Abstract
The demand for water for civil and industrial use is diminishing the availability of such a valuable environmental resource for agricultural purposes. Thus, for the next generation, it is imperative to find alternative water sources for crop irrigation. The citrus agroindustry utilizes a large amount of water for processing fruit (e.g., essential oil extraction, fruit washing). Wastewaters produced by citrus industry (CWWs) are rich in organic matter and mineral nutrients, thus making them potentially usable for crop irrigation. Conversely, due to their high content of organic acids and low pH, they may increase the availability of soluble metals, in the form of both plant nutrients and contaminants. The aim of this study was to evaluate the effect of CWWs on the dynamics of soil water-soluble metals and pH. To this end, CWWs from the processing of lemons, oranges, and tangerines at three different doses were used. CWWs were analyzed to investigate type and amount of organic acids. Soil water-soluble metals (Na, Mg, Al, K, Ca, Fe, Co, Ni, Cu, Zn, and Cd) and pH were determined at days 1, 3, 7, 21, and 28 after the addition of CWWs. Citric, ascorbic, oxalic, tartaric, acetic, and malic acids were found in CWWs, with citric acid being two orders of magnitude more concentrated than the other acids. After the addition of CWWs, soil pH promptly decreased from 7.2 to at least 5.3 depending on the type and concentration of CWWs. Concurrently, the concentration of almost all investigated metals sharply increased within 7 days after the addition of CWWs. Then, it decreased, reaching values similar to that of the control. The increase in metals availability as a consequence of pH decrease was ascribed to different causes: the exchange reaction between H+ and cations adsorbed onto colloid surfaces, the addition of organic matter by CWWs that stimulated microbial activity, and the quantity and type of organic acids added via CWWs. In conclusion, the obtained results suggest that the use of citrus wastewater for irrigation purposes could be a valid solution, with them being rich in plant nutrients and easily mobilized elements such as Ca, Mg, K, and Na. Further research is needed to refine the understanding of the impact of CWWs in the long term and to develop targeted strategies for managing industrial wastewater in agriculture.
1. Introduction
Over a third of humanity lives in arid and semi-arid lands, which constitute around 41% of the planet’s terrestrial surface [1]. Such lands are distinguished by a harsh climate, which includes minimal precipitation, intense sun radiation, and strong wind speeds for most of the year [2]. These issues and the expanding population inexorably cause water to become scarce and limited, which in turn reduces crop yields and occasionally causes complete crop failure. Therefore, it has become increasingly indispensable to identify alternative sources of water that may be used for irrigation in an economical and efficient manner [3]. Due to excessive urbanization, population increase, industrial development, and agricultural expansion, the scarcity of water resources has worsened. In addition, climate change poses a significant risk, since it is influencing crops and water supplies. The major consumer activity of freshwater, accounting for around 70% of consumption, is agriculture [4]. Treated or not and diluted or not, wastewater could be used to irrigate crops in many arid and semi-arid regions [5]. The reuse of citrus wastewaters (CWWs) is in line with the principles of the circular economy approach, as it provides the recovery of resources considered lost, while achieving a reduction in waste disposal and the development of value-added products [6,7]. The use of treated wastewater is an efficient way to counteract water scarcity, save a significant amount of drinking water, and reduce the use of chemical fertilizers because of nutrients held in wastewater, thereby improving crop yields and protecting the environment [8].
On average, more than 120 million tons of citrus fruits are produced annually, of which about 20% are for industrial processing [4]. The citrus industry uses a huge amount of water, specifically approximately 0.8 m3 of potable water per ton of fruits (Eurofood, Messina, Sicily, Italy; personal communication) that, if recovered, may represent a valid alternative to freshwater used for agricultural purposes.
CWWs are a blend of water from the industrial process and from fruit constitution. They are rich in organic matter, have low pH, and contain traces of essential oils, nutrients such as N, P, K, Ca, and Mg, and metallic elements (Cu, Zn, Fe, Mn, etc.) [5,9,10].
Since CWWs derive from potable water and edible fruits, it would be interesting to reuse them in agriculture for crop irrigation, either as they are or diluted with freshwater, within the circular economy principle [5,10,11]. The availability of water for crop irrigation is a critical consideration, especially in arid and semi-arid regions where water scarcity is a pressing issue. Repurposing CWWs can help alleviate the demand for freshwater resources, contributing to sustainable water management practices.
The production of CWWs is very variable, from a 1:1 fruit–water ratio (v/v) in the case of small local companies located in Sicily [10] to 1:17 in reports by Corsino et al. [12].
Recently, Ioppolo et al. [10] assessed the impact of lemon, orange, and tangerine processing wastewaters diluted at 1/3, 2/3, and 3/3 on soil chemical and biochemical properties under laboratory conditions. They incubated a sandy clay soil moistened with such CWWs for 56 days. At the end of the incubation, they found that the total and labile C pools increased, and soil microbial activity and biomass were stimulated. Overall, CWWs improved the biological soil fertility, thus suggesting a positive role of CWWs for sustainable agriculture.
However, since CWWs generally have pH < 3.5, their use for crop irrigation may affect the availability of macronutrients and metallic micronutrients in soils. Indeed, soil pH is among the main factors affecting the availability of metals [13,14]; in alkaline soils, metal solubility decreases, whereas in acidic soils, solubility increases [15]. Kicińska et al. [16] studied the extraction rate of Cd, Pb, and Zn from highly contaminated and uncontaminated soils after gradual acidification. The authors demonstrated that even the slightest decrease in soil pH increased metal concentration in different extractant solutions. Furthermore, metal mobility significantly increased with soil acidification in the following order: Cd > Zn > Pb. Thus, CWWs, due to their low pH, may enhance metal solubility in soils if used for crop irrigation.
On the other hand, CWWs are rich in organic acids such as citric, oxalic, malonic, acetic, and tartaric acids, which are responsible for the low pH and may affect the availability of metals also via complexation [17]. Following the addition of CWWs and, consequently, the introduction of organic acids, the ensuing chemical interactions lead to the formation of complexes with metals already existing in the soil. According to Huang and Keller [18] and Boudot et al. [19], among all organic acids, citrate possesses the capability to dissolve minerals through reactions with polyvalent cations (e.g., Al3+, Fe3+), owing to its three carboxylate groups, resulting in the formation of water-soluble metallic–organic complexes, commonly known as chelates. This process facilitates the extraction of metals, resulting in the formation of soluble salts within the aqueous fraction (solution) of the soil. Thus, by introducing a substantial amount of citrate through CWWs, it may be possible to release and solubilize a large quantity of metals, forming complexes. However, once microorganisms degrade citrate linked to metals, it is crucial to understand the fate of these metals. Terzano et al. [20] demonstrated that the degradation of citrate facilitates the co-precipitation of copper within aluminum (hydr)oxides in calcareous soils. Their findings support the hypothesis that copper (Cu) solubility in soil can be considerably reduced through the formation of co-precipitates with aluminum (Al) (hydr)oxides. The observed precipitates highlight the role of soil microorganisms in decomposing aluminum– and copper–citrate complexes.
Overall, the exploitation of CWW for crop irrigation presents an exciting opportunity for innovation and sustainable resource management within the agricultural sector. However, despite the potential benefits of utilizing CWWs for agricultural irrigation, such an opportunity has not been previously explored.
Thus, based on the above findings, the aim of this study was to evaluate the dynamics of soil water-soluble metals following the addition of CWWs with a pH lower than 3.5, coming from the processing of orange, lemon, and tangerine fruits. We hypothesized that moistening soil with CWWs lowers soil reaction activity and, in turn, owing also to the presence of organic acids, increases the availability of metals. Moreover, we hypothesized that the higher the concentration of CWWs, the lower the pH and, thus, the greater the availability of metals in soil.
To test these hypotheses, diluted and undiluted CWWs were added to soil, bringing it up to 50% of its water-holding capacity. Then, soil reaction and water-soluble metal concentrations were monitored during the following 28 days of incubation.
2. Materials and Methods
2.1. Citrus Wastewaters
Citrus wastewaters were obtained from three citrus species: Citrus sinensis (L.) Osbeck (Orange), Citrus limon (L.) Osbeck (Lemon), and Citrus reticulata Blanco (Tangerine). The CWWs, obtained via the cold-pressing method (FMC Technology by Althaller Italia s.r.l., MI, Italy), were provided by a local industry (EuroFood, ME, Sicily, Italy).
The CWWs were analyzed to determine their main chemical properties. Density was determined through the mass/volume ratio by filling a volumetric flask of 50 mL and weighing it. CWWs’ reactivity (pH) and electrical conductivity (EC) were determined in undiluted forms after filtration through Whatman paper #42 by a pHmeter (FiveEasy, Mettler Toledo Spa, Milan, Italy) and a conductometer (HI5321, Hanna Instruments Italia srl, Padua, Italy), respectively. Ashes were determined after stove heating at 550 °C. Organic carbon and total nitrogen were determined via the oxidation method with potassium dichromate [21] and via Kjeldahl digestion with sulfuric acid and distillation with boric acid [22], respectively. Total carbohydrates were determined via the anthrone method after the acid hydrolysis of CWW [23], while the soluble monosaccharides by titration with the Fehling’s reagent as described by Yebra et al. [24]. Total fibers were determined according to the method described by Prosky et al. [25].
Total phosphorus was determined on an aliquot of the CWWs ashes by a spectrophotometer (UVmini-1240, Shimadzu Italia srl, Milan Italy) using ammonium molybdate solution [26]. The total contents of Ca, Mg, K, Na, Al, Cd, Co, Cu, Fe, Ni, and Zn were determined via ICP–MS (Agilent 7500-ce; Agilent Technologies Italia SpA, Milan, Italy) after acidifying the CWWs with ultrapure nitric acid. All the reagents were provided by Merck KGaA (Darmstadt, Germany).
2.2. Experimental Set-Up
The experiment was carried out at the laboratory scale. The topsoil (0–15 cm) of an olive orchard was used (Figure 1; 38°02′15.7″ N 14°36′31.3″ E).
Figure 1.
Soil sampling location.
The main characteristics of the soil were reported in Table 1. After sampling, the soil was air dried and sieved at <2 mm. Then, 500 g of soil were placed in 1 L PET bottles and moistened up to 50% of the water-holding capacity (WHC) by applying CWWs or distilled water only (control, CTR). The CWWs were applied at three different doses to reach 1/3, 2/3, and 3/3 of the 50% of soil WHC. Totals doses of 39, 78, and 117 mL of each CWW were added to 500 g of air-dried soil, respectively. Soils were then incubated in the dark at a constant temperature (22.0 ± 0.5 °C). Four replicates per treatment were run for a total of 40 samples (3 CWWs × 3 doses × 4 replicates plus 4 controls (water)).

Table 1.
Main physical and chemical properties of the soil used in this study.
2.3. Organic Acids Determinations by High Performance Liquid Chromatography (HPLC)
Organic acids in CWWs were determined by high-performance liquid chromatography. CWWs were centrifuged at 3000× g for 10 min, then the supernatant was filtered through Whatman paper #42 and diluted 1:50 or 1:5 for the determination of citric acid or other acids, respectively. Each CWW was analyzed twice from different aliquots and in duplicates. Standards of citric, malic, tartaric, oxalic, ascorbic, and acetic acid were purchased from Merck (Milan, Italy). A mixed standard stock solution was prepared containing 2000 mg L−1 citric acid, 200 mg L−1 malic acid, 160 mg L−1 tartaric acid, 100 mg L−1 acetic and ascorbic acids, and 60 mg L−1 oxalic acid. The stock solution and the corresponding dilutions were prepared in ultrapure water and stored in the dark between the experiments, at low temperature (+4 °C). The organic acids were detected and quantified with external calibration graphs. The HPLC analysis was performed with an HPLC Ultimate 3000 (Thermo Scientific, Germany). For the simultaneous detection of the six analytes, the detector was set at λ = 254 nm for ascorbic acid and λ = 214 nm for the other five organic acids. The determinations were collected under isocratic conditions, at 20 °C, using a mobile phase made of 50 mM phosphate solution at pH = 2.8. The flow rate of the mobile phase was 0.7 mL min−1 for all the chromatographic separations. The volume injected was 5 μL for either the prepared sample or standard solution.
2.4. Soil Analyses
The soil pH was determined soon after (day 0) the addition of CWWs and also at 1, 3, 7, 21, and 28 days after addition using a pHmeter (FiveEasy, Mettler Toledo Spa, Milan, Italy) in 1:2.5 (w/v) soil–distilled water suspension. The suspension 1:5 (w/v) soil–distilled water was filtered through Whatman paper #42 and advisably diluted to be analyzed by ICP-MS (Agilent 7500-ce; Agilent Technologies Italia SpA, Milan, Italy) for water-soluble metals determination. All instrumental parameters were optimized for the analyses of all the investigated elements. Each solution was measured three times, and ICP-MS analyses were carried out with a classical external calibration approach, from 1 to 10,000 µg L−1 for each investigated element, using 103Rh (1000 pg mL−1) as an internal standard to compensate for any signal instability. Instrument and measurement parameters were as follows: forward power, 1550 W; nebulizer gas flow, 1.0 L min−1; auxiliary gas flow, 0.80 L min−1; and plasma gas flow, 15 L min−1.
3. Results
3.1. Chemical Compositions of CWWs
The three CWWs had similar densities (1.02 g cm−3 on average) but quite different chemical properties (Table 2). Orange wastewater (OWW) had the highest content of C and N, more than twice the concentration compared to lemon wastewater (LWW) and tangerine wastewater (TWW) (Table 2). The C/N ratio of CWWs ranged from 8.9 to 19.9, being the lowest in the LWW and the highest in TWW. Moreover, OWW had the highest amount of total carbohydrates, total fibers, total P, and the four basic macronutrients (Ca, Mg, K, and Na) and the lowest ash content. The pH of CWWs ranged from 2.8 to 3.5 and was the lowest in LWW and the highest in OWW (Table 3). Following the dilution with distilled water, the pH of the three CWWs did not show significant differences compared to the respective undiluted CWW.

Table 2.
Main physical and chemical properties of lemon, orange, and tangerine wastewaters (LWW, OWW, and TWW, respectively). Along the rows, different letters indicate significant differences among the three citrus wastewaters at p < 0.05.

Table 3.
Reactivity (pH) of diluted and undiluted lemon, orange, and tangerine wastewaters (LWW, OWW, and TWW, respectively). In each column, different capital letters indicate significant differences among citrus wastewaters at the same concentration at p < 0.05, whereas lowercase letters indicate significant differences among doses within the same citrus wastewater at p < 0.05.
3.2. Metal Content of CWWs
The concentration of metals in CWWs spanned from mg L−1 to μg L−1. The most abundant metal was K, ranging from 206 mg L−1 in OWW to 65 mg L−1, on average, in LWW and TWW. The second most abundant metal was Ca, followed by Mg and Na, with the latter being one order of magnitude lower than Ca and Mg. The concentrations of Ca, Mg, K, and Na were the highest in OWW (Table 2). Of the same order of magnitude as Na was Al, ranging from 132 to 244 mg L−1. Then, Al was followed, in decreasing order, by Fe > Cu > Zn > Ni > Co > Cd.
3.3. Amount of Metals Added to Soil by CWWs
The amount of metals supplied by CWWs to soil is reported in Table 4. Regardless of the type of CWW, K was the metal supplied in the highest amount, followed by Ca and then by Mg, with both of at least one order of magnitude lower than K; Na was the metal supplied at the lowest amount (<0.3 mg kg−1 of soil). With regard to the other metals, Al was supplied at the same order of magnitude as Na, followed, in decreasing order, by Fe, Cu, Zn, Ni, Co, and Cd. Regardless of the type of metal, the amount of metals supplied proportionally decreased with an increasing dilution of CWWs (Table 4).

Table 4.
Amount of metals added by diluted and undiluted lemon, orange, and tangerine wastewaters (LWW, OWW, and TWW, respectively).
3.4. Organic Acids in CWWs
Citric acid was the most abundant organic acid found in CWWs, having a concentration at least of two orders of magnitude greater than the other acids (Table 5). LWW had the highest content of citric acid, followed by OWW and TWW. The content of the other organic acids never exceeded 0.43 g L−1. LWW had the highest concentrations of most organic acids, followed by OWW and TWW, except for tartaric acid, which was more abundant in OWW.

Table 5.
Organic acids assessed in lemon, orange, and tangerine wastewaters (LWW, OWW, and TWW, respectively). Within each column, different lowercase letters indicate significant differences among citrus wastewaters at p < 0.05.
3.5. Effect of CWWs Application on Soil pH
Two hours after the application of CWWs at the lowest dose (1/3), soil pH decreased from 7.2 (pH of the control soil) to between 5.5 (OWW) and 6.3 (TWW), while at the highest dose (3/3) it ranged from 4.1 (LWW) to 5.4 (TWW) (Figure 2). Then, soil pH increased and, at the lowest CWWs concentration, reached the same value of the control soil after one week, while at the highest concentration for LWW and OWW, the value was reached after 21 days. Notably, TWW at any concentration reached control soil pH after just one week. After 21 days, no further variations in soil pH were observed compared to control soil.
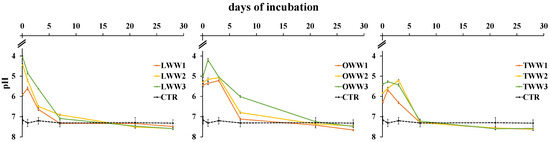
Figure 2.
Soil reaction following the addition of lemon, orange, and tangerine wastewater (LWW, OWW, and TWW, respectively) and in the control soil (CTR) at three different concentrations (1/3, 2/3, and 3/3) during the 28 days experiment. Bars are standard deviations (n = 4).
3.6. Effect of CWWs Application on Soil Water-Soluble Metals
The concentration of metals, determined on soil water extracts after the addition of CWWs, was generally affected by both the type of CWWs and dilution. The concentrations of Ca2+, Mg2+ (Figure 3a,b), Co2+, and Ni2+ (Figure 3c,d) during the incubation showed similar patterns. They increased soon after the addition of CWWs, reaching the maximum concentration in soil at day 1 or 3. Such an increase was inversely related to CWWs dilution. Then, they decreased, reaching, by the end of the incubation, the same concentration as that in the control soil, except for the treatment with OWW, where the concentration of Ca2+ was still higher than in the control, and the concentration of Mg2+ was higher than in the control soil only at the highest concentration of OWW.
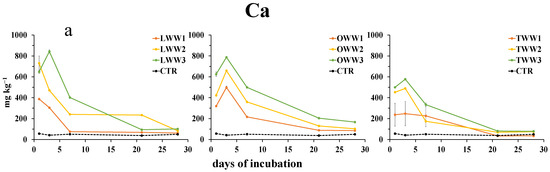
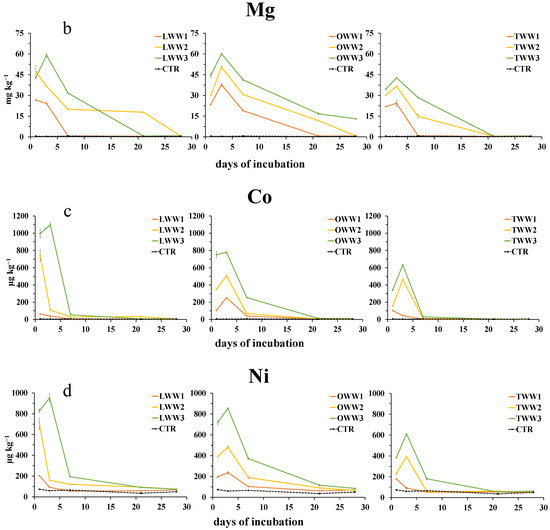
Figure 3.
Dynamics of water-extractable calcium (a), magnesium (b), cobalt (c), and nickel (d) in soil following the addition of lemon, orange, and tangerine wastewater (LWW, OWW, and TWW, respectively) and in the control soil (CTR) at three different concentrations (1/3, 2/3, and 3/3) during the 28 days experiment. Bars are standard deviations (n = 4).
Also, the concentrations of Na+ and K+ (Figure 4a,b) were generally increased by the addition of CWWs, although to a lesser extent by TWW.
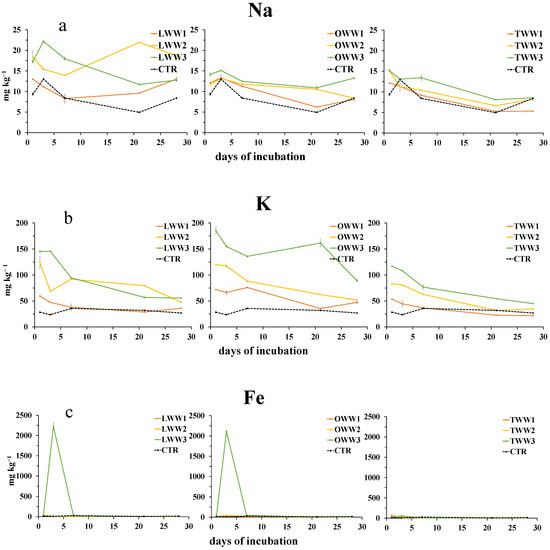
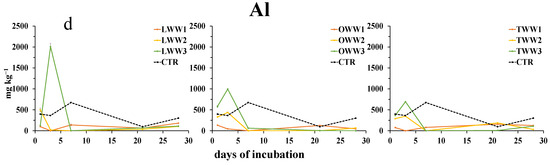
Figure 4.
Dynamics of water-extractable sodium (a), potassium (b), iron (c), and aluminum (d) in soil following the addition of lemon, orange, and tangerine wastewater (LWW, OWW, and TWW, respectively) and in the control soil (CTR) at three different concentrations (1/3, 2/3, and 3/3) during the 28 days experiment. Bars are standard deviations (n = 4).
However, during the incubation, their patterns did not follow a clear trend. The concentration of Fe3+ and Al3+ (Figure 4c,d and Figure 5) showed the highest values at day 3, when soil was treated with LWW and OWW, whereas the addition of TWW did not affect their concentration. After day 7, the concentrations of both cations were similar to or lower than that in the control soil.

Figure 5.
Zoom of water-extractable iron in soil following the addition of lemon, orange, and tangerine wastewater (LWW, OWW, and TWW, respectively) at three different concentrations (1/3, 2/3, and 3/3) during the 28 days experiment. Bars are standard deviations (n = 4).
The concentration of Cu2+ (Figure 6a) was higher than that in the control only at day 3 and for LWW, while for other two CWWs, it was equal or lesser than the control soil. The concentration of Zn2+ (Figure 6b) was affected by both the type and dilution of CWWs. LWW elevated the concentration of Zn2+ only when applied at the highest concentration on day 3. In OWW and TWW, Zn2+ showed an increase after day 1, exceeding the control, but by day 3, the concentration decreased.
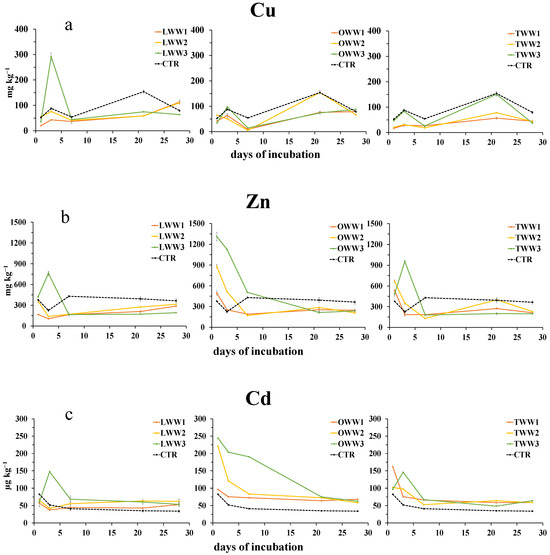
Figure 6.
Dynamics of water-extractable copper (a), zinc (b), and cadmium (c) in soil following the addition of lemon, orange, and tangerine wastewater (LWW, OWW, and TWW, respectively) and in the control soil (CTR) at three different concentrations (1/3, 2/3, and 3/3) during the 28 days experiment. Bars are standard deviations (n = 4).
Irrespective of CWWs’ type and dilution, at the conclusion of incubation, Zn2+ concentration was lower than that of the control. Also, the concentration of Cd2+ was significantly affected by both the type and dilution of CWWs. LWW and TWW sharply increased the concentration of Cd2+ (Figure 6c) compared to that in the control during the first five days of incubation. Then, Cd2+ concentration decreased but remained higher than that of the control during all the incubation. Instead, OWW, much more so than LWW and TWW, increased the concentration of Cd2+, especially at the highest concentration, remaining higher than that of the control also at the end of the incubation.
4. Discussion
4.1. Water Dilution Effect on CWWs Characteristics
Except for pH, the characteristics of CWWs fell within the range of waters suitable for agricultural irrigation [27]. The low pH values were out of range compared to those generally used for crop irrigation and did not change following their dilution with distilled water, likely due to the good buffering capacity of CWWs. Such an ability arises from the presence of weak polycarboxylic organic acids along with their salts that provide for their conjugate bases in the solution [28].
However, according to Zaman et al. [27], the pH of irrigation water is not always a reliable indicator of water quality, and most crops can tolerate a wide pH range; this is also because soils have a good buffering capacity due to organic and inorganic colloids [29].
Generally, OWW had the highest amount of macronutrient cations, i.e., Ca, Mg, K, and Na. Instead, LWW contained more Al and Fe than the other CWWs. Cu, Zn, Co, Ni, and Cd contents were similar in all three CWWs. However, these contents are within the limits set by law for water intended for agriculture, above which it is considered that there is a risk to human health and to living species in associated ecosystems [30].
4.2. CWWs Effects on Soil pH
Soil pH before the addition of CWWs was equal to 7.2. Following the addition of undiluted CWWs, it decreased down to 5.3 within the first 2 h (Day 0). Then, after day 1, it started to increase, reaching the initial value 7 days after the addition of CWWs. Such a behavior has been also observed by Mekki et al. [31] who, after the addition of untreated olive mill wastewater at a pH of 5.0, did not find any difference between initial pH and that determined during the six months of the experiment.
Undiluted LWW had the strongest immediate effect on soil pH, but on day 7, the pH reached the initial value before the application. On the other hand, with the application of OWW, the maximal lowering of soil pH was delayed by 1 day. However, soil irrigated with OWW recovered the pH value more slowly, i.e., a long-lasting effect of OWW occurred. TWW only slightly affected soil pH, which was lowered to 5.3 by the highest dose. Such different effects on soil pH likely were due to the initial pH of CWWs that increased according to the order LWW< TWW < OWW, but they were also due to the content of organic acids and, thus, buffering power, which were in the order LWW > OWW > TWW. Such results suggest the CWWs can be successfully used for crop irrigation in soil with good buffering capacity, such as those rich in carbonates, organic matter, and clay. The buffering capacity of these soils allows them to absorb the acidity from the water, gradually bringing the pH to a more neutral level. However, the temporary drop in soil pH may be useful to aid in the plant uptake of some minor nutrients such as iron in calcareous soils.
4.3. CWWs Effects on Soil Metals Availability
The availability of various soil metals following the addition of CWWs did not show a similar pattern. The concentration of almost of all investigated metals sharply increased within 7 days after the addition of CWWs. Then, it decreased, reaching values similar to that of the control. Such behavior may be ascribed mainly to the rapid decrease in soil pH, which occurred soon after the addition of CWWs, and, to a lesser extent, to the quantity of metals supplied by CWWs. Indeed, the first hypothesis is reasonable, since the increase in each metal during the first 7 days of incubation is much higher than the quantity of each metal supplied.
The increase in metals’ hydro-solubility/availability as a consequence of pH decrease may be linked to many concurrent factors [29]. The increased concentration of H+ in soil solution could promote an exchange reaction with exchangeable metallic cations adsorbed onto colloids’ surfaces, thus releasing them in soil solution. The study conducted by Najafi and Jalali [32] investigated the differential influence of pH on the solubility of Ca, Mg, and K. The research focused on the specific mechanisms governing the binding of these elements and their competition with protons. The results suggest a higher sensitivity to pH changes for Ca and Mg compared to K. This is probably due to the lower competition with protons for K compared to Ca and Mg [33]. This is in accordance with our results; indeed, the increase in the amount of Ca and Mg compared to K is more pronounced with the decrease in pH.
Another cause for the increase in metals availability could be the addition of organic matter by CWWs. The supply of fresh organic matter stimulates microbial activity [10], thus producing organic acids that enhance metal solubility under acidic conditions [34,35]. Moreover, with the increase in organic matter by the addition of CWWs, metals may form soluble complexes with organic functional groups, thus becoming more available [36,37].
The mechanisms involved in retaining metals by organic matter seem to encompass both complexation and adsorption. In other words, inner sphere reactions may occur along with ion exchange [38]. This duality is reflected in the literature, where some authors describe metal–organic matter interactions in terms of ion exchange and complexation [39,40]. Functional groups, such as phenol, carboxyl(-ate), and amino groups, are active components in metal binding [41].
However, given that metals availability generally increased by decreasing the dilution of CWWs and that the CWWs’ dilution had a negligible effect on their pH, this suggests that there is another factor besides pH that influences metal availability. Such an other factor is likely the quantity and type of organic acids added by CWWs. Metal complexation by organic acids (acetic, ascorbic, citric, malic, oxalic, and tartaric acids) commonly found in CWWs may be predicted from complexation constants (Table 6). The latter depends on the type of organic acid (mono-, di-, and tricarboxylic) and on the oxidation state of the metal.
For example, considering citric acid, a tricarboxylic acid, the complexation constant increases by increasing the metal oxidation state; Na and K, with an oxidation state of +1, have a lower complexation constant compared to metals with an oxidation state of +2, such as Ca, Mg, Cu, Zn, Mn, Ni, and Cd. The highest complexation constants are found with metals having an oxidation state of +3, namely Al and Fe. On the other hand, the complexation constants between acetic acid (monocarboxylic acid) and metals are only slightly affected by metals’ oxidation state, falling within the range of 1.18–1.92.
Therefore, the type and quantity of organic acids may affect the availability of some metals in soil [42,43,44]. Indeed, in this study, by applying LWW, which has the highest content of organic acids, particularly citric acid, the concentration of water-soluble metals increases; therefore, this concentration is higher than that found when OWW and TWW are applied.
CWWs’ dilution influenced the availability of metals not because of the low amounts of metals they supplied, but more likely because of the different quantity of organic acids added. Indeed, the concentration of metals in CWWs was not so high as to justify their increase after the addition. On the other hand, the quantity of organic acids added to the soil depended on CWWs’ dilution. Among the organic acids within CWWs, the most abundant was citric acid, in the following order: LWW > OWW > TWW. Citric acid, being tricarboxylic, is very able to complex metals in the soil. The resulting citrate salts are soluble in water, being more soluble at low value of pH [44,45].
Thus, within the following 7 days after the addition of CWWs to soil, the concentration of almost all metals in water increased. Thereafter, when pH started to increase, the water-soluble fraction of most metals decreased; however, it remained equal to or greater than the control.
Instead, the concentrations of Al, Fe, Cu, and Zn were, after day 7, lower than the control, regardless of the type and dilution of CWWs. Such results can be due to the fate of the organo–metal complex. Citric acid, among the organic acids, exhibits the highest complexation constants for Al and Fe (log K = 7.87 and 11.2, respectively, Martell and Smith [46]). According to findings by Terzano et al. [20] and Huang and Keller [47], the stability of organic aluminum complexes persists in solution until the organo–metal complex undergoes disruption through microbial degradation. In our study, such a hypothesis is supported by the results from Ioppolo et al. [10], who observed that shortly after the addition of CWWs, there was an increase in both the quantity and the activity (soil respiration) of soil microorganisms. Finally, the rise in pH due to soil buffer capacity led to the formation of aluminum and iron hydroxides, which co-precipitated with copper and zinc [48,49].


Table 6.
Stability constants (log K) of main organic acids present in CWWs and metals. Reported values are derived from Martell and Smith [46,50,51] a, Zelanina and Zelinin [52] b, Chandrathilaka et al. [53] c, Kim et al. [17] d, and Zhang et al. [54] e. Experimental conditions are not always the same.
Table 6.
Stability constants (log K) of main organic acids present in CWWs and metals. Reported values are derived from Martell and Smith [46,50,51] a, Zelanina and Zelinin [52] b, Chandrathilaka et al. [53] c, Kim et al. [17] d, and Zhang et al. [54] e. Experimental conditions are not always the same.
| Organic Acid | ||||||
|---|---|---|---|---|---|---|
| Metal | Citric | Ascorbic | Oxalic | Tartaric | Acetic | Malic |
| Ca | 3.18–3.50 a | 1.66–3.00 a | 0.45–1.18 a | 1.67–2.72 a | ||
| Mg | 3.27–3.37 a | 2.76–3.43 a | 0.50–1.27 a | 1.42–1.71 a | ||
| K | 0.59 a–0.84 b | 0.48 b | 0.11–0.4 a | |||
| Na | 0.89b | 1.06 b | 0.17–0.29 a | |||
| Al | 7.87 a | 3.86 c | 11.09 a | 1.51 a | ||
| Fe | 11.2 a | 7.59 a | 1.40 a | |||
| Cu | 5.90 d | 7.56 c | 4.84 d | 1.89 d | 3.33–3.60 a | |
| Zn | 4.98 d –5.90 a | 3.88 d | 3.31 a | 1.10 d–1.57 a | ||
| Mn | 4.15 a | 3.35 e | 1.94 e | 1.40 e | ||
| Ni | 5.35 a | 4.69 e | 2.41 e | 1.44 e | ||
| Cd | 4.54 a | 3.74 c | 3.35 e | 2.15 e | 1.92 e | |
Thus, at the beginning of the experiment, the concentration of metals, especially aluminum, was notably high, favoring strong complexation by citrate. However, at the end of the experiment, the concentrations of aluminum, iron, zinc, and copper were lower than the control, suggesting the formation of insoluble co-precipitates due to the degradation of organo–metal complexes and the pH increase. This phenomenon is more pronounced with the application of LWW, which contain a higher concentration of citric acid compared to OWW and TWW.
5. Conclusions
The guiding principle of the circular economy model, focusing on resource recovery from wastes while reducing their disposal and encouraging the development of products with added value, encompasses the reuse of wastewater. In the present study, it was found that the use of citrus wastewater for irrigation purposes could be a valid solution, with them being rich in plant macronutrients and easily mobilizing micronutrients such as Fe, Cu, Mn, and Zn.
While the characteristics of CWWs adhered to criteria for agricultural irrigation suitability, the pH values, although outside commonly accepted standards, demonstrated high stability despite the dilution of CWW.
When applied to soil, CWWs contribute to the mobilization of metals, thus increasing their potential bioavailability. The presence of organic acids, particularly citric acid, has shown a significant influence on metal complexation in the soil, leading to the formation of soluble complexes. However, the decrease in concentrations of some metals at the end of the experiment suggests the formation of insoluble co-precipitates of Al and Fe with Cu and Zn.
Furthermore, citric acid in CWWs initially complexes with metals in the soil, followed by subsequent release due to microbial degradation, which is consistent with the literature. The generally low pH of CWWs, once added to soil, may be easily counterbalanced by the native soil buffer capacity. However, the temporary drop in soil pH may be useful to aid in the plant uptake of some minor nutrients such as iron in calcareous soils.
This study contributes significantly to understanding the impacts of industrial wastewaters on the agricultural environment and emphasizes the importance of carefully considering their composition and soil dynamics in implementing sustainable irrigation practices. Further research is needed to refine the understanding of such impacts in the long term and to develop targeted strategies for managing industrial wastewater in agriculture.
Author Contributions
Conceptualization, V.A.L. and E.P.; methodology, V.A.L. and E.P.; formal analysis, D.P. and A.I.; investigation, D.P. and A.I.; data curation, D.P. and A.I.; writing—original draft preparation, V.A.L., F.S., E.P., D.P. and A.I.; writing—review and editing, V.A.L., F.S., E.P., D.P., L.B. and A.I.; supervision, V.A.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors thank Anna Micalizzi for her support during soil analyses.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mortimore, M.; Anderson, S.; Cotula, L.; Davies, J.; Faccer, K.; Hesse, C.; John Morton, J.; Nyangena, W.; Skinner, J.; Wolfangel, C. Dryland Opportunities: A New Paradigm for People, Ecosystems and Development; IUCN: Gland, Switzerland, 2009; p. 86. [Google Scholar]
- Modarres, R.; da Silva, V.R. Rainfall Trends in Arid and Semi-Arid Regions of Iran. J. Arid Environ. 2007, 70, 344–355. [Google Scholar] [CrossRef]
- Kulkarni, S.J. Recycle and Reuse of Water-A Review. Int. J. Res. 2014, 1, 802–805. [Google Scholar]
- FAO. Citrus Fruit Fresh and Processed Statistical Bulletin 2020; FAO: Roma, Italy, 2021. [Google Scholar]
- Lucia, C.; Laudicina, V.A.; Badalucco, L.; Galati, A.; Palazzolo, E.; Torregrossa, M.; Viviani, G.; Corsino, S.F. Challenges and opportunities for citrus wastewater management and valorisation: A review. J. Environ. Manag. 2022, 321, 115924. [Google Scholar] [CrossRef] [PubMed]
- Corsino, S.F.; Di Trapani, D.; Capodici, M.; Torregrossa, M.; Viviani, G. Optimization of acetate production from citrus wastewater fermentation. Water Resour. Ind. 2021, 25, 100140. [Google Scholar] [CrossRef]
- Lee, W.S.; Chua, A.S.M.; Yeoh, H.K.; Ngoh, G.C. A review of the production and applications of waste-derived volatile fatty acids. Chem. Eng. J. 2014, 235, 83–99. [Google Scholar] [CrossRef]
- Ungureanu, N.; Vlăduț, V.; Dincă, M.; Zăbavă, B.Ș. Reuse of wastewater for irrigation, a sustainable practice in arid and semi-arid regions. In Proceedings of the 7th International Conference on Thermal Equipment, Renewable Energy and Rural Development (TE-RE-RD), Drobeta-Turnu Severin, Romania, 31 May–2 June 2018; pp. 379–384. [Google Scholar]
- Zema, D.A.; Bombino, G.; Andiloro, S.; Zimbone, S.M. Irrigation of energy crops with urban wastewater: Effects on biomass yields, soils and heating values. Agric. Water Manag. 2012, 115, 55–65. [Google Scholar] [CrossRef]
- Ioppolo, A.; Laudicina, V.A.; Badalucco, L.; Saiano, F.; Palazzolo, E. Wastewaters from citrus processing industry as natural biostimulants for soil microbial community. J. Environ. Manag. 2020, 273, 111137. [Google Scholar] [CrossRef]
- Bastida, F.; Torres, I.F.; Abadía, J.; Romero-Trigueros, C.; Ruiz-Navarro, A.; Alarcón, J.J.; García, C.; Nicolás, E. Comparing the impacts of drip irrigation by freshwater and reclaimed wastewater on the soil microbial community of two citrus species. Agric. Water Manag. 2018, 203, 53–62. [Google Scholar] [CrossRef]
- Corsino, S.F.; Di Trapani, D.; Torregrossa, M.; Viviani, G. Aerobic granular sludge treating high strength citrus wastewater: Analysis of pH and organic loading rate effect on kinetics, performance and stability. J. Environ. Manag. 2018, 214, 23–35. [Google Scholar] [CrossRef]
- Yadav, K.K.; Gupta, N.; Kumar, A.; Reece, L.M.; Singh, N.; Rezania, S.; Khan, S.A. Mechanistic understanding and holistic approach of phytoremediation: A review on application and future prospects. Ecol. Eng. 2018, 120, 274–298. [Google Scholar] [CrossRef]
- Gupta, N.; Yadav, K.K.; Kumar, V.; Kumar, S.; Chadd, R.P.; Kumar, A. Trace elements in soil-vegetables interface: Translocation, bioaccumulation, toxicity and amelioration—A review. Sci. Total Environ. 2019, 6512, 2927–2942. [Google Scholar] [CrossRef] [PubMed]
- Sheoran, V.; Sheoran, A.S.; Poonia, P. Factors affecting phytoextraction: A review. Pedosphere 2016, 26, 148–166. [Google Scholar] [CrossRef]
- Kicińska, A.; Pomykała, R.; Izquierdo-Diaz, M. Changes in soil pH and mobility of heavy metals in contaminated soils. Eur. J. Soil Sci. 2022, 73, 13203. [Google Scholar] [CrossRef]
- Kim, J.O.; Lee, Y.W.; Chung, J. The role of organic acids in the mobilization of heavy metals from soil. KSCE J. Civ. Eng. 2013, 17, 1596–1602. [Google Scholar] [CrossRef]
- Huang, W.H.; Keller, W.D. Organic acids as agents of chemical weathering of silicate minerals. Nat. Phys. Sci. 1972, 239, 149–151. [Google Scholar] [CrossRef]
- Boudot, J.P.; Bel, H.; Brahim, A.; Steiman, R.; Seigle-Murandi, F. Biodegradation of Synthetic Organo-Metallic Complexes of Iron and Aluminum with Selected Metal to Carbon Ratios. Soil Biol. Biochem. 1989, 21, 961–966. [Google Scholar] [CrossRef]
- Terzano, R.; Cuccovillo, G.; Pascazio, S.; Crecchio, C.; Lettino, A.; Fiore, S.; Tomasi, N.; Pinton, R.; Mimmo, T.; Cesco, S. Degradation of citrate promotes copper co-precipitation within aluminium-hydroxides in calcareous soils. Biol. Fertil. Soils 2017, 53, 115–128. [Google Scholar] [CrossRef]
- Walkley, A.; Black, C.A. An examination of degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–83. [Google Scholar] [CrossRef]
- Bremner, J.M. Nitrogen—Total. In Methods of Soil Analysis. Part 3, Chemical Methods; Sparks, D.L., Ed.; SSSA Book Series No. 5; Soil Science Society of America & American Society of Agronomy: Madison, WI, USA, 1996; pp. 1085–1121. [Google Scholar]
- Hedge, J.E.; Hofreiter, B.T. Carbohydrate Chemistry 17; Whistler, R.L., Be Miller, J.N., Eds.; Academic Press: New York, NY, USA, 1962. [Google Scholar]
- Yebra, M.C.; Gallego, M.; Valcárcel, M. Automatic determination of reducing sugars by atomic absorption spectrometry. Anal. Chim. Acta 1993, 276, 385–391. [Google Scholar] [CrossRef]
- Prosky, L.; Asp, N.G.; Schweizer, T.F.; Devries, J.W.; Furda, I. Determination of insoluble, soluble, and total dietary fiber in foods and Food products: Interlaboratory study. J. Assoc. Off. Anal. Chem. 1988, 71, 5. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Zaman, M.; Shahid, S.A.; Heng, L. Irrigation water quality. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; p. 5. [Google Scholar]
- Jones, D.L.; Dennis, P.G.; Owen, A.G.; van Hees, P.A.W. Organic acid behaviour in soils-misconceptions and knowledge gaps. Plant Soil 2003, 248, 31–41. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soil, 14th ed.; Prentice-Hall: Upper Saddle River, NJ, USA, 2008. [Google Scholar]
- Regulation EU 2020/741, 2020. Regulation EU 2020/741, Minimum Requirements for Water Reuse. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020R0741 (accessed on 28 February 2024).
- Mekki, A.; Dhouib, A.; Sayadi, S. Review: Effects of olive mill wastewater application on soil properties and plants growth. Int. J. Recycl. Org. Waste Agric. 2013, 2, 15. [Google Scholar] [CrossRef]
- Najafi, S.; Jalali, M. Effect of heavy metals on pH buffering capacity and solubility of Ca, Mg, K, and P in non-spiked and heavy metal-spiked soils. Environ. Monit. Assess 2016, 1886, 342. [Google Scholar] [CrossRef] [PubMed]
- Almas, A.R.; Lofts, S.; Mulder, J.; Tipping, E. Solubility of major cations and Cu, Zn and Cd in soil extracts of some contaminated agricultural soils near a zinc smelter in Norway: Modelling with a multi surface extension of WHAM. Eur. J. Soil Sci. 2007, 58, 1074–1086. [Google Scholar] [CrossRef]
- Uroz, S.; Oger, P.; Lepleux, C.; Collignon, C.; Frey-Klett, P.; Turpault, M.P. Bacterial weathering and its contribution to nutrient cycling in temperate forest ecosystems. Res. Microbiol. 2011, 1629, 820–831. [Google Scholar] [CrossRef] [PubMed]
- Kirchman, D.L. Microbial growth, biomass production, and controls. In Processes in Microbial Ecology, 2nd ed.; Oxford University Press: New York, NY, USA, 2018; pp. 133–153. [Google Scholar]
- Mortensen, J.L. Complexing of Metals by Soil Organic Matter. Soil Sci. Soc. Am. J. 1963, 272, 179–186. [Google Scholar] [CrossRef]
- Rieuwerts, J.S.; Thornton, I.; Farago, M.E.; Ashmore, M.R. Factors influencing metal bioavailability in soils: Preliminary investigations for the development of a critical loads approach for metals. Chem. Speciat. Bioavailab. 1998, 102, 61–75. [Google Scholar] [CrossRef]
- Evans, L.J. Chemistry of metal retention by soils. Environ. Sci. Technol. 1989, 23, 1046–1056. [Google Scholar] [CrossRef]
- Borggaard, O.K.; Holm, P.E.; Strobel, B.W. Potential of dissolved organic matter DOM to extract As, Cd, Co, Cr, Cu, Ni, Pb and Zn from polluted soils: A review. Geoderma 2019, 343, 235–246. [Google Scholar] [CrossRef]
- Liu, X.; Tournassat, C.; Grangeon, S.; Kalinichev, A.; Takahashi, Y.; Fernandes, M. Molecular-level understanding of metal ion retention in clay-rich materials. Nat. Rev. Earth. Environ. 2022, 3, 461–476. [Google Scholar] [CrossRef]
- Javanbakht, V.; Alavi, S.A.; Zilouei, H. Mechanisms of heavy metal removal using microorganisms as biosorbent. Water Sci.Technol. 2014, 69, 1775–1787. [Google Scholar] [CrossRef] [PubMed]
- Renella, G.; Landi, L.; Nannipieri, P. Degradation of low molecular weight organic acids complexed with heavy metals in soil. Geoderma 2004, 122, 311–315. [Google Scholar] [CrossRef]
- Schwab, A.P.; Zhu, D.S.; Banks, M.K. Influence of organic acids on the transport of heavy metals in soil. Chemosphere 2008, 72, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Adeleke, R.; Nwangburuka, C.; Oboirien, B. Origins, roles and fate of organic acids in soils: A review. S. Afr. J. Bot. 2017, 108, 393–406. [Google Scholar] [CrossRef]
- Goss, S.L.; Lemons, K.A.; Kerstetter, J.E.; Bogner, R.H. Determination of calcium salt solubility with changes in pH and PCO2, simulating varying gastrointestinal environments. J. Pharm. Pharmacol. 2007, 59, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.M.; Martell, A.E. Critical Stability Constants; Plenum Press: New York, NY, USA, 1974; Volume 1, p. 135. [Google Scholar]
- Huang, W.H.; Keller, W.D. Geochemical mechanisms for the dissolution, transport, and deposition of aluminum in the zone of weathering. Clay Clay Min. 1972, 20, 69–74. [Google Scholar] [CrossRef]
- McBride, M.B. Cu2+ adsorption characteristics of aluminum hydroxide and oxyhydroxides. Clay Clay Min. 1982, 30, 21–28. [Google Scholar] [CrossRef]
- Martinez, C.E.; McBride, M.B. Aging of coprecipitated Cu in alumina: Changes in structural location, chemical form, and solubility. Geochim. Cosmochim. Acta 2000, 64, 1729–1736. [Google Scholar] [CrossRef]
- Smith, R.M.; Martell, A.E. Critical Stability Constants; Plenum Press: New York, NY, USA, 1977; Volume 3, p. 512. [Google Scholar]
- Smith, R.M.; Martell, A.E. Critical Stability Constants; Plenum Press: New York, NY, USA, 1989; Volume 6, p. 643. [Google Scholar]
- Zelenina, T.E.; Zelenin, O.Y. Complexation of citric and tartaric acids with Na and K ions in aqueous solution. Russ. J. Coord. Chem. 2005, 31, 235–242. [Google Scholar] [CrossRef]
- Chandrathilaka, A.M.D.S.; Ileperuma, O.A.; Hettiarachchi, C.V. Spectrophotometric and pH-metric studies on Pb(II), Cd(II), Al(III) and Cu(II) complexes of paracetamol and ascorbic acid. J. Natl. Sci. Found. 2013, 41, 337–344. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, R.; Lu, T.; Qi, W.; Zhu, Y.; Lu, M.; Qi, Z.; Chen, W. Enhanced transport of heavy metal ions by low-molecular-weight organic acids in saturated porous media: Link complex stability constants to heavy metal mobility. Chemosphere 2022, 290, 133339. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).