The Influence of Triphenyltin Exposure on the Osmoregulatory Capacity of Marine Medaka (Oryzias melastigma) at Different Salinities
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Test Chemicals
2.2. Experimental Design and Sample Collection
2.3. Biochemical Analysis
2.4. Quantitative Real-Time PCR (qPCR) Assay
2.5. Statistical Analysis
3. Results and Discussion
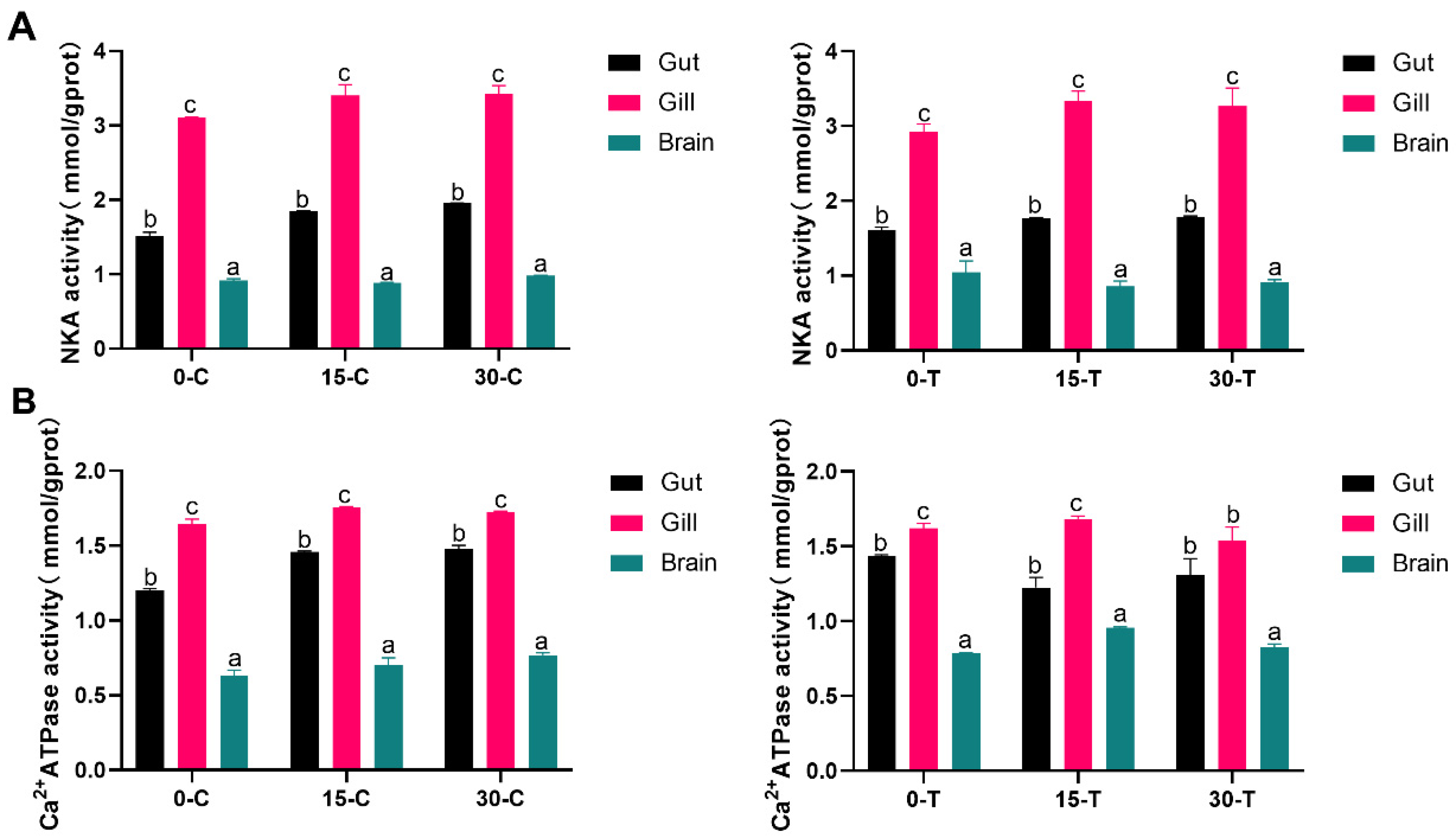
3.1. Changes in Ion-Transporting Proteins
3.2. Disturbances in the Concentration of Different Ions
3.3. Differences in Expression Levels of Related Genes
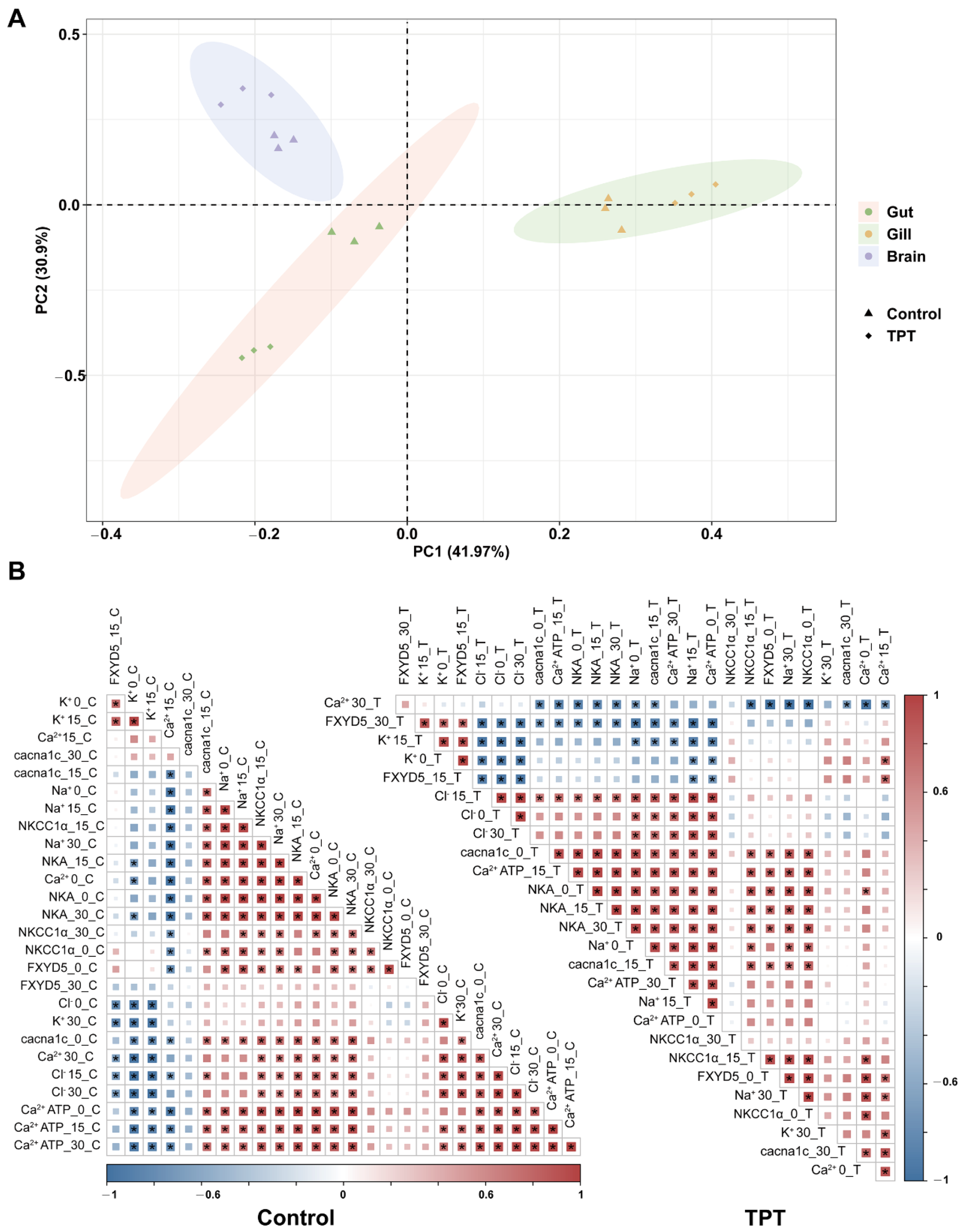
3.4. PCA and Correlation Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- He, S.W.; Li, P.; Li, Z.H. Review on endocrine disrupting toxicity of triphenyltin from the perspective of species evolution: Aquatic, amphibious and mammalian. Chemosphere 2021, 269, 128711. [Google Scholar] [CrossRef]
- Li, P.; Li, Z.H. Environmental co-exposure to TBT and Cd caused neurotoxicity and thyroid endocrine disruption in zebrafish, a three-generation study in a simulated environment. Environ. Pollut. 2020, 259, 113868. [Google Scholar] [CrossRef]
- Li, P.; Li, Z.H.; Zhong, L.Q. Parental exposure to triphenyltin inhibits growth and disrupts thyroid function in zebrafish larvae. Chemosphere 2020, 240, 124936. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Hu, J.Y.; Zhen, H.J.; Wu, X.Q.; Huang, C. Reproductive Inhibition and Transgenerational Toxicity of Triphenyltin on Medaka (Oryzias latipes) at Environmentally Relevant tip Levels. Environ. Sci. Technol. 2008, 42, 8133–8139. [Google Scholar] [CrossRef]
- Sun, L.B.; Zhang, J.L.; Zuo, Z.H.; Chen, Y.X.; Wang, X.H.; Huang, X.; Wang, C.G. Influence of triphenyltin exposure on the hypothalamus-pituitary-gonad axis in male Sebastiscus marmoratus. Aquat. Toxicol. 2011, 104, 263–269. [Google Scholar] [CrossRef]
- Yi, X.L.; Leung, K.M.Y. Assessing the toxicity of triphenyltin to different life stages of the marine medaka Oryzias melastigma through a series of life-cycle based experiments. Mar. Pollut. Bull. 2017, 124, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; Li, P. Effects of the tributyltin on the blood parameters, immune responses and thyroid hormone system in zebrafish. Environ. Pollut. 2021, 268, 115707. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Wang, T.; Hsieh, C.Y.; Tien, C.J. Organotin contamination in fishes with different living patterns and its implications for human health risk in Taiwan. Environ. Pollut. 2005, 137, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.Y.; Zhen, H.J.; Wan, Y.; Gao, J.M.; An, W.; An, L.H.; Jin, F.; Jin, X.H. Trophic magnification of triphenyltin in a marine food web of Bohai Bay, north China: Comparison to tributyltin. Environ. Sci. Technol. 2006, 40, 3142–3147. [Google Scholar] [CrossRef] [PubMed]
- Sham, R.C.T.; Ho, K.K.Y.; Zhou, G.J.; Li, Y.Y.; Wang, X.H.; Leung, K.M.Y. Occurrence, ecological and human health risks of phenyltin compounds in the marine environment of Hong Kong. Mar. Pollut. Bull. 2020, 154, 111093. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.J.; Slater, M.J.; Kunzmann, A. What metabolic, osmotic and molecular stress responses tell us about extreme ambient heatwave impacts in fish at low salinities: The case of European seabass, Dicentrarchus labrax. Sci. Total Environ. 2020, 749, 141458. [Google Scholar] [CrossRef]
- Liu, D.R.; Zhang, Z.W.; Song, Y.K.; Yang, J.Y.; Lu, Y.Y.; Lai, W.J.; Wu, Z.Y.; Zhao, D.D.; Lin, H.R.; Zhang, Y.; et al. Effects of salinity on growth, physiology, biochemistry and gut microbiota of juvenile grass carp (Ctenopharyngodon idella). Aquat. Toxicol. 2023, 258, 106482. [Google Scholar] [CrossRef]
- Laiz-Carrión, R.; Sangiao-Alvarellos, S.; Guzmán, J.M.; del Río, M.P.M.; Soengas, J.L.; Mancera, J.M. Growth performance of gilthead sea bream Sparus aurata in different osmotic conditions: Implications for osmoregulation and energy metabolism. Aquaculture 2005, 250, 849–861. [Google Scholar] [CrossRef]
- Zhu, J.H.; Wang, X.D.; Bu, X.Y.; Wang, C.L.; Pan, J.Y.; Li, E.C.; Shi, Q.C.; Zhang, M.L.; Qin, J.G.; Chen, L.Q. Relationship between myo-inositol synthesis and carbohydrate metabolism changes in Mozambique tilapia (Oreochromis mossambicus) under acute hypersaline stress. Aquaculture 2021, 532, 736005. [Google Scholar] [CrossRef]
- Fang, Z.R.; Li, X.J.; Wang, Y.P.; Lu, W.; Hou, J.C.; Cheng, J. Steroidogenic Effects of Salinity Change on the Hypothalamus-Pituitary-Gonad (HPG) Axis of Male Chinese Sea Bass (Lateolabrax maculatus). Int. J. Mol. Sci. 2022, 23, 10905. [Google Scholar] [CrossRef]
- Hedén, I.; Sundell, K.; Jönsson, E.; Sundh, H. The role of environmental salinity on Na+-dependent intestinal amino acid uptake in rainbow trout (Oncorhynchus mykiss). Sci. Rep. 2022, 12, 10. [Google Scholar] [CrossRef]
- Cao, Q.Q.; Blondeau-Bidet, E.; Lorin-Nebel, C. Intestinal osmoregulatory mechanisms differ in Mediterranean and Atlantic European sea bass: A focus on hypersalinity. Sci. Total Environ. 2022, 804, 150208. [Google Scholar] [CrossRef]
- da Silva, A.O.F.; Martinez, C.B.R. Acute effects of cadmium on osmoregulation of the freshwater teleost Prochilodus lineatus: Enzymes activity and plasma ions. Aquat. Toxicol. 2014, 156, 161–168. [Google Scholar] [CrossRef]
- Wen, J.J.; Cui, X.Y.; Gibson, M.; Li, Z.Y. Water quality criteria derivation and ecological risk assessment for triphenyltin in China. Ecotoxicol. Environ. Saf. 2018, 161, 397–401. [Google Scholar] [CrossRef]
- Saoud, I.P.; Kreydiyyeh, S.; Chalfoun, A.; Fakih, M. Influence of salinity on survival, growth, plasma osmolality and gill Na+-K+-ATPase activity in the rabbitfish Siganus rivulatus. J. Exp. Mar. Biol. Ecol. 2007, 348, 183–190. [Google Scholar] [CrossRef]
- Chandrasekar, S.; Nich, T.; Tripathi, G.; Sahu, N.P.; Pal, A.K.; Dasgupta, S. Acclimation of brackish water pearl spot (Etroplus suratensis) to various salinities: Relative changes in abundance of branchial Na+/K+-ATPase and Na+/K+/2Cl− co-transporter in relation to osmoregulatory parameters. Fish Physiol. Biochem. 2014, 40, 983–996. [Google Scholar] [CrossRef]
- Bradford, M.M. Rapid and Sensitive Method for Quantitation of Microgram Quantities of Protein Utilizing Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Marshall, W.S. Na+, Cl−, Ca2+ and Zn2+ transport by fish gills: Retrospective review and prospective synthesis. J. Exp. Zool. 2002, 293, 264–283. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, S.; Chainy, G.B.N.; Jena, J.K. Characterization of Ca2+-ATPase activity in gill microsomes of freshwater mussel, Lamellidens marginalis (Lamarck) and heavy metal modulations. Aquaculture 2007, 270, 443–450. [Google Scholar] [CrossRef]
- Loro, V.L.; Wood, C.M. The roles of calcium and salinity in protecting against physiological symptoms of waterborne zinc toxicity in the euryhaline killifish (Fundulus heteroclitus). Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2022, 261, 109422. [Google Scholar] [CrossRef]
- Kulac, B.; Atli, G.; Canli, M. Response of ATPases in the osmoregulatory tissues of freshwater fish Oreochromis niloticus exposed to copper in increased salinity. Fish Physiol. Biochem. 2013, 39, 391–401. [Google Scholar] [CrossRef]
- Sinha, A.K.; Limbaugh, N.; Renukdas, N.; Bishop, W.M.; Romano, N. Modulating effect of elevated water hardness on growth performance, ammonia dynamics and ion-regulatory capacity in channel catfish (Ictalurus punctatus) following chronic challenge with high environmental ammonia and salinity stress. Aquaculture 2022, 560, 738489. [Google Scholar] [CrossRef]
- Sardella, B.A.; Brauner, C.J. The effect of elevated salinity on ‘California’ Mozambique tilapia (Oreochromis mossambicus × O-urolepis hornorum) metabolism. Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2008, 148, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.K.; Hsu, A.D.; Kang, C.K.; Lai, I.P.; Liao, P.S.; Lee, T.H. Intestinal FXYD12 and sodium-potassium ATPase: A comparative study on two euryhaline medakas in response to salinity changes. PLoS ONE 2018, 13, 16. [Google Scholar] [CrossRef]
- Allen, P.J.; McEnroe, M.; Forostyan, T.; Cole, S.; Nicholl, M.M.; Hodge, B.; Cech, J.J. Ontogeny of salinity tolerance and evidence for seawater-entry preparation in juvenile green sturgeon, Acipenser medirostris. J. Comp. Physiol. B-Biochem. Syst. Environ. Physiol. 2011, 181, 1045–1062. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.Y.; Li, P.; He, S.W.; Cao, Z.H.; Wang, X.; Cao, X.Q.; Liu, B.; Chen, C.Z.; You, H.; Li, Z.H. Physiological responses in Nile tilapia (Oreochromis niloticus) induced by combined stress of environmental salinity and triphenyltin. Mar. Environ. Res. 2022, 180, 105736. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.A.; Jefferson, L.; Sudan, N.; Lloyd, T.C.; Ren, J. Acetaldehyde depresses myocardial contraction and cardiac myocyte shortening in spontaneously hypertensive rats role of intracellular Ca2+. Cell. Mol. Biol. 1999, 45, 453–465. [Google Scholar] [PubMed]
- Sandmann, S.; Min, J.Y.; Meissner, A.; Unger, T. Effects of the calcium channel antagonist mibefradil on haemodynamic parameters and myocardial Ca2+-handling in infarct-induced heart failure in rats. Cardiovasc. Res. 1999, 44, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Matsui, H. Effects of triphenyltin on cytosolic Na+ and Ca2+ response to glucose and acetylcholine in pancreatic β-cells from hamster. Toxicol. Appl. Pharmacol. 2001, 174, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.Y.; Kwon, H.B.; Ahn, J.C.; Kang, D.; Kwon, S.H.; Park, J.A.; Kim, K.W. Functional and developmental analysis of the blood-brain barrier in zebrafish. Brain Res. Bull. 2008, 75, 619–628. [Google Scholar] [CrossRef]
- Diao, X.Z.; Han, H.; Li, B.L.; Guo, Z.; Fu, J.; Wu, W.H. The Rare Marine Bioactive Compounds in Neurological Disorders and Diseases: Is the Blood-Brain Barrier an Obstacle or a Target? Mar. Drugs 2023, 21, 406. [Google Scholar] [CrossRef]
- Kennedy, C.J.; Tierney, K.B.; Mittelstadt, M. Inhibition of P-glycoprotein in the blood-brain barrier alters avermectin neurotoxicity and swimming performance in rainbow trout. Aquat. Toxicol. 2014, 146, 176–185. [Google Scholar] [CrossRef]
- Li, Z.H.; Xu, H.Y.; Zheng, W.L.; Lam, S.H.; Gong, Z.Y. RNA-sequencing analysis of TCDD-induced responses in zebrafish liver reveals high relatedness to in vivo mammalian models and conserved biological pathways. PLoS ONE 2013, 8, e77292. [Google Scholar] [CrossRef] [PubMed]
- Cutler, C.P.; Cramb, G. Two isoforms of the Na+/K+/2CI− cotransporter are expressed in the European eel (Anguilla anguilla). Biochim. Biophys. Acta-Biomembr. 2002, 1566, 92–103. [Google Scholar] [CrossRef]
- Horng, J.L.; Yu, L.L.; Liu, S.T.; Chen, P.Y.; Lin, L.Y. Potassium Regulation in Medaka (Oryzias latipes) Larvae Acclimated to Fresh Water: Passive Uptake and Active Secretion by the Skin Cells. Sci. Rep. 2017, 7, 14. [Google Scholar] [CrossRef]
- Kang, C.K.; Tsai, H.J.; Liu, C.C.; Lee, T.H.; Hwang, P.P. Salinity-dependent expression of a Na+, K+, 2Cl− cotransporter in gills of the brackish medaka Oryziasdancena: A molecular correlate for hyposmoregulatory endurance. Comp. Biochem. Physiol. A-Mol. Integr. Physiol. 2010, 157, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Gotliv, I.L. FXYD5: Na+/K+-ATPase Regulator in Health and Disease. Front. Cell. Dev. Biol. 2016, 4, 26. [Google Scholar] [CrossRef]
- Miller, T.J.; Davis, P.B. FXYD5 modulates Na+ absorption and is increased in cystic fibrosis airway epithelia. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2008, 294, L654–L664. [Google Scholar] [CrossRef] [PubMed]


| Gene Name | Sequences of Primers (5′−3′) |
|---|---|
| NKCC1αF | TCAAACCAGTGAGGGAGAATACAAC |
| NKCC1αR | TTCTTGTTCATTTTAAGGGCGTCGG |
| FXYD5F | CCAATGATAGAAGGCAAAGAGT |
| FXYD5R | ATGGAGCTTGTTCACAGGA |
| cacna1cF | GTTTGTGGCAATCAGGACCAT |
| cacna1cR | AAAACTTCCCCTTAAAGAGTTGCA |
| 18SF | GACAAATCGCTCCACCAACT |
| 18SR | CCTGCGGCTTAATTTGACCC |
| Group | Ca2+ | Cl− | K+ | Na+ | NKA | Ca2+ATP | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | DF | p | F | DF | p | F | DF | p | F | DF | p | F | DF | p | F | DF | p | |
| 0-C | 95.29 | 2; 6 | <0.01 | 94.27 | 2; 6 | <0.01 | 132.25 | 2; 6 | <0.01 | 104.41 | 2; 6 | <0.01 | 975.527 | 2; 6 | <0.01 | 272.459 | 2; 6 | <0.01 |
| 15-C | 95.29 | 2; 6 | <0.01 | 718.86 | 2; 6 | <0.01 | 418.94 | 2; 6 | <0.01 | 372.63 | 2; 6 | <0.01 | 224.382 | 2; 6 | <0.01 | 374.482 | 2; 6 | <0.01 |
| 30-C | 171.44 | 2; 6 | <0.01 | 1790.96 | 2; 6 | <0.01 | 41.22 | 2; 6 | <0.01 | 92.48 | 2; 6 | <0.01 | 384.036 | 2; 6 | <0.01 | 1049.785 | 2; 6 | <0.01 |
| 0-T | 418.11 | 2; 6 | <0.01 | 465.38 | 2; 6 | <0.01 | 111.61 | 2; 6 | <0.01 | 160.27 | 2; 6 | <0.01 | 77.229 | 2; 6 | <0.01 | 448.870 | 2; 6 | <0.01 |
| 15-T | 101.48 | 2; 6 | <0.01 | 2226.26 | 2; 6 | <0.01 | 45.24 | 2; 6 | <0.01 | 388.67 | 2; 6 | <0.01 | 194.268 | 2; 6 | <0.01 | 84.878 | 2; 6 | <0.01 |
| 30-T | 114.77 | 2; 6 | <0.01 | 905.14 | 2; 6 | <0.01 | 2.99 | 2; 6 | 0.126 | 767.51 | 2; 6 | <0.01 | 68.404 | 2; 6 | <0.01 | 19.359 | 2; 6 | 0.02 |
| Group | NKCC1α | FXYD5 | cacna1c | ||||||
|---|---|---|---|---|---|---|---|---|---|
| F | DF | p | F | DF | p | F | DF | p | |
| 0-C | 55.22 | 2; 6 | <0.01 | 77.39 | 2; 6 | <0.01 | 12.57 | 2; 6 | 0.07 |
| 15-C | 53.39 | 2; 6 | <0.01 | 12.29 | 2; 6 | 0.08 | 0.23 | 2; 6 | 0.013 |
| 30-C | 3.66 | 2; 6 | 0.91 | 0.68 | 2; 6 | 0.543 | 0.01 | 2; 6 | 0.429 |
| 0-T | 200.65 | 2; 6 | <0.01 | 50.86 | 2; 6 | <0.01 | 2.75 | 2; 6 | <0.01 |
| 15-T | 24.29 | 2; 6 | <0.01 | 152.03 | 2; 6 | <0.01 | 0.40 | 2; 6 | <0.01 |
| 30-T | 1.02 | 2; 6 | 0.416 | 41.44 | 2; 6 | <0.01 | 0.15 | 2; 6 | 0.092 |
| Factors/Interactions | NKA | Ca2+ ATPase | Na+ | Cl− | Ca2+ | K+ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DF | F | p | F | p | F | p | F | p | F | p | F | p | |
| Tissue | 2 | 928.95 | 0.000 | 666.15 | 0.000 | 1151.39 | 0.000 | 3115.98 | 0.000 | 68.94 | 0.000 | 223.27 | 0.000 |
| Salinity | 2 | 7.90 | 0.001 | 5.26 | 0.010 | 10.48 | 0.000 | 167.84 | 0.000 | 334.82 | 0.000 | 23.083 | 0.000 |
| TPT | 1 | 1.89 | 0.177 | 0.01 | 0.945 | 1.58 | 0.217 | 10.20 | 0.003 | 10.80 | 0.002 | 41.063 | 0.000 |
| Tissue * Salinity | 4 | 3.88 | 0.10 | 1.90 | 0.131 | 58.58 | 0.000 | 115.55 | 0.000 | 168.37 | 0.000 | 110.46 | 0.000 |
| Tissue * TPT | 2 | 0.95 | 0.396 | 14.95 | 0.000 | 25.90 | 0.000 | 37.78 | 0.000 | 186.79 | 0.000 | 5.14 | 0.011 |
| Salinity * TPT | 2 | 0.94 | 0.401 | 10.47 | 0.000 | 39.82 | 0.000 | 26.75 | 0.000 | 1.73 | 0.192 | 48.86 | 0.000 |
| Tissue * Salinity * TPT | 4 | 0.47 | 0.760 | 5.78 | 0.001 | 51.06 | 0.000 | 85.39 | 0.000 | 139.03 | 0.000 | 17.72 | 0.000 |
| Factors/Interactions | NKCC1α | FXYD5 | cacna1c | ||||
|---|---|---|---|---|---|---|---|
| DF | F | p | F | p | F | p | |
| Tissue | 2 | 176.79 | 0.000 | 72.056 | 0.000 | 64.43 | 0.000 |
| Salinity | 2 | 39.88 | 0.000 | 4.12 | 0.000 | 18.44 | 0.000 |
| TPT | 1 | 0.27 | 0.607 | 48.79 | 0.024 | 10.46 | 0.003 |
| Tissue * Salinity | 4 | 33.073 | 0.000 | 43.36 | 0.000 | 21.37 | 0.000 |
| Tissue * TPT | 2 | 4.96 | 0.013 | 12.27 | 0.000 | 17.60 | 0.000 |
| Salinity * TPT | 2 | 10.48 | 0.000 | 130.7 | 0.000 | 5.335 | 0.009 |
| Tissue * Salinity * TPT | 4 | 7.74 | 0.000 | 12.47 | 0.000 | 8.338 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.-Z.; Chen, C.-Z.; Xing, S.-Y.; Liu, L.; Li, P.; Li, Z.-H. The Influence of Triphenyltin Exposure on the Osmoregulatory Capacity of Marine Medaka (Oryzias melastigma) at Different Salinities. Water 2024, 16, 921. https://doi.org/10.3390/w16070921
Li T-Z, Chen C-Z, Xing S-Y, Liu L, Li P, Li Z-H. The Influence of Triphenyltin Exposure on the Osmoregulatory Capacity of Marine Medaka (Oryzias melastigma) at Different Salinities. Water. 2024; 16(7):921. https://doi.org/10.3390/w16070921
Chicago/Turabian StyleLi, Teng-Zhou, Cheng-Zhuang Chen, Shao-Ying Xing, Ling Liu, Ping Li, and Zhi-Hua Li. 2024. "The Influence of Triphenyltin Exposure on the Osmoregulatory Capacity of Marine Medaka (Oryzias melastigma) at Different Salinities" Water 16, no. 7: 921. https://doi.org/10.3390/w16070921
APA StyleLi, T.-Z., Chen, C.-Z., Xing, S.-Y., Liu, L., Li, P., & Li, Z.-H. (2024). The Influence of Triphenyltin Exposure on the Osmoregulatory Capacity of Marine Medaka (Oryzias melastigma) at Different Salinities. Water, 16(7), 921. https://doi.org/10.3390/w16070921







