature of Manganese in Drinking Water. Sci. Total Environ. 2013, 447, 10–16. [Google Scholar] [CrossRef]
- Bastidas, D.M.; Criado, M.; Fajardo, S.; La Iglesia, V.M.; Cano, E.; Bastidas, J.M. Copper Deterioration: Causes, Diagnosis and Risk Minimisation. Int. Mater. Rev. 2010, 55, 99–127. [Google Scholar] [CrossRef]
- Sarin, P.; Snoeyink, V.L.; Bebee, J.; Jim, K.K.; Beckett, M.A.; Kriven, W.M.; Clement, J.A. Iron Release from Corroded Iron Pipes in Drinking Water Distribution Systems: Effect of Dissolved Oxygen. Water Res. 2004, 38, 1259–1269. [Google Scholar] [CrossRef] [PubMed]
| Parameter | DS(I) | DS(II) |
|---|---|---|
| Average annual water production in m3/d | 819 | 695 |
| Estimated number of people supplied with water from the water supply system | 5642 | 3065 |
| Current status of the water supply network in km: | ||
| • With household connections | 143.50 | 75.28 |
| • Without connections | 82.64 | 38.94 |
| Type of construction material | PVC, PE | PVC, PE |
| Parameter | Unit | Method/Device |
|---|---|---|
| Temperature | °C | Digital HACCP thermometer (Hendi, Robakowo, Poland) |
| pH | - | Petameter Hach-Lange HQ 40d Multi (Hach-Lange, Düsseldorf, Germany) |
| Conductivity | µS/cm | |
| Turbidity | NTU | EUTECHTM TN-100 Turbidimeter (Thermo Scientific™, Gdańsk, Poland) |
| Color | mg Pt/L | Colorimetric method |
| Ammonium nitrogen | mg N-NH4+/L | Spectrophotometric method Hach-Lange DR 6000 spectrophotometer (Hach-Lange, Düsseldorf, Germany); (Spectrophotometric method: 8038; LCK341; 8039; LCK 348, 8051) |
| Nitrite nitrogen | mg N-NO2−/L | |
| Nitrate nitrogen | mg N-NO3−/L | |
| Phosphorus | mg P-PO43−/L | |
| Sulfates | mg SO4−/L | |
| Alkalinity | mg CaCO3/L | Titration method |
| Hardness | mg CaCO3/L | |
| Chlorides | mg Cl−/L | |
| Free/Total chlorine | mg Cl/L | Photometer PC MULTIDirect (Lovibond® Water Testing, Dortmund, Germany) |
| Dissolved organic carbon (DOC), Biodegradable dissolved organic carbon (BDOC) * | mg C/L | Total organic carbon analyzer TOC-L, (Shimadzu, Markham, ON, Canada) |
| Mn, Fe, Cu, Cd, Cr, Zn, Pb, Ca, Na, K, Mg | ppm/L; ppb/L | Agilent 8900 ICP-MS Triple Quad (Agilent, Santa Clara, CA, USA) |
| Equation | Water Feature |
|---|---|
| Langelier Saturation Index (IL) | |
| IL = pH-pHs pHs = (9.3 + A + B) − (C + D) Where: pH = pH measured in situ. pHs = pH at saturation pHs A = (log10 [TDS*] − 1)/10, B = –13.12 × log10 (°C + 273) + 34.55, C = log10 [Ca+2 mg/L as CaCo3] − 0.4, D = log10 [Alkalinity as CaCo3], TDS = Total Dissolved Solids, TDS = E × ke, E − conductivity [µS/cm], ke = 0.55–0.8, assumed: 0.64 | IL > 0; Water can dissolve calcium compounds and its corrosion properties are enhanced. −0.5 < IL < +0.5; Water is stable: it does not tend to precipitate or dissolve calcium carbonate, and the corrosion properties are weakened. IL < 0; Water can precipitate lime and its corrosion properties are weakened. |
| Ryznar Stability Index (IR) | |
| IR = 2 pHs-pH Where: pHs = pH at saturation pH = pH measured in situ. | IR < 5.5 Heavy scales likely to form 5.5 < IR < 6.2 Moderate scale-forming 6.2 < IR < 6.8 is considered neutral 6.8 < IR < 8.5 Low corrosion IR > 8.5 High corrosion |
| Larson Skold Index (ILS) | |
Cl−, SO42−, HCO3−—concentrations of chlorides, sulfates, and bicarbonates (total alkalinity of water) expressed in mval/L. | <0.8; chlorides and sulfates do not participate in the formation of natural layers protecting steel surfaces (they are not part of them and do not cause corrosion) 0.8 ÷ 1.2; chlorides and sulfates may participate in the formation of natural layers on steel surfaces and the corrosion rate may be increased >1.2; a significant rate of local corrosion is expected |
| DS(I) | |||||||||||||||||
| Parameter | Unit | WTP(I) | P-1 | P-2 | P-3 | ||||||||||||
| MIN | MAX | Mean | SD | MIN | MAX | Mean | SD | MIN | MAX | Mean | SD | MIN | MAX | Mean | SD | ||
| Temperature | °C | 10.10 | 11.80 | 10.72 | 0.63 | 17.00 | 18.90 | 17.68 | 0.70 | 16.80 | 18.50 | 17.60 | 0.60 | 15.90 | 18.00 | 17.08 | 0.69 |
| Ph | - | 7.29 | 7.35 | 7.31 | 0.02 | 7.30 | 7.40 | 7.33 | 0.04 | 7.30 | 7.40 | 7.33 | 0.03 | 7.29 | 7.33 | 7.31 | 0.01 |
| Conductivity | µS/cm | 481 | 525 | 481 | 12.93 | 491 | 522 | 506 | 11.61 | 481 | 533 | 508 | 17.55 | 475 | 535 | 502 | 19.15 |
| Turbidity | NTU | 0.52 | 0.82 | 0.68 | 0.10 | 0.57 | 1.37 | 0.82 | 0.24 | 0.60 | 0.97 | 0.79 | 0.14 | 0.64 | 0.82 | 0.69 | 0.06 |
| Ammonium nitrogen | mg N-NH4+/L | 0.02 | 0.22 | 0.10 | 0.06 | 0.00 | 0.16 | 0.03 | 0.05 | 0.00 | 0.13 | 0.03 | 0.04 | 0.00 | 0.09 | 0.03 | 0.03 |
| Nitrite nitrogen | mg N-NO2−/L | 0.01 | 0.02 | 0.01 | 0.00 | 0.01 | 0.02 | 0.01 | 0.00 | 0.00 | 0.09 | 0.02 | 0.03 | 0.01 | 0.02 | 0.01 | 0.00 |
| Nitrate nitrogen | mg N-NO3−/L | 0.40 | 2.40 | 0.84 | 0.66 | 0.60 | 2.40 | 1.13 | 0.75 | 0.70 | 2.20 | 1.31 | 0.71 | 0.70 | 2.70 | 1.35 | 0.79 |
| Phosphorus | mg P-PO43−/L | 0.00 | 0.05 | 0.02 | 0.02 | 0.00 | 0.04 | 0.01 | 0.01 | 0.00 | 0.04 | 0.01 | 0.01 | 0.00 | 0.06 | 0.03 | 0.02 |
| Alkalinity | mg CaCO3/L | 255 | 280 | 272 | 8.43 | 250 | 285 | 271 | 10.61 | 248 | 285 | 272 | 10.81 | 255 | 280 | 274 | 8.35 |
| Hardness | mg CaCO3/L | 275 | 303 | 294 | 9.82 | 280 | 298 | 293 | 5.94 | 280 | 308 | 297 | 8.43 | 278 | 313 | 299 | 11.18 |
| Chlorides | mg Cl−/L | 10.65 | 17.75 | 13.98 | 2.41 | 10.65 | 17.75 | 14.20 | 2.68 | 10.65 | 24.85 | 14.64 | 4.42 | 10.65 | 21.30 | 13.76 | 3.52 |
| Sulfates | mg SO42−/L | 34.00 | 39.00 | 37.17 | 1.72 | 34.00 | 39.00 | 36.50 | 1.76 | 36.00 | 38.00 | 37.00 | 0.89 | 35.00 | 37.00 | 36.17 | 0.75 |
| Free chlorine | mg Cl/L | 0.00 | 0.35 | 0.12 | 0.12 | 0.03 | 0.16 | 0.09 | 0.04 | 0.03 | 0.09 | 0.06 | 0.02 | 0.04 | 0.09 | 0.07 | 0.02 |
| Total chlorine | mg Cl/L | 0.05 | 0.31 | 0.17 | 0.10 | 0.15 | 0.26 | 0.19 | 0.04 | 0.17 | 0.22 | 0.20 | 0.02 | 0.13 | 0.27 | 0.18 | 0.06 |
| DOC | mg C/L | 0.56 | 23.85 | 12.53 | 9.38 | 0.13 | 3.30 | 0.93 | 1.05 | 0.04 | 4.29 | 1.58 | 1.58 | 0.48 | 5.97 | 1.87 | 1.99 |
| BDOC | mg C/L | 0.06 | 2.53 | 1.33 | 0.99 | 0.01 | 0.35 | 0.10 | 0.11 | 0.00 | 0.45 | 0.17 | 0.17 | 0.05 | 0.63 | 0.20 | 0.21 |
| Escherichia coli | CFU/100 mL | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| DS(II) | |||||||||||||||||
| Parameter | Unit | WTP(II) | P-I | P-II | P-III | ||||||||||||
| Temperature | °C | 10.10 | 11.80 | 10.78 | 0.73 | 14.80 | 17.70 | 15.88 | 0.99 | 15.60 | 19.00 | 17.97 | 1.23 | 16.40 | 18.90 | 17.42 | 1.11 |
| Ph | - | 7.19 | 7.30 | 7.23 | 0.03 | 7.21 | 7.29 | 7.24 | 0.03 | 7.23 | 7.35 | 7.27 | 0.05 | 7.27 | 7.33 | 7.29 | 0.02 |
| Conductivity | µS/cm | 609 | 653 | 609 | 18.23 | 618 | 669 | 639.5 | 21.33 | 606 | 669 | 637.3 | 24.63 | 621 | 675 | 644.9 | 23.28 |
| Turbidity | NTU | 0.58 | 0.75 | 0.65 | 0.07 | 0.55 | 0.96 | 0.73 | 0.14 | 0.62 | 0.94 | 0.74 | 0.11 | 0.55 | 0.82 | 0.68 | 0.10 |
| Ammonium nitrogen | mg N-NH4+/L | 0.00 | 0.08 | 0.04 | 0.03 | 0.00 | 0.15 | 0.07 | 0.05 | 0.00 | 0.05 | 0.02 | 0.02 | 0.00 | 0.08 | 0.03 | 0.03 |
| Nitrite nitrogen | mg N-NO2−/L | 0.00 | 0.01 | 0.01 | 0.00 | 0.01 | 0.03 | 0.02 | 0.01 | 0.00 | 0.04 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.00 |
| Nitrate nitrogen | mg N-NO3−/L | 2.30 | 4.70 | 3.53 | 0.89 | 2.10 | 4.64 | 3.41 | 0.98 | 2.20 | 5.80 | 3.48 | 1.37 | 1.80 | 4.86 | 3.55 | 1.20 |
| Phosphorus | mg P-PO43−/L | 0.00 | 0.07 | 0.03 | 0.03 | 0.00 | 0.17 | 0.07 | 0.05 | 0.00 | 0.11 | 0.05 | 0.04 | 0.00 | 0.10 | 0.07 | 0.03 |
| Alkalinity | mg CaCO3/L | 315 | 325 | 319 | 4.43 | 315 | 325 | 319 | 4.17 | 310 | 330 | 318 | 7.43 | 300 | 325 | 316 | 8.02 |
| Hardness | mg CaCO3/L | 343 | 373 | 360 | 10.04 | 238 | 375 | 348 | 45.27 | 353 | 380 | 367 | 8.96 | 365 | 375 | 369 | 3.45 |
| Chlorides | mg Cl−/L | 19.53 | 28.40 | 23.52 | 3.11 | 17.75 | 28.40 | 23.74 | 3.14 | 19.53 | 28.40 | 24.41 | 2.46 | 19.53 | 28.40 | 24.09 | 2.87 |
| Sulfates | mg SO42−/L | 50.00 | 54.00 | 51.67 | 1.37 | 48.00 | 55.00 | 52.00 | 2.45 | 50.00 | 53.00 | 51.50 | 1.05 | 51.00 | 54.00 | 52.40 | 1.14 |
| Free chlorine | mg Cl/L | 0.04 | 0.09 | 0.07 | 0.02 | 0.00 | 0.23 | 0.07 | 0.08 | 0.03 | 0.11 | 0.06 | 0.03 | 0.00 | 0.09 | 0.04 | 0.03 |
| Total chlorine | mg Cl/L | 0.17 | 0.29 | 0.22 | 0.04 | 0.09 | 0.24 | 0.18 | 0.04 | 0.14 | 0.20 | 0.17 | 0.02 | 0.00 | 0.21 | 0.11 | 0.08 |
| DOC | mg C/L | 0.37 | 21.89 | 15.71 | 8.74 | 0.35 | 6.64 | 2.17 | 2.01 | 0.11 | 9.20 | 3.18 | 2.89 | 0.01 | 12.61 | 3.01 | 4.53 |
| BDOC | mg C/L | 0.04 | 2.32 | 1.66 | 0.93 | 0.04 | 0.70 | 0.23 | 0.21 | 0.01 | 0.97 | 0.23 | 0.31 | 0.00 | 1.35 | 0.31 | 0.48 |
| Escherichia coli | CFU/100 mL | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Metal | Unit | DS(I) | |||||||||||||||
| WTP(I) | P-1 | P-2 | P-3 | ||||||||||||||
| Min | Max | Mean | SD | Min | Max | Mean | SD | Min | Min | Mean | SD | Min | Max | Mean | SD | ||
| Na | ppm/L | 12.95 | 17.34 | 16.19 | 1.23 | 12.53 | 17.34 | 16.19 | 1.54 | 13.24 | 17.14 | 16.08 | 1.23 | 13.74 | 18.80 | 16.43 | 1.38 |
| Mg | ppm/L | 12.42 | 16.50 | 14.69 | 1.28 | 11.80 | 15.87 | 14.83 | 1.31 | 12.31 | 16.06 | 14.83 | 1.16 | 12.79 | 15.98 | 14.95 | 0.97 |
| K | ppm/L | 3.62 | 4.21 | 3.89 | 0.20 | 3.56 | 4.08 | 3.92 | 0.17 | 3.63 | 4.07 | 3.87 | 0.16 | 3.75 | 4.11 | 3.92 | 0.10 |
| Ca | ppm/L | 97.16 | 141.20 | 111.83 | 13.59 | 104.00 | 133.38 | 112.20 | 9.17 | 99.02 | 112.55 | 107.69 | 4.51 | 106.96 | 131.43 | 112.05 | 7.99 |
| Zn | ppm/L | 0.02 | 0.10 | 0.05 | 0.03 | 0.04 | 0.12 | 0.06 | 0.03 | 0.04 | 0.09 | 0.05 | 0.02 | 0.05 | 0.52 | 0.26 | 0.18 |
| Fe | ppm/L | 0.03 | 0.07 | 0.05 | 0.02 | 0.03 | 0.08 | 0.05 | 0.02 | 0.01 | 0.13 | 0.05 | 0.03 | 0.02 | 0.08 | 0.04 | 0.02 |
| Mn | ppb/L | 7.17 | 11.82 | 8.78 | 1.56 | 4.85 | 11.24 | 6.02 | 2.13 | 5.85 | 9.80 | 7.29 | 1.33 | 5.23 | 11.08 | 6.55 | 1.93 |
| Cr | ppb/L | 0.17 | 0.92 | 0.48 | 0.23 | 0.12 | 1.02 | 0.51 | 0.30 | 0.26 | 1.25 | 0.55 | 0.34 | 0.11 | 0.66 | 0.39 | 0.19 |
| Ni | ppb/L | 0.07 | 0.63 | 0.25 | 0.20 | 0.30 | 1.75 | 0.68 | 0.50 | 0.07 | 1.81 | 0.41 | 0.58 | 0.10 | 0.33 | 0.22 | 0.09 |
| Cu | ppb/L | 2.98 | 14.62 | 4.59 | 3.84 | 3.88 | 6.81 | 5.42 | 1.04 | 1.64 | 19.10 | 4.76 | 5.82 | 4.51 | 18.24 | 8.91 | 5.88 |
| Cd | ppb/L | 0.00 | 0.11 | 0.02 | 0.04 | 0.00 | 0.02 | 0.01 | 0.01 | 0.00 | 1.12 | 0.16 | 0.39 | 0.00 | 0.30 | 0.05 | 0.10 |
| Pb | ppb/L | 0.25 | 0.70 | 0.44 | 0.10 | 0.36 | 0.65 | 0.48 | 0.10 | 0.19 | 0.89 | 0.40 | 0.24 | 0.35 | 1.80 | 0.77 | 0.52 |
| Metal | Unit | DS(II) | |||||||||||||||
| WTP(II) | P-I | P-II | P-III | ||||||||||||||
| 17.91 | 21.44 | 20.33 | 1.17 | 21.13 | 22.11 | 21.61 | 0.37 | 19.19 | 21.55 | 20.55 | 0.90 | 19.44 | 21.55 | 20.32 | 0.85 | ||
| Mg | ppm/L | 18.44 | 20.51 | 19.90 | 0.68 | 19.96 | 21.35 | 20.60 | 0.48 | 18.47 | 20.74 | 19.68 | 0.79 | 18.60 | 20.98 | 19.66 | 0.83 |
| K | ppm/L | 3.97 | 4.30 | 4.22 | 0.12 | 4.14 | 4.44 | 4.31 | 0.10 | 3.87 | 4.40 | 4.20 | 0.16 | 3.88 | 4.31 | 4.18 | 0.16 |
| Ca | ppm/L | 119.50 | 128.37 | 124.52 | 2.71 | 119.77 | 134.01 | 125.78 | 4.67 | 111.98 | 128.53 | 122.84 | 5.27 | 114.46 | 127.36 | 122.90 | 4.55 |
| Zn | ppm/L | 0.01 | 0.33 | 0.09 | 0.11 | 0.02 | 0.12 | 0.06 | 0.04 | 0.01 | 0.08 | 0.04 | 0.02 | 0.03 | 0.23 | 0.10 | 0.07 |
| Fe | ppm/L | 0.02 | 0.09 | 0.03 | 0.03 | 0.03 | 0.09 | 0.04 | 0.03 | 0.01 | 0.09 | 0.04 | 0.03 | 0.01 | 0.08 | 0.04 | 0.02 |
| Mn | ppb/L | 1.43 | 2.43 | 1.74 | 0.34 | 1.71 | 2.16 | 1.84 | 0.17 | 1.35 | 2.25 | 1.76 | 0.30 | 1.55 | 2.49 | 1.85 | 0.33 |
| Cr | ppb/L | 0.27 | 1.22 | 0.69 | 0.32 | 0.24 | 0.81 | 0.60 | 0.21 | 0.54 | 1.26 | 0.77 | 0.25 | 0.25 | 1.08 | 0.67 | 0.29 |
| Ni | ppb/L | 1.52 | 6.31 | 2.80 | 1.64 | 2.19 | 4.02 | 2.85 | 0.70 | 1.63 | 4.80 | 2.92 | 1.11 | 1.61 | 5.43 | 2.85 | 1.60 |
| Cu | ppb/L | 1.56 | 17.55 | 6.86 | 6.60 | 3.09 | 12.00 | 5.21 | 3.40 | 3.28 | 86.18 | 22.68 | 29.55 | 20.11 | 92.68 | 37.11 | 27.96 |
| Cd | ppb/L | 0.02 | 1.87 | 0.31 | 0.69 | 0.01 | 4.91 | 0.86 | 1.98 | 0.02 | 0.29 | 0.10 | 0.12 | 0.01 | 0.30 | 0.09 | 0.11 |
| Pb | ppb/L | 0.19 | 2.44 | 0.77 | 0.76 | 0.34 | 1.45 | 0.77 | 0.41 | 0.27 | 1.57 | 0.84 | 0.47 | 0.83 | 1.77 | 1.38 | 0.32 |
| N | BDOC | P | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | Number of Samples < 0.2 mg Ninorg/L [%] | Min | Max | Mean | Number of Samples < 0.25 mg C/L [%] | Min | Max | Mean | Number of Samples < 0.01 mg P-PO43−/L [%] | |
| DS(I) | ||||||||||||
| WTP(I) | 0.56 | 2.53 | 0.96 | 0 | 0.06 | 2.53 | 1.33 | 25 | 0.00 | 0.05 | 0.02 | 50 |
| P-1 | 0.61 | 2.42 | 1.18 | 0 | 0.01 | 0.35 | 0.10 | 87.5 | 0.00 | 0.04 | 0.01 | 62.5 |
| P-2 | 0.74 | 2.25 | 1.18 | 0 | 0.00 | 0.45 | 0.17 | 75 | 0.00 | 0.04 | 0.01 | 62.5 |
| P-3 | 0.76 | 2.73 | 1.39 | 0 | 0.05 | 0.63 | 0.20 | 75 | 0.00 | 0.06 | 0.03 | 37.5 |
| DS(II) | ||||||||||||
| WTP(II) | 2.3 | 4.73 | 3.58 | 0 | 0.36 | 2.28 | 1.66 | 0 | 0.00 | 0.07 | 0.03 | 37.5 |
| P-I | 2.27 | 4.52 | 3.50 | 0 | 0.05 | 0.70 | 0.23 | 62.5 | 0.00 | 0.17 | 0.07 | 12.5 |
| P-II | 2.26 | 5.81 | 3.50 | 0 | 0.01 | 0.97 | 0.23 | 87.5 | 0.00 | 0.11 | 0.05 | 12.5 |
| P-III | 1.85 | 4.88 | 3.59 | 0 | 0.00 | 1.35 | 0.32 | 71.4 | 0.00 | 0.10 | 0.07 | 14.3 |
| Langelier Index | Ryznar Index | Larson–Skold Index | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | Min | Max | Mean | Min | Max | Mean | |
| DS(I) | |||||||||
| WTP(I) | −0.09 | 0.09 | −0.03 | 7.12 | 7.50 | 7.37 | 0.12 | 0.16 | 0.14 |
| P-1 | 0.07 | 0.18 | 0.13 | 6.93 | 7.18 | 7.07 | 0.13 | 0.16 | 0.14 |
| P-2 | 0.04 | 0.20 | 0.11 | 7.00 | 7.22 | 7.11 | 0.12 | 0.20 | 0.15 |
| P-3 | 0.07 | 0.16 | 0.10 | 6.97 | 7.18 | 7.18 | 0.12 | 0.18 | 0.14 |
| DS(II) | |||||||||
| WTP(II) | −0.04 | 0.04 | 0.00 | 7.18 | 7.27 | 7.23 | 0.16 | 0.20 | 0.18 |
| P-I | 0.07 | 0.14 | 0.10 | 6.99 | 7.07 | 7.03 | 0.17 | 0.21 | 0.19 |
| P-II | 0.12 | 0.26 | 0.18 | 6.83 | 6.99 | 6.92 | 0.17 | 0.21 | 0.19 |
| P-III | 0.17 | 0.24 | 0.19 | 6.84 | 6.94 | 6.91 | 0.18 | 0.21 | 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domoń, A.; Kowalska, B.; Papciak, D.; Wojtaś, E.; Kamińska, I. Safety of Tap Water in Terms of Changes in Physical, Chemical, and Biological Stability. Water 2024, 16, 1221. https://doi.org/10.3390/w16091221
Domoń A, Kowalska B, Papciak D, Wojtaś E, Kamińska I. Safety of Tap Water in Terms of Changes in Physical, Chemical, and Biological Stability. Water. 2024; 16(9):1221. https://doi.org/10.3390/w16091221
Chicago/Turabian StyleDomoń, Andżelika, Beata Kowalska, Dorota Papciak, Edyta Wojtaś, and Iwona Kamińska. 2024. "Safety of Tap Water in Terms of Changes in Physical, Chemical, and Biological Stability" Water 16, no. 9: 1221. https://doi.org/10.3390/w16091221
APA StyleDomoń, A., Kowalska, B., Papciak, D., Wojtaś, E., & Kamińska, I. (2024). Safety of Tap Water in Terms of Changes in Physical, Chemical, and Biological Stability. Water, 16(9), 1221. https://doi.org/10.3390/w16091221








