Mercury Bioconcentration and Translocation in Rooted Macrophytes (Paspalum repens Berg.) from Floodplain Lakes in the Araguaia River Watershed, Brazilian Savanna
Abstract
1. Introduction
2. Materials and Methods
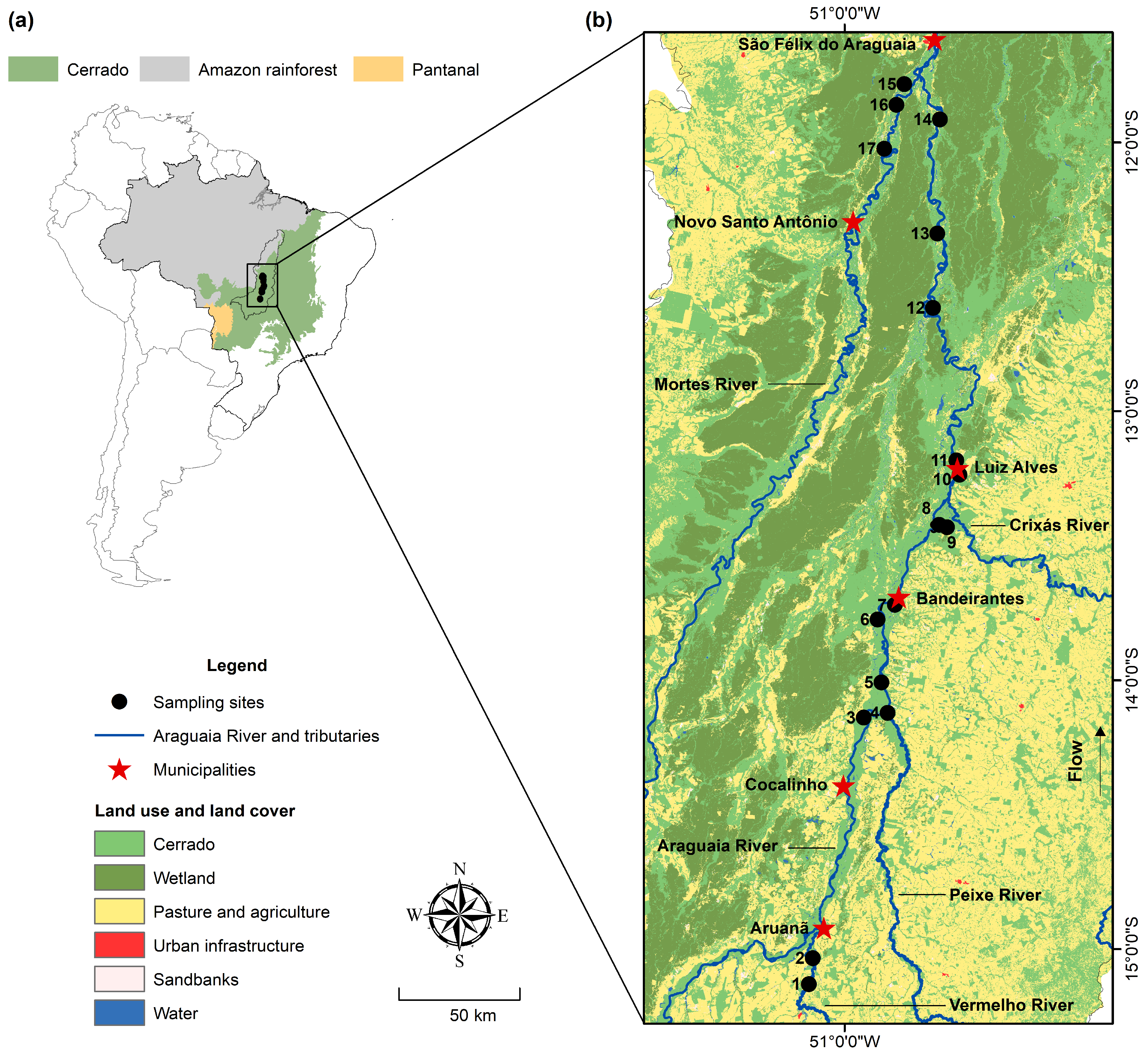
2.1. Study Area
2.2. Sample Collection and Processing
2.3. Quantification of Total Mercury (THg) in Water and Macrophytes
2.4. THg Bioconcentration and Translocation in Macrophytes
2.5. Characterization of Environmental Conditions and Land Use and Cover
2.6. Data Analysis
2.7. Geostatistical Analysis
3. Results
3.1. THg Concentrations in Water and Environmental Characterization
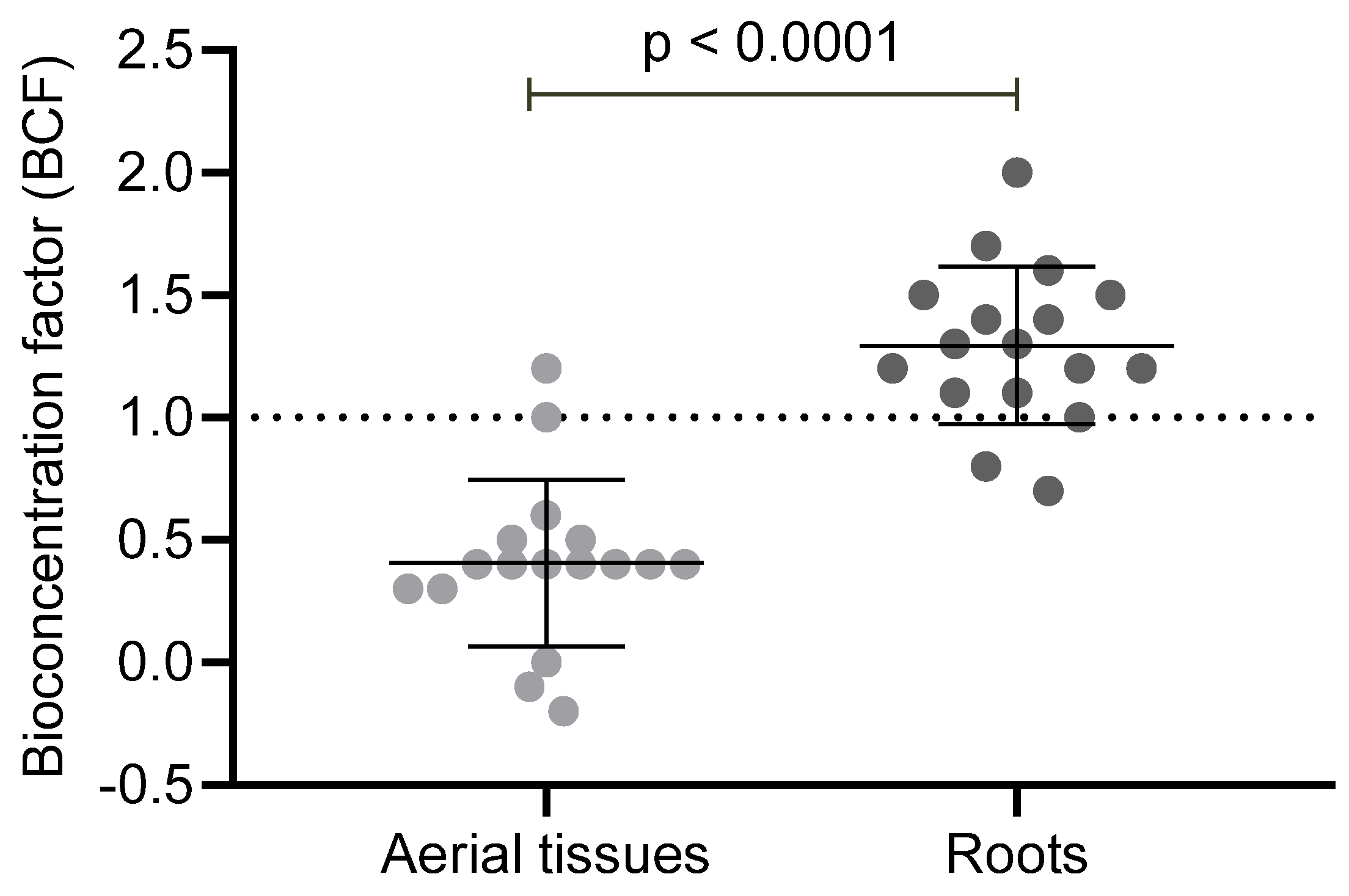
3.2. THg Bioconcentration and Translocation in Macrophytes
3.3. Association between THg Concentrations, Environmental Conditions, and Land Use and Cover
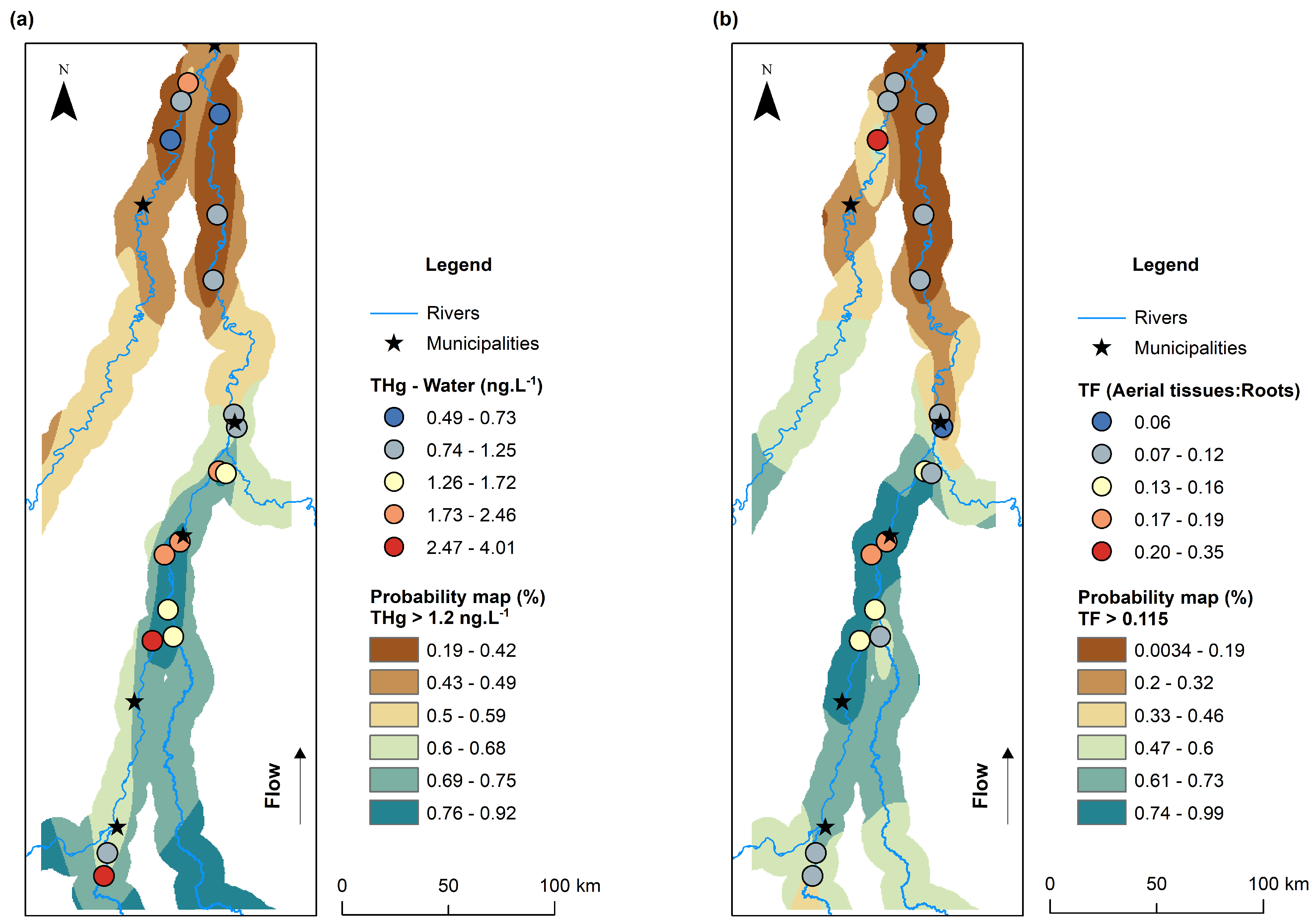
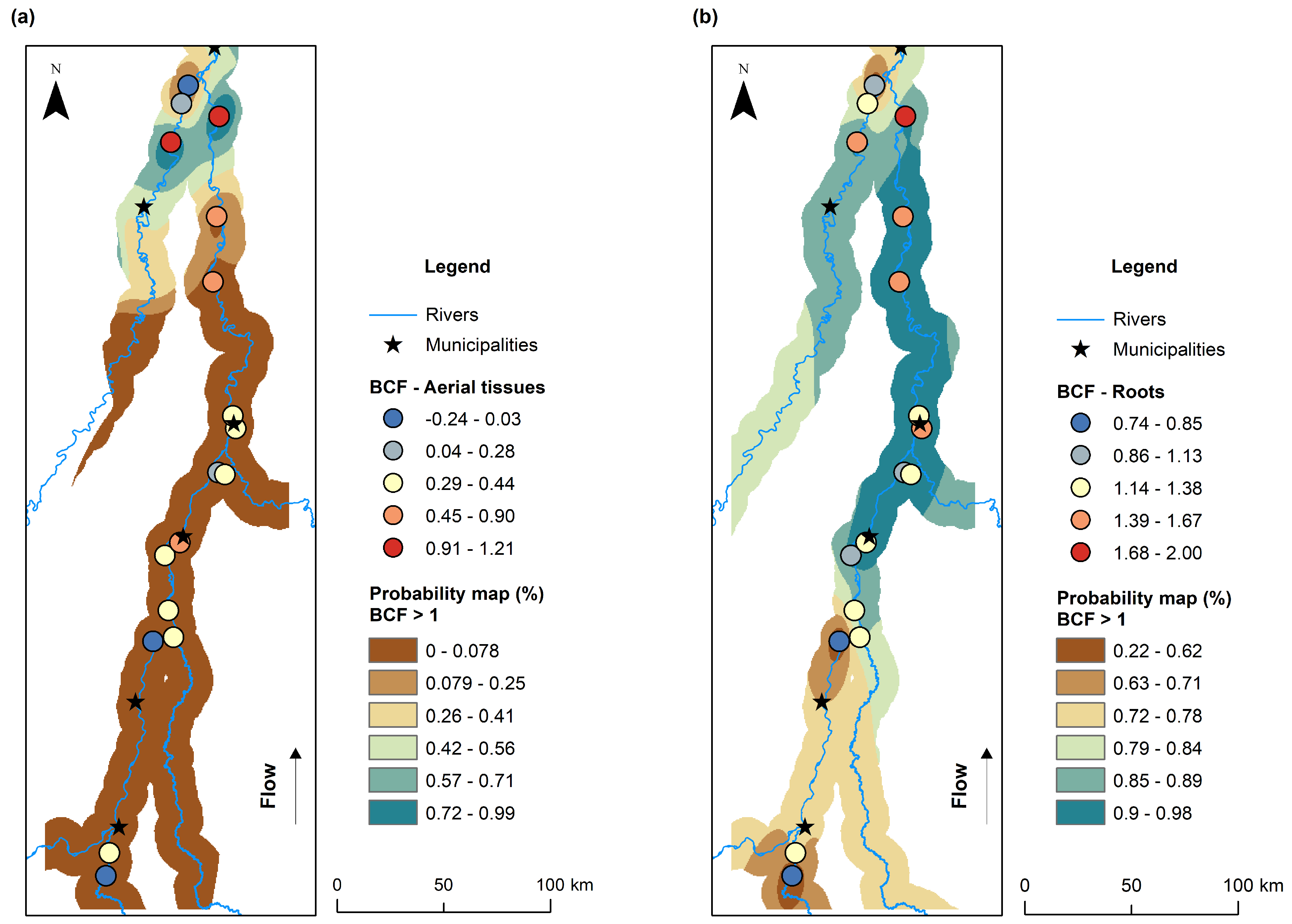
3.4. Spatial Distribution of THg Concentrations in Water and Bioconcentration and Translocation Factors in Macrophytes
4. Discussion
4.1. Influence of Land Use and pH on THg Concentrations in Water
4.2. Bioconcentration, Translocation, and the Influence of Environmental Conditions on THg Accumulation in Macrophytes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ziegler, J.P.; Solomon, C.T.; Finney, B.P.; Gregory-Eaves, I. Macrophyte biomass predicts food chain length in shallow lakes. Ecosphere 2015, 6, 1–16. [Google Scholar] [CrossRef]
- Nõges, T.; Luup, H.; Feldmann, T. Primary production of aquatic macrophytes and their epiphytes in two shallow lakes (Peipsi and Võrtsjärv) in Estonia. Aquat. Ecol. 2010, 44, 83–92. [Google Scholar] [CrossRef]
- Brothers, S.M.; Hilt, S.; Meyer, S.; Köhler, J. Plant community structure determines primary productivity in shallow, eutrophic lakes. Freshw. Biol. 2013, 58, 2264–2276. [Google Scholar] [CrossRef]
- Holmroos, H.; Horppila, J.; Niemistö, J.; Nurminen, L.; Hietanen, S. Dynamics of dissolved nutrients among different macrophyte stands in a shallow lake. Limnology 2015, 16, 31–39. [Google Scholar] [CrossRef]
- Lu, J.; Bunn, S.E.; Burford, M.A. Nutrient release and uptake by littoral macrophytes during water level fluctuations. Sci. Total Environ. 2018, 622, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Madsen, J.D.; Chambers, P.A.; James, W.F.; Koch, E.W.; Westlake, D.F. The interaction between water movement, sediment dynamics and submersed macrophytes. Hydrobiologia 2001, 444, 71–84. [Google Scholar] [CrossRef]
- Lopes, T.M.; Cunha, E.R.; Silva, J.C.B.; Behrend, R.D.; Gomes, L.C. Dense macrophytes influence the horizontal distribution of fish in floodplain lakes. Environ. Biol. Fishes 2015, 98, 1741–1755. [Google Scholar] [CrossRef]
- Alvim, E.A.; Kisaka, T.B.; Nardoto, G.B.; de Mendonca-Galvao, L.; Fonseca, B.M.; Bustamante, M.M. Trophic relationships between primary producers and associated fauna in a pristine Cerrado pond. J. Limnol. 2019, 78, 310–322. [Google Scholar] [CrossRef]
- Polechońska, L.; Klink, A. Macrophytes as passive bioindicators of trace element pollution in the aquatic environment. Wiley Interdiscip. Rev. Water 2023, 10, e1630. [Google Scholar] [CrossRef]
- Branfireun, B.A.; Cosio, C.; Poulain, A.J.; Riise, G.; Bravo, A.G. Mercury cycling in freshwater systems—An updated conceptual model. Sci. Total Environ. 2020, 745, 140906. [Google Scholar] [CrossRef]
- Regier, N.; Larras, F.; Bravo, A.G.; Ungureanu, V.G.; Amouroux, D.; Cosio, C. Mercury bioaccumulation in the aquatic plant Elodea nuttallii in the field and in microcosm: Accumulation in shoots from the water might involve copper transporters. Chemosphere 2013, 90, 595–602. [Google Scholar] [CrossRef]
- Guimaraes, J.R.D.; Meili, M.; Hylander, L.D.; e Silva, E.D.C.; Roulet, M.; Mauro, J.B.N.; de Lemos, R.A. Mercury net methylation in five tropical flood plain regions of Brazil: High in the root zone of floating macrophyte mats but low in surface sediments and flooded soils. Sci. Total Environ. 2000, 261, 99–107. [Google Scholar] [CrossRef]
- Bravo, A.G.; Cosio, C.; Amouroux, D.; Zopfi, J.; Chevalley, P.A.; Spangenberg, J.E.; Ungureanu, V.G.; Dominik, J. Extremely elevated methyl mercury levels in water, sediment and organisms in a Romanian reservoir affected by release of mercury from a chlor-alkali plant. Water Res. 2014, 49, 391–405. [Google Scholar] [CrossRef]
- Gomez, F.H.; Collivignarelli, M.C.; Masoud, A.M.N.; Carnevale Miino, M.; Torres, K.C.; Quintero, J.A.; Sorlini, S.; Vaccari, M. Mercury Removal from Mining Wastewater by Phytoaccumulation in Autochthonous Aquatic Plant Species. Clean Technol. 2023, 5, 839–851. [Google Scholar] [CrossRef]
- Correia, R.R.S.; de Oliveira, D.C.M.; Guimarães, J.R.D. Total mercury distribution and volatilization in microcosms with and without the aquatic macrophyte Eichhornia crassipes. Aquat. Geochem. 2012, 18, 421–432. [Google Scholar] [CrossRef]
- Bonanno, G.; Vymazal, J.; Cirelli, G.L. Translocation, accumulation and bioindication of trace elements in wetland plants. Sci. Total Environ. 2018, 631, 252–261. [Google Scholar] [CrossRef]
- Cintrón, M.L.B.; Broomandi, P.; Cárdenas-Escudero, J.; Cáceres, J.O.; Galán-Madruga, D. Elucidating Best Geospatial Estimation Method Applied to Environmental Sciences. Bull. Environ. Contam. Toxicol. 2024, 112, 6. [Google Scholar] [CrossRef]
- Rodrigues, Y.O.S.; Dórea, J.G.; Landim, P.M.B.; Bernardi, J.V.E.; Monteiro, L.C.; Souza, J.P.R.; Pinto, L.C.M.; Fernandes, I.O.; Souza, J.V.V.; Sousa, A.R.; et al. Mercury spatiality and mobilization in roadside soils adjacent to a savannah ecological reserve. Environ. Res. 2022, 205, 112513. [Google Scholar] [CrossRef]
- Yevugah, L.L.; Osei Jnr, E.M.; Bak, J.L.; Nkansah, M.A.; Darko, G. Geospatial distribution of mercury in surface soils across Ghana. Chem. Afr. 2023, 6, 3119–3129. [Google Scholar] [CrossRef]
- Forsythe, K.W.; Marvin, C.H.; Valancius, C.J.; Watt, J.P.; Aversa, J.M.; Swales, S.J.; Jakubek, D.J.; Shaker, R.R. Geovisualization of Mercury Contamination in Lake St. Clair Sediments. J. Mar. Sci. Eng. 2016, 4, 19. [Google Scholar] [CrossRef]
- Kim, S.M.; Choi, Y.; Yi, H.; Park, H.D. Geostatistical prediction of heavy metal concentrations in stream sediments considering the stream networks. Environ. Earth Sci. 2017, 76, 72. [Google Scholar] [CrossRef]
- Monteiro, L.C.; Vieira, L.C.G.; Bernardi, J.V.E.; Moraes, L.C.; Rodrigues, Y.O.S.; de Souza, J.P.R.; Souza, J.R.; Bastos, W.R.; Passos, C.J.S.; Dórea, J.G. Ecological risk of mercury in bottom sediments and spatial correlation with land use in Neotropical savanna floodplain lakes, Araguaia River, Central Brazil. Environ. Res. 2023, 238, 117231. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Bayer, M.; Assis, P.C.; Suizu, T.M.; Gomes, M.C. Mudança no uso e cobertura da terra na bacia hidrográfica do rio Araguaia e seus reflexos nos recursos hídricos, o trecho médio do rio Araguaia em Goiás. Confins Rev. Fr.-Brésilienne Geogr. 2020, 48, 48. [Google Scholar] [CrossRef]
- Teixeira, A.S.; Vieira, L.C.G.; Souza, C.A.; Bernardi, J.V.E.; Monteiro, L.C. Evidence of water surface and flow reduction in the main hydrographic basin of the Brazilian savannah (Cerrado biome): The Araguaia river. Hydrobiologia 2024, 851, 2503–2518. [Google Scholar] [CrossRef]
- Monteiro, L.C.; Vieira, L.C.G.; Bernardi, J.V.E.; Bastos, W.R.; Souza, J.P.R.; Recktenvald, M.C.N.N.; Nery, A.F.C.; Oliveira, I.A.S.; Cabral, C.S.; Moraes, L.C.; et al. Local and landscape factors influencing mercury distribution in water, bottom sediment, and biota from lakes of the Araguaia River floodplain, Central Brazil. Sci. Total Environ. 2024, 908, 168336. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.D.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Gomes, E.P.; Blanco, C.J.C.; Pessoa, F.C.L. Identification of homogeneous precipitation regions via Fuzzy c-means in the hydrographic region of Tocantins–Araguaia of Brazilian Amazonia. Appl. Water Sci. 2019, 9, 6. [Google Scholar] [CrossRef]
- Junk, W.J.; Piedade, M.T.F.; Lourival, R.; Wittmann, F.; Kandus, P.; Lacerda, L.D.; Bozelli, R.L.; Esteves, F.A.; Nundes da Cunha, C.; Maltchik, L.; et al. Brazilian wetlands: Their definition, delineation, and classification for research, sustainable management, and protection. Aquat. Conserv. Mar. Freshw. Ecosyst 2014, 24, 5–22. [Google Scholar] [CrossRef]
- Aquino, S.; Latrubesse, E.M.; de Souza Filho, E.E. Caracterização hidrológica e geomorfológica dos afluentes da Bacia do Rio Araguaia. Rev. Bras. Geomorfol. 2009, 10, 43–54. [Google Scholar] [CrossRef]
- Irion, G.; Nunes, G.M.; Nunes da Cunha, C.; de Arruda, E.C.; Santos-Tambelini, M.; Dias, A.P.; Morais, J.O.; Junk, W.J. Araguaia river floodplain: Size, age, and mineral composition of a large tropical savanna wetland. Wetlands 2016, 36, 945–956. [Google Scholar] [CrossRef]
- Latrubesse, E.M.; Amsler, M.L.; de Morais, R.P.; Aquino, S. The geomorphologic response of a large pristine alluvial river to tremendous deforestation in the South American tropics: The case of the Araguaia River. Geomorphology 2009, 113, 239–252. [Google Scholar] [CrossRef]
- Arruda, E.C.; Nunes da Cunha, C.; Junk, W.J. Área Alagável do Rio Araguaia: Classificação dos Macrohabitat de uma Grande Área Úmida Savânica Tropical. Biodivers. Bras. 2023, 13, 1–24. [Google Scholar] [CrossRef]
- Moraes, L.; Bernardi, J.V.E.; de Souza, J.P.R.; Portela, J.F.; Vieira, L.C.G.; Sousa Passos, C.J.; de Souza, J.R.; Bastos, W.R.; Monteiro, L.C.; Rodrigues, Y.O.S.; et al. Sediment Mercury, Geomorphology and Land Use in the Middle Araguaia River Floodplain (Savanna Biome, Brazil). Soil Syst. 2023, 7, 97. [Google Scholar] [CrossRef]
- Coelho-Souza, S.A.; Guimarães, J.R.D.; Miranda, M.R.; Poirier, H.; Mauro, J.B.; Lucotte, M.; Mergler, D. Mercury and flooding cycles in the Tapajós river basin, Brazilian Amazon: The role of periphyton of a floating macrophyte (Paspalum repens). Sci. Total Environ. 2011, 409, 2746–2753. [Google Scholar] [CrossRef]
- U. S. EPA—Environment Protection Agency. Method 1631, Revision E: Mercury in Water by Oxidation, Purge and Trap, and Cold Vapor Atomic Fluorescence Spectrometry; U. S. EPA: Washington, DC, USA, 2002; 45p.
- Arnot, J.A.; Gobas, F.A. A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Environ. Rev. 2006, 14, 257–297. [Google Scholar] [CrossRef]
- Stoltz, E.; Greger, M. Accumulation properties of As, Cd, Cu, Pb and Zn by four wetland plant species growing on submerged mine tailings. Environ. Exp. Bot. 2002, 47, 271–280. [Google Scholar] [CrossRef]
- MapBiomas Project. Coleção 8 da Série Anual de Mapas de Cobertura e Uso da Terra do Brasil. 2023. Available online: https://plataforma.brasil.mapbiomas.org (accessed on 11 January 2024).
- Marcionilio, S.M.L.O.; Machado, K.B.; Carneiro, F.M.; Ferreira, M.E.; Carvalho, P.; Vieira, L.C.G.; Moraes, V.L.; Nabout, J.C. Environmental factors affecting chlorophyll-a concentration in tropical floodplain lakes, Central Brazil. Environ. Monit. Assess. 2016, 188, 611. [Google Scholar] [CrossRef]
- Ometto, J.P.H.; Martinelli, L.A.; Ballester, M.V.; Gessner, A.; Krusche, A.V.; Victoria, R.L.; Williams, M. Effects of land use on water chemistry and macroinvertebrates in two streams of the Piracicaba river basin, south-east Brazil. Freshw. Biol. 2001, 44, 327–337. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2023. Available online: https://www.R-project.org (accessed on 11 January 2024).
- Bernardi, J.V.E.; Neira, M.P.; Manzatto, A.G.; De Holanda, I.B.B.; Almeida, R.; Bastos, W.R.; Dórea, J.G.; Landim, P.M.B.; Vieira, L.C.G. Aplicação da análise geoestatística para modelagem espacial do mercúrio e matéria orgânica em solos florestais na Amazônia Ocidental. Front. J. Soc. Technol. Environ. Res. 2015, 4, 31–46. [Google Scholar] [CrossRef]
- Goovaerts, P. Geostatistics for NATURAL Resources Evaluation; Oxford University Press: Oxford, UK, 1997; 496p, ISBN 01-9511-538-4. [Google Scholar]
- Cambardella, C.A.; Moorman, T.B.; Novak, J.M.; Parkin, T.B.; Karlen, D.L.; Turco, R.F.; Konopka, A.E. Field-scale variability of soil properties in central Iowa soils. Sci. Soc. Am. J. 1994, 58, 1501–1511. [Google Scholar] [CrossRef]
- Lininger, K.B.; Latrubesse, E.M. Flooding hydrology and peak discharge attenuation along the middle Araguaia River in central Brazil. Catena 2016, 143, 90–101. [Google Scholar] [CrossRef]
- Malvić, T.; Bastaić, B. Reducing variogram uncertainties using the ‘jack-knifing’ method, a case study of the Stari Gradac–Barcs-Nyugat field. Bull. Hung. Geol. Soc. 2008, 138, 165–174. [Google Scholar]
- Arétouyap, Z.; Njandjock Nouck, P.; Nouayou, R.; Ghomsi Kemgang, F.E.; Piépi Toko, A.D.; Asfahani, J. Lessening the adverse effect of the semivariogram model selection on an interpolative survey using kriging technique. SpringerPlus 2016, 5, 549. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.R.D.; Silva, J.R.; Baumann, L.R.F.; Miziara, F.; Ferreira, L.G.; Merelles, L.R.D.O. Tecnologia e degradação de pastagens na pecuária no Cerrado brasileiro. Soc. Nat. 2022, 32, 585–596. [Google Scholar] [CrossRef]
- Lima, A.F.L.; Campos, M.C.C.; Enck, B.F.; Simões, W.S.; Araújo, R.M.; Santos, L.A.C.; Cunha, J.M. Physical soil attributes in areas under forest/pasture conversion in northern Rondônia, Brazil. Environ. Monit. Assess. 2022, 194, 34. [Google Scholar] [CrossRef] [PubMed]
- Merten, G.H.; Minella, J.P. The expansion of Brazilian agriculture: Soil erosion scenarios. Int. Soil Water Conserv. Res. 2013, 1, 37–48. [Google Scholar] [CrossRef]
- Lacerda, L.D.; Bastos, W.R.; Almeida, M.D. The impacts of land use changes in the mercury flux in the Madeira River, Western Amazon. An. Acad. Bras. Ciênc. 2012, 84, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Godoy, J.M.; Padovani, C.R.; Guimarães, J.R.D.; Pereira, J.C.; Vieira, L.M.; Carvalho, Z.L.; Galdino, S. Evaluation of the siltation of River Taquari, Pantanal, Brazil, through 210Pb geochronology of floodplain lake sediments. J. Braz. Chem. Soc. 2002, 13, 71–77. [Google Scholar] [CrossRef]
- Roulet, M.; Lucotte, M.; Canuel, R.; Farella, N.; Goch, Y.G.F.; Peleja, J.R.P.; Guimarães, J.R.D.; Mergler, D.; Amorim, M. Spatio-temporal geochemistry of mercury in waters of the Tapajós and Amazon rivers, Brazil. Limnol. Oceanogr. 2001, 46, 1141–1157. [Google Scholar] [CrossRef]
- Lino, A.S.; Kasper, D.; Guida, Y.S.; Thomaz, J.R.; Malm, O. Total and methyl mercury distribution in water, sediment, plankton and fish along the Tapajós River basin in the Brazilian Amazon. Chemosphere 2019, 235, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Aquino, S.; Latrubesse, E.; Bayer, M. Assessment of wash load transport in the Araguaia River (Aruana gauge station), Central Brazil. Lat. Am. J. Sedimentol. Basin Anal. 2009, 16, 119–128. [Google Scholar]
- Maia, P.D.; Maurice, L.; Tessier, E.; Amouroux, D.; Cossa, D.; Pérez, M.; Moreira-Turcq, P.; Rhéault, I. Mercury distribution and exchanges between the Amazon River and connected floodplain lakes. Sci. Total Environ. 2009, 407, 6073–6084. [Google Scholar] [CrossRef] [PubMed]
- Attua, E.M.; Ayamga, J.; Pabi, O. Relating land use and land cover to surface water quality in the Densu River basin, Ghana. Int. J. River Basin Manag. 2014, 12, 57–68. [Google Scholar] [CrossRef]
- Kocman, D.; Kanduč, T.; Ogrinc, N.; Horvat, M. Distribution and partitioning of mercury in a river catchment impacted by former mercury mining activity. Biogeochemistry 2011, 104, 183–201. [Google Scholar] [CrossRef]
- Córdoba-Tovar, L.; Marrugo-Negrete, J.; Barón, P.A.R.; Calao-Ramos, C.R.; Díez, S. Toxic metal (loids) levels in the aquatic environment and nuclear alterations in fish in a tropical river impacted by gold mining. Environ. Res. 2023, 224, 115517. [Google Scholar] [CrossRef]
- Lago-Vila, M.; Arenas-Lago, D.; Rodríguez-Seijo, A.; Andrade, M.L.; Vega, F.A. Ability of Cytisus scoparius for phytoremediation of soils from a Pb/Zn mine: Assessment of metal bioavailability and bioaccumulation. J. Environ. Manag. 2019, 235, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Pott, V.J.; Pott, A. Plantas Aquáticas do Pantanal; Embrapa Comunicação para Transferência de Tecnologia: Brasília, Brazil, 2000; 404p, ISBN 85-7383-091-3. [Google Scholar]
- Amaral, M.C.E.; Bittrich, V.; Faria, A.D.; Anderson, L.O.; Aona, L.Y.S. Guia de Campo para Plantas Aquáticas e Palustres do Estado de São Paulo; Holos Editora: Ribeirão Preto, Brasil, 2008; 452p, ISBN 85-8669-964-0. [Google Scholar]
- Núñez, S.R.; Negrete, J.M.; Rios, J.A.; Hadad, H.R.; Maine, M.A. Hg, Cu, Pb, Cd, and Zn accumulation in macrophytes growing in tropical wetlands. Water Air Soil Pollut. 2011, 216, 361–373. [Google Scholar] [CrossRef]
- Colonnello, G. Biomass production of Eichhornia crassipes and Paspalum repens in two contrasting environment of the Orinoco River delta (Venezuela). Int. Ver. Theor. Angew. Limnol. Verhandlungen 1998, 26, 1827–1829. [Google Scholar] [CrossRef]
- Aula, I.; Braunschweiler, H.; Malin, I. The watershed flux of mercury examined with indicators in the Tucurui reservoir in Para, Brazil. Sci. Total Environ. 1995, 175, 97–107. [Google Scholar] [CrossRef]
- Polechońska, L.; Samecka-Cymerman, A.; Dambiec, M. Changes in growth rate and macroelement and trace element accumulation in Hydrocharis morsus-ranae L. during the growing season in relation to environmental contamination. Environ. Sci. Pollut. Res. 2017, 24, 5439–5451. [Google Scholar] [CrossRef] [PubMed]
- Narayan, A.; Mora, A.; Sánchez, L.; Rosales, J. Temporal and spatial variability of heavy metals in bottom sediments and the aquatic macrophyte Paspalum repens of the Orinoco River floodplain lagoons impacted by industrial activities. Environ. Sci. Pollut. Res. 2020, 27, 37074–37086. [Google Scholar] [CrossRef] [PubMed]
- Dórea, J.G.; Monteiro, L.C.; Bernardi, J.V.E.; Fernandes, I.O.; Oliveira, S.F.B.; de Souza, J.P.R.; Rodrigues, Y.O.S.; Vieira, L.C.G.; Souza, J.R. Land use impact on mercury in sediments and macrophytes from a natural lake in the Brazilian savanna. Environ. Pollut. 2023, 337, 122414. [Google Scholar] [CrossRef] [PubMed]
- Frohne, T.; Rinklebe, J.; Langer, U.; Du Laing, G.; Mothes, S.; Wennrich, R. Biogeochemical factors affecting mercury methylation rate in two contaminated floodplain soils. Biogeosciences 2012, 9, 493–507. [Google Scholar] [CrossRef]
- Myrbo, A.; Swain, E.B.; Johnson, N.W.; Engstrom, D.R.; Pastor, J.; Dewey, B.; Monson, P.; Brenner, J.; Shore, M.D.; Peters, E.B. Increase in nutrients, mercury, and methylmercury as a consequence of elevated sulfate reduction to sulfide in experimental wetland mesocosms. J. Geophys. Res. Biogeosciences 2017, 122, 2769–2785. [Google Scholar] [CrossRef]
- Yu, R.Q.; Reinfelder, J.R.; Hines, M.E.; Barkay, T. Syntrophic pathways for microbial mercury methylation. ISME J. 2018, 12, 1826–1835. [Google Scholar] [CrossRef]
- Mauro, J.; Guimaraes, J.; Hintelmann, H.; Watras, C.; Haack, E.; Coelho-Souza, S. Mercury methylation in macrophytes, periphyton, and water–comparative studies with stable and radio-mercury additions. Anal. Bioanal. Chem. 2002, 374, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.P.; Huang, C.P.A.; Chee, N.; Terry, N. Rhizosphere bacteria enhance the accumulation of selenium and mercury in wetland plants. Planta 1999, 209, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Canredon, A.; Anschutz, P.; Buquet, D.; Charbonnier, C.; Amouroux, D.; Tessier, E.; Poirier, D.; Bujan, S.; Devaux, L.; Gouillieux, B.; et al. Lake sediment mercury biogeochemistry controlled by sulphate input from drainage basin. Appl. Geochem. 2019, 104, 135–145. [Google Scholar] [CrossRef]
- Lázaro, W.L.; Díez, S.; da Silva, C.J.; Ignácio, Á.R.; Guimarães, J.R. Waterscape determinants of net mercury methylation in a tropical wetland. Environ. Res. 2016, 150, 438–445. [Google Scholar] [CrossRef]
- Brito, B.C.; Forsberg, B.R.; Kasper, D.; Amaral, J.H.; Vasconcelos, M.R.; Sousa, O.P.; Cunha, F.A.G.; Bastos, W.R. The influence of inundation and lake morphometry on the dynamics of mercury in the water and plankton in an Amazon floodplain lake. Hydrobiologia 2017, 790, 35–48. [Google Scholar] [CrossRef]
- Kasper, D.; Forsberg, B.R.; Amaral, J.H.; Py-Daniel, S.S.; Bastos, W.R.; Malm, O. Methylmercury modulation in Amazon rivers linked to basin characteristics and seasonal flood-pulse. Environ. Sci. Technol. 2017, 51, 14182–14191. [Google Scholar] [CrossRef]
- Pestana, I.A.; Azevedo, L.S.; Bastos, W.R.; de Souza, C.M.M. The impact of hydroelectric dams on mercury dynamics in South America: A review. Chemosphere 2019, 219, 546–556. [Google Scholar] [CrossRef]
- Silva, G.S.D.; Bisinoti, M.C.; Fadini, P.S.; Magarelli, G.; Jardim, W.F.; Fostier, A.H. Major aspects of the mercury cycle in the Negro River Basin, Amazon. J. Braz. Chem. Soc. 2009, 20, 1127–1134. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, L.; Zhou, Y.; Zhang, Y.; Guo, J.; Han, Y.; Zhang, Y.; Hu, L.; Jang, K.S.; Spencer, R.G.M.; et al. Terrestrial dissolved organic matter inputs accompanied by dissolved oxygen depletion and declining pH exacerbate CO2 emissions from a major Chinese reservoir. Water Res. 2024, 251, 121155. [Google Scholar] [CrossRef]
- Wang, R.; Wong, M.H.; Wang, W.X. Mercury exposure in the freshwater tilapia Oreochromis niloticus. Environ. Pollut. 2010, 158, 2694–2701. [Google Scholar] [CrossRef] [PubMed]
- Jardine, T.D.; Kidd, K.A.; O’Driscoll, N. Food web analysis reveals effects of pH on mercury bioaccumulation at multiple trophic levels in streams. Aquat. Toxicol. 2013, 132, 46–52. [Google Scholar] [CrossRef]
- Wang, S.; Xing, D.; Jia, Y.; Li, B.; Wang, K. The distribution of total mercury and methyl mercury in a shallow hypereutrophic lake (Lake Taihu) in two seasons. Appl. Geochem. 2012, 27, 343–351. [Google Scholar] [CrossRef]
- Kelly, C.A.; Rudd, J.W.; Holoka, M.H. Effect of pH on mercury uptake by an aquatic bacterium: Implications for Hg cycling. Environ. Sci. Technol. 2003, 37, 2941–2946. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, M. Interactions between mercury and dissolved organic matter—A review. Chemosphere 2004, 55, 319–331. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, M.; Li, B.; Xing, D.; Wang, X.; Wei, C.; Jia, Y. Comparison of mercury speciation and distribution in the water column and sediments between the algal type zone and the macrophytic type zone in a hypereutrophic lake (Dianchi Lake) in Southwestern China. Sci. Total Environ. 2012, 417, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Mauro, J.B.; Guimarães, J.R.D.; Melamed, R. Mercury methylation in a tropical macrophyte: Influence of abiotic parameters. Appl. Organomet. Chem. 1999, 13, 631–636. [Google Scholar] [CrossRef]

| Mean | SD | Min | Max | CV | |
|---|---|---|---|---|---|
| [THg] Water (ng L−1) | 1.68 | 0.95 | 0.5 | 4 | 57 |
| Physicochemical water parameters | |||||
| pH | 6.6 | 0.5 | 5.7 | 7.4 | 7 |
| Oxidation–reduction potential (ORP, mV) | 209.9 | 42.9 | 152 | 337 | 20 |
| Transparency (m) | 0.81 | 0.40 | 0.3 | 1.8 | 50 |
| Depth (m) | 7.05 | 2.11 | 3.8 | 11.2 | 3 |
| Turbidity (NTU) | 6.75 | 6.49 | 0 | 21.4 | 96 |
| Dissolved oxygen (OD, mg L−1) | 2.46 | 0.61 | 1.02 | 3.33 | 25 |
| Sulfate (mg L−1) | 0.0161 | 0.0165 | 0.001 | 0.046 | 102 |
| Land use and cover (10 km) | |||||
| Cerrado (%) * | 50.5 | 15.7 | 29.6 | 75.2 | 31. |
| Wetlands (%) | 20.8 | 21.4 | 1.7 | 56.1 | 103 |
| Pasture and agriculture (%) | 22.0 | 16.7 | 4.5 | 65.7 | 76 |
| Urban infrastructure (%) | 0.05 | 0.1 | 0 | 0.3 | 223 |
| Land Use Index (LUI) | 44.3 | 33.4 | 9.0 | 131.5 | 75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteiro, L.C.; Vieira, L.C.G.; Bernardi, J.V.E.; Rodrigues, Y.O.S.; de Mesquita, L.P.B.; Souza, J.P.R.d.; Sena, G.; Oliveira, I.A.d.S.; Cabral, C.d.S.; Gonçalves Júnior, J.F.; et al. Mercury Bioconcentration and Translocation in Rooted Macrophytes (Paspalum repens Berg.) from Floodplain Lakes in the Araguaia River Watershed, Brazilian Savanna. Water 2024, 16, 1199. https://doi.org/10.3390/w16091199
Monteiro LC, Vieira LCG, Bernardi JVE, Rodrigues YOS, de Mesquita LPB, Souza JPRd, Sena G, Oliveira IAdS, Cabral CdS, Gonçalves Júnior JF, et al. Mercury Bioconcentration and Translocation in Rooted Macrophytes (Paspalum repens Berg.) from Floodplain Lakes in the Araguaia River Watershed, Brazilian Savanna. Water. 2024; 16(9):1199. https://doi.org/10.3390/w16091199
Chicago/Turabian StyleMonteiro, Lucas Cabrera, Ludgero Cardoso Galli Vieira, José Vicente Elias Bernardi, Ygor Oliveira Sarmento Rodrigues, Lígia Pereira Borges de Mesquita, João Pedro Rudrigues de Souza, Guilherme Sena, Iuri Aparecida da Silva Oliveira, Cássio da Silva Cabral, José Francisco Gonçalves Júnior, and et al. 2024. "Mercury Bioconcentration and Translocation in Rooted Macrophytes (Paspalum repens Berg.) from Floodplain Lakes in the Araguaia River Watershed, Brazilian Savanna" Water 16, no. 9: 1199. https://doi.org/10.3390/w16091199
APA StyleMonteiro, L. C., Vieira, L. C. G., Bernardi, J. V. E., Rodrigues, Y. O. S., de Mesquita, L. P. B., Souza, J. P. R. d., Sena, G., Oliveira, I. A. d. S., Cabral, C. d. S., Gonçalves Júnior, J. F., de Souza, J. R., & Bastos, W. R. (2024). Mercury Bioconcentration and Translocation in Rooted Macrophytes (Paspalum repens Berg.) from Floodplain Lakes in the Araguaia River Watershed, Brazilian Savanna. Water, 16(9), 1199. https://doi.org/10.3390/w16091199











