Microplastics in the Danube River and Its Main Tributaries—Ingestion by Freshwater Macroinvertebrates
Abstract
1. Introduction
2. Materials and Methods
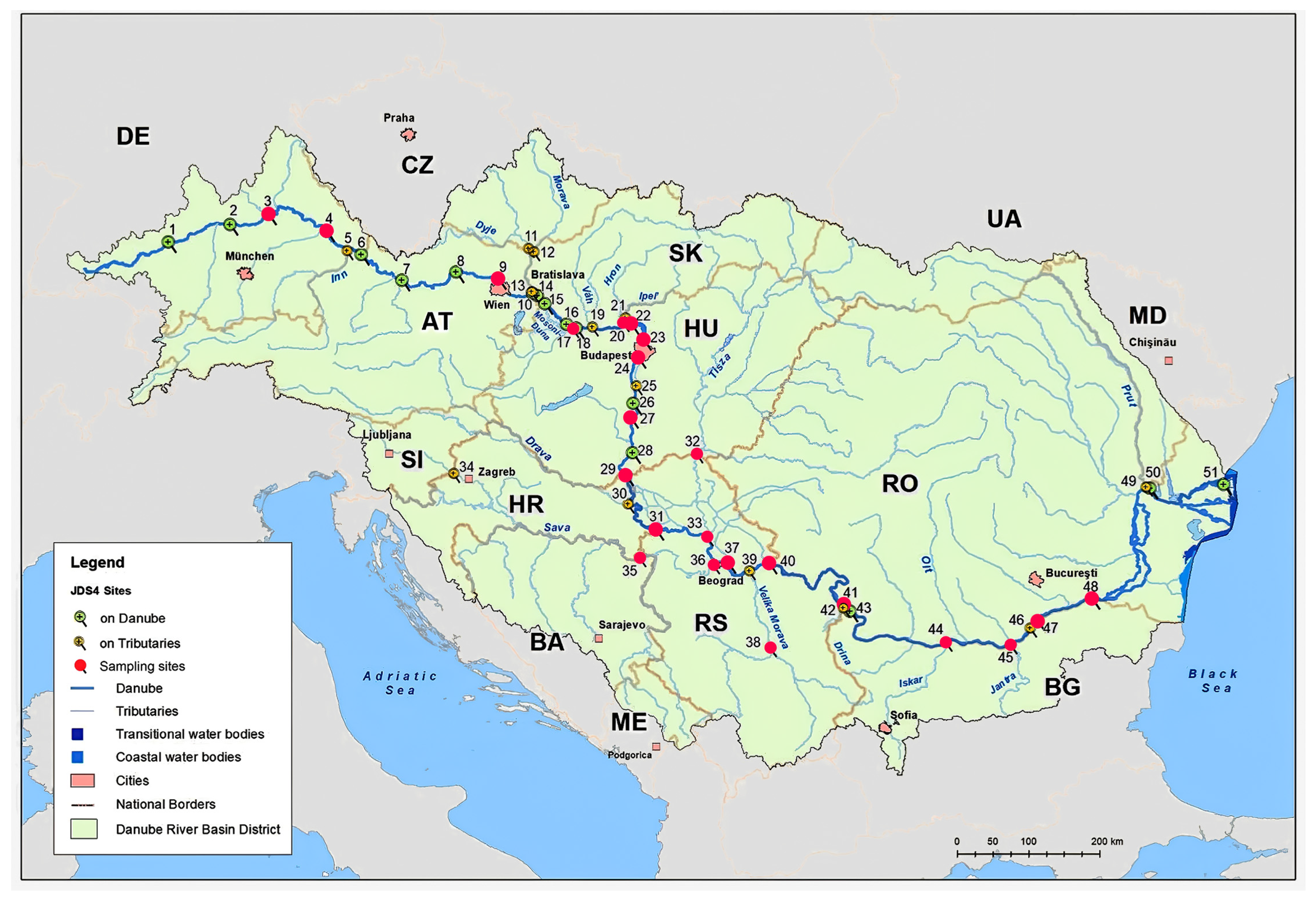
2.1. Sampling Sites and Procedure
2.2. Preparation of the Samples for MP Isolation
2.3. Isolation of MPs
2.4. Particle Analysis
2.5. Micro-Fourier Transform Infrared Spectroscopy (µFTIR)
2.6. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hale, R.C.; Seeley, M.E.; La Guardia, M.J.; Mai, L.; Zeng, E.Y. A Global Perspective on Microplastics. J. Geophys. Res. Ocean. 2020, 125, e2018JC014719. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the Marine Environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Lambert, S.; Wagner, M. Microplastics Are Contaminants of Emerging Concern in Freshwater Environments: An Overview. In Freshwater Microplastics: Emerging Environmental Contaminants; Wagner, M., Lambert, S., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–23. ISBN 978-3-319-61615-5. [Google Scholar]
- Gregory, M.R.; Andrady, A.L. Plastics in the Marine Environment. In Plastics and the Environment; John Wiley & Sons: Hoboken, NJ, USA, 2003; pp. 379–401. ISBN 9780471721550. [Google Scholar]
- Bowmer, T.; Kershaw, P.J. (Eds.) GESAMP. In Proceedings of the GESAMP International Workshop on Plastic Particles as a Vector in Transporting Persistent, Bio-Accumulating and Toxic Substances in the Oceans, Paris, France, 28–30 June 2010; GESAMP Reports & Studies No. 82. pp. 1–68. [Google Scholar]
- Lebreton, L.C.M.; van der Zwet, J.; Damsteeg, J.-W.; Slat, B.; Andrady, A.; Reisser, J. River Plastic Emissions to the World’s Oceans. Nat. Commun. 2017, 8, 15611. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Everaert, G.; Van Cauwenberghe, L.; De Rijcke, M.; Koelmans, A.A.; Mees, J.; Vandegehuchte, M.; Janssen, C.R. Risk Assessment of Microplastics in the Ocean: Modelling Approach and First Conclusions. Environ. Pollut. 2018, 242, 1930–1938. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, R.M.; Jovanović, B. From Nanoplastic to Microplastic: A Bibliometric Analysis on the Presence of Plastic Particles in the Environment. Mar. Pollut. Bull. 2021, 163, 111926. [Google Scholar] [CrossRef] [PubMed]
- Katare, Y.; Singh, P.; Sankhla, M.S.; Singhal, M.; Jadhav, B.E.; Parihar, K.; Nikalje, B.T.; Trpathi, A.; Bhardwaj, L. Microplastics in Aquatic Environments: Sources, Ecotoxicity, Detection & Remediation. Biointerface Res. Appl. Chem. 2022, 12, 3407–3428. [Google Scholar]
- Dris, R.; Gasperi, J.; Rocher, V.; Saad, M.; Renault, N.; Tassin, B. Microplastic Contamination in an Urban Area: A Case Study in Greater Paris. Environ. Chem. 2015, 12, 592–599. [Google Scholar] [CrossRef]
- Lechner, A.; Keckeis, H.; Lumesberger-Loisl, F.; Zens, B.; Krusch, R.; Tritthart, M.; Glas, M.; Schludermann, E. The Danube so Colourful: A Potpourri of Plastic Litter Outnumbers Fish Larvae in Europe’s Second Largest River. Environ. Pollut. 2014, 188, 177–181. [Google Scholar] [CrossRef]
- Morritt, D.; Stefanoudis, P.V.; Pearce, D.; Crimmen, O.A.; Clark, P.F. Plastic in the Thames: A River Runs through It. Mar. Pollut. Bull. 2014, 78, 196–200. [Google Scholar] [CrossRef]
- Eriksen, M.; Mason, S.; Wilson, S.; Box, C.; Zellers, A.; Edwards, W.; Farley, H.; Amato, S. Microplastic Pollution in the Surface Waters of the Laurentian Great Lakes. Mar. Pollut. Bull. 2013, 77, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, P.L.; Norris, T.; Ceccanese, T.; Walzak, M.J.; Helm, P.A.; Marvin, C.H. Hidden Plastics of Lake Ontario, Canada and Their Potential Preservation in the Sediment Record. Environ. Pollut. 2015, 204, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Biginagwa, F.J.; Mayoma, B.S.; Shashoua, Y.; Syberg, K.; Khan, F.R. First Evidence of Microplastics in the African Great Lakes: Recovery from Lake Victoria Nile Perch and Nile Tilapia. J. Great Lakes Res. 2016, 42, 146–149. [Google Scholar] [CrossRef]
- Imhof, H.K.; Ivleva, N.P.; Schmid, J.; Niessner, R.; Laforsch, C. Contamination of Beach Sediments of a Subalpine Lake with Microplastic Particles. Curr. Biol. 2013, 23, R867–R868. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Worch, E.; Knepper, T.P. Occurrence and Spatial Distribution of Microplastics in River Shore Sediments of the Rhine-Main Area in Germany. Environ. Sci. Technol. 2015, 49, 6070–6076. [Google Scholar] [CrossRef]
- Zbyszewski, M.; Corcoran, P.L. Distribution and Degradation of Fresh Water Plastic Particles Along the Beaches of Lake Huron, Canada. Water Air Soil Pollut. 2011, 220, 365–372. [Google Scholar] [CrossRef]
- Gregory, M.R. Environmental Implications of Plastic Debris in Marine Settings—Entanglement, Ingestion, Smothering, Hangers-on, Hitch-Hiking and Alien Invasions. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2009, 364, 2013–2025. [Google Scholar] [CrossRef] [PubMed]
- Masó, M.; Garcés, E.; Pagès, F.; Camp, J. Drifting Plastic Debris as a Potential Vector for Dispersing Harmful Algal Bloom (HAB) Species. Sci. Mar. 2003, 67, 107–111. [Google Scholar] [CrossRef]
- McCormick, A.; Hoellein, T.J.; Mason, S.A.; Schluep, J.; Kelly, J.J. Microplastic Is an Abundant and Distinct Microbial Habitat in an Urban River. Environ. Sci. Technol. 2014, 48, 11863–11871. [Google Scholar] [CrossRef]
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the “Plastisphere”: Microbial Communities on Plastic Marine Debris. Environ. Sci. Technol. 2013, 47, 7137–7146. [Google Scholar] [CrossRef]
- Sommerwerk, N.; Hein, T.; Schneider-Jacoby, M.; Baumgartner, C.; Ostojić, A.; Siber, R.; Bloesch, J.; Paunović, M.; Tockner, K. Chapter 3—The Danube River Basin. In Rivers of Europe; Tockner, K., Uehlinger, U., Robinson, C.T., Eds.; Academic Press: London, UK, 2009; pp. 59–112. ISBN 978-0-12-369449-2. [Google Scholar]
- Scherer, C.; Brennholt, N.; Reifferscheid, G.; Wagner, M. Feeding Type and Development Drive the Ingestion of Microplastics by Freshwater Invertebrates. Sci. Rep. 2017, 7, 17006. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bao, L.; Wei, Y.; Zhao, W.; Wang, F.; Liu, X.; Su, H.; Zhang, R. Occurrence, Bioaccumulation, and Risk Assessment of Microplastics in the Aquatic Environment: A Review. Water 2023, 15, 1768. [Google Scholar] [CrossRef]
- Foekema, E.M.; De Gruijter, C.; Mergia, M.T.; van Franeker, J.A.; Murk, A.J.; Koelmans, A.A. Plastic in North Sea Fish. Environ. Sci. Technol. 2013, 47, 8818–8824. [Google Scholar] [CrossRef]
- Horton, A.A.; Jürgens, M.D.; Lahive, E.; van Bodegom, P.M.; Vijver, M.G. The Influence of Exposure and Physiology on Microplastic Ingestion by the Freshwater Fish Rutilus Rutilus (Roach) in the River Thames, UK. Environ. Pollut. 2018, 236, 188–194. [Google Scholar] [CrossRef]
- Setälä, O.; Norkko, J.; Lehtiniemi, M. Feeding Type Affects Microplastic Ingestion in a Coastal Invertebrate Community. Mar. Pollut. Bull. 2016, 102, 95–101. [Google Scholar] [CrossRef]
- Bour, A.; Haarr, A.; Keiter, S.; Hylland, K. Environmentally Relevant Microplastic Exposure Affects Sediment-Dwelling Bivalves. Environ. Pollut. 2018, 236, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Sfriso, A.A.; Tomio, Y.; Rosso, B.; Gambaro, A.; Sfriso, A.; Corami, F.; Rastelli, E.; Corinaldesi, C.; Mistri, M.; Munari, C. Microplastic Accumulation in Benthic Invertebrates in Terra Nova Bay (Ross Sea, Antarctica). Environ. Int. 2020, 137, 105587. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Liu, Q.; Jiang, R.; Li, W.; Sun, X.; Lin, H.; Jiang, S.; Huang, H. Microplastic Pollution and Ecological Risk Assessment in an Estuarine Environment: The Dongshan Bay of China. Chemosphere 2021, 262, 127876. [Google Scholar] [CrossRef]
- Bertoli, M.; Pastorino, P.; Lesa, D.; Renzi, M.; Anselmi, S.; Prearo, M.; Pizzul, E. Microplastics Accumulation in Functional Feeding Guilds and Functional Habit Groups of Freshwater Macrobenthic Invertebrates: Novel Insights in a Riverine Ecosystem. Sci. Total Environ. 2022, 804, 150207. [Google Scholar] [CrossRef]
- Nel, H.A.; Froneman, P.W. Presence of Microplastics in the Tube Structure of the Reef-Building Polychaete Gunnarea Gaimardi (Quatrefages 1848). Afr. J. Mar. Sci. 2018, 40, 87–89. [Google Scholar] [CrossRef]
- Windsor, F.M.; Durance, I.; Horton, A.A.; Thompson, R.C.; Tyler, C.R.; Ormerod, S.J. A Catchment-Scale Perspective of Plastic Pollution. Glob. Change Biol. 2019, 25, 1207–1221. [Google Scholar] [CrossRef] [PubMed]
- Yıldız, D.; Yalçın, G.; Jovanović, B.; Boukal, D.S.; Vebrová, L.; Riha, D.; Stanković, J.; Savić-Zdraković, D.; Metin, M.; Akyürek, Y.N.; et al. Effects of a Microplastic Mixture Differ across Trophic Levels and Taxa in a Freshwater Food Web: In Situ Mesocosm Experiment. Sci. Total Environ. 2022, 836, 155407. [Google Scholar] [CrossRef] [PubMed]
- Paunović, M.; Csányi, B.; Simonović, P.; Zorić, K. Invasive Alien Species in the Danube. In The Danube River Basin; Liska, I., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 389–409. ISBN 978-3-662-47739-7. [Google Scholar]
- Li, J.; Green, C.; Reynolds, A.; Shi, H.; Rotchell, J.M. Microplastics in Mussels Sampled from Coastal Waters and Supermarkets in the United Kingdom. Environ. Pollut. 2018, 241, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Paunović, M.; Csányi, B.; Knežević, S.; Simić, V.; Nenadić, D.; Jakovčev-Todorović, D.; Stojanović, B.; Cakić, P. Distribution of Asian Clams Corbicula Fluminea (Müller, 1774) and C. Fluminalis (Müller, 1774) in Serbia. Aquat. Invasions 2007, 2, 99–106. [Google Scholar] [CrossRef]
- Rodriguez, P.; Reynoldson, T.B. The Pollution Biology of Aquatic Oligochaetes, 1st ed.; Springer: Dordrecht, The Netherlands, 2011; pp. 225–261. ISBN 978-94-007-1717-6. [Google Scholar]
- Atanacković, A.D.; Šporka, F.; Csányi, B.; Vasiljević, B.M.; Tomović, J.M.; Paunović, M.M. Oligochaeta of the Danube River—A Faunistical Review. Biologia 2013, 68, 269–277. [Google Scholar] [CrossRef]
- Pinder, L.C. V Biology of Freshwater Chironomidae. Annu. Rev. Entomol. 1986, 31, 1–23. [Google Scholar] [CrossRef]
- Stanković, J.; Milošević, D.; Savić-Zdraković, D.; Yalçın, G.; Yildiz, D.; Beklioğlu, M.; Jovanović, B. Exposure to a Microplastic Mixture Is Altering the Life Traits and Is Causing Deformities in the Non-Biting Midge Chironomus Riparius Meigen (1804). Environ. Pollut. 2020, 262, 114248. [Google Scholar] [CrossRef] [PubMed]
- AQEM. Consortium Manual for the Application of the AQUEM System. A Compr. Method Assess Eur. Streams Using Benthic Macroinvertebrates Dev. Purp. Water Framew. Dir. 2002, 1, 202. [Google Scholar]
- EN 27828:1994; Water Quality—Methods of Biological Sampling—Guidance on Handnet Sampling of Aquatic Benthic MacroInvertebrates (ISO 7828:1985). European Committee for Standardisation: Brussels, Belgium, 1994.
- Liška, I.; Wagner, F.; Sengl, M.; Deutsch, K.; Slobodník, J. Joint Danube Survey 3: A Comprehensive Analysis of Danube Water Quality; International Commission for the Protection of the Danube River (ICPDR): Vienna, Austria, 2015; pp. 1–369. [Google Scholar]
- Moller Pillot, H.K.M. De Larven Der Nederlandse Chironomiae (Diptera). (Inleiding, Tanypodinae En Chironominitle). Ned. Faun. Meded. 1984, 1A, 1–277. [Google Scholar]
- Moller Pillot, H.K.M. De Larven Der Nederlandse Chironomiae (Diptera) (Orthocladiinae Sensu Lato). Ned. Faun. Meded. 1984, 1B, 1–175. [Google Scholar]
- Vallenduuk, H.J.; Moller Pillot, H.K.M. Chironomidae Larvae of the Netherlands and Adjacent Lowlands: General Ecology and Tanypodinae; Chironomidae Larvae of the Netherlands and Adjacent Lowlands; KNNV: Zeist, The Netherlands, 2007; ISBN 9789050112598. [Google Scholar]
- Pfleger, V. Molluscs; Field guide in colour to; Silverdale Books: Devon, UK, 2000; ISBN 9781856054492. [Google Scholar]
- Timm, T. A Guide to the Freshwater Oligochaeta and Polychaeta of Northern and Central Europe. Lauterbornia 2009, 66, 1–235. Available online: https://www.researchgate.net/publication/285323193_A_guide_to_the_freshwater_Oligochaeta_and_Polychaeta_of_Northern_and_Central_Europe (accessed on 4 March 2024).
- Li, L.; Su, L.; Cai, H.; Rochman, C.M.; Li, Q.; Kolandhasamy, P.; Peng, J.; Shi, H. The Uptake of Microfibers by Freshwater Asian Clams (Corbicula Fluminea) Varies Based upon Physicochemical Properties. Chemosphere 2019, 221, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Webb, H.; Lindeque, P.K.; Fileman, E.S.; Halsband, C.; Galloway, T.S. Isolation of Microplastics in Biota-Rich Seawater Samples and Marine Organisms. Sci. Rep. 2014, 4, 4528. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the Marine Environment: A Review of the Methods Used for Identification and Quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Cai, H.; Kolandhasamy, P.; Wu, C.; Rochman, C.M.; Shi, H. Using the Asian Clam as an Indicator of Microplastic Pollution in Freshwater Ecosystems. Environ. Pollut. 2018, 234, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Güven, O.; Gökdağ, K.; Jovanović, B.; Kıdeyş, A.E. Microplastic Litter Composition of the Turkish Territorial Waters of the Mediterranean Sea, and Its Occurrence in the Gastrointestinal Tract of Fish. Environ. Pollut. 2017, 223, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Naji, A.; Nuri, M.; Vethaak, A.D. Microplastics Contamination in Molluscs from the Northern Part of the Persian Gulf. Environ. Pollut. 2018, 235, 113–120. [Google Scholar] [CrossRef]
- Hohenblum, P.; Liebmann, B.; Liedermann, M. Plastic and Microplastic in the Environmen; Report REP-0551; Umweltbundesamt GmbH: Vienna, Austria, 2015; ISBN 978-3-99004-363-9. Available online: https://www.umweltbundesamt.at/fileadmin/site/publikationen/rep0551.pdf (accessed on 4 March 2024).
- Hurley, R.R.; Woodward, J.C.; Rothwell, J.J. Ingestion of Microplastics by Freshwater Tubifex Worms. Environ. Sci. Technol. 2017, 51, 12844–12851. [Google Scholar] [CrossRef]
- Lin, C.-T.; Chiu, M.-C.; Kuo, M.-H. Effects of Anthropogenic Activities on Microplastics in Deposit-Feeders (Diptera: Chironomidae) in an Urban River of Taiwan. Sci. Rep. 2021, 11, 400. [Google Scholar] [CrossRef]
- Stanton, T.; Johnson, M.; Nathanail, P.; MacNaughtan, W.; Gomes, R.L. Freshwater and Airborne Textile Fibre Populations Are Dominated by ‘Natural’, Not Microplastic, Fibres. Sci. Total Environ. 2019, 666, 377–389. [Google Scholar] [CrossRef] [PubMed]
- TextileExchange. 2020 Annual Report. Texas, USA, 2020. pp. 1–7. Available online: http://textileexchange.org/ (accessed on 4 March 2024).
- Lu, G.; Xue, Q.; Ling, X.; Zheng, X. Toxic Interactions between Microplastics and the Antifungal Agent Ketoconazole in Sediments on Limnodrilus Hoffmeistteri. Process Saf. Environ. Prot. 2023, 172, 250–261. [Google Scholar] [CrossRef]
- Oliveira, P.; Barboza, L.G.A.; Branco, V.; Figueiredo, N.; Carvalho, C.; Guilhermino, L. Effects of Microplastics and Mercury in the Freshwater Bivalve Corbicula Fluminea (Müller, 1774): Filtration Rate, Biochemical Biomarkers and Mercury Bioconcentration. Ecotoxicol. Environ. Saf. 2018, 164, 155–163. [Google Scholar] [CrossRef]
- Rochman, C.M.; Parnis, J.M.; Browne, M.A.; Serrato, S.; Reiner, E.J.; Robson, M.; Young, T.; Diamond, M.L.; Teh, S.J. Direct and Indirect Effects of Different Types of Microplastics on Freshwater Prey (Corbicula Fluminea) and Their Predator (Acipenser Transmontanus). PLoS ONE 2017, 12, e0187664. [Google Scholar] [CrossRef]
- Silva, C.J.M.; Silva, A.L.P.; Gravato, C.; Pestana, J.L.T. Ingestion of Small-Sized and Irregularly Shaped Polyethylene Microplastics Affect Chironomus Riparius Life-History Traits. Sci. Total Environ. 2019, 672, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.J.M.; Beleza, S.; Campos, D.; Soares, A.M.V.M.; Patrício Silva, A.L.; Pestana, J.L.T.; Gravato, C. Immune Response Triggered by the Ingestion of Polyethylene Microplastics in the Dipteran Larvae Chironomus Riparius. J. Hazard. Mater. 2021, 414, 125401. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Xue, Y.; Li, L.; Yang, D.; Kolandhasamy, P.; Li, D.; Shi, H. Microplastics in Taihu Lake, China. Environ. Pollut. 2016, 216, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, A.K.; Spanjer, A.R.; Rosen, M.R.; Thom, T. Microplastics in Lake Mead National Recreation Area, USA: Occurrence and Biological Uptake. PLoS ONE 2020, 15, e0228896. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Du, F.; Li, L.; Li, B.; Li, J.; Shi, H. A Practical Approach Based on FT-IR Spectroscopy for Identification of Semi-Synthetic and Natural Celluloses in Microplastic Investigation. Sci. Total Environ. 2019, 669, 692–701. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Janssen, C.R. Microplastics in Bivalves Cultured for Human Consumption. Environ. Pollut. 2014, 193, 65–70. [Google Scholar] [CrossRef]
- Oehlmann, J.; Schulte-Oehlmann, U.; Kloas, W.; Jagnytsch, O.; Lutz, I.; Kusk, K.O.; Wollenberger, L.; Santos, E.M.; Paull, G.C.; Van Look, K.J.W.; et al. A Critical Analysis of the Biological Impacts of Plasticizers on Wildlife. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2047–2062. [Google Scholar] [CrossRef] [PubMed]
- Endo, S.; Takizawa, R.; Okuda, K.; Takada, H.; Chiba, K.; Kanehiro, H.; Ogi, H.; Yamashita, R.; Date, T. Concentration of Polychlorinated Biphenyls (PCBs) in Beached Resin Pellets: Variability among Individual Particles and Regional Differences. Mar. Pollut. Bull. 2005, 50, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Ogata, Y.; Takada, H.; Mizukawa, K.; Hirai, H.; Iwasa, S.; Endo, S.; Mato, Y.; Saha, M.; Okuda, K.; Nakashima, A.; et al. International Pellet Watch: Global Monitoring of Persistent Organic Pollutants (POPs) in Coastal Waters. 1. Initial Phase Data on PCBs, DDTs, and HCHs. Mar. Pollut. Bull. 2009, 58, 1437–1446. [Google Scholar] [CrossRef]
- Hirai, H.; Takada, H.; Ogata, Y.; Yamashita, R.; Mizukawa, K.; Saha, M.; Kwan, C.; Moore, C.; Gray, H.; Laursen, D.; et al. Organic Micropollutants in Marine Plastics Debris from the Open Ocean and Remote and Urban Beaches. Mar. Pollut. Bull. 2011, 62, 1683–1692. [Google Scholar] [CrossRef] [PubMed]
- Sousa, R.; Antunes, C.; Guilhermino, L. Ecology of the Invasive Asian Clam Corbicula Fluminea (Müller, 1774) in Aquatic Ecosystems: An Overview. Ann. Limnol. Int. J. Lim. 2008, 44, 85–94. [Google Scholar] [CrossRef]
- dos Santos, K.C.; Martinez, C.B.R. Genotoxic and Biochemical Effects of Atrazine and Roundup®, Alone and in Combination, on the Asian Clam Corbicula Fluminea. Ecotoxicol. Environ. Saf. 2014, 100, 7–14. [Google Scholar] [CrossRef]
- Cid, A.; Picado, A.; Correia, J.B.; Chaves, R.; Silva, H.; Caldeira, J.; de Matos, A.P.A.; Diniz, M.S. Oxidative Stress and Histological Changes Following Exposure to Diamond Nanoparticles in the Freshwater Asian Clam Corbicula Fluminea (Müller, 1774). J. Hazard. Mater. 2015, 284, 27–34. [Google Scholar] [CrossRef]



| Corbicula spp. | L. hoffmeisteri | P. nubeculosum | |
|---|---|---|---|
| TL ± SD | 14.23 ± 3.78 | 8.52 ± 6.17 | 5.56 ± 2.07 |
| BW ± SD | 340 ± 0.21 | 0.76 ± 0.88 | 0.45 ± 0.74 |
| Corbicula spp. | L. hoffmeisteri | P. nubeculosum | ||||
|---|---|---|---|---|---|---|
| Min | Max | Min | Max | Min | Max | |
| Fibres | 0.08 | 4.67 | 0.049 | 4.61 | 0.031 | 4.13 |
| Fragments | 0.02 | 3.22 | 0.018 | 0.288 | 0.016 | 0.0241 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanković, J.; Milošević, D.; Paunović, M.; Jovanović, B.; Popović, N.; Tomović, J.; Atanacković, A.; Radulović, K.; Lončarević, D.; Raković, M. Microplastics in the Danube River and Its Main Tributaries—Ingestion by Freshwater Macroinvertebrates. Water 2024, 16, 962. https://doi.org/10.3390/w16070962
Stanković J, Milošević D, Paunović M, Jovanović B, Popović N, Tomović J, Atanacković A, Radulović K, Lončarević D, Raković M. Microplastics in the Danube River and Its Main Tributaries—Ingestion by Freshwater Macroinvertebrates. Water. 2024; 16(7):962. https://doi.org/10.3390/w16070962
Chicago/Turabian StyleStanković, Jelena, Djuradj Milošević, Momir Paunović, Boris Jovanović, Nataša Popović, Jelena Tomović, Ana Atanacković, Katarina Radulović, Davor Lončarević, and Maja Raković. 2024. "Microplastics in the Danube River and Its Main Tributaries—Ingestion by Freshwater Macroinvertebrates" Water 16, no. 7: 962. https://doi.org/10.3390/w16070962
APA StyleStanković, J., Milošević, D., Paunović, M., Jovanović, B., Popović, N., Tomović, J., Atanacković, A., Radulović, K., Lončarević, D., & Raković, M. (2024). Microplastics in the Danube River and Its Main Tributaries—Ingestion by Freshwater Macroinvertebrates. Water, 16(7), 962. https://doi.org/10.3390/w16070962












