Aminated Spherical SiO2 Synthesized from Fly Ash and Its Application for Pb2+ and Cu2+ Sorption
Abstract
1. Introduction
2. Experiments
2.1. Materials
2.2. Preparation of the Samples
2.3. Characterization
2.4. Sorption Experiments
3. Results and Discussion
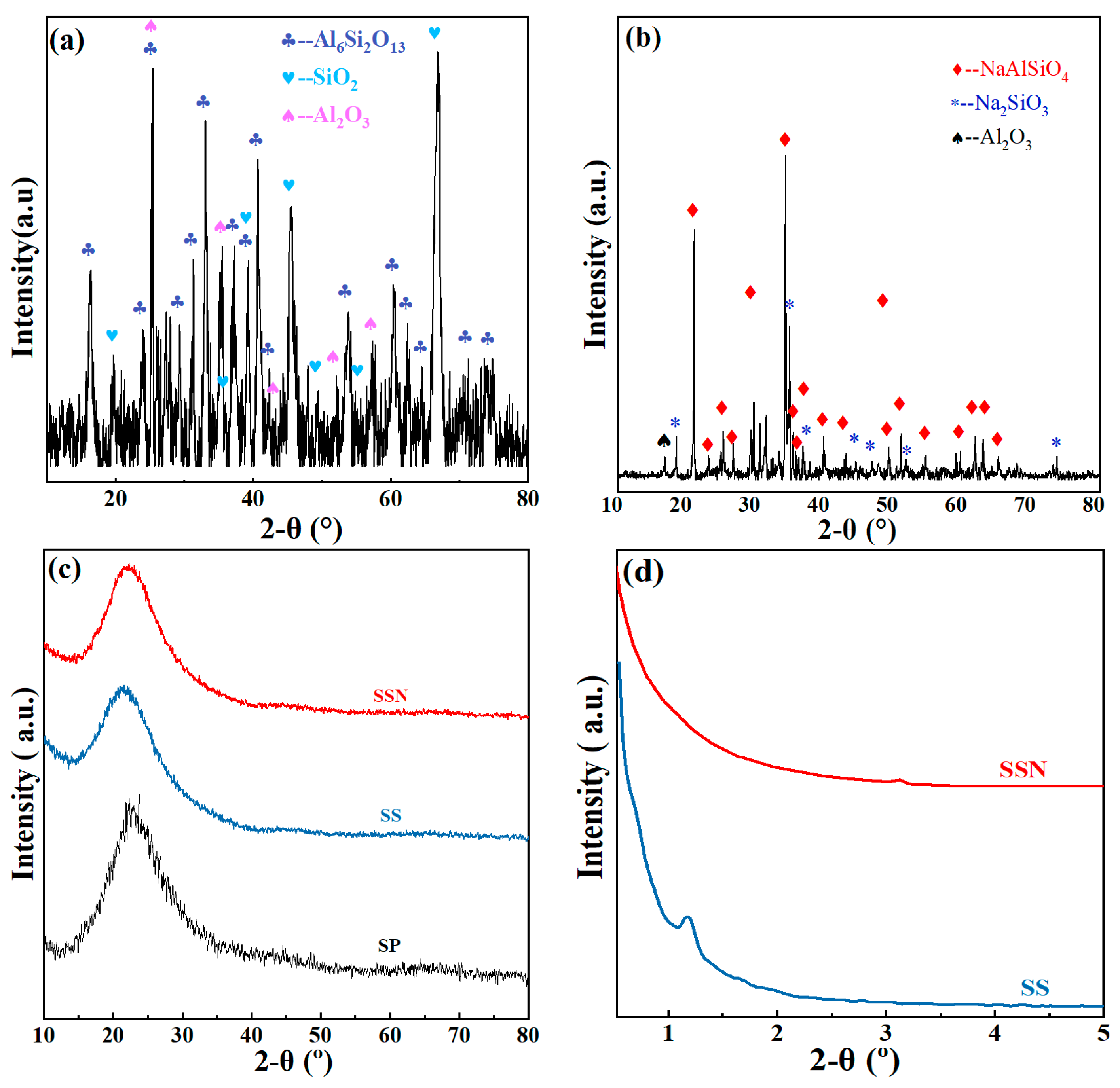
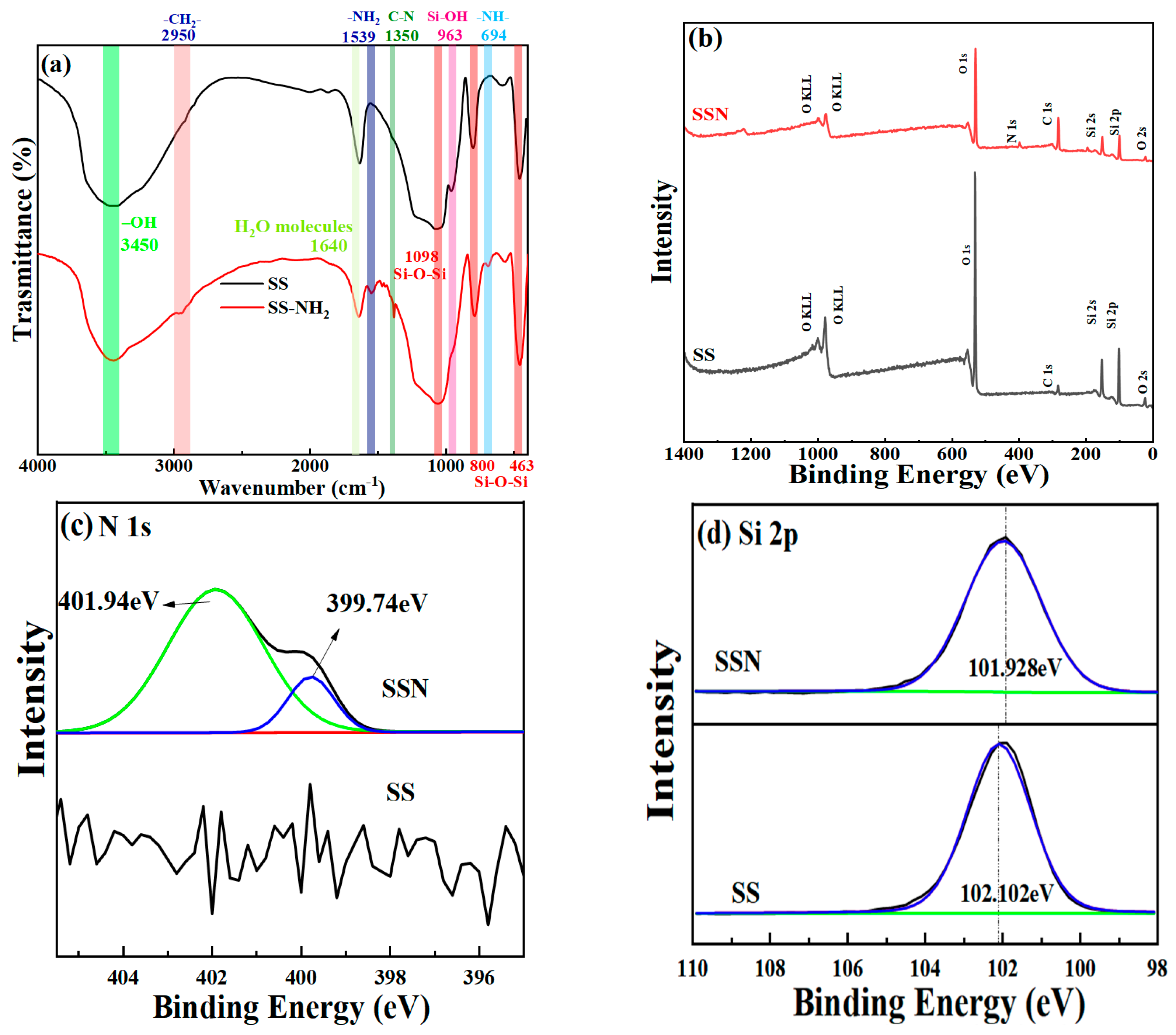
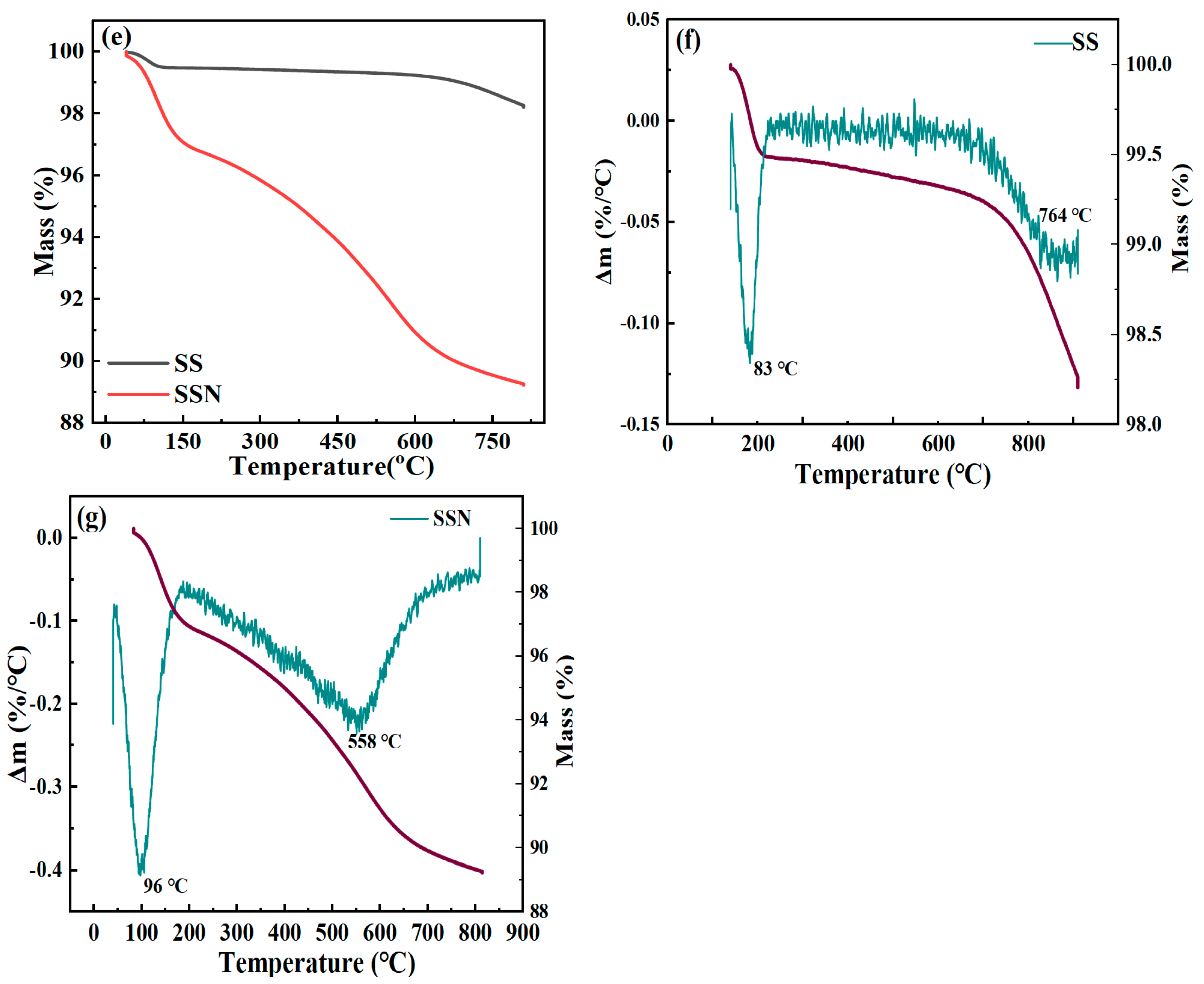
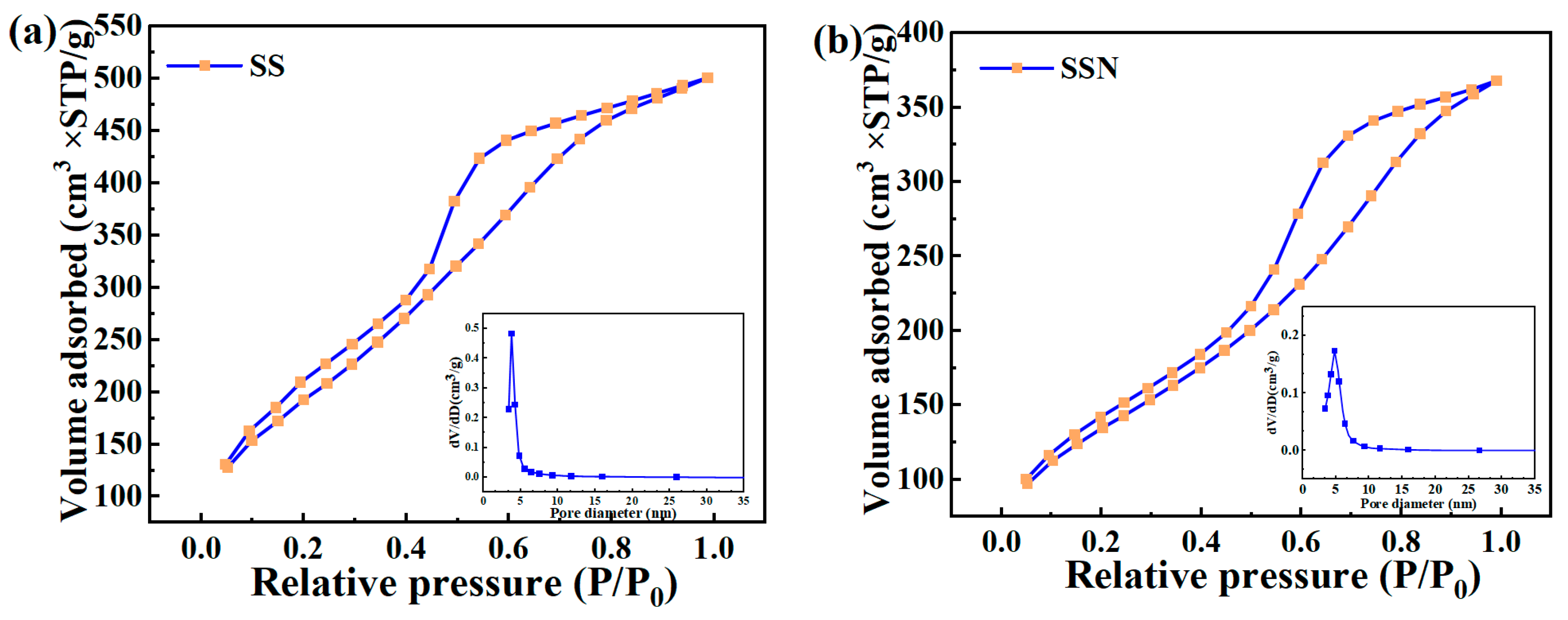
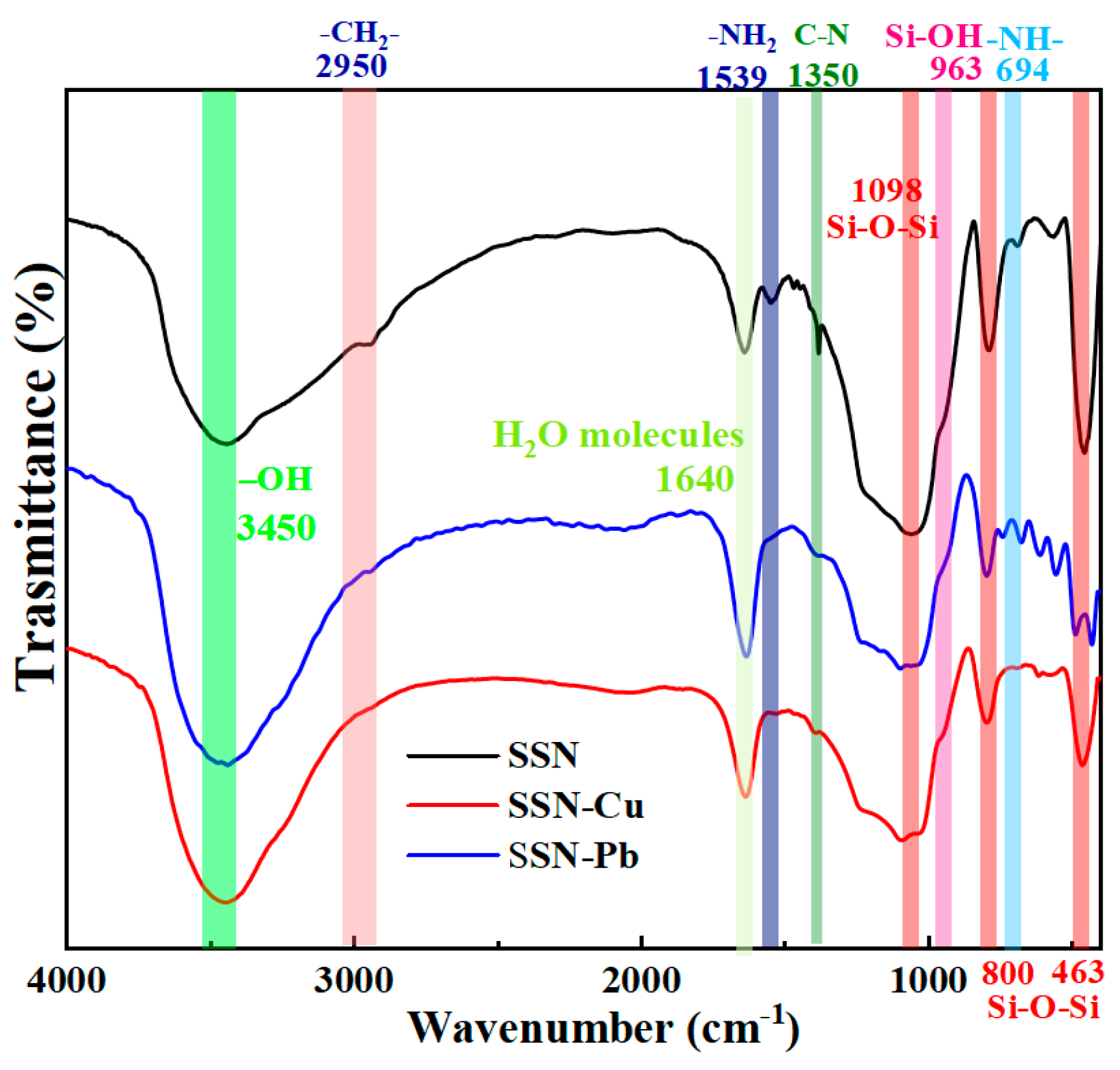
3.1. Physicochemical Properties
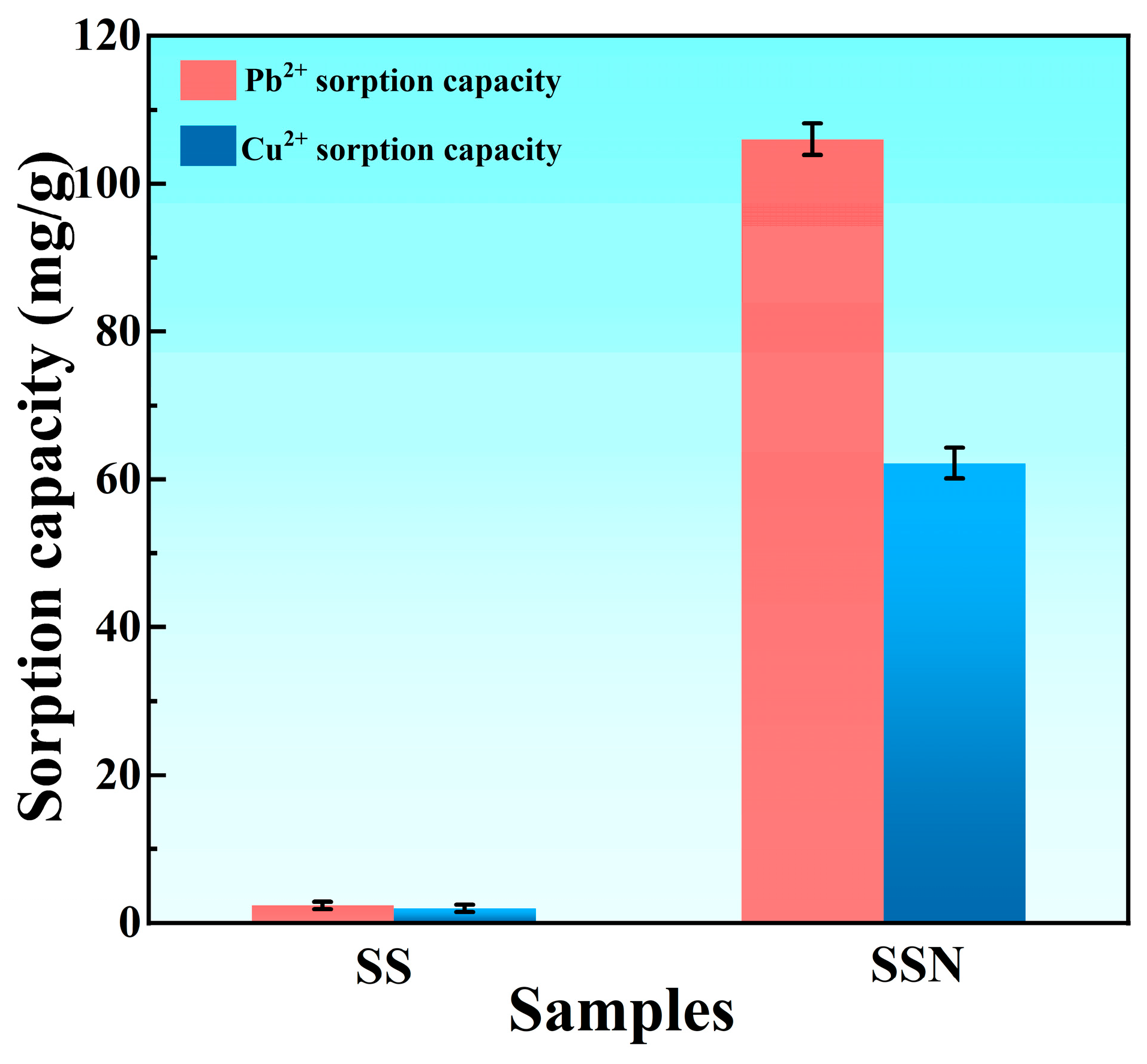
3.2. Sorption Ability and Behavior
3.2.1. Batch Sorption Experiment
3.2.2. Further Sorption Experiments
Effect of Different Factors
Isotherm and Kinetics of Sorption
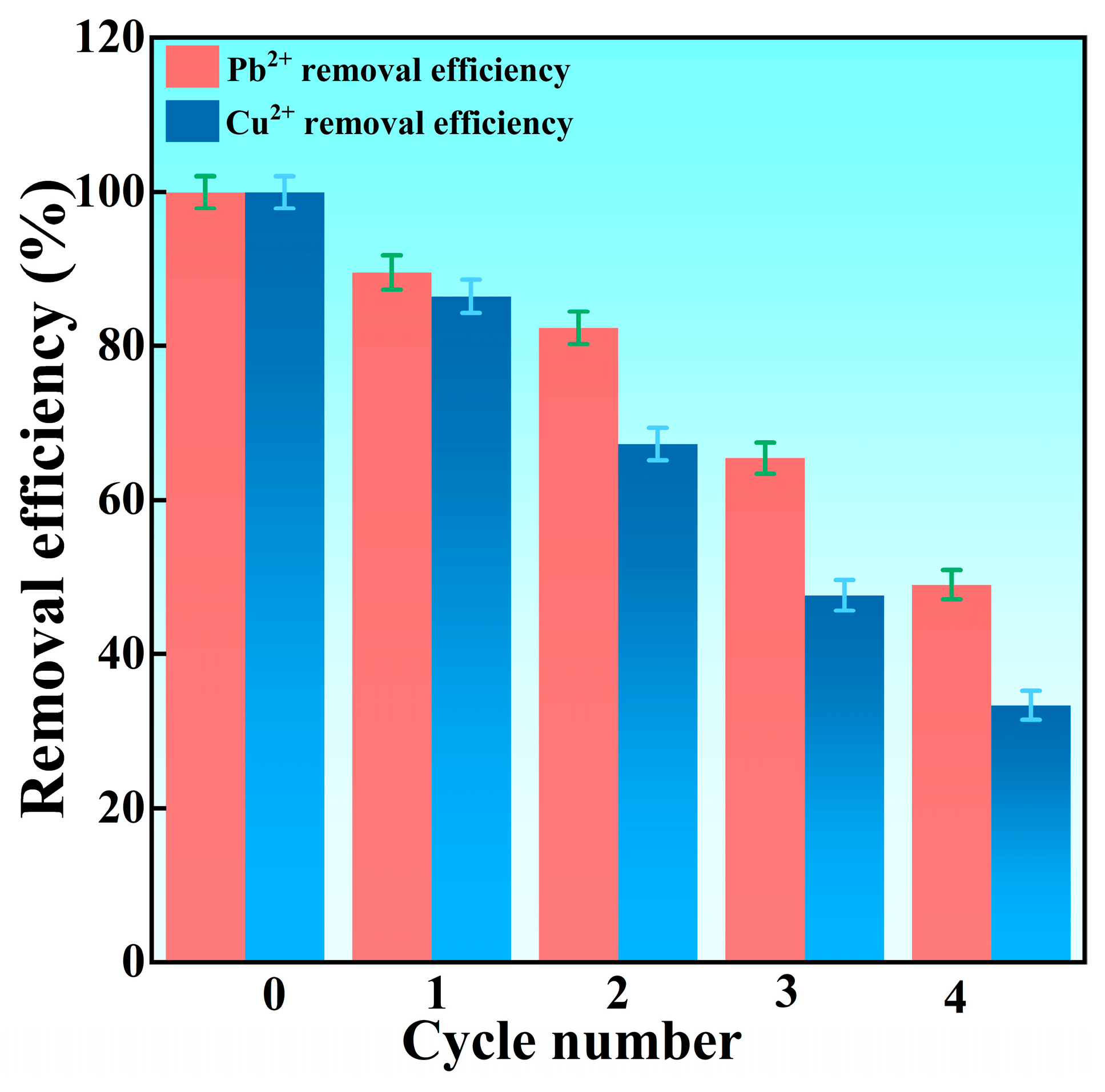
Recycling Performance of SSN
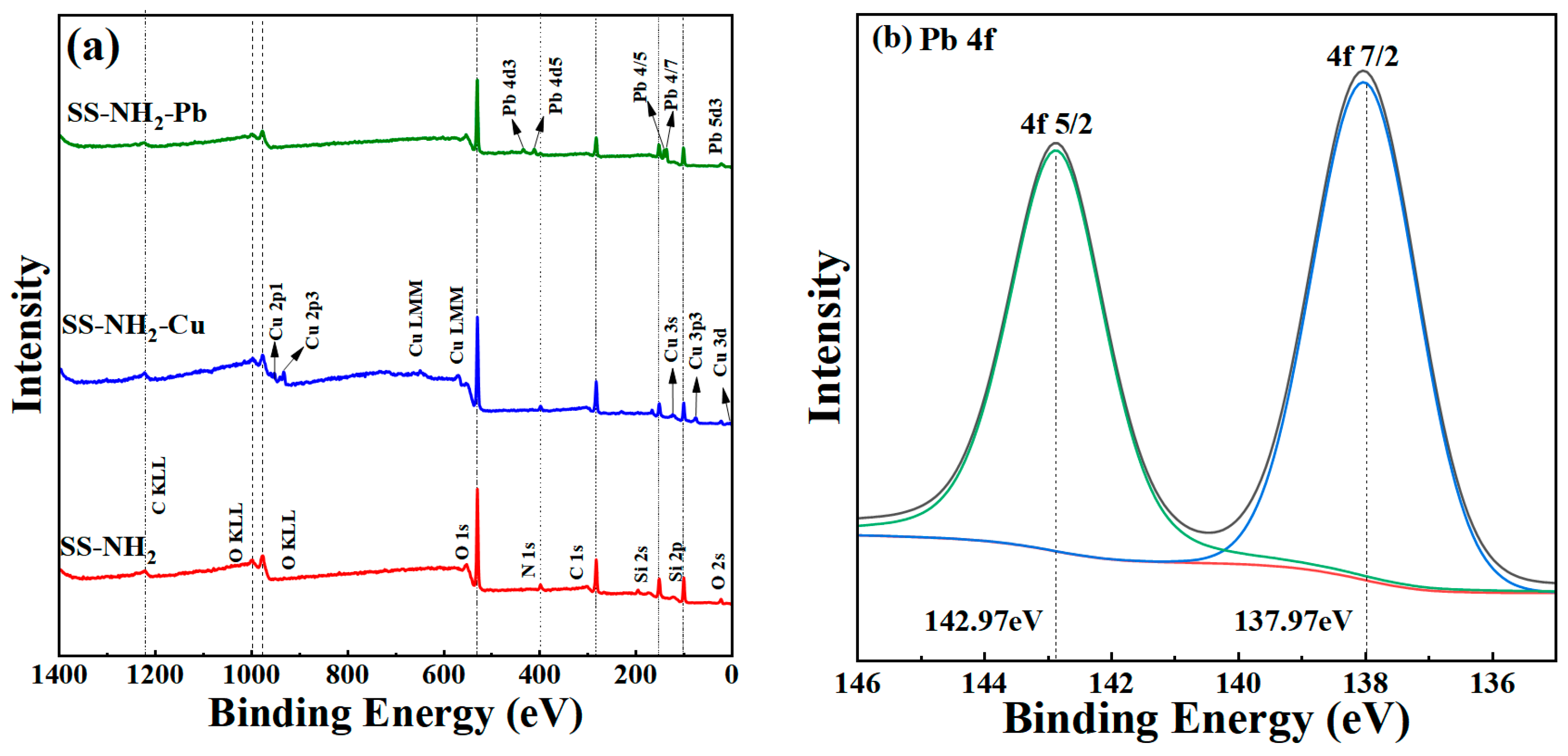
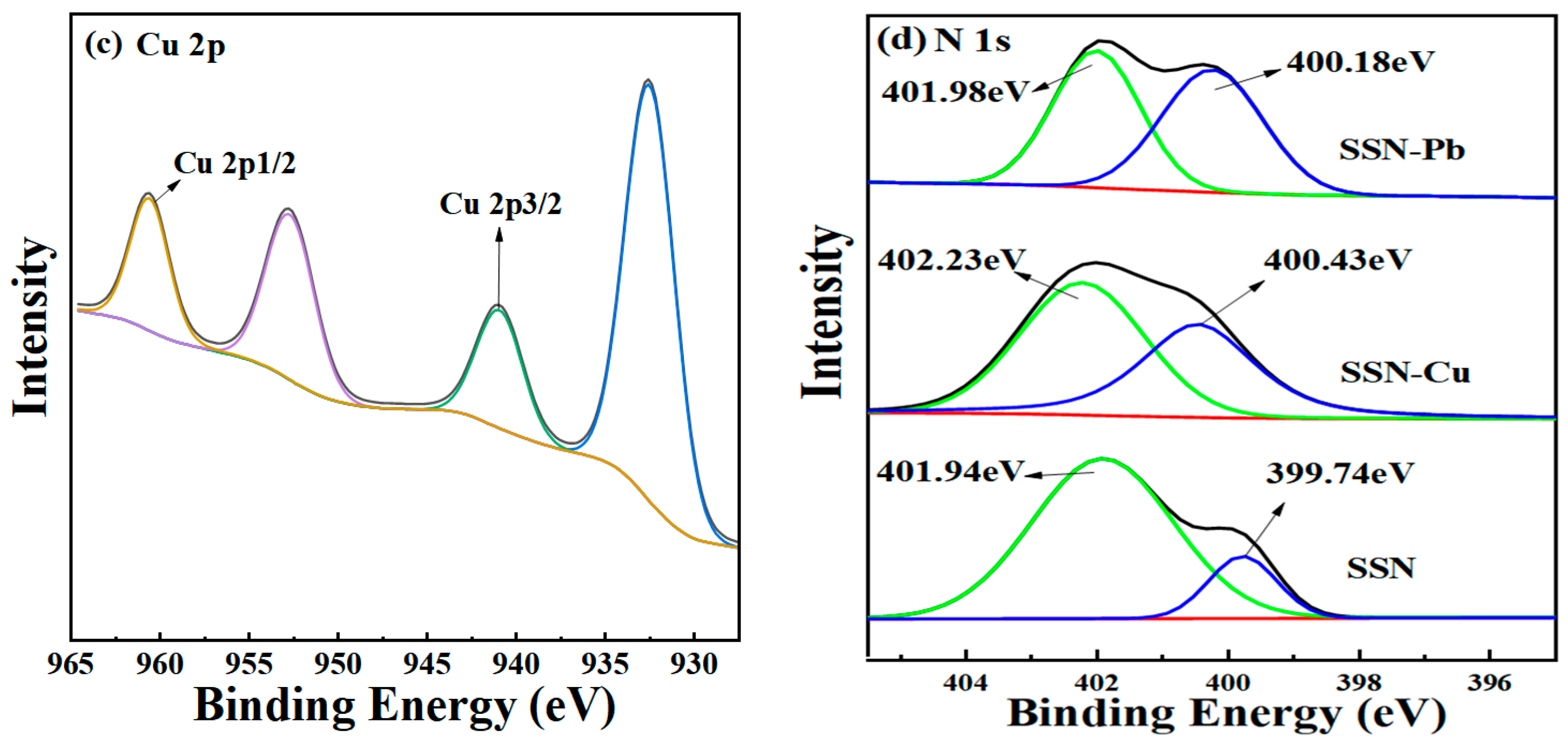
3.3. Mechanisms for Sorption
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rahman, A. Promising and Environmentally Friendly Removal of Copper, Zinc, Cadmium, and Lead from Wastewater Using Modified Shrimp-Based Chitosan. Water 2024, 16, 184. [Google Scholar] [CrossRef]
- Shah, K.J.; Yu, J.; Zhang, T.; You, Z. Y-Type Zeolite Synthesized from an Illite Applied for Removal of Pb(II) and Cu(II) Ions from Aqueous Solution: Box-Behnken Design and Kinetics. Water 2023, 15, 1171. [Google Scholar] [CrossRef]
- Wadhawan, S.; Jain, A.; Nayyar, J.; Mehta, S.K. Role of nanomaterials as adsorbents in heavy metal ion removal from waste water: A review. J. Water Process Eng. 2019, 33, 101038. [Google Scholar] [CrossRef]
- Guimarães, T.G.S.; Barros, L.A.; Silva, R.S.; Gonzalez, M.H.; Carrilho, E.N.V.M.; Labuto, G. Synthesis and characterization of biochars modified with natural deep eutectic solvent (NADES) for dipyrone removal from aqueous medium. Sustain. Chem. Pharm. 2023, 35, 101205. [Google Scholar] [CrossRef]
- Shafiq, M.; Alazba, A.A.; Amin, M.T. Preparation of ZnMgAl-Layered Double Hydroxide and Rice Husk Biochar Composites for Cu(II) and Pb(II) Ions Removal from Synthetic Wastewater. Water 2023, 15, 2207. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Z. Lead sorption performance on active silica derived from fly ash. Water Sci. Technol. 2015, 71, 661–666. [Google Scholar] [CrossRef][Green Version]
- Zeng, Q.; Huang, Y.; Huang, L.; Hu, L.; Sun, W.; Zhong, H.; He, Z. High adsorption capacity and super selectivity for Pb() by a novel adsorbent: Nano humboldtine/almandine composite prepared from natural almandine. Chemosphere 2020, 253, 126650. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, J.; Feng, B.; Xu, H.; Sun, Y.; Gu, X.; Hu, X.; Naushad, M.; Gao, B.; Ren, H. Delamination of multilayer Ti3C2Tx MXene alters its adsorpiton and reduction of heavy metals in water. Environ. Pollut. 2023, 330, 121777. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, L.; Su, N.; Zhu, S.; Wu, D. Preparation of amino-functionalized fly ash based tobermorite for enhanced removal of Cr(VI). Environ. Sci. Pollut. Res. 2023, 30, 54547–54555. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Qian, W.; Chen, Y.; Xu, P.; Li, J.; Yang, J. A new treasure in industrial solid waste—Coal fly ash for effective oil/water separation. J. Taiwan Inst. Chem. Eng. 2020, 118, 196–203. [Google Scholar] [CrossRef]
- Pattanaik, S.; Parhi, P.K.; Das, D.; Samal, A.K. Acacia concinna: A natural dispersant for stabilization and transportation of fly ash-water slurry. J. Taiwan Inst. Chem. Eng. 2019, 99, 193–200. [Google Scholar] [CrossRef]
- Misran, H.; Singh, R.; Begum, S.; Yarmo, M.A. Processing of mesoporous silica materials (MCM-41) from coal fly ash. J. Mater. Process. Technol. 2007, 186, 8–13. [Google Scholar] [CrossRef]
- Joseph, I.V.; Tosheva, L.; Doyle, A.M. Simultaneous removal of Cd(II), Co(II), Cu(II), Pb(II), and Zn(II) ions from aqueous solutions via adsorption on FAU-type zeolites prepared from coal fly ash. J. Environ. Chem. Eng. 2020, 8, 103895. [Google Scholar] [CrossRef]
- Guo, Y.; Tan, C.; Wang, P.; Sun, J.; Yan, J.; Li, W.; Zhao, C.; Lu, P. Kinetic study on CO2 adsorption behaviors of amine-modified co-firing fly ash. J. Taiwan Inst. Chem. Eng. 2018, 96, 374–381. [Google Scholar] [CrossRef]
- Liu, X.; Liu, N.; Li, X.; Luo, Y.; Li, X.; Hu, B.; He, X.; Liu, C.; He, S. Effect of aluminum modified mesoporous SBA-15 on surface properties and arsenic adsorption performance. J. Porous Mater. 2022, 30, 127–140. [Google Scholar] [CrossRef]
- McManamon, C.; Burke, A.M.; Holmes, J.D.; Morris, M.A. Amine-functionalised SBA-15 of tailored pore size for heavy metal adsorption. J. Colloid Interface Sci. 2011, 369, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xie, J.; Zheng, M.; Zhu, J.; Shi, C. Preparation of Mesoporous Biochar from Cornstalk for the Chromium (VI) Elimination by Using One-Step Hydrothermal Carbonation. J. Anal. Methods Chem. 2021, 2021, 3418887. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Yan, L.; Wang, R.; Li, G.; Rao, P.; Ju, M.; Jian, L.; Guo, X.; Che, L. Recyclable nitrogen-doped biochar via low-temperature pyrolysis for enhanced lead(II) removal. Chemosphere 2021, 286, 131666. [Google Scholar] [CrossRef] [PubMed]
- Kasera, N.; Kolar, P.; Hall, S.G. Nitrogen-doped biochars as adsorbents for mitigation of heavy metals and organics from water: A review. Biochar 2022, 4, 17. [Google Scholar] [CrossRef]
- Cheng, S.; Zhao, S.; Guo, H.; Xing, B.; Liu, Y.; Zhang, C.; Ma, M. High-efficiency removal of lead/cadmium from wastewater by MgO modified biochar derived from crofton weed. Bioresour. Technol. 2021, 343, 126081. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Chen, H.; Feng, Q.; Yao, D.; Chen, H.; Wang, H.; Zhou, Z.; Li, H.; Tian, Y.; Lu, X. Effect of phosphoric acid on the surface properties and Pb(II) adsorption mechanisms of hydrochars prepared from fresh banana peels. J. Clean. Prod. 2017, 165, 221–230. [Google Scholar] [CrossRef]
- Jung, K.-W.; Lee, S.Y.; Choi, J.-W.; Lee, Y.J. A facile one-pot hydrothermal synthesis of hydroxyapatite/biochar nanocomposites: Adsorption behavior and mechanisms for the removal of copper(II) from aqueous media. Chem. Eng. J. 2019, 369, 529–541. [Google Scholar] [CrossRef]
- Li, X.; Hu, B.; Liu, N.; Liu, X.; Liu, C.; He, X.; He, S. Extraction of alumina from high-alumina fly ash by ammonium sulfate: Roasting kinetics and mechanism. RSC Adv. 2022, 12, 33229–33238. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, W.; Li, Z.; Ding, K.; Jin, Z. Investigation on into the adsorption of Cu(II), Pb(II) and Cr(VI) on hollow mesoporous silica using microcalorimetry. J. Therm. Anal. Calorim. 2019, 137, 1443–1450. [Google Scholar] [CrossRef]
- Alsalbokh, M.; Fakeri, N.; Lawson, S.; Rownaghi, A.A.; Rezaei, F. Adsorption of iodine from aqueous solutions by aminosilane-grafted mesoporous alumina. Chem. Eng. J. 2021, 415, 128968. [Google Scholar] [CrossRef]
- Li, Y.; Li, T.; Jin, Z. Stabilization of Fe0 nanoparticles with silica fume for enhanced transport and remediation of hexavalent chromium in water and soil. J. Environ. Sci. 2011, 23, 1211–1218. [Google Scholar] [CrossRef]
- Fang, W.; Jiang, X.; Luo, H.; Geng, J. Synthesis of graphene/SiO2@polypyrrole nanocomposites and their application for Cr(VI) removal in aqueous solution. Chemosphere 2018, 197, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, H.; Ahmadian-Alam, L.; Molavi, H. Grafting of sulfonated monomer onto an amino-silane functionalized 2-aminoterephthalate metal − organic framework via surface-initiated redox polymerization: Proton-conducting solid electrolytes. Polym. Int. 2015, 64, 1578–1584. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, N.; Li, X.; Wang, Y.; Xiong, Y.; Meng, R.; Liu, L.; He, S. Single-step hydrothermal synthesis of biochar from waste industrial hemp stalk core for Pb2+ sorption: Characterization and mechanism studies. Sustain. Chem. Pharm. 2023, 36, 101316. [Google Scholar] [CrossRef]
- Kang, S.; Chung, Y.G.; Kang, J.H.; Song, H. CO2 absorption characteristics of amino group functionalized imidazolium-based amino acid ionic liquids. J. Mol. Liq. 2019, 297, 111825. [Google Scholar] [CrossRef]
- Bao, S.; Yang, W.; Wang, Y.; Yu, Y.; Sun, Y.; Li, K. PEI grafted amino-functionalized graphene oxide nanosheets for ultrafast and high selectivity removal of Cr(VI) from aqueous solutions by adsorption combined with reduction: Behaviors and mechanisms. Chem. Eng. J. 2020, 399, 125762. [Google Scholar] [CrossRef]
- Luo, L.; Cheng, S.; Yue, L.; You, Z.; Cai, J. N-doped biochar from chitosan gel-like solution: Effect of hydrothermal temperature and superior aqueous Cr (VI) removal performance. Colloids Surf. A 2022, 641, 128426. [Google Scholar] [CrossRef]
- Han, C.; Xie, J.; Wei, K.; Liang, L.; Yang, T.; He, S.; Shi, Q. Simultaneous removal of Rhodamine B and Cu(II) by Fe0(1 1 0)-decorated ZSM-5: Cu(II) role, reactivity and mechanism. Chem. Eng. Sci. 2023, 282, 119225. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, T.; Zhu, N.; Li, D.; Chen, Z.; Lang, Q.; Liu, Z.; Jiao, W. Enhanced adsorption of Pb(II) onto modified hydrochar: Modeling and mechanism analysis. Bioresour. Technol. 2019, 288, 121593. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wu, J.; Wang, J.; Xu, M.; Zhou, W.; Du, Y.; Li, Y.; Li, H. Fabricating hydroxyapatite functionalized biochar composite using steel slag and Hami melon peel for Pb(II) and Cd(II) removal. Colloids Surf. A 2023, 666, 131310. [Google Scholar] [CrossRef]
- Zhu, F.; Li, L.; Xing, J. Selective adsorption behavior of Cd(II) ion imprinted polymers synthesized by microwave-assisted inverse emulsion polymerization: Adsorption performance and mechanism. J. Hazard. Mater. 2016, 321, 103–110. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, C.; Zhang, Z.; Wang, H.; Zhou, S.; Zhou, W. A comparative study on fly ash, geopolymer and faujasite block for Pb removal from aqueous solution. Fuel 2016, 185, 81–189. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, H.; Zhang, G.; Li, J.; Yang, Y.; Ji, P. Potential of removing Cd(II) and Pb(II) from contaminated water using a newly modified fly ash. Chemosphere 2019, 242, 125148. [Google Scholar] [CrossRef] [PubMed]
- Harja, M.; Buema, G.; Lupu, N.; Chiriac, H.; Herea, D.D.; Ciobanu, G. Fly Ash Coated with Magnetic Materials: Improved Adsorbent for Cu (II) Removal from Wastewater. Materials 2020, 14, 63. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Feng, X.; Huang, K.; Ren, X.; Zhao, Y.; Xing, P. Synthesis and adsorption performance of fly ash/steel slag-based geopolymer for removal of Cu (II) and methylene blue. Int. J. Appl. Ceram. Technol. 2023, 20, 1591–1605. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, X.; Zhang, K.; Xue, Y. Desorption of calcium-rich crayfish shell biochar for the removal of lead from aqueous solutions. J. Colloid Interface Sci. 2019, 554, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Zhang, W.; Zhao, C.; Xu, J. Evaluation of Removal Efficiency of Ni(II) and 2,4-DCP Using in Situ Nitrogen-Doped Biochar Modified with Aquatic Animal Waste. ACS Omega 2019, 4, 19366–19374. [Google Scholar] [CrossRef] [PubMed]



| Sample | Component (%) | ||||
|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | TiO2 | |
| FA | 37.40 | 46.22 | 3.05 | 5.17 | 3.51 |
| SP | 79.65 | 4.46 | 0.12 | 0.43 | 2.27 |
| Sample | SSA (m2/g) | PV (cm3/g) | PD (nm) | pHpzc |
|---|---|---|---|---|
| SS | 677.5 | 0.023 | 3.42 | 2.62 |
| SSN | 351.4 | 0.012 | 3.07 | 3.64 |
| Adsorbate | qe(exp) (mg/g) | Pseudo-First-Order Model | Pseudo-Second-Order Model | |||||
|---|---|---|---|---|---|---|---|---|
| k1 (min−1) | qe (mg/g) | R2 | k2 (g/mg·min−1) | qe (mg/g) | R2 | |||
| SSN | Pb2+ | 106.032 | 0.0088 | 63.8924 | 0.9018 | 0.0106 | 94.3396 | 0.9901 |
| SSN | Cu2+ | 62.212 | 0.0303 | 30.7349 | 0.8478 | 0.0177 | 56.4972 | 0.9983 |
| Adsorbent | Adsorbate | Isotherm | Parameters | R2 |
|---|---|---|---|---|
| SSN | Pb2+ | Langmuir | qm = 185.1852 mg/g, b = 0.0008 L/mg | 0.9018 |
| SSN | Pb2+ | Freundlich | n = 1.3277, kf = 2.7063 | 0.9901 |
| SSN | Cu2+ | Langmuir | qm = 86.2069 mg/g, b = 0.0009 L/mg | 0.8397 |
| SSN | Cu2+ | Freundlich | n = 1.6432, kf = 2.2991 | 0.9901 |
| Adsorbate | Dosage (g/L) | pH | qe/(mg/g) | Ref | |
|---|---|---|---|---|---|
| Activated coal fly ash | Pb2+ | 4 | 3.0 | 49.8 | [37] |
| Silica synthesized from fly ash | Pb2+ | 1 | 5.0 | 90.0 | [6] |
| NaOH- activated-coal fly ash | Pb2+ | 2 | 5.0 | 126.6 | [38] |
| Aminated spherical SiO2 synthesized from fly ash | Pb2+ | 1 | Natural pH | 185.2 | This work |
| Fly ash/magnetite material | Cu2+ | 10 | 5.0 | 17.4 | [39] |
| Fly ash/steel slag-based geopolymer | Cu2+ | 2 | Natural pH | 38.4 | [40] |
| Fly ash-derived calcium silicate hydrate | Cu2+ | 1 | Natural pH | 57.8 | [13] |
| Aminated spherical SiO2 synthesized from fly ash | Cu2+ | 1 | Natural pH | 86.2 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Liu, N.; Wang, Y.; Li, X.; Zhang, Z.; Liu, L.; Dou, Z.; He, S. Aminated Spherical SiO2 Synthesized from Fly Ash and Its Application for Pb2+ and Cu2+ Sorption. Water 2024, 16, 1149. https://doi.org/10.3390/w16081149
Chen J, Liu N, Wang Y, Li X, Zhang Z, Liu L, Dou Z, He S. Aminated Spherical SiO2 Synthesized from Fly Ash and Its Application for Pb2+ and Cu2+ Sorption. Water. 2024; 16(8):1149. https://doi.org/10.3390/w16081149
Chicago/Turabian StyleChen, Jiahui, Nengsheng Liu, Yunzhu Wang, Xiang Li, Zheren Zhang, Le Liu, Zhaoyang Dou, and Sufang He. 2024. "Aminated Spherical SiO2 Synthesized from Fly Ash and Its Application for Pb2+ and Cu2+ Sorption" Water 16, no. 8: 1149. https://doi.org/10.3390/w16081149
APA StyleChen, J., Liu, N., Wang, Y., Li, X., Zhang, Z., Liu, L., Dou, Z., & He, S. (2024). Aminated Spherical SiO2 Synthesized from Fly Ash and Its Application for Pb2+ and Cu2+ Sorption. Water, 16(8), 1149. https://doi.org/10.3390/w16081149





