1. Introduction
The geographic extent and intensity of harmful algal blooms (HABs) and their associated ecological and economic impacts have grown over the last several decades [
1,
2,
3]. A major driver of these trends is the cultural eutrophication of aquatic habitats caused by inputs of nutrients from agricultural and developed lands [
4,
5].
Prymnesium parvum is an HAB-forming member of the phylum Haptophyta that is found in brackish waters worldwide and whose toxins can be lethal to aquatic organisms including fishes, amphibians, crustaceans, shellfish, and other algae [
6]. Although
P. parvum is responsible for some of the worst HAB-associated ecological catastrophes in inland waters [
6], field methods to control its blooms are presently unavailable.
Much of the recent research on HAB control methods in standing waters has focused on natural, plant-derived products due to their high biodegradability, short half-lives, and effectiveness as inhibitors of algal growth [
7,
8,
9,
10]. However, little research has been carried out on the use of natural products to control
P. parvum blooms. Earlier studies explored the effects of barley straw (
Hordeum vulgare) preparations on
P. parvum growth, but the results obtained were not promising [
11,
12]. More recently, a methanolic extract of giant reed (
Arundo donax) was shown to suppress
P. parvum growth in a concentration-dependent manner [
13]. A follow-up study reported that an indole alkaloid found in giant reed, ellipticine [
14], has highly potent algicidal activity against
P. parvum [
15]. Various extract preparations from giant reed can also inhibit growth of other HAB species, such as the cyanobacterium
Microcystis aeruginosa [
14,
16,
17,
18] and the marine dinoflagellate
Karenia brevis [
19]. These observations suggest that giant reed products may have the advantage over other natural products of being broadly applicable to the control of some of the most important prokaryotic and eukaryotic HABs.
There is some evidence indicating that, among microalgae, growth inhibition in the presence of giant reed extracts is preferentially observed in HAB species (i.e.,
M. aeruginosa) over green microalgae [
16]; however, no information is available concerning the effects of giant reed products on aquatic fauna. This information is needed to evaluate the unintended environmental consequences of any bloom control method.
The goal of this study is to facilitate the development of natural and environmentally friendly methods of HAB control. The working hypothesis is that giant reed extracts and ellipticine will preferentially impair growth of P. parvum over selected nontarget species. We first determined the effect of giant reed dried chips (directly immersed in culture media) and leachate (aqueous extract) on P. parvum growth. We then determined the effects of leachate and ellipticine on the green microalga Chlorella sorokiniana, the planktonic crustacean Daphnia pulex, and the teleost fish Danio rerio. These nontarget species are widely used in basic and applied (including ecotoxicological) studies, and the large amount of information available for them facilitates inferences about environmental impacts based on laboratory studies.
2. Materials and Methods
2.1. General Culture Methods
Prymnesium parvum (UTEX-2797) was obtained from UTEX Culture Collection of Algae (The University of Texas at Austin, TX, USA). Stock cultures were maintained as previously described [
15]. Briefly, nonaxenic cultures were maintained in modified UTEX artificial seawater medium at 5 psu and f/2 levels of nutrients (modified ASM) and placed in a plant germination rack with two full-spectrum LED lights. The photoperiod was set to 12:12 h light: dark with an average light intensity of ~84 μmol photons m
−2 s
−1 and average daily temperature of ~22 °C. Culture flasks were gently swirled once daily for several seconds. Stock cultures in late exponential growth phase (day 15) were used to prepare new stock cultures and as source of inoculum for experimental cultures.
Chlorella sorokiniana (UTEX-1230) was obtained from UTEX Culture Collection of Algae. Stock cultures were maintained in BG-11 medium (UTEX) following culture conditions previously described [
20]. Briefly, nonaxenic cultures were maintained on an orbital shaker at 120 rpm under two full-spectrum LED lights. The photoperiod was set to 16:8 h light: dark with an average light intensity of ~100 µmol photons m
−2 s
−1 and average daily temperature of 25 °C. Stock cultures in exponential growth phase (day 3) were used to prepare new stock cultures and as source of inoculum for experimental cultures.
Daphnia pulex was acquired from Carolina Biological Supply Company (Burlington, NC, USA) and maintained as previously described [
21]. Briefly, organisms were maintained in COMBO medium in a controlled Precision Low Temp BOD Refrigerated Incubator (VWR, Radnor, PN, USA). The photoperiod was set to 14:10 h light: dark with an average light intensity of ~7000 lux and an average daily temperature of 20 °C. Stock cultures were maintained in glass jars, and organisms were gently transferred to new jars with fresh media once every 3 days to prevent accumulation of waste. Daphnids were fed with
Scenedesmus acutus inoculated at a concentration of 10
5 cells mL
−1. They were allowed two months of acclimation prior to experimentation.
Adult, wild-type 5D-strain
D. rerio were originally obtained from Robyn Tanguay’s laboratory at Oregon State University and maintained as previously described [
22]. Briefly, fish were maintained in a recirculating system (Aquaneering, San Diego, CA, USA) at a photoperiod of 14:10 h light: dark with an average light intensity of ~6900 lux, average daily temperature of ~28 °C, and dissolved oxygen of 80 percent saturation. Ammonia, nitrite, and nitrate levels were measured weekly and were consistently below 0.1 mg/L, 0.05 mg/L, and 2 mg/L, respectively. Fish were fed twice daily with commercially available dry food (Gemma Micro 300, Skretting, UT, USA).
For the purpose of D. rerio embryo collection, 3 adult males were paired with 5 adult females in each of three 6 L tanks equipped with false bottoms the night before breeding; these tanks were placed within the recirculating system. On the day of breeding, fertilized eggs were collected within 4 to 6 h post-spawn. Embryos were transferred to egg water media (60 mg of Instant Ocean Salt (Instant Ocean, Blacksburg, VA, USA) per liter of deionized water). Zebrafish procedures were conducted under Texas Tech University IACUC permit #20012-02.
2.2. Experimental Reagents
Giant reed with stems of approximately 2 m in height and without flowering plumes were selected for this study. Plants were collected from an urban stand in Lubbock (TX, USA), rinsed with tap water, and air-dried for 24 h. The stems and leaves were first chopped into small pieces with an electric garden chipper (GS70015, Earthwise, Indianapolis, IN, USA) and then cut into fine chips using a kitchen chopper (Nj100Gr, Ninja, Needham, MA, USA). Chips were placed in a heater at 60 °C for 48 h and the dried material (
Figure 1) was stored at 4 °C in a desiccating chamber. For use in cultures, the appropriate amount of chips was placed in sterilized (autoclaved), drawstring tea filter bags and immersed in the experimental flasks. In initial experiments, cultures were started (inoculated) upon immersion of the bags in the flasks. In later experiments, chips were allowed to leach for 24 h at 4 °C and the leachate was collected and filtered sequentially with 0.45 and 0.22 µm syringe filters (SLHAM33S, SLGSR33SS, Sigma-Aldrich, St. Louis, MO, USA) before use. The latter procedure was applied to minimize the presence of debris and bacterial contamination in the cultures.
Ellipticine (324,688, Sigma-Aldrich) was prepared following procedures previously described [
15]. Dimethyl sulfoxide (DMSO) (D4540, Sigma-Aldrich) was used as carrier solution at a final concentration of 0.05% in experimental media.
2.3. Giant Reed Exposures
A range-finding test was conducted to determine the effective concentration of dried chips that inhibits
P. parvum growth. Concentrations ranged from 0 to 16 g L
−1 in modified ASM. Algal cells were inoculated into 100 mL of modified ASM in 250 mL flasks at a starting density of 50,000 cells mL
−1, and one teabag containing the appropriate amount of chips was directly added to each flask. Ambient culture conditions were the same as described for stock cultures. Cells were counted at 24 h of incubation by hemocytometry [
15]. Each exposure treatment was conducted in triplicate flasks.
Based on the estimated 24 h IC
50 (concentration that inhibits growth by 50%) for chips (see Results), leachate was prepared in modified ASM at concentrations of 0 and 3 g chips L
−1 (hereafter, g L
−1) in an initial experiment with
P. parvum, and 0 and 1 g L
−1 in a subsequent experiment. The lower leachate concentration was used to serve as reference for experiments with
D. pulex and
D. rerio, where at least one of the endpoints measured was affected at 3 g L
−1 (see Results).
Prymnesium parvum was inoculated in 100 mL of leachate, cultured, and counted as described above. Cell density was determined at 24 h [
15].
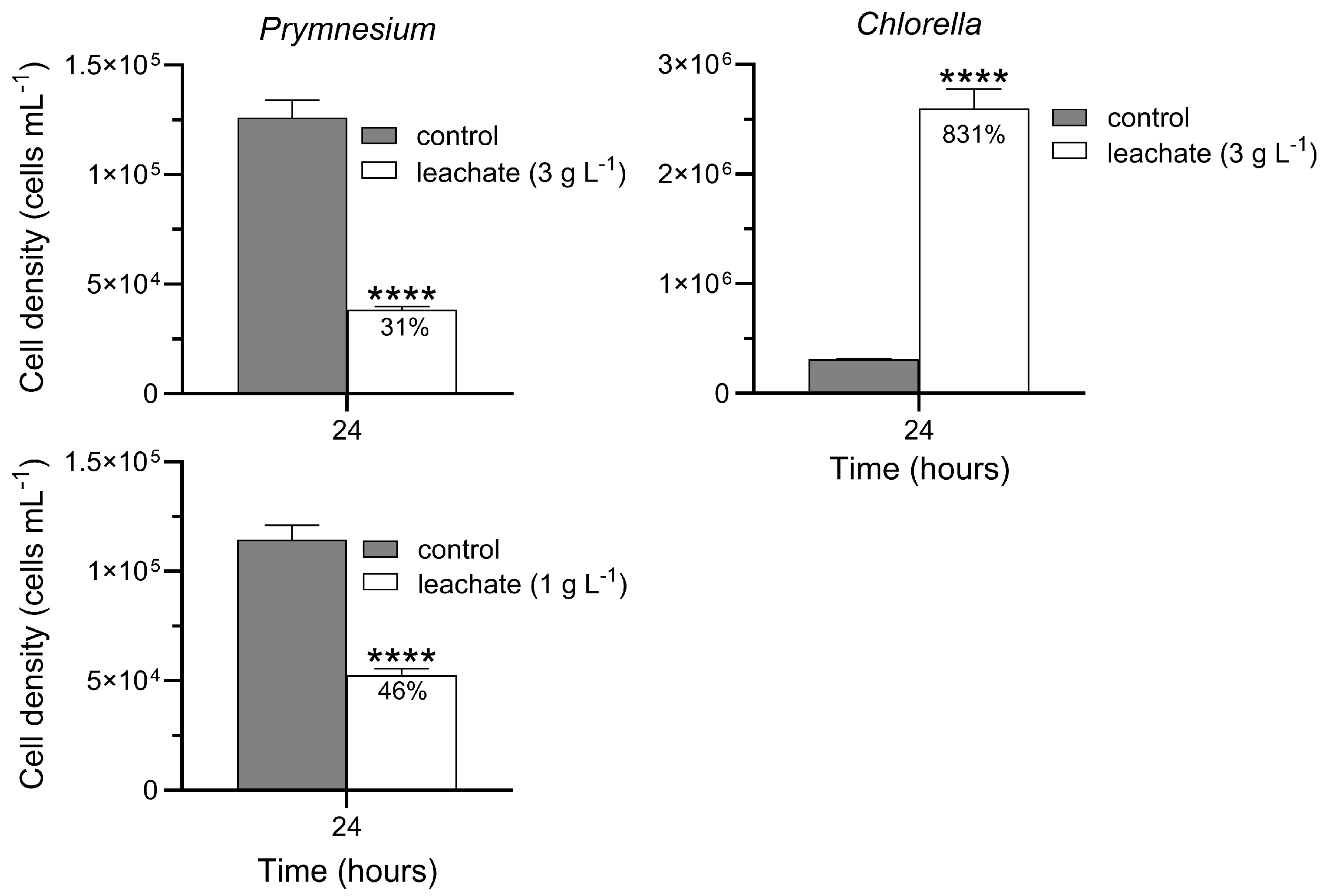
For C. sorokiniana, leachate (in BG-11 media) was prepared at concentrations of 0 and 3 g L−1. Cultures were inoculated in 20 mL of leachate at an initial density of 50,000 cells mL−1. Ambient and culture conditions were the same as described for stock cultures. Cell density was determined at 24 h by hemocytometry, except cells were counted on a computer monitor using an Olympus DP74 camera mounted on a compound microscope. Each exposure treatment was conducted in triplicate flasks.
For
D. pulex, leachate (in COMBO media) was prepared at concentrations of 0 and 3 g L
−1 in an initial experiment and 0 and 1 g L
−1 in a subsequent experiment.
Daphnia pulex neonates (≤24 h) were placed in 24-well plates (one daphnid per well), with each well containing 2 mL of appropriate treatment medium. One full plate (
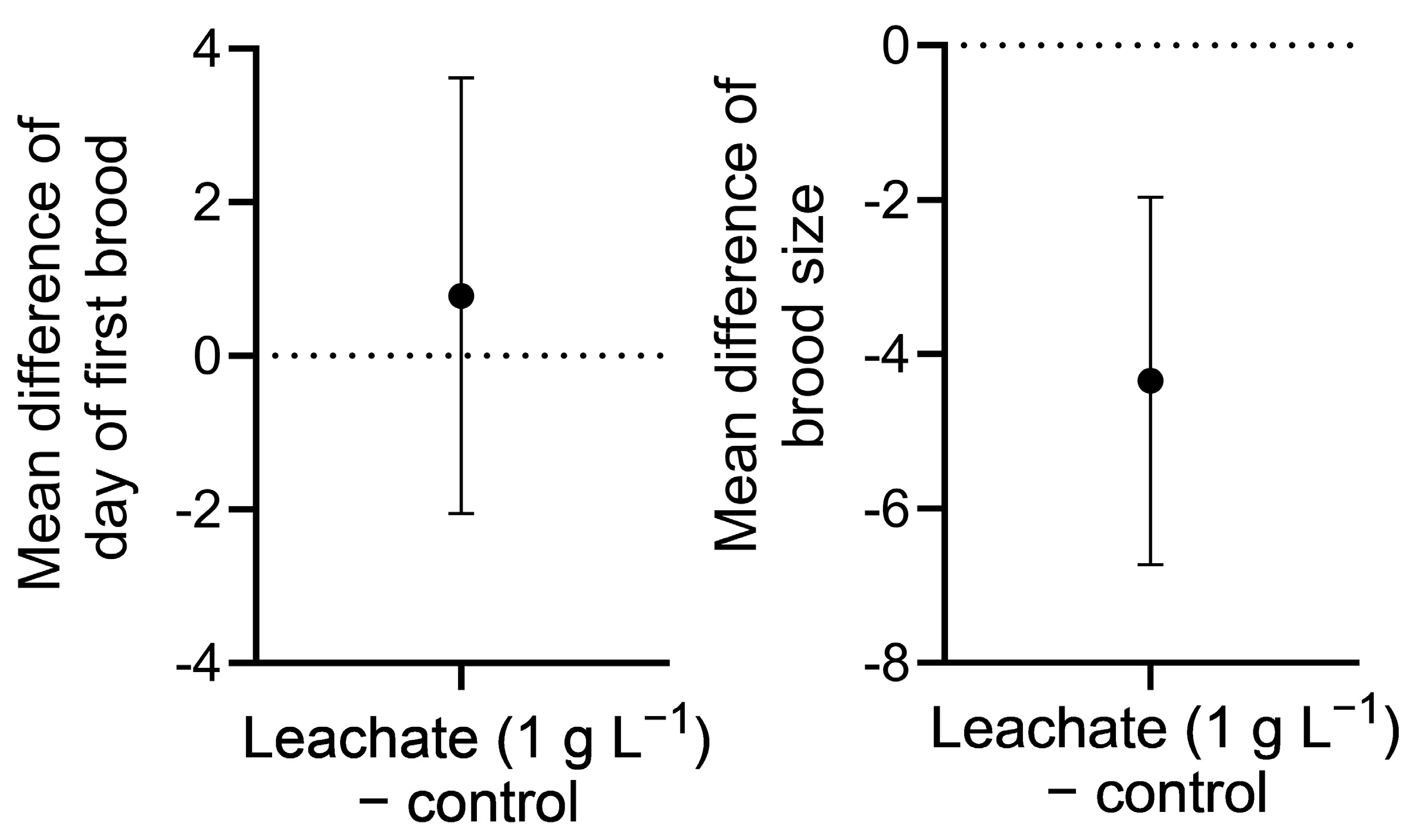
n = 24) was assigned to each treatment concentration, and two independent experiments (trials) were conducted. Ambient culture conditions were the same as described for stock cultures. Cultures were continued until day 21 to determine exposure effects on long-term survival and on two reproductive indices: day of first brood production and cumulative brood size [
23]. Survival was monitored every 24 h until the end of the culture. To minimize accumulation of waste and bacterial growth,
D. pulex was gently transferred to new plates with fresh media and diet (2.2 × 10
5 S. acutus cells mL
−1) every other day with a transfer pipette.
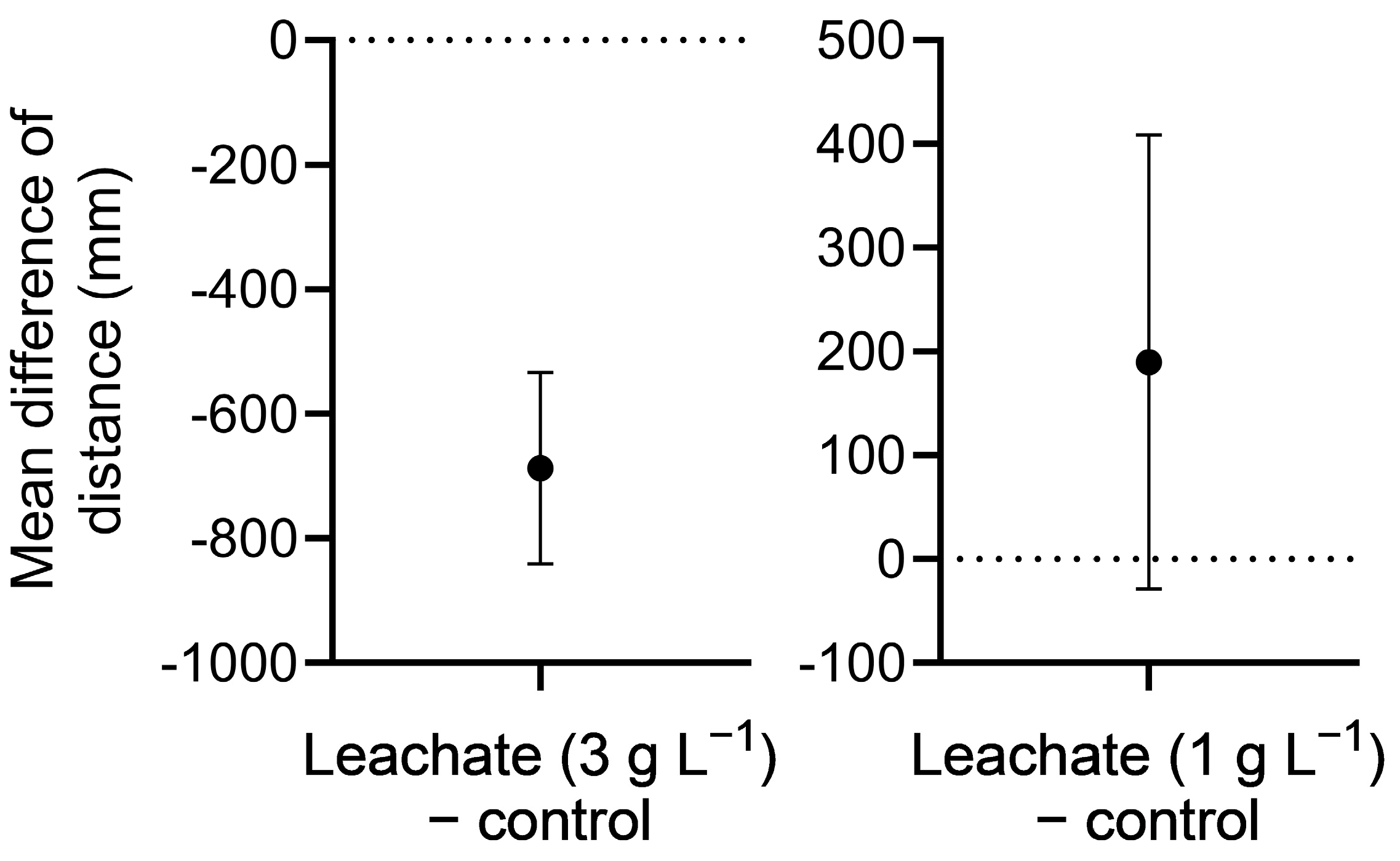
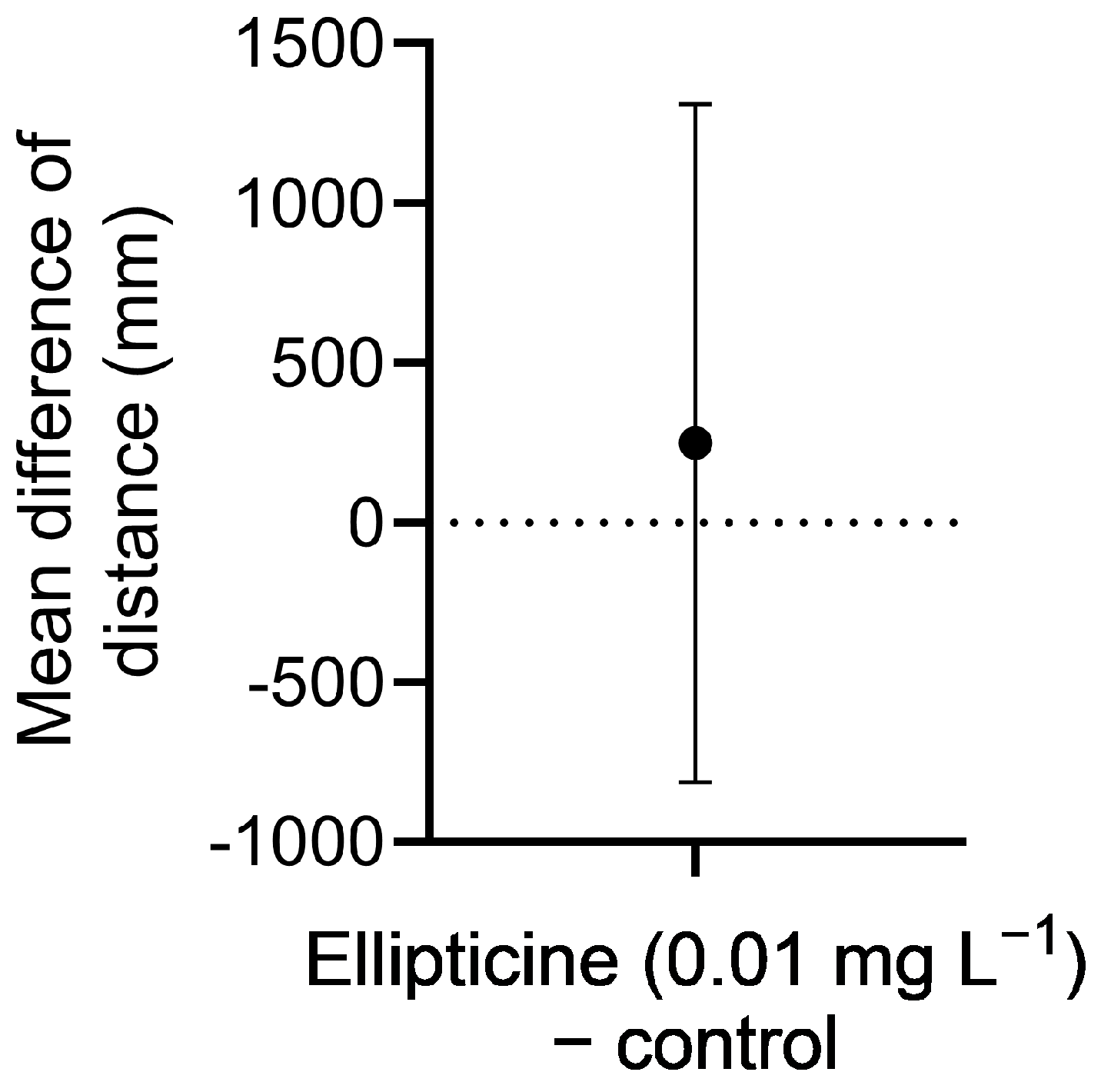
For D. rerio, leachate (in egg water media) was prepared at concentrations of 0 and 3 g L−1 in an initial experiment, and 0 and 1 g L−1 in a subsequent experiment. Embryos were placed in 24-well plates (one embryo per well), each containing 1 mL of appropriate treatment medium. One full plate (n = 24) was assigned to each treatment concentration, and two independent experiments (trials) were conducted. Embryo cultures were maintained in a Fisher Scientific Isotemp Refrigerated Incubator (Fisher Scientific 3720, Hampton, NH, USA) for a total of 5 days at a temperature of 28.5 °C, photoperiod of 12:12 h light: dark, and light intensity of ~5100 lux. To minimize accumulation of waste and bacterial growth, culture media were replaced gently with a transfer pipette on days 2 and 4. Survival was checked every 24 h for 120 h under a stereomicroscope. Hatch rate was determined at 72 h, and eleutheroembryo (post-hatch) survival was recorded at 120 h. Swimming distance (startle response to light stimulation) was also determined at 120 h. For this measurement, well-plates containing the larvae were placed in a DanioVision™ (Noldus, Reston, VA, USA) and, after a 10 min acclimation period, larval behavior was videotaped for 20 min, during which larvae were stimulated with light for 10 min and kept in the dark for the next 10 min. Total swimming distance during this time period was estimated using Ethovision®XT software version 13 (Noldus, Reston, VA, USA).
2.4. Ellipticine Exposures
The 3-day IC
50 value for ellipticine in
P. parvum was previously estimated at ~0.01 mg L
−1 [
15], and in this study, ellipticine was prepared in modified ASM at concentrations of 0 and 0.01 mg L
−1.
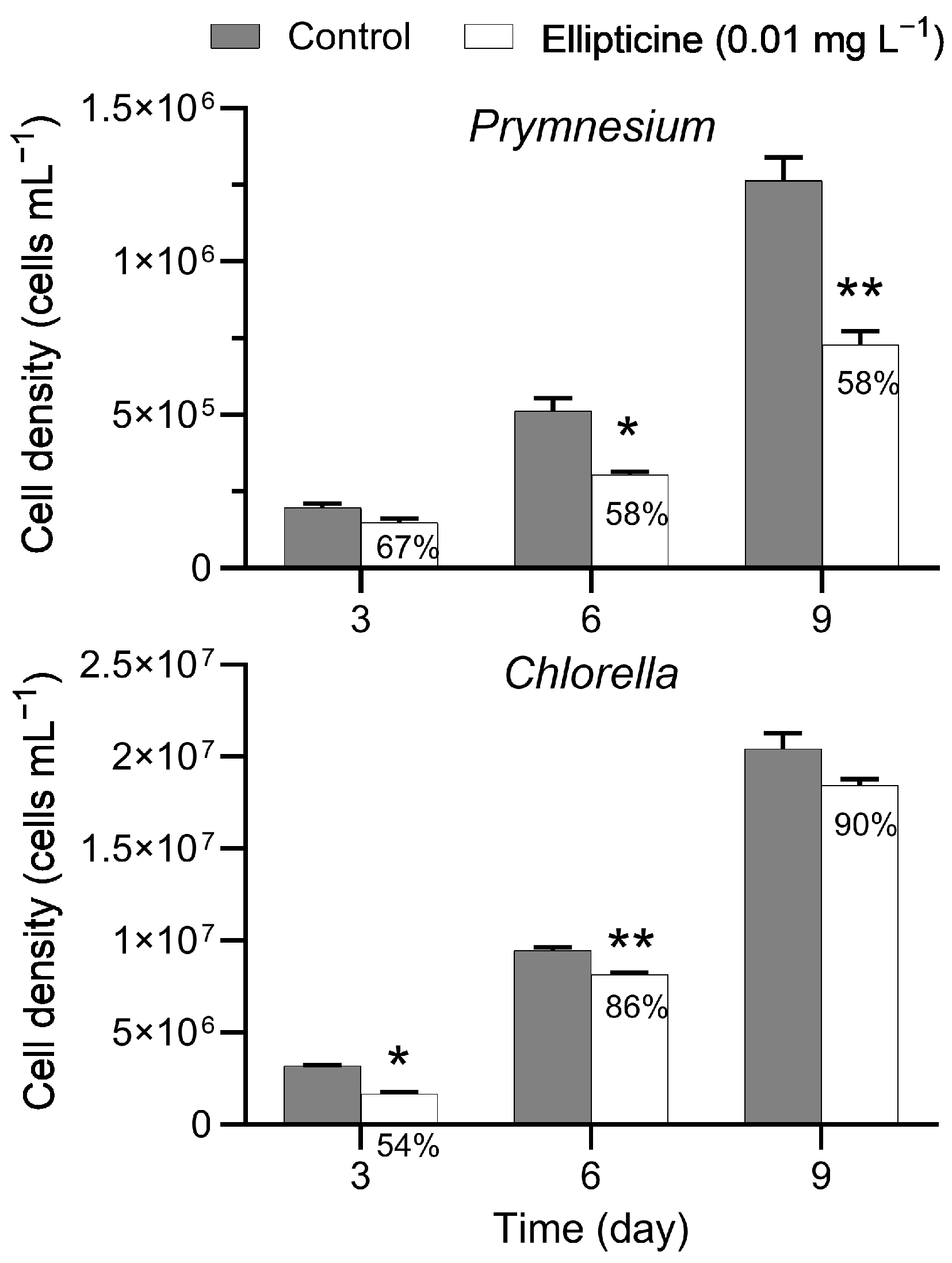
Prymnesium parvum was inoculated and cultured as described earlier, and cell density was determined every 3 days for 9 days (exposure effects through day 21 were previously reported [
15]). Each exposure treatment was conducted in triplicate flasks.
For C. sorokiniana, ellipticine was prepared in BG-11 media at concentrations of 0 and 0.01 mg L−1. Chlorella sorokiniana was inoculated and cultured as described earlier, and cell density was determined every 3 days through day 21. Each exposure treatment was conducted in triplicate flasks.
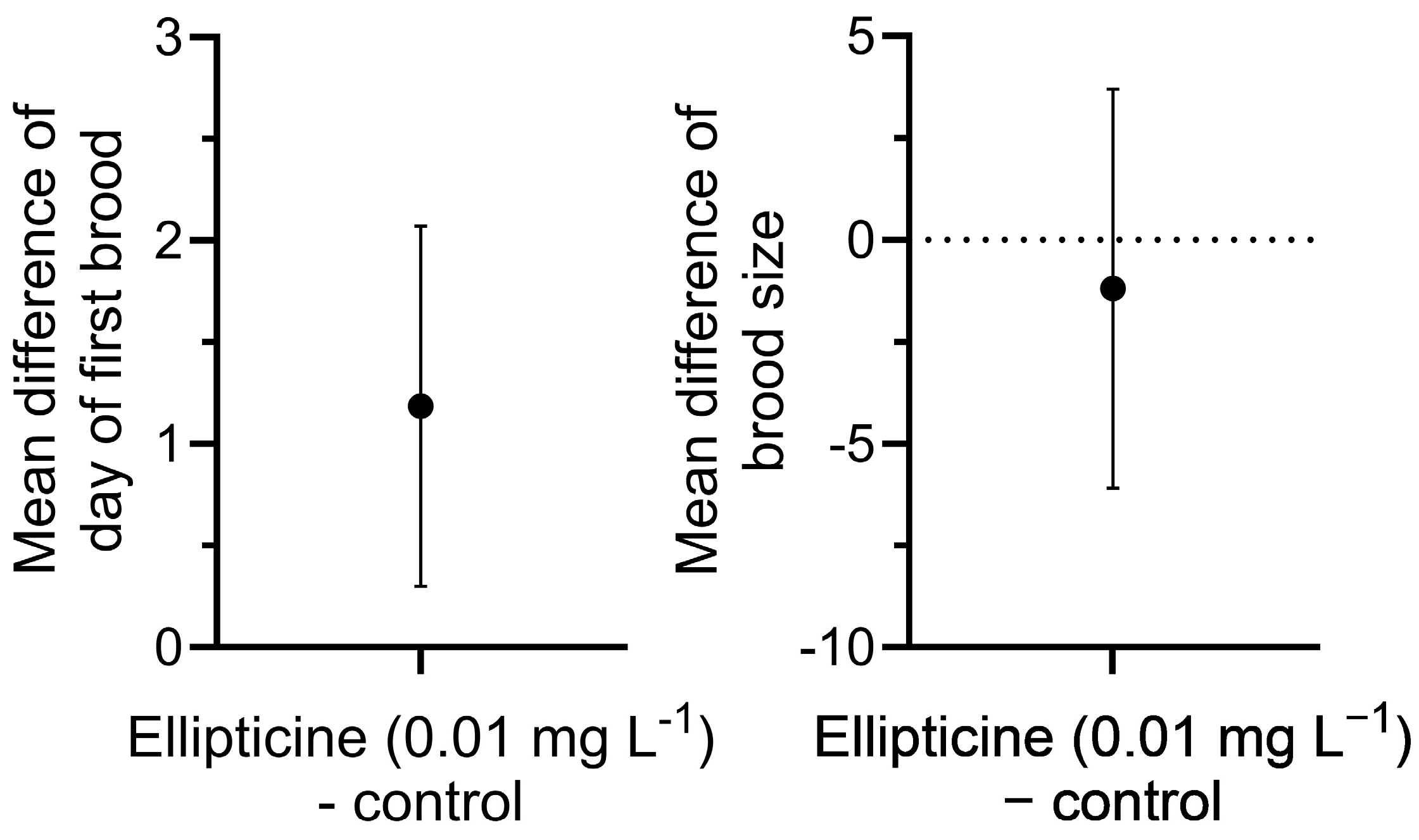
For D. pulex and D. rerio, ellipticine was prepared in COMBO media and egg water media, respectively, at concentrations of 0 and 0.01 mg L−1. All other experimental conditions and endpoints measured were the same as described earlier. One full plate (n = 24) was assigned to each treatment concentration for each species, and two independent experiments (trials) were conducted.
2.5. Data Analysis
The IC
50 of dried chips for
P. parvum growth was estimated using a symmetrical–sigmoidal dose–response algorithm, as previously described [
15]. In the leachate experiments with
P. parvum and
C. sorokiniana, differences in growth between control and respective treatment groups at 24 h were evaluated with Student’s
t-tests (two-tailed) using log-transformed values. In the ellipticine experiments, 2-way repeated measures (RM) ANOVA (treatment by culture time; RM factor, time; sphericity was not assumed, and the Geisser–Greenhouse correction was applied) was followed by Sidak’s multiple comparison tests on algal counts at each sampling day using log-transformed values.
Kaplan–Meier’s survival analysis was applied to
D. pulex data, and comparisons between treatment and control groups were made using log-rank (Mantel–Cox) tests. Differences in
D. rerio hatch rates were determined using Fisher’s exact test. Post-hatch survival was 100% for
D. rerio regardless of treatment (see Results), and larval survival analysis was unnecessary. All analyses were performed separately on each trial. Time to first brood production (time to first reproduction) and cumulative brood size in
D. pulex, and larval behavior (swimming distance) in
D. rerio were evaluated using nested
t-test (trials nested into treatment). Prior to analysis, the ROUT test (Q = 1; ref. [
24]) was used to identify and exclude outliers in behavior data, and the D’Agostino–Pearson test was used to assess normality of data distribution.
GraphPad Prism version 10.0 (GraphPad Software, Boston, MA, USA) was used for all analysis, and the α threshold for statistical significance was 0.05.
4. Discussion
Earlier studies reported that methanolic extracts of giant reed suppress growth of
P. parvum [
13] and
M. aeruginosa [
17], that volatile oil extracts inhibit growth of
M. aeruginosa [
18], and that aqueous extracts (leachate) have algicidal activity against
M. aeruginosa [
14,
16]. This study found that giant reed leachate suppresses growth of
P. parvum and additionally characterized the unintended effects of leachate and ellipticine—a potent anti-
P. parvum allelochemical found in giant reed [
15]—on nontarget aquatic organisms. The results obtained indicate that when applied at the appropriate concentrations, giant reed products can inhibit growth of
P. parvum with little or no harm to nontarget species.
When directly immersed in medium at the time of inoculation, giant reed chips acutely inhibited growth of
P. parvum with a 24 h IC
50 of ~3 g L
−1. When prepared over a 24 h period prior to inoculation, leachate seemed to have a stronger effect; namely, compared to control cultures, 3 g L
−1 of leachate inhibited growth of
P. parvum by ~70%. One reason for the stronger effect of preprepared leachate may be that allelochemicals are already present in culture media—and at relatively high concentrations—from the moment the cultures begin. The potency of giant reed leachate against
P. parvum growth seems somewhat stronger than against
M. aeruginosa, where 5 g L
−1 achieved ~30% growth inhibition at 24 h [
14].
Giant reed leachate at 3 g L
−1 was not toxic to
C. sorokiniana. On the contrary, leachate enhanced the growth of this species by a large margin compared to control cultures. Giant reed leachate reportedly also stimulates growth of at least two other species of freshwater green algae [
16]. Although it seems perplexing that giant reed leachate can suppress the growth of harmful species such as
P. parvum (haptophyte) and
M. aeruginosa (cyanobacteria) but enhance the growth of some green algal species, the phenomenon of species specificity is typical of allelopathy [
8]. Selective inhibition of cyanobacterial growth, sometimes but not always coupled with stimulation of green algal growth, has been reported for extracts and allelochemicals derived from various aquatic macrophytes [
8,
26]. One advantage of giant reed leachate over other natural or synthetic products as an HAB control tool seems to be that, based on current knowledge, it selectively suppresses growth of harmful prokaryotes (
M. aeruginosa) and harmful eukaryotes (
P. parvum).
Leachate at 3 g L−1 considerably reduced survival of D. pulex at 24 h and was fully lethal by 72 h. Although no significant effects of leachate at 3 g L−1 were observed on hatching or survival of D. rerio, swimming behavior (distance) in 5-day-old larvae in response to light stimulation was significantly reduced. At the lower concentration of 1 g L−1, leachate retained full algistatic activity against P. parvum at 24 h but had no effects on survival or age of first reproduction of D. pulex, and only moderately reduced its brood size. In addition, leachate at 1 g L−1 had no effects on D. rerio hatching, survival, or swimming behavior. It appears, therefore, that giant reed leachate at 1 g L−1 could be used to control P. parvum blooms without major impacts to the survival and various key indices of performance of nontarget species.
Ellipticine is a plant-derived indole alkaloid found in giant reed leachate [
14]. An earlier study of
P. parvum reported a 3-day IC
50 on growth of 0.01 mg L
−1 [
15], which is among the highest known allelochemical potencies against harmful algae. In the present study, ellipticine at 0.01 mg L
−1 suppressed growth of
P. parvum in a time-dependent manner. Cell density was similar to the control group on day 3 but growth was significantly reduced compared to the control group from day 6 through day 9. This finding is consistent with results of the earlier study with
P. parvum [
15], which found that growth suppression by ellipticine is time-dependent and irreversible. Although ellipticine inhibited growth of
C. sorokiniana on day 3 and day 6, the magnitude of the inhibition was lower on day 6 than on day 3 and, moreover, growth inhibition was no longer observed from day 9 through day 21. These observations indicate that although ellipticine has an acute effect on the growth of
C. sorokiniana, the effect is short-lived. In addition, ellipticine at 0.01 mg L
−1 had no major adverse effects on
D. pulex and
D. rerio—only a slight delay in the day of first reproduction of
D. pulex was recorded (~1 day). Overall, this study confirmed the high potency of ellipticine against
P. parvum growth reported earlier [
15], and also established its specificity against
P. parvum relative to various nontarget organisms.
Mechanisms of action were not examined in this study. However, ellipticine was previously shown to reduce chlorophyll content, increase cell volume, and impair swimming behavior in
P. parvum [
15]. Reduction in chlorophyll content and increased cell swelling have also been observed in
M. aeruginosa exposed to various plant-derived allelochemicals [
27,
28,
29] and bacterial toxins [
30]. In
Chlamydomonas reinhardtii, a synthetic derivative of ellipticine reduced growth, perhaps by disrupting DNA topoisomerase activities [
31]. The chemical composition of leachate is likely to be complex, and so the cellular and physiological mechanisms responsible for its growth-inhibiting activity against
P. parvum are likely to be more complex than those of ellipticine. A study of the transcriptomic response of
P. parvum to giant reed leachate suggested that pathways and processes associated with photosynthesis and cell division are likely involved in the suppression of growth [
32].
Water managers may need to weigh the benefits of an HAB control treatment against its unintended ecological impacts. Treatment cost and application feasibility are also important considerations. The present study found that giant reed leachate at a concentration of 1 g chips L
−1 and ellipticine at a concentration of 0.01 mg L
−1 blocked or reduced growth of
P. parvum with minimal effects on nontarget aquatic flora and fauna. Ellipticine seemed to have the fewest side effects but, at the present time, its availability and cost may be discouraging for field application purposes. Leachate, on the other hand, is easier and less costly to prepare. It should be mentioned that giant reed leachate impairs growth of not only
P. parvum (present study) but also the cyanobacterium
M. aeruginosa [
16], two of the most important HAB species found in inland waters [
6]. Growth of the harmful marine dinoflagellate
K. brevis can be suppressed with a pyrolytic extract of giant reed [
19], but the effects of leachate on this species are unknown.
Although the present laboratory results indicate that giant reed products have promise as tools for HAB control in standing inland waters, mesocosm and field trials would be useful to better understand the products’ limitations and adaptively adjust treatments. Future research on ellipticine could explore its potential use in combination with allelochemical sustained-release technologies to enhance its HAB control efficiency at low additional cost [
10]. Research to determine the risk of giant reed establishment in non-native habitats may be necessary before directly using dried chips in the field. The presence of an intact stem node seems necessary for the vegetative propagation of this highly invasive plant [
33] but, to our knowledge, the ability of dried node fragments (part of the material used in the present study;
Figure 1) to sprout has not been examined. The use of leachate or leachate derivatives may be preferable. For large water bodies where treatment application over their entire surface is not feasible or practical, knowledge of the spatial sources and seasonal timing of propagule production would facilitate spot treatment strategies to stop blooms from forming and spreading. Spot treatments could also be used to create refugia for fishes and other aquatic organisms during active toxic blooms. The potential of giant reed products to control the growth of multiple HAB species may serve as incentive for further exploration.