Using the Hydraulic Properties of Zeolite to Grow Desert Willow—A Case Study to Rehabilitate Riparian Areas of Semi-Arid Environments
Abstract
1. Introduction
2. Materials and Methods
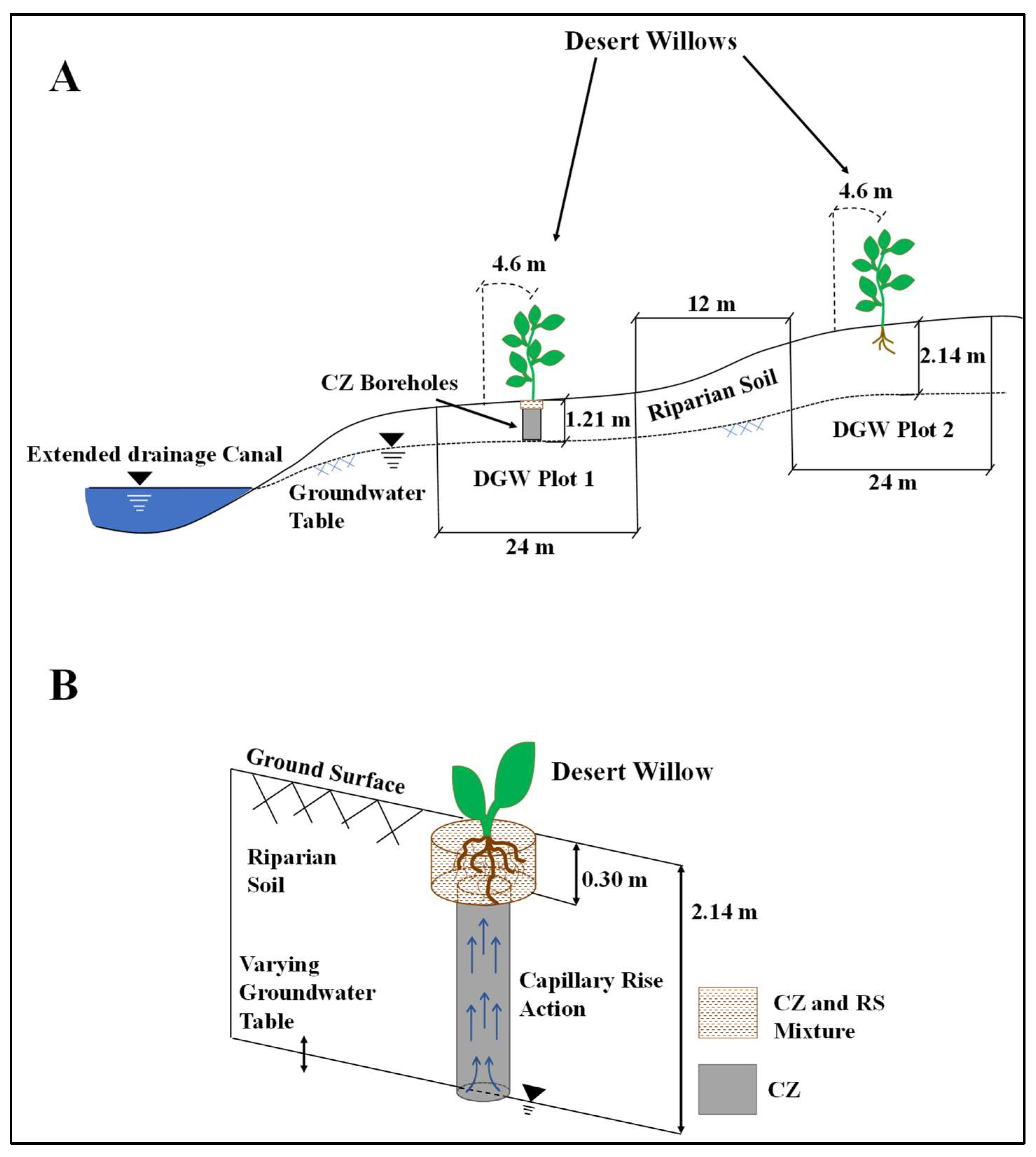
2.1. Study Location and Experimental Setup
2.2. Stem Water Potential and Climate Measurements
2.3. Statistical Analysis
3. Results
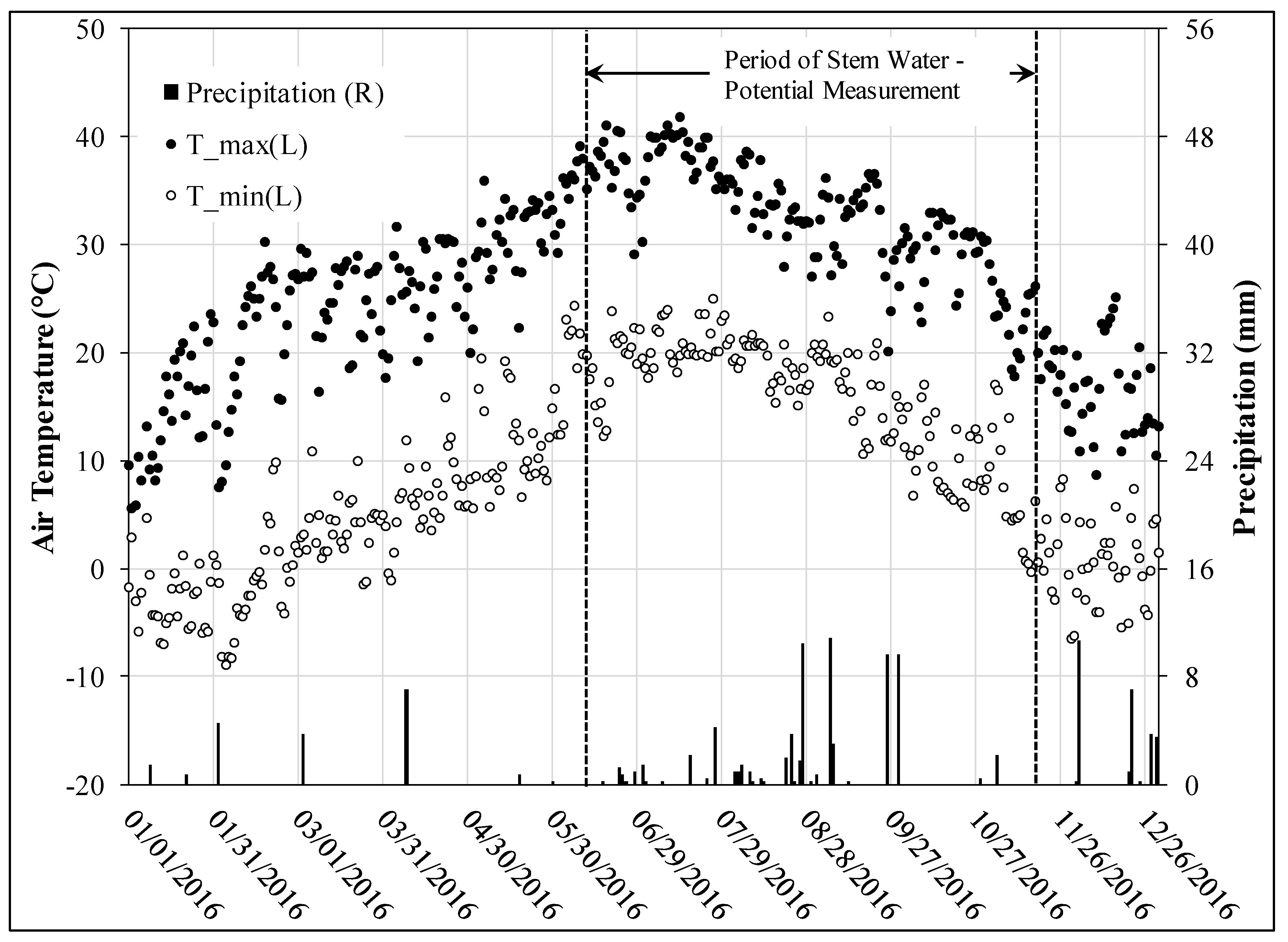
3.1. Climate
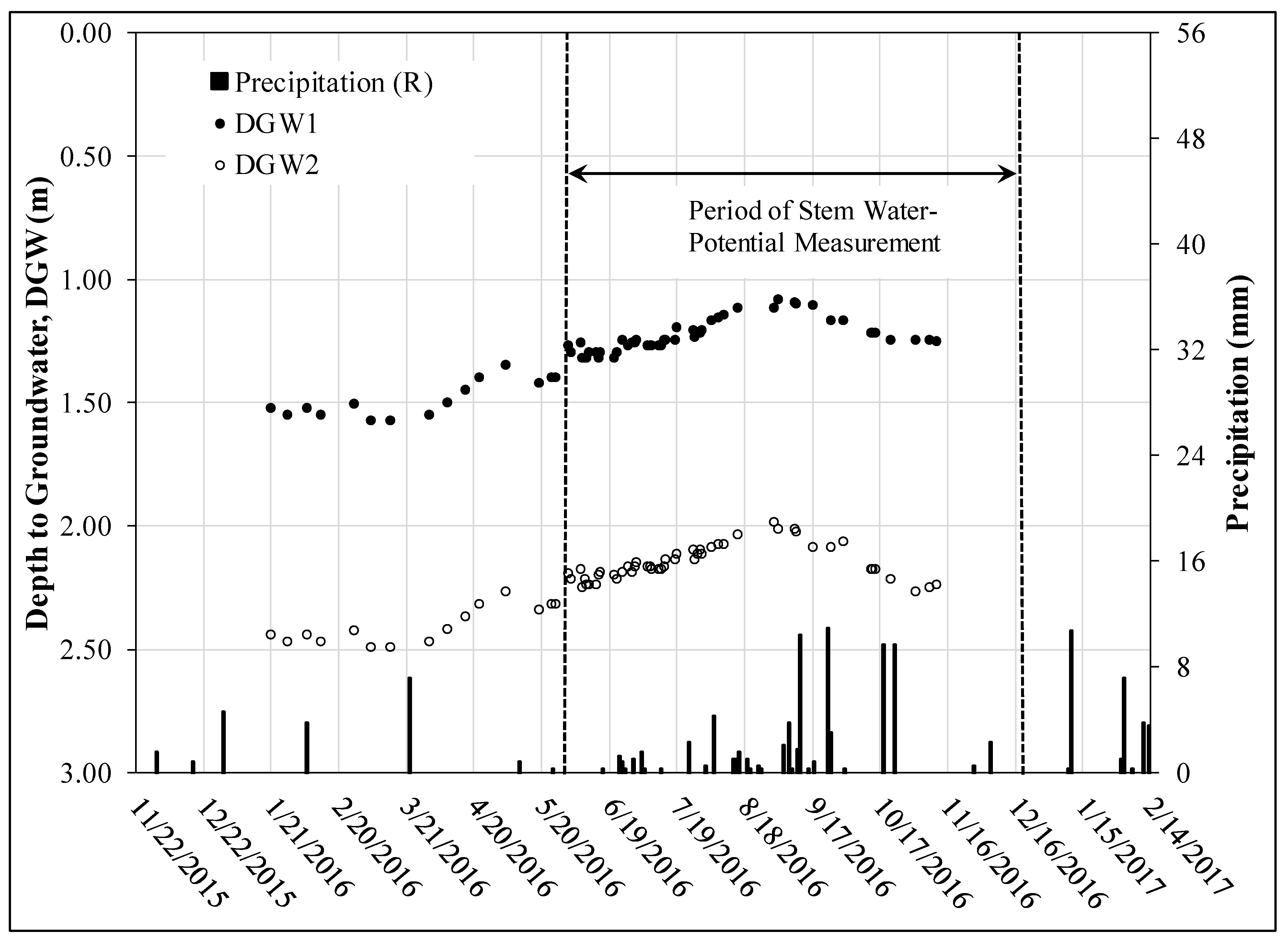
3.2. Groundwater
3.3. Stem Water Potential
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, R.; Tiwari, A.K.; Singh, G.S. Managing Riparian Zones for River Health Improvement: An Integrated Approach. Landsc. Ecol. Eng. 2021, 17, 195–223. [Google Scholar] [CrossRef]
- Young-Mathews, A.; Culman, S.W.; Sánchez-Moreno, S.; Toby O’Geen, A.; Ferris, H.; Hollander, A.D.; Jackson, L.E. Plant-Soil Biodiversity Relationships and Nutrient Retention in Agricultural Riparian Zones of the Sacramento Valley, California. Agroforest Syst. 2010, 80, 41–60. [Google Scholar] [CrossRef]
- Lyons, J.; Thimble, S.W.; Paine, L.K. Grass versus Trees: Managing Riparian Areas to Benefit Streams of Central North America. J. Am. Water. Resour. Assoc. 2000, 36, 919–930. [Google Scholar] [CrossRef]
- Schaff, S.D.; Pezeshki, S.R.; Shields, F.D. Effects of Soil Conditions on Survival and Growth of Black Willow Cuttings. Environ. Manag. 2003, 31, 748–763. [Google Scholar] [CrossRef] [PubMed]
- McKergow, L.A.; Matheson, F.E.; Quinn, J.M. Riparian Management: A Restoration Tool for New Zealand Streams. Eco. Manag. Restor. 2016, 17, 218–227. [Google Scholar] [CrossRef]
- Hunter, M.L.; Acuña, V.; Bauer, D.M.; Bell, K.P.; Calhoun, A.J.K.; Felipe-Lucia, M.R.; Fitzsimons, J.A.; González, E.; Kinnison, M.; Lindenmayer, D.; et al. Conserving Small Natural Features with Large Ecological Roles: A Synthetic Overview. Biol. Conserv. 2017, 211, 88–95. [Google Scholar] [CrossRef]
- González, E.; Felipe-Lucia, M.R.; Bourgeois, B.; Boz, B.; Nilsson, C.; Palmer, G.; Sher, A.A. Integrative Conservation of Riparian Zones. Biol. Conserv. 2017, 211, 20–29. [Google Scholar] [CrossRef]
- Munshower, F.F. Practical Handbook of Disturbed Land Revegetation; CRC Press: New York, NY, USA, 2017; ISBN 9781351084376. [Google Scholar]
- Taylor, J.P.; McDaniel, K.C. Restoration of Saltcedar (Tamarix spp.)-Infested Floodplains on the Bosque Del Apache National Wildlife Refuge. Weed Technol. 1998, 12, 345–352. [Google Scholar] [CrossRef]
- Salinas, M.J.; Guirado, J. Riparian Plant Restoration in Summer-Dry Riverbeds of Southeastern Spain. Restor. Ecol. 2002, 10, 695–702. [Google Scholar] [CrossRef]
- Dreesen, D.R.; Fenchel, G.A. Deep-Planting Techniques to Establish Riparian Vegetation in Arid and Semiarid Regions. Nativ. Plants J. 2010, 11, 15–22. [Google Scholar] [CrossRef]
- Lopez, E.M. Using St. Cloud Clinoptilolite Zeolite as a Wicking Material to Sustain Riparian Vegetation. Masters’ Thesis, New Mexico State University, Las Cruces, NM, USA, 2009. [Google Scholar]
- Dung, T.T.; Bawazir, A.S.; Shukla, M.K.; Bandini, P. Some Hydraulic and Wicking Properties of St. Cloud Zeolite and Zeolite-Soil Mixtures. Appl. Eng. Agric. 2011, 27, 955–967. [Google Scholar] [CrossRef]
- Piñón-Villarreal, A.R.; Bawazir, A.S.; Shukla, M.K.; Samani, Z.A.; King, J.P. Modeling Capillary Rise in Clinoptilolite Zeolite and Riparian Soils to Sustain Vegetation in Water-Scarce Areas. J. Irrig. Drain Eng. 2017, 143, 04017044. [Google Scholar] [CrossRef]
- Fryer, J.L. Chilopsis Linearis, Desert Willow. Available online: www.fs.usda.gov/database/feis/plants/tree/chilin/all.html (accessed on 9 January 2024).
- Desert Willow Chilopsis Linearis (Cav.) Sweet, Plant Fact Sheet. Available online: https://plants.usda.gov/DocumentLibrary/plantguide/pdf/pg_chli2.pdf (accessed on 10 January 2024).
- Dodson, C.; Ivey, R.D. A Guide to Plants of the Northern Chihuahuan Desert; University of New Mexico Press: Albuquerque, NM, USA, 2012; ISBN 9780826350213. [Google Scholar]
- Gilman, E.F.; Watson, D.G. Chilopsis Linearis: Desert Willow. Available online: https://edis.ifas.ufl.edu/publication/ST159 (accessed on 20 November 2023).
- Breck, D.W. Zeolite Molecular Sieves: Structure, Chemistry, and Use; Wiley: New York, NY, USA, 1973; ISBN 9781523114528. [Google Scholar]
- Mumpton, F.A. La Roca Magica: Uses of Natural Zeolites in Agriculture and Industry. Proc. Natl. Acad. Sci. USA 1999, 96, 3463–3470. [Google Scholar] [CrossRef]
- Polat, E.; Karaca, M.; Demir, H.; Onus, A.N. Use of Natural Zeolite (Clinoptilolite) in Agriculture. J. Fruit Ornam. Plant Res. 2004, 12, 183–189. [Google Scholar]
- Eyde, T.; Holmes, D. Zeolite. In Industrial Minerals & Rocks: Commodities, Markets, and Uses; Kogel, J.E., Trivedi, N.C., Barker, J.M., Krukowski, S.T., Eds.; Society for Mining, Metallurgy, and Exploration: Littleton, CO, USA, 2006; ISBN 9780873352338. [Google Scholar]
- Malm, N.R. Climate Guide, Las Cruces, 1892–2000; New Mexico State University, Agricultural Experiment Station: Las Cruces, NM, USA, 2003. [Google Scholar]
- Miyamoto, S.; White, J.M. Foliar Salt Damage of Landscape Plant Induced by Sprinkler Irrigation; Texas Water Resources Institute TR -1202; Texas Agricultural Experiment Station, Agricultural Research and Extension Center at El Paso, Texas Water Resources Institute: El Paso, TX, USA, 2002. [Google Scholar]
- Dreesen, D.; Harrington, J.; Subirge, T.; Stewart, P.; Fenchel, G. Riparian Restoration in the Southwest: Species Selection, Propagation, Planting Methods, and Case Studies. In National Proceedings: Forest and Conservation Nursery Associations, 1999, 2000, and 2001; Dumroese, R.K., Riley, L.E., Landis, T.D., Eds.; U.S. Department of Agriculture Forest, Rocky Mountain Research Station: Odgen, UT, USA, 2002; pp. 253–272. [Google Scholar]
- Nortes, P.A.; Pérez-Pastor, A.; Egea, G.; Conejero, W.; Domingo, R. Comparison of Changes in Stem Diameter and Water Potential Values for Detecting Water Stress in Young Almond Trees. Agric. Water Manag. 2005, 77, 296–307. [Google Scholar] [CrossRef]
- Choné, X. Stem Water Potential Is a Sensitive Indicator of Grapevine Water Status. Ann. Bot. 2001, 87, 477–483. [Google Scholar] [CrossRef]
- De Swaef, T.; Steppe, K.; Lemeur, R. Determining Reference Values for Stem Water Potential and Maximum Daily Trunk Shrinkage in Young Apple Trees Based on Plant Responses to Water Deficit. Agric. Water Manag. 2009, 96, 541–550. [Google Scholar] [CrossRef]
- Mirás-Avalos, J.M.; Trigo-Córdoba, E.; Bouzas-Cid, Y. Does Predawn Water Potential Discern between Irrigation Treatments in Galician White Grapevine Cultivars? OENO One 2014, 48, 123. [Google Scholar] [CrossRef]
- Deb, S.K.; Shukla, M.K.; Mexal, J.G. Estimating Midday Leaf and Stem Water Potentials of Mature Pecan Trees from Soil Water Content and Climatic Parameters. HortScience 2012, 47, 907–916. [Google Scholar] [CrossRef]
- Othman, Y.; Steele, C.; VanLeeuwen, D.; Heerema, R.; Bawazir, S.; St. Hilaire, R. Remote Sensing Used to Detect Moisture Status of Pecan Orchards Grown in a Desert Environment. Int. J. Remote Sens. 2014, 35, 949–966. [Google Scholar] [CrossRef]
- Fulton, A.; Grant, J.; Buchner, R.; Connell, J. Using the Pressure Chamber for Irrigation Management in Walnut, Almond and Prune; University of California, Agriculture and Natural Resources: Davis, CA, USA, 2014; ISBN 9781601078582. [Google Scholar]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration: Guidelines for Computing Crop Water Requirements; FAO irrigation and drainage paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 1998; ISBN 9789251042199. [Google Scholar]
- Allen, R.G.; Walter, I.A.; Elliot, R.; Howell, T.; Itensfisu, D.; Jensen, M.; Snyder, R. The ASCE Standardized Reference Evapotranspiration Equation; American Society of Civil Engineers: Reston, VA, USA, 2005; ISBN 9780784408056. [Google Scholar]
- Kuehl, R.O.; Kuehl, R.O. Design of Experiments: Statistical Principles of Research Design and Analysis, 2nd ed.; Duxbury/Thomson Learning: Pacific Grove, CA, USA, 2000; ISBN 9780534368340. [Google Scholar]
- Dowdy, S.M.; Wearden, S.; Chilko, D.M. Statistics for Research, 3rd ed.; Wiley series in probability and statistics; Wiley-Interscience: Hoboken, NJ, USA, 2004; ISBN 9780471267355. [Google Scholar]
- Sheppard, P.; Comrie, A.; Packin, G.; Angersbach, K.; Hughes, M. The Climate of the US Southwest. Clim. Res. 2002, 21, 219–238. [Google Scholar] [CrossRef]
- DePree, E.; Ludwig, J.A. Vegetative and Reproductive Growth Patterns in Desert Willow (Chilopsis linearis (Cav.) Sweet). Southwest. Nat. 1978, 23, 239. [Google Scholar] [CrossRef]
- Odening, W.R.; Strain, B.R.; Oechel, W.C. The Effect of Decreasing Water Potential on Net CO2 Exchange of Intact Desert Shrubs. Ecology 1974, 55, 1086–1095. [Google Scholar] [CrossRef]
- Macro, K. Stem Water Potential in Desert Willow Grown in Clinoptilolite Zeolite and In-Situ Riparian Soil. Bachelor’s Thesis, State University of New York College of Environmental Science and Forestry, Syracuse, NY, USA, 2017. [Google Scholar]


| Month | T_max | T_min | RH | VPD | ETSO | Precip. |
|---|---|---|---|---|---|---|
| °C | °C | % | kPa | mm | mm | |
| January | 23.6 | −6.9 | 55.54 | 0.73 | 55.8 | 2.3 |
| February | 30.3 | −8.9 | 42.66 | 1.26 | 83.9 | 4.6 |
| March | 29.6 | −1.5 | 35.45 | 1.61 | 140.4 | 3.8 |
| April | 31.7 | −1.1 | 40.43 | 1.73 | 158.9 | 7.1 |
| May | 35.9 | 5.6 | 37.70 | 2.24 | 202.9 | 1.0 |
| June † | 41.1 | 12.3 | 39.79 | 3.05 | 218.0 | 3.6 |
| July † | 41.9 | 17.6 | 42.32 | 3.29 | 217.3 | 9.1 |
| August † | 38.6 | 15.1 | 53.79 | 2.20 | 174.6 | 24.1 |
| September † | 36.5 | 10.6 | 53.35 | 2.02 | 143.9 | 34.3 |
| October † | 33.0 | 5.8 | 49.31 | 1.89 | 110.3 | 0.5 |
| November † | 28.2 | −6.5 | 50.74 | 1.08 | 76.5 | 2.3 |
| December | 25.1 | −6.2 | 58.41 | 0.73 | 51.8 | 26.7 |
| Total | 1634.2 | 119.4 |
| DGW Plots | Treatment | N Samples | Ψmd ± SE (MPa) |
|---|---|---|---|
| DGW1: 1.21 m | CZ1 | 33 | −0.91 ± 0.046 a |
| RS1 | 50 | −0.81 ± 0.029 a | |
| DGW2: 2.14 m | CZ2 | 45 | −0.75 ± 0.032 a |
| RS2 | 12 | −2.03 ± 0.170 b |
| Treatment | N Samples | Mean Ranks | Z-Value | p-Value |
|---|---|---|---|---|
| CZ1 | 33 | 36.00 | −1.847 * | 0.032 |
| RS1 | 50 | 45.96 | ||
| CZ1 | 33 | 28.36 | −4.548 ** | <0.001 |
| RS2 | 12 | 8.25 | ||
| CZ1 | 33 | 32.03 | −2.499 * | 0.006 |
| CZ2 | 45 | 44.98 | ||
| CZ2 | 45 | 51.87 | −1.301 | 0.097 |
| RS1 | 50 | 44.52 | ||
| CZ2 | 45 | 34.83 | −5.15 ** | <0.001 |
| RS2 | 12 | 7.13 | ||
| RS1 | 50 | 37.31 | −5.189 ** | <0.001 |
| RS2 | 12 | 7.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solis, J.C.; Bawazir, A.S.; Piñon-Villarreal, A.R. Using the Hydraulic Properties of Zeolite to Grow Desert Willow—A Case Study to Rehabilitate Riparian Areas of Semi-Arid Environments. Water 2024, 16, 932. https://doi.org/10.3390/w16070932
Solis JC, Bawazir AS, Piñon-Villarreal AR. Using the Hydraulic Properties of Zeolite to Grow Desert Willow—A Case Study to Rehabilitate Riparian Areas of Semi-Arid Environments. Water. 2024; 16(7):932. https://doi.org/10.3390/w16070932
Chicago/Turabian StyleSolis, Juan C., A. Salim Bawazir, and Aldo R. Piñon-Villarreal. 2024. "Using the Hydraulic Properties of Zeolite to Grow Desert Willow—A Case Study to Rehabilitate Riparian Areas of Semi-Arid Environments" Water 16, no. 7: 932. https://doi.org/10.3390/w16070932
APA StyleSolis, J. C., Bawazir, A. S., & Piñon-Villarreal, A. R. (2024). Using the Hydraulic Properties of Zeolite to Grow Desert Willow—A Case Study to Rehabilitate Riparian Areas of Semi-Arid Environments. Water, 16(7), 932. https://doi.org/10.3390/w16070932






