Abstract
Human exposure to p-phenylenediamine derivatives (PPDs) may induce hepatotoxicity and altered glycolipid metabolism. Recent studies have demonstrated the wide presence of PPDs in environmental matrixes. However, until now, the occurrence of PPDs in tap water has not been well known. This study analyzed nine PPDs in tap water collected from Hangzhou and Taizhou, China. The results showed that seven PPDs were detected in tap water samples from Hangzhou (n = 131), with the concentration of total detected PPDs ranging from 0.29 to 7.9 ng/L (mean: 1.6 ng/L). N-(1, 3-dimethylbutyl)-N′-phenyl-p-phenylenediamine (6PPD; mean: 0.79 ng/L, <LOD−5.7 ng/L) was the predominant PPD in tap water from Hangzhou, followed by N, N′-di-2-butyl-p-phenylenediamine (44PD; 0.39 ng/L, <LOD−2.2 ng/L) and N-isopropyl-N′-phenyl-1, 4-phenylenediamine (IPPD; 0.31 ng/L, <LOD−1.4 ng/L). Five PPDs were detected in tap water collected from Taizhou (n = 30). N-phenyl-N′-cyclohexyl-p-phenylenediamine (CPPD; mean: 1.0 ng/L, <LOD−4.2 ng/L) was the predominant PPD in tap water from Taizhou, followed by 6PPD (0.93 ng/L, <LOD−2.6 ng/L) and 44PD (0.78 ng/L, <LOD−1.8 ng/L). The mean daily intake (DI) of PPDs for adults and children in Hangzhou was estimated to be 4.9–24 and 6.4–32 pg/kg bw/day, respectively. Meanwhile, the mean DI of PPDs for adults and children living in Taizhou was 11–31 and 14–40 pg/kg bw/day, respectively. To our knowledge, this study provides the first data on the occurrence of PPDs in tap water, which is vital for human exposure risk assessment.
1. Introduction
p-Phenylenediamines derivatives (PPDs) have been widely added to many rubber-related industrial and consumer products, such as rubber tires, gloves, cables, and belts [1,2,3]. These chemicals are effective in preventing the oxidative cracking, thermal degradation, and the aging of rubber-related products [4,5]. The most commonly used PPDs in the rubber industry mainly include N-(1,3-dimethylbutyl)-N′-phenyl-p-phenylenediamine (6PPD), N-isopropyl-N′-phenyl-1,4-phenylenediamine (IPPD), and N, N-bis(1,4-dimethylpentyl)-p-phenylenediamine (77PD) [6]. In North America, the annual production of 6PPD has reached 23,000–45,000 tons and has been listed as a High Yield Compound [7]. In Europe, the production volume of N, N′-diphenyl-p-phenylenediamine (DPPD), IPPD, and 77PD is in the range of 10–10,000 tons/year [8]. The massive application of PPDs has resulted in their inevitable release into the environment [9,10]. Consequently, many kinds of PPDs, including 6PPD, 77PD, N-phenyl-N′-cyclohexyl-p-phenylenediamine (CPPD), IPPD, and DPPD, have been detected in various environmental matrixes, such as water, sediment, dust, soil, and air particles [11,12,13,14,15].
Considering the wide presence of PPDs in the environment, humans are inevitably exposed to various PPDs [8]. The preliminary human exposure pathways for PPDs include inhalation, oral intake, and dermal contact [10]. This has attracted increasing concerns owing to the possible toxic effects of exposure to PPDs on human health [16]. For example, exposure to DPPD has been linked to a notably extended gestation period and potentially even dystocia in female rats [17]. Fang et al. [18] reported that 6PPD exposure induced hepatotoxicity (increased liver weight) and altered glycolipid metabolism and glutathione metabolism in mice. In addition, some metabolites of PPDs (e.g., PPDs-derived quinones) in the human body may be more toxic than their parent PPDs [4,19]. For instance, the oxidized product of 6PPD (LC50, 250 μg/L), known as 6PPD-quinone (0.82 μg/L), has been observed to induce acute mortality in coho salmon [20]. Based on the aforementioned in vitro and in vivo studies, it can be inferred that exposure to PPD may have adverse health effects on human health.
Tap water quality is greatly affected by human activity, industrial expansion, and agricultural development [21]. More than 875 million people in the world do not have access to clean tap water [22]. From a public health standpoint, it is crucial to identify the contaminants present in tap water [23,24]. Ingesting contaminants in tap water can lead to both acute and chronic adverse human health effects that may persist for many years [25,26]. For example, the ingestion of elevated levels of lead and arsenic in tap water can result in acute and chronic poisoning, potentially leading to cancer in humans [27,28,29]. Moreover, tap water intake is an important source of human exposure to a variety of pollutants, such as organophosphate flame retardants and bisphenol analogues [30,31,32]. For the general population, the uptake of some pollutants through plants that are irrigated by contaminated water should also be a concern [33,34,35]. Considering the wide presence of PPDs in environmental surface water, PPDs may occur in tap water sources, such as freshwater rivers and reservoirs [11,36]. For example, Zhang et al. [14] analyzed PPDs in a catchment providing tap water to Guangzhou city, China, and reported the wide presence of 6PPD and IPPD. Additionally, removal efficiencies of various PPDs in the wastewater treatment plant were around 49−77%, suggesting that a considerable amount of these PPDs can not be removed through wastewater treatment technology [37]. However, the knowledge on the occurrence of PPDs in tap water is still scarce. Such data are important for conducting human exposure risk assessments.
In this study, tap water samples were collected from households in urban residential regions of Hangzhou and Taizhou, China, and were analyzed for nine kinds of PPDs. The occurrence and concentration profiles of PPDs in tap water were investigated. The daily intake of PPDs through tap water ingestion in the general population was also estimated. To our knowledge, this is the first study investigating the occurrence of PPDs in tap water.
2. Materials and Methods
2.1. Standards and Reagents
Nine target PPDs were analyzed in this study, and their chemical structure, full name, and CAS number are listed in Table S1 of Supplementary Materials. These target PPDs were always among the most frequently detected PPDs reported in previous studies [11,12,14,15]. Specifically, 44PD (N, N′-di-2-butyl-p-phenylenediamine), 6PPD, 7PPD ((1,4-dimethylpentyl)-N′-phenylbenzene-1,4-diamine), and 77PD were purchased from Ehrenstorfer GmbH (Augsburg, Germany). CPPD, DNPD (N, N′-di-2-naphthyl-p-phenylenediamine), DPPD, DTPD (N, N′-di(o-tolyl)-p-phenylenediamine), and IPPD were obtained from TRC (Burlington, ON, Canada) and Anpel Scientific (Shanghai, China). 13C isotopically labeled 6PPD (i.e., 13C6-6PPD; internal standard) was purchased from Cambridge Isotope Laboratories (Andover, MA, USA). Formic acid, HPLC-grade water, and acetonitrile were obtained from Merck (Darmstadt, Germany).
2.2. Tap Water Sample Collection
During March−June 2023, tap water (i.e., tap water) samples were collected from different households in Hangzhou (n = 131) and Taizhou (n = 30). Tap water samples were collected in three different districts of Hangzhou city and in one district of Taizhou city, as shown in Supplementary Materials Table S2. Hangzhou (population 12 million), located in eastern China, is the capital city of Zhejiang province [38]. Taizhou city, located in southeastern Zhejiang province, has a population exceeding 6.5 million [39]. The location of sampling regions is shown in Supplementary Materials Figure S1. Tap water in Hangzhou and Taizhou was supplied by water treatment plants. Tap water treatment technologies, including coagulation, flocculation, sedimentation, filtration, and disinfection (chlorination), are commonly applied in these water treatment plants. Collected tap water in Hangzhou originated from Tiaoxi River, Qiantang River, and Fuchunjiang River. The collected tap water in Taizhou was from Lingjiang River.
Tap water samples (3 L each) were directly collected from the stopcock in the kitchen room of a household. Three subsamples (1 L each) of tap water were collected within one week in the same household and then were pooled to obtain one real sample. Collected tap water samples were transferred to brown glass containers, mixed with formic acid (1% of total volume), and then stored at -60 °C until extraction. Blank field samples (3 L of pure water) were also transported with the collected tap water in each sampling campaign.
2.3. Sample Extraction
Tap water samples were extracted, following the methods of previous studies [11,40,41]. In brief, tap water samples (1000 mL) were firstly spiked with the internal standard (5 ng of 13C6-6PPD), acidified with 1% formic acid, and then loaded onto HLB cartridges (6.0 mL, 250 mg; Oasis, Waters, MA, USA). These cartridges were preconditioned with 6 mL of acetonitrile and 6 mL of pure water. Following tap water sample loading, these HLB cartridges were rinsed with 4 mL of 5%/95% acetonitrile/pure water, vacuum-dried for 10 min, and then eluted with 6 mL of acetonitrile. The eluent solutions were evaporated to dryness and then redissolved in 50 μL of acetonitrile for instrumental analysis.
2.4. Instrumental Analysis
Target PPDs in the sample extracts were analyzed using a Waters ACQUITY liquid chromatography system coupled with a Waters XEVO_TQS tandem mass spectrometry (Waters; Milford, MA, USA). Ten microliters of sample extract were injected onto an Eclipse Plus C18 column (1.8 µm, 3.0 mm × 100 mm; Agilent Technologies, Santa Clara, CA, USA) for chromatographic separation. Mobile phases A and B were 0.1% formic acid in pure water and acetonitrile, respectively. Flow rate of the mobile phase was 0.2 mL/min. The C18 column was kept at 40 °C, and the linear gradient elution started at 10% B, which was increased to 50% B over 2 min to 95% B over 8 min, followed by the isocratic elution with 95% B for 2 min, and then kept at 10% B for 4 min before the next injection. The ion source was operated in the positive ionization mode. Multiple reaction monitoring (MRM) mode was used to detect PPDs. MRM transition parameters for the detection of PPDs are described in the Supplementary Materials Table S3.
2.5. Daily Intake Estimation
Daily intake (DI; pg/kg bw/day) of PPDs through the ingestion of tap water was estimated for the local residents using the following equation [42]:
where CPPDs is the concentration of individual PPD in tap water (ng/L). IR means the human daily ingestion rate of tap water and is assumed to be 2 L/day for adults and 1 L/day for children [32,43]. BW means the body weight (bw, kg) of local residents and is set at 65 kg for adults and 25 kg for children [36,44].
DI = 1000 × (CPPDs × IR)/BW
2.6. QA/QC
Brown glass containers were pre-rinsed using methanol and acetonitrile before use. All of the used solvents and field blank samples were checked for background contamination of PPDs, and no PPDs were detected. One procedural blank and one solvent blank were analyzed between every ten tap water samples in order to monitor the background and cross-over contamination of PPDs. We acknowledge that our study was based on the sampling of tap water from a single tap in each location. We selected representative sampling points based on population density and water usage patterns. Three subsamples of tap water were collected within one week in the same household and then were pooled to obtain one real tap water sample. This sampling strategy can improve the representativeness of the tap water. In addition, point source pollution could happen in the collected tap water samples, while field blank samples were also transported with the collected tap water in each sampling campaign to monitor the possible point source pollution.
Identification of the target PPDs involved a comparison of the peak ratio between the quantification ion and the qualification ion with their respective authentic standards, all exhibiting variations below 20%. Concentrations of PPDs in collected tap water samples were quantified using an internal standard approach. Calibration curve (six concentration points; 0.5−100 ng/mL) was built for each target analyte. The correlation coefficient (R2) for the calibration curve of each specific target analyte consistently exceeded 0.995. Limits of detection (LODs) of PPDs were defined as the mean concentration of PPDs in procedural blanks plus three times the standard deviation. Calculated LODs of PPDs in tap water were in the range of 0.019 (CPPD)−0.070 (DNPD) ng/L. Extraction recovery experiments were carried out to evaluate the effectiveness of the extraction method used in this study. Tap water samples were spiked with PPDs at 0.1, 1, or 15 ng/L and then analyzed using the current method. Mean extraction recoveries of PPDs in tap water samples were in the range of 81−109%. LODs and extraction recoveries of PPDs in tap water are shown in the Supplementary Materials Table S4. Matrix effects (MEs) in the analysis of PPDs were assessed by comparing the concentrations of the target PPDs in tap water sample extracts (n = 5) with that detected in the acetonitrile [45]. Calculated MEs of PPDs in this study ranged from 94 to 107%, indicating no obvious suppression and strength for the signal response of PPDs.
2.7. Statistical Analysis
Quantified concentrations of PDDs below their LODs were reported as <LOD. In tap water samples, mean concentrations of PDDs were only calculated if their detection frequencies were higher than 50%. Mann–Whitney U was used to assess the difference in concentrations among individual PPDs in tap water samples from Hangzhou or Taizhou. Spearman′s correlation analysis was conducted to evaluate the correlation among concentrations of various PPDs detected in tap water samples from Hangzhou or Taizhou. Statistical analysis in this study was performed using SPSS software (version 21; IBM, Ontario, CA, USA). A p value of <0.05 indicates statistical significance.
3. Results and Discussion
3.1. PPDs in Tap Water from Hangzhou
Seven PPDs were detected in tap water samples from Hangzhou, China (Table 1). Among detected PPDs, 6PPD, IPPD, 44PD, and CPPD had detection frequencies of >50%. 7PPD, 77PD, and DTPD were less frequently detected, with detection frequencies lower than 40%. The concentrations of total detected PPDs (∑PPDs) were 0.29−7.9 ng/L (mean 1.6 ng/L). 6PPD (mean 0.79 ng/L, <LOD−5.7 ng/L) was the predominant PPD in tap water from Hangzhou, followed by 44PD (0.39 ng/L, <LOD−2.2 ng/L) and IPPD (0.31 ng/L, <LOD−1.4 ng/L). 6PPD accounted for a mean of 48% of ∑PPDs in tap water samples from Hangzhou (Figure 1). Consistently, 6PPD was always the dominant PPD in urban runoff from Chinese cities and surface water from Chinese rivers [11,14,36]. Possibly, this is owing to the greater application of 6PPD than other PPDs in the rubber industry [7,8]. 44PD and IPPD were detected in human urine collected from Quzhou city, China, with concentrations of <LOD−2.9 ng/L and <LOD−0.93 ng/L (mean 0.14 ng/L), respectively [46]. Dust samples were also reported to contain 44PD and IPPD [47]. The solubility of PPDs may greatly affect their occurrence in tap water. Organic chemicals with relatively higher water solubility may have higher concentrations in tap water. PPDs had relatively low solubility in the water phase. For example, the solubility of 6PPD in water is only around 0.1 g/L [48], which may result in its adsorption to the particles in the water and greatly decrease their levels in the tap water.

Table 1.
Concentrations (ng/L) of PPDs in tap water samples from Hangzhou and Taizhou, China.
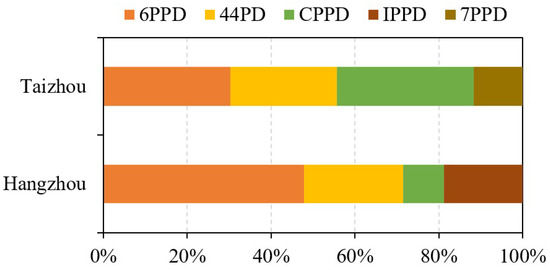
Figure 1.
Concentration profile of PPDs in tap water from Hangzhou and Taizhou, China.
Comparatively, urban runoff water from Hong Kong, Huizhou, and Dongguan cities contained much higher levels of PPDs than that reported in this study [11,36]. The mean concentration of 6PPD in tap water (0.79 ng/L) from Hangzhou city is higher than that reported in surface water from Zhujiang River (mean 0.56 ng/L) and Dongjiang River (0.47 ng/L) in Guangdong, China [36]. The mean concentration of IPPD in tap water from Hangzhou is comparable to that reported in surface water from Liuxi River (0.31 ng/L) [14]. Based on this comparison, despite the levels of PPDs in the source water of the collected tap water being unknown, we speculate that PPDs cannot be effectively removed after the tap water treatment, mainly including coagulation, flocculation, sedimentation, filtration, and disinfection (chlorination).
Concentrations of various PPDs in tap water from Hangzhou were barely correlated with each other (Spearman’s correlation coefficient, rs = 0.15−0.40; p > 0.19; Supplementary Materials Table S5). This contrasts with the previous observation in dust [49] and surface water [14], in which detected PPDs (such as 6PPD, IPPD, and CPPD) were always correlated with one another. This may be because of the great influence of the tap water treatment process (e.g., chlorination, advanced oxidation, and activated carbon adsorption) on the occurrence of PPDs in tap water. Previous studies have demonstrated the oxidative degradation of 6PPD (e.g., ozonation and photooxidation) in the water environment [50,51].
In addition, we investigated the differences in concentrations and concentration profiles of PPDs in tap water from different districts of Hangzhou city. The results showed that the mean concentrations of ∑PPDs in tap water from Linping (1.8 ng/L) and Fuyang (1.7 ng/L) districts are comparable but are higher than that from Xiaoshan district (1.4 ng/L, p = 0.04) (Figure 2). Concentration profiles of PPDs in tap water from Linping and Xiaoshan districts are similar, but tap water from Fuyang district had a comparatively higher composition of 6PPD (mean 61% of ∑PPDs). This may be partially because the tap water in these three Hangzhou districts is from different sources (i.e., Tiaoxi River, Qiantang River, and Fuchunjiang River). The pollution characteristics of PPDs in these three rivers may be different, but this warrants further studies to confirm. Alternatively, different tap water treatment processes may also contribute to this discrepancy.
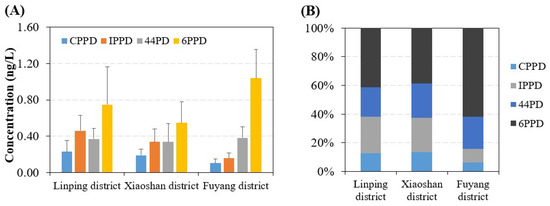
Figure 2.
(A) Concentrations (mean ± SD) and (B) concentration profiles of PPDs in tap water samples collected from different districts of Hangzhou city.
3.2. PPDs in Tap Water from Taizhou
Five PPDs were detected in tap water collected from Taizhou, China, with the concentrations of ∑PPDs ranging from 0.091 to 6.6 ng/L (Table 1). 6PPD, CPPD, 44PD, and 7PPD were detected in 63−83% of tap water samples from Taizhou. IPPD was detected in only 37% of Taizhou tap water samples. CPPD (mean 1.0 ng/L, <LOD−4.2 ng/L) was the predominant PPD in tap water from Taizhou, followed by 6PPD (0.93 ng/L, <LOD−2.6 ng/L) and 44PD (0.78 ng/L, <LOD−1.8 ng/L). This concentration profile is different from that observed in tap water from Hangzhou (Figure 1). Comparatively, concentrations of PPDs in tap water from Taizhou are higher than that from Hangzhou, especially for 6PPD (mean 0.93 and 0.79 ng/L, respectively; p < 0.01) and CPPD (1.0 and 0.16 ng/L, respectively; p < 0.01) (Supplementary Materials Figure S2). These differences are partially due to the different pollution characteristics of PPDs in the source water of tap water. The tap water collected from Taizhou city was mainly from Lingjiang River, which is contaminated due to rapid urbanization and industrial growth. The water pollution of Lingjiang River mainly stems from industrial wastewater, agricultural runoff, and urban discharge [52,53]. Concentrations of various PPDs in tap water from Taizhou city were barely correlated with each other (Spearman’s correlation coefficient, rs = 0.17−0.42; p > 0.084; Supplementary Materials Table S6).
We further compared the concentrations of PPDs in Taizhou tap water with that of other organic contaminants in tap water. Comparatively, concentrations of PPDs in tap water from Taizhou are lower than that of perfluorobutanoic acid (median 18 ng/L) in Chinese tap water, but the median concentration of 44PD and 6PPD is comparable to that of perfluorooctanoic acid (median 0.74 ng/L) [54]. The detection frequencies of PPDs are higher than that of bisphenol A (detection frequency 40%) in tap water from China [32]. The most commonly detected organophosphate flame retardants, including tris(2-butoxyethyl) phosphate (mean 70.1 ng/L), triphenyl phosphate (40.0 ng/L), and tris(2-chloroisopropyl) phosphate (33.4 ng/L), in tap water from China also had higher mean concentrations than PPDs [55]. Despite their comparatively low concentrations, the wide distribution of PPDs in tap water still suggests that more concerns should be paid to the potential risks of human exposure to PPDs through tap water ingestion.
3.3. Human Daily Intake Estimation
The estimated DI of PPDs for local residents living in Hangzhou and Taizhou through the ingestion of tap water is shown in Table 2. The mean DIs of PPDs for adults and children in Hangzhou were estimated to be 4.9–24 and 6.4–32 pg/kg bw/day, respectively. Meanwhile, the mean DIs of PPDs for adults and children living in Taizhou were 11–31 and 14–40 pg/kg bw/day, respectively. These data suggest that residents living in Hangzhou had higher exposure doses of PPDs than those in Taizhou, and children had higher amounts of PPD exposure than adults. This suggests that children were more susceptible to PPD exposure than adults via tap water ingestion, possibly due to their lower body weight and higher ingestion rate of tap water. Among PPDs, 6PPD (24–32 pg/kg bw/day) had the highest mean DI for residents in Hangzhou, followed by 44PD (12–16 pg/kg bw/day) and IPPD (10–12 pg/kg bw/day). Meanwhile, CPPD (31–40 pg/kg bw/day) had the highest mean DI for residents in Taizhou, followed by 6PPD (29–37 pg/kg bw/day) and IPPD (24–31 pg/kg bw/day). DIs calculated in this study provide the baseline data to estimate the total amount of human exposure to PPDs.

Table 2.
Estimated daily intake (pg/kg bw/day) of PPDs through tap water ingestion for adults and children.
Previous studies have estimated the DIs of PPDs through other human exposure pathways. For example, Wang et al. [56] reported that the human daily intake of PPDs through the inhalation of PM2.5 was in the range of 0.19–1.41 ng/kg bw/day. Cao et al. [11] estimated that the mean daily intake of PPDs through the oral ingestion of roadside soil dust was 1–10 ng/kg bw/day. Comparatively, the DIs of PPDs through tap water ingestion are much lower than those through inhalation of PM2.5 and oral ingestion of roadside soil dust. A European risk assessment study on synthetic turf rubber infill proposed the reference dose of 26,000 ng/kg bw/day for 6PPD in terms of cancer risk [57], which is much higher than the DIs of PPDs through tap water ingestion reported in this study. This suggests that the risk of human exposure to PPDs through tap water ingestion is relatively low. Despite that, toxic effects caused by long-term exposure to PPDs through tap water ingestion should still be of concern. In addition, as predicted by ADMETlab 3.0 software, 6PPD, CPPD, and IPPD (possibility 0.74−0.91) had higher potentials of inducing human skin sensitization than 44PD and 7PPD (possibility < 0.5). Hence, other toxic effects induced by human exposure to PPDs should also be investigated.
4. Conclusions
This study examined the occurrence of PPDs in tap water samples for the first time [14,58]. The results showed that 6PPD was the predominant PPD in tap water from Hangzhou, followed by 44PD and IPPD, while CPPD was the predominant PPD in tap water from Taizhou, followed by 6PPD and 44PD. In addition, DIs of PPDs through tap water ingestion for adults and children were estimated in this study. More studies are needed to examine the concentration of PPDs in human urine. It is important to acknowledge the challenges associated with determining the origin of PPDs due to their complex sources and transport pathways. Additionally, proposing effective measures for environmental control or water purification presents significant difficulties. Future studies should focus on addressing these challenges to better understand and mitigate the impact of PPDs on public health. The detection of PPDs in tap water highlights a previously overlooked exposure route. This finding underscores the importance of monitoring PPDs in water sources to assess human health risks. It prompts the need for regulatory measures to ensure safe tap water and emphasizes the importance of public health initiatives to raise awareness of potential health effects. Future research should focus on elucidating the health impacts of PPD exposure, identifying sources of contamination, and developing effective mitigation strategies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w16081128/s1, Table S1. List of p-Phenylenediamine Antioxidants Monitored in This Study and Their Abbreviation, Full Name, CAS Number, and Chemical Structure; Table S2. Source of the Collected Drinking Water in Hangzhou and Taizhou; Table S3. MRM Transition Parameters for Analyzing PPDs and Internal Standard; Table S4. LODs and Extraction Recoveries of Target Analytes in Human Urine; Table S5. Correlations among Concentrations (ng/L) of Various PPDs in Hangzhou Drinking Water; Table S6. Correlations among Concentrations (ng/L) of Various PPDs in Taizhou Drinking Water; Figure S1. The map of sampling regions in this study, Hangzhou city and Taizhou city; Figure S2. Concentrations (mean ± SD) of detected PPDs in drinking water samples from Hangzhou and Taizhou. Different alphabets mean the significant difference in concentrations.
Author Contributions
Software, J.Z. and S.J.; Validation, F.R. and H.J.; Formal analysis, R.G.; Investigation, J.Z.; Resources, S.J.; Writing—review & editing, H.J.; Visualization, R.G.; Supervision, H.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of Zhejiang Province (LR23D030001; LY21B070006) and the National Natural Science Foundation of China (21806139).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Dorofeev, A.; Zemskii, D. Oxypropylated aromatic diamines-stabilisers for tyre rubbers. Int. Polym. Sci. Technol. 2017, 44, 27–30. [Google Scholar] [CrossRef]
- Poldushova, G.; Kandyrin, K.; Reznichenko, S. The effect of the structure of p-phenylenediamine antiagers on the physicomechanical and hysteresis properties of filled rubber compounds. Int. Polym. Sci. Technol. 2016, 43, 19–22. [Google Scholar] [CrossRef]
- Xu, J.; Hao, Y.; Yang, Z.; Li, W.; Xie, W.; Huang, Y.; Wang, D.; He, Y.; Liang, Y.; Matsiko, J.J. Rubber antioxidants and their transformation products: Environmental occurrence and potential impact. Int. J. Environ. Res. 2022, 19, 14595. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Wang, D. Tire-rubber related pollutant 6-PPD quinone: A review of its transformation, environmental distribution, bioavailability, and toxicity. J. Hazard. Mater. 2023, 459, 132265. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Y.; Koenig, J. A review of rubber oxidation. Rubber Chem. Technol. 2005, 78, 355–390. [Google Scholar] [CrossRef]
- Lv, Y. Analysis of technology progress and market demand of para-phenylenediamine rubber antioxidants. China Rubber Sci. Technol. 2010, 1, 223–225. [Google Scholar]
- US EPA ChemView. 2022. Available online: https://chemview.epa.gov/chemview/ (accessed on 11 June 2022).
- Jin, R.; Venier, M.; Chen, Q.; Yang, J.; Liu, M.; Wu, Y. Amino antioxidants: A review of their environmental behavior, human exposure, and aquatic toxicity. Chemosphere 2023, 317, 137913. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Zhang, J.; Wang, W.; Wu, P.; Ru, Y.; Cai, Z. Mass spectrometry analysis of a ubiquitous tire rubber-derived quinone in the environment. TrAC Trends Anal. Chem. 2022, 157, 116756. [Google Scholar] [CrossRef]
- Chen, X.; He, T.; Yang, X.; Gan, Y.; Qing, X.; Wang, J.; Huang, Y. Analysis, environmental occurrence, fate and potential toxicity of tire wear compounds 6PPD and 6PPD-quinone. J. Hazard. Mater. 2023, 452, 131245. [Google Scholar] [CrossRef]
- Cao, G.; Wang, W.; Zhang, J.; Wu, P.; Zhao, X.; Yang, Z.; Hu, D.; Cai, Z. New Evidence of Rubber-Derived Quinones in Water, Air, and Soil. Environ. Sci. Technol. 2022, 56, 4142–4150. [Google Scholar] [CrossRef]
- Wang, W.; Cao, G.; Zhang, J.; Chen, Z.; Dong, C.; Chen, J.; Cai, Z. p-Phenylenediamine-Derived Quinones as New Contributors to the Oxidative Potential of Fine Particulate Matter. Environ. Sci. Technol. Lett. 2022, 9, 712–717. [Google Scholar] [CrossRef]
- Zeng, L.; Li, Y.; Sun, Y.; Liu, L.Y.; Shen, M.; Du, B. Widespread Occurrence and Transport of p-Phenylenediamines and Their Quinones in Sediments across Urban Rivers, Estuaries, Coasts, and Deep-Sea Regions. Environ. Sci. Technol. 2023, 57, 2393–2403. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, S.; Liu, X.; Tian, L.; Mo, Y.; Yi, X.; Liu, S.; Liu, J.; Li, J.; Zhang, G. Aquatic environmental fates and risks of benzotriazoles, benzothiazoles, and p-phenylenediamines in a catchment providing water to a megacity of China. Environ. Res. 2023, 216 Pt 4, 114721. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Guo, R.; Jiang, S.; Wu, P.; Jin, H. Occurrence of p-phenylenediamine antioxidants (PPDs) and PPDs-derived quinones in indoor dust. Sci. Total Environ. 2024, 169325. [Google Scholar] [CrossRef]
- Guo, Z.; Cheng, Z.; Zhang, S.; Zhu, H.; Zhao, L.; Baqar, M.; Wang, L.; Sun, H. Unexpected Exposure Risks to Emerging Aromatic Amine Antioxidants and p-Phenylenediamine Quinones to Residents: Evidence from External and Internal Exposure as Well as Hepatotoxicity Evaluation. Environ. Health 2024. [Google Scholar] [CrossRef]
- Matsumoto, M.; Yamaguchi, M.; Yoshida, Y.; Senuma, M.; Takashima, H.; Kawamura, T.; Kato, H.; Takahashi, M.; Hirata-Koizumi, M.; Ono, A. An antioxidant, N, N′-diphenyl-p-phenylenediamine (DPPD), affects labor and delivery in rats: A 28-day repeated dose test and reproduction/developmental toxicity test. Food Chem. Toxicol. 2013, 56, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Fang, C.; Di, S.; Yu, Y.; Wang, C.; Wang, X.; Jin, Y. Oral exposure to tire rubber-derived contaminant 6PPD and 6PPD-quinone induce hepatotoxicity in mice. Sci. Total Environ. 2023, 869, 161836. [Google Scholar] [CrossRef]
- Bohara, K.; Timilsina, A.; Adhikari, K.; Kafle, A.; Basyal, S.; Joshi, P.; Yadav, A.K. A mini review on 6PPD quinone: A new threat to aquaculture and fisheries. Environ. Pollut. 2024, 340 Pt 2, 122828. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Zhao, H.; Peter, K.T.; Gonzalez, M.; Wetzel, J.; Wu, C.; Hu, X.; Prat, J.; Mudrock, E.; Hettinger, R. A ubiquitous tire rubber-derived chemical induces acute mortality in coho salmon. Science 2021, 371, 185–189. [Google Scholar] [CrossRef]
- Akhtar, N.; Syakir Ishak, M.I.; Bhawani, S.A.; Umar, K. Various natural and anthropogenic factors responsible for water quality degradation: A review. Water 2021, 13, 2660. [Google Scholar] [CrossRef]
- Liu, J.; Bridget, R. Food-energy-water nexus for multi-scale sustainable development. Resour. Conserv. Recycl. 2020, 154, 104565. [Google Scholar]
- Babuji, P.; Thirumalaisamy, S.; Duraisamy, K.; Periyasamy, G. Human health risks due to exposure to water pollution: A review. Water 2023, 15, 2532. [Google Scholar] [CrossRef]
- Liu, M.; Graham, N.; Wang, W.; Zhao, R.; Lu, Y.; Elimelech, M.; Yu, W. Spatial assessment of tap-water safety in China. Nat. Sustain. 2022, 5, 689–698. [Google Scholar] [CrossRef]
- Evans, S.; Campbell, C.; Naidenko, O.V. Cumulative risk analysis of carcinogenic contaminants in United States drinking water. Heliyon 2019, 5, e02314. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Duttagupta, S.; Mukherjee, A. Emerging organic contaminants in global community drinking water sources and supply: A review of occurrence, processes and remediation. J. Environ. Chem. Eng. 2022, 10, 107560. [Google Scholar] [CrossRef]
- Dahiya, V. Heavy metal toxicity of drinking water: A silent killer. GSC Biol. Pharm. Sci. 2022, 19, 020–025. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Naseri, K.; Esform, A.; Aramjoo, H.; Naghizadeh, A. Drinking water heavy metal toxicity and chronic kidney diseases: A systematic review. Rev. Environ. Health 2021, 36, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Verma, A.K. Arsenic: It’s toxicity and impact on human health. Int. J. Biol. Innov. 2021, 3, 38–47. [Google Scholar] [CrossRef]
- Lee, S.; Jeong, W.; Kannan, K.; Moon, H.B. Occurrence and exposure assessment of organophosphate flame retardants (OPFRs) through the consumption of drinking water in Korea. Water Res. 2016, 103, 182–188. [Google Scholar] [CrossRef]
- Madhav, S.; Ahamad, A.; Singh, A.K.; Kushawaha, J.; Chauhan, J.S.; Sharma, S.; Singh, P. Water pollutants: Sources and impact on the environment and human health. In Sensors in Water Pollutants Monitoring: Role of Material; Springer: Singapore, 2020; pp. 43–62. [Google Scholar]
- Zhang, H.; Zhang, Y.; Li, J.; Yang, M. Occurrence and exposure assessment of bisphenol analogues in source water and drinking water in China. Sci. Total Environ. 2019, 655, 607–613. [Google Scholar] [CrossRef]
- Al-Nasir, F.; Hijazin, T.J.; Al-Alawi, M.M.; Jiries, A.; Mayyas, A.; Al-Dalain, S.A.; Al-Dmour, R.; Alahmad, A.; Al-Madanat, O.Y.; Batarseh, M.I. Accumulation, Source Identification, and Cancer Risk Assessment of Polycyclic Aromatic Hydrocarbons (PAHs) in Different Jordanian Vegetables. Toxics 2022, 10, 643. [Google Scholar] [CrossRef] [PubMed]
- Jiries, A.; Al-Nasir, F.; Hijazin, T.J.; Al-Alawi, M.; El Fels, L.; Mayyas, A.; Al-Dmour, R.; Al-Madanat, O.Y. Polycyclic aromatic hydrocarbons in citrus fruit irrigated with fresh water under arid conditions: Concentrations, sources, and risk assessment. Arab. J. Chem. 2022, 15, 104027. [Google Scholar] [CrossRef]
- Zhang, C.; Feng, Y.; Liu, Y.; Chang, H.; Li, Z.; Xue, J. Uptake and translocation of organic pollutants in plants: A review. J. Integr. Agric. 2017, 16, 1659–1668. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Huang, Z.; Liu, Y.H.; Hu, L.X.; He, L.Y.; Liu, Y.S.; Zhao, J.L.; Ying, G.G. Occurrence and risks of 23 tire additives and their transformation products in an urban water system. Environ. Int. 2023, 171, 107715. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Wang, W.; Zhang, J.; Wu, P.; Qiao, H.; Li, H.; Huang, G.; Yang, Z.; Cai, Z. Occurrence and Fate of Substituted p-Phenylenediamine-Derived Quinones in Hong Kong Wastewater Treatment Plants. Environ. Sci. Technol. 2023, 57, 15635–15643. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Dong, X.; Zhao, N.; Zhao, M.; Jin, H. Polyhalogenated carbazoles in indoor dust from Hangzhou, China. Sci. Total Environ. 2023, 859 Pt 1, 159971. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, Y.; Li, T.; Chen, W.; Wang, W. Gap to End-TB targets in eastern China: A joinpoint analysis from population-based notification data in Zhejiang Province, China, 2005–2018. Int. J. Infect. Dis. 2021, 104, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Kryuchkov, F.; Foldvik, A.; Sandodden, R.; Uhlig, S. Presence of 6PPD-quinone in runoff water samples from Norway using a new LC-MS/MS method. Front. Environ. Chem. 2023, 4, 1194664. [Google Scholar] [CrossRef]
- Seiwert, B.; Nihemaiti, M.; Troussier, M.; Weyrauch, S.; Reemtsma, T. Abiotic oxidative transformation of 6-PPD and 6-PPD quinone from tires and occurrence of their products in snow from urban roads and in municipal wastewater. Water Res. 2022, 212, 118122. [Google Scholar] [CrossRef]
- Zuccarello, P.; Ferrante, M.; Cristaldi, A.; Copat, C.; Grasso, A.; Sangregorio, D.; Fiore, M.; Oliveri Conti, G. Exposure to microplastics (<10 mum) associated to plastic bottles mineral water consumption: The first quantitative study. Water Res. 2019, 157, 365–371. [Google Scholar]
- Kahn, H.D.; Stralka, K. Estimated daily average per capita water ingestion by child and adult age categories based on USDA’s 1994–1996 and 1998 continuing survey of food intakes by individuals. J. Expo. Sci. Environ. Epidemiol. 2009, 19, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Wang, Y.; Xia, W.; He, Z.; Xu, S. Neonicotinoids in raw, finished, and tap water from Wuhan, Central China: Assessment of human exposure potential. Sci. Total Environ. 2019, 675, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Yang, S.; Wang, P.G. Matrix effects and application of matrix effect factor. Bioanalysis 2017, 9, 1839–1844. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Jin, H.; Guo, R.; Chen, P.; Zhong, S.; Wu, X. Occurrence of p-phenylenediamine antioxidants in human urine. Sci. Total Environ. 2024, 914, 170045. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Li, J.; Du, B.; Pan, Z.; Liu, L.Y.; Zeng, L. E-Waste recycling emits large quantities of emerging aromatic amines and organophosphites: A poorly recognized source for another two classes of synthetic antioxidants. Environ. Sci. Technol. Lett. 2022, 9, 625–631. [Google Scholar] [CrossRef]
- PubChem. 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/N-_1_3-Dimethylbutyl_-N_-phenyl-p-phenylenediamine#section=Solubility (accessed on 23 January 2022).
- Deng, C.; Huang, J.; Qi, Y.; Chen, D.; Huang, W. Distribution patterns of rubber tire-related chemicals with particle size in road and indoor parking lot dust. Sci. Total Environ. 2022, 844, 157144. [Google Scholar] [CrossRef]
- Hu, X.; Zhao, H.N.; Tian, Z.; Peter, K.T.; Dodd, M.C.; Kolodziej, E.P. Transformation product formation upon heterogeneous ozonation of the tire rubber antioxidant 6PPD (N-(1, 3-dimethylbutyl)-N′-phenyl-p-phenylenediamine). Environ. Sci. Technol. Lett. 2022, 9, 413–419. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Yin, S.; Wang, Q.; Li, Y.; Liu, Q.; Liu, L.; Luo, X.; Chen, L.; Zheng, H. First insights into 6PPD-quinone formation from 6PPD photodegradation in water environment. J. Hazard. Mater. 2023, 459, 132127. [Google Scholar] [CrossRef]
- Wang, C.Y.; Zhou, B.; Huang, B. A continuing 30-year decline in water quality of Jiaojiang Estuary, China. Water Sci. Eng. 2015, 8, 20–29. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, Y.; Wu, J.; Su, F. Surface water quality and health risk assessment in Taizhou City, Zhejiang Province (China). Expo. Health 2022, 14, 1–16. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Zhang, L.; Huang, Z.; Liu, Y.; Wu, N.; He, J.; Zhang, Z.; Zhang, Y.; Niu, Z. Perfluoroalkyl acids in drinking water of China in 2017: Distribution characteristics, influencing factors and potential risks. Environ. Int. 2019, 123, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, N.; Zhang, B.; Jin, L.; Li, M.; Hu, M.; Zhang, X.; Wei, S.; Yu, H. Occurrence of organophosphate flame retardants in drinking water from China. Water Res. 2014, 54, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cao, G.; Zhang, J.; Wu, P.; Chen, Y.; Chen, Z.; Qi, Z.; Li, R.; Dong, C.; Cai, Z. Beyond substituted p-Phenylenediamine antioxidants: Prevalence of their quinone derivatives in PM2.5. Environ. Sci. Technol. 2022, 56, 10629–10637. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.; Bierwisch, A.; Kaiser, E. ERASSTRI-European risk assessment study on synthetic turf rubber infill-Part 3: Exposure and risk characterisation. Sci. Total Environ. 2020, 718, 137721. [Google Scholar] [CrossRef]
- Rauert, C.; Vardy, S.; Daniell, B.; Charlton, N.; Thomas, K.V. Tyre additive chemicals, tyre road wear particles and high production polymers in surface water at 5 urban centres in Queensland, Australia. Sci. Total Environ. 2022, 852, 158468. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).