Synthesis of MgO-Coated Canna Biochar and Its Application in the Treatment of Wastewater Containing Phosphorus
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Biochar Preparation
2.3. Adsorption Performance Tests
2.3.1. Adsorption Kinetics Experiments
2.3.2. Adsorption Isotherm Experiment
2.4. Adsorption of Real Wastewater Experiment
2.5. Characterization of Biochar
2.6. Statistical Analysis
3. Results and Discussion
3.1. Yields, Ash, and pH of Modified Biochar (MBC) and Unmodified Biochar (BC)
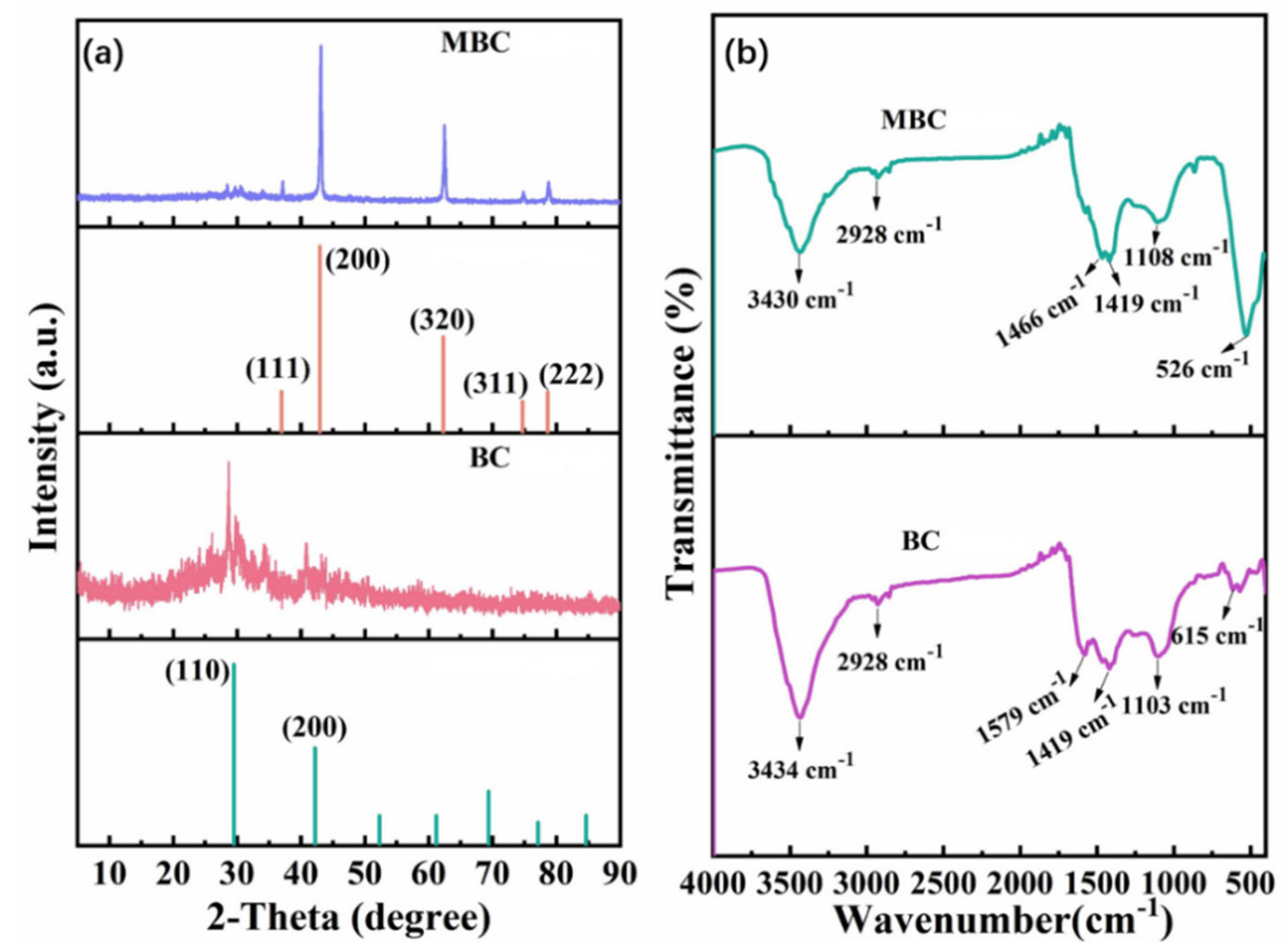
3.2. Composition and Structure of the BC and MBC
3.2.1. Element Composition and Pore Structure Analysis
3.2.2. Biochar Microstructures
3.3. Adsorption Isotherms and Kinetics
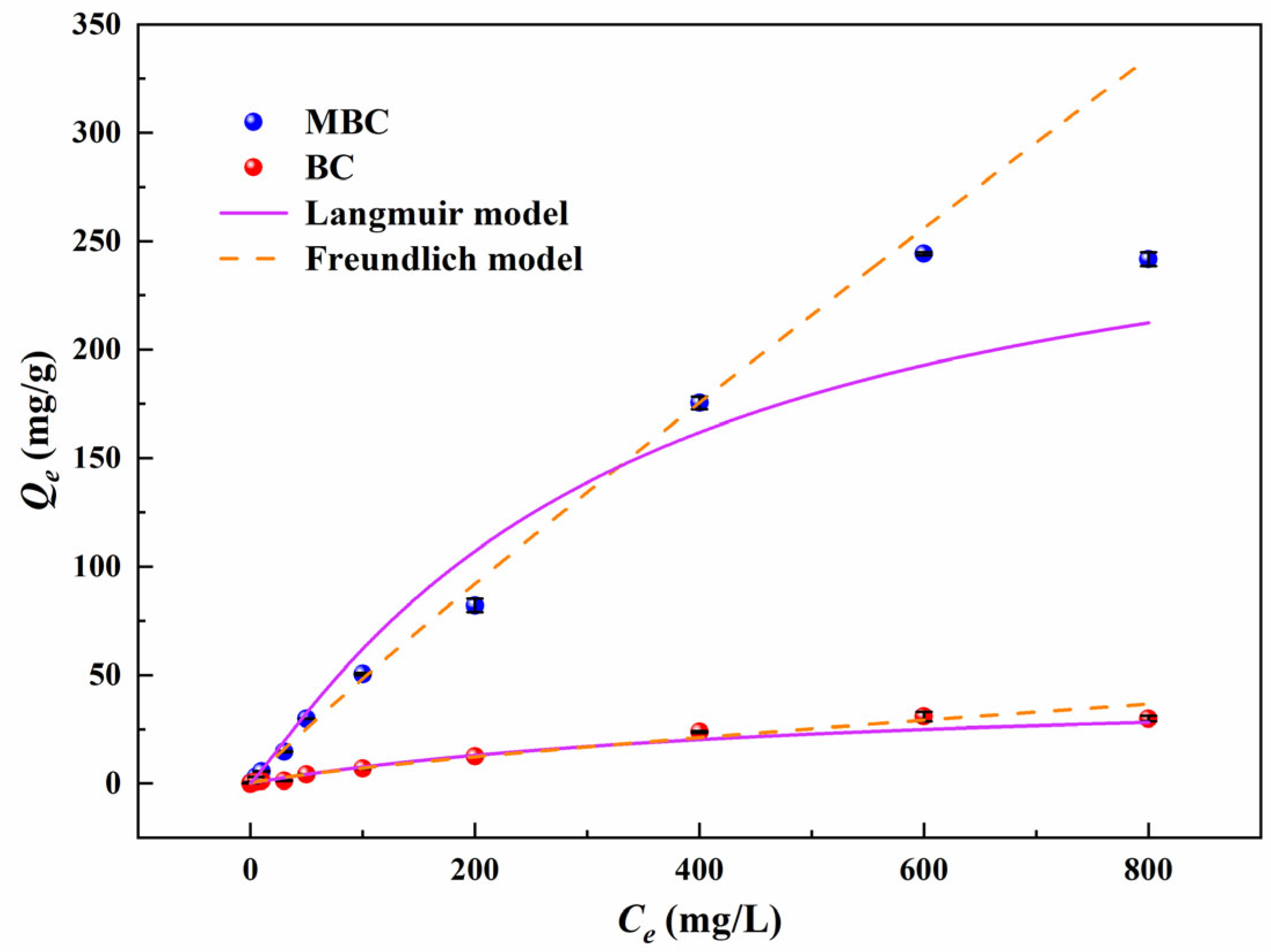
3.3.1. Adsorption Isotherm
3.3.2. Adsorption Kinetics
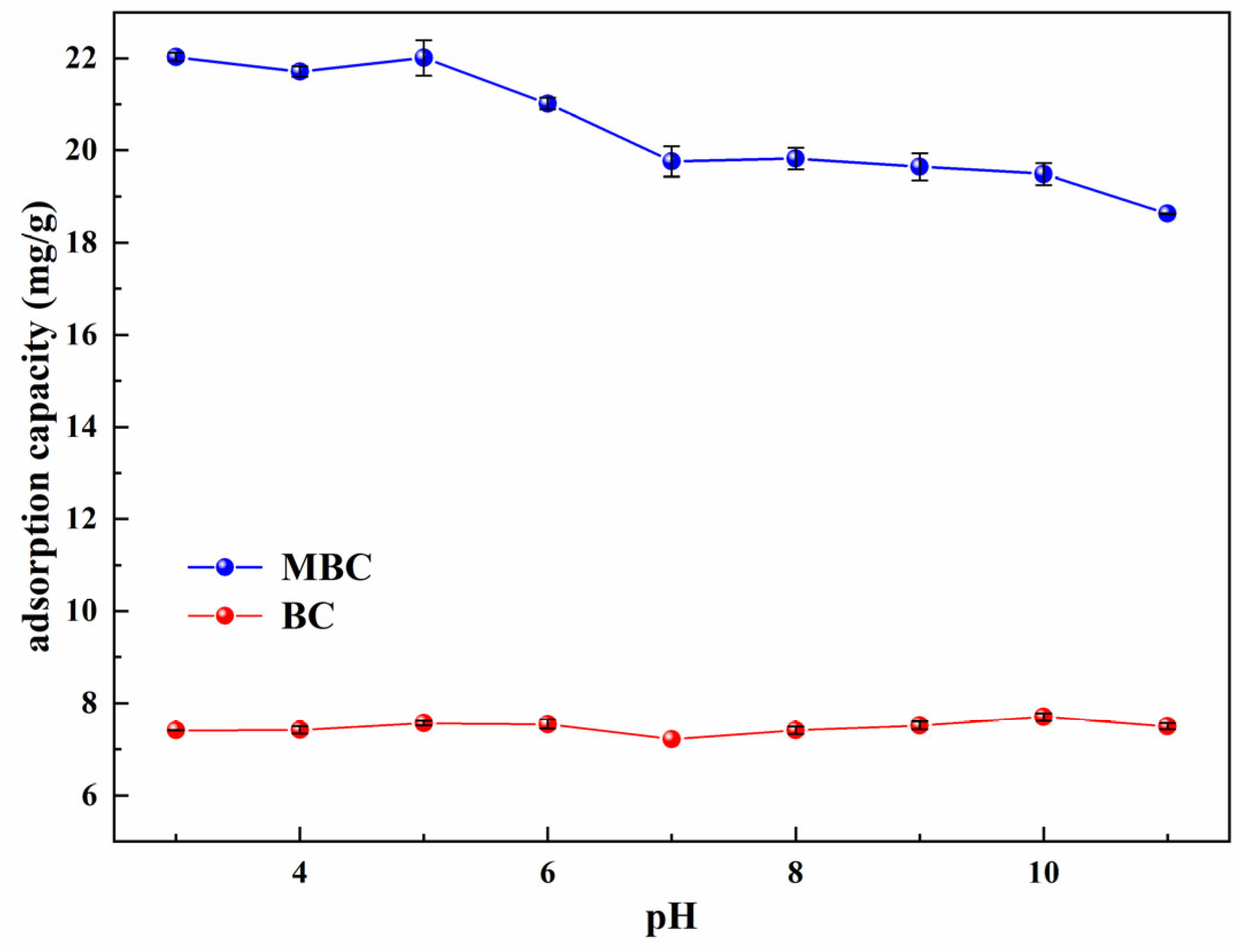
3.4. Effect of pH
3.5. The Removal Effect on Actual Wastewater Treatment
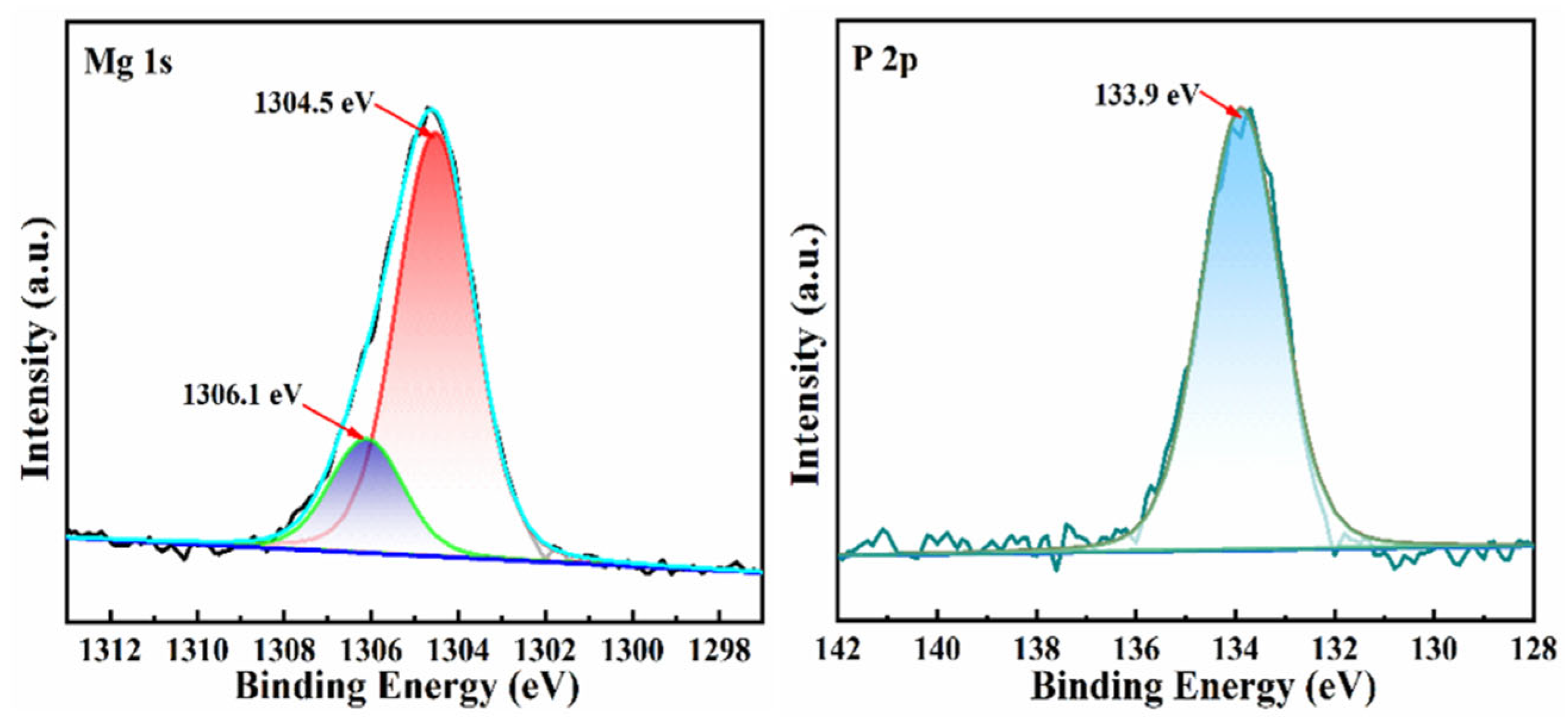
3.6. Mechanism of Phosphorus Adsorption by MBC
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jellali, S.; Hadroug, S.; Al-Wardy, M.; Al-Nadabi, H.; Nassr, N.; Jeguirim, M. Recent Developments in Metallic-Nanoparticles-Loaded Biochars Synthesis and Use for Phosphorus Recovery from Aqueous Solutions. A Critical Review. J. Environ. Manag. 2023, 342, 118307. [Google Scholar] [CrossRef] [PubMed]
- Priya, E.; Kumar, S.; Verma, C.; Sarkar, S.; Maji, P.K. A Comprehensive Review on Technological Advances of Adsorption for Removing Nitrate and Phosphate from Waste Water. J. Water Process. Eng. 2022, 49, 103159. [Google Scholar] [CrossRef]
- Pereira, A.C.; Mulligan, C.N. Practices for Eutrophic Shallow Lake Water Remediation and Restoration: A Critical Literature Review. Water 2023, 15, 2270. [Google Scholar] [CrossRef]
- Jadhav, S.V.; Bringas, E.; Yadav, G.D.; Rathod, V.K.; Ortiz, I.; Marathe, K.V. Arsenic and Fluoride Contaminated Groundwaters: A Review of Current Technologies for Contaminants Removal. J. Environ. Manag. 2015, 162, 306–325. [Google Scholar] [CrossRef]
- Ahmed, S.; Ashiq, M.N.; Li, D.; Tang, P.; Leroux, F.; Feng, Y. Recent Progress on Adsorption Materials for Phosphate Removal. Recent Pat. Nanotechnol. 2019, 13, 3–16. [Google Scholar] [CrossRef]
- Huang, Y.; He, Y.; Zhang, H.; Wang, H.; Li, W.; Li, Y.; Xu, J.; Wang, B.; Hu, G. Selective Adsorption Behavior and Mechanism of Phosphate in Water by Different Lanthanum Modified Biochar. J. Environ. Chem. Eng. 2022, 10, 107476. [Google Scholar] [CrossRef]
- Chen, W.; Chen, F.; Ren, Y. Preparation of Magnolia grandiflora Linn leaves biochar-loaded Mg oxide composite adsorbent and its adsorption performance for phosphorous solution. J. Henan Univ. Sci. Technol. (Nat. Sci.) 2022, 43, 99–104. [Google Scholar]
- Meng, Q.R.; Cui, X.H.; Zhu, Y.; Chu, X.L.; Zhang, Q. Characterization of MgO-loaded aquatic plants biochar and its adsorption capacity of phosphorus in aqueous solution. Acta Sci. Circumstantiae 2017, 37, 2960–2967. [Google Scholar]
- Yin, Q.; Liu, M.; Li, Y.; Li, H.; Wen, Z. Computational Study of Phosphate Adsorption on Mg/Ca Modified Biochar Structure in Aqueous Solution. Chemosphere 2021, 269, 129374. [Google Scholar] [CrossRef]
- Ok, Y.S.; Chang, S.X.; Gao, B.; Chung, H.-J. SMART Biochar Technology—A Shifting Paradigm towards Advanced Materials and Healthcare Research. Environ. Technol. Innov. 2015, 4, 206–209. [Google Scholar] [CrossRef]
- Ren, J.; Li, N.; Li, L.; An, J.-K.; Zhao, L.; Ren, N.-Q. Granulation and Ferric Oxides Loading Enable Biochar Derived from Cotton Stalk to Remove Phosphate from Water. Bioresour. Technol. 2015, 178, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, X.; Luo, W.; Sun, J.; Xu, Q.; Chen, F.; Zhao, J.; Wang, S.; Yao, F.; Wang, D.; et al. Effectiveness and Mechanisms of Phosphate Adsorption on Iron-Modified Biochars Derived from Waste Activated Sludge. Bioresour. Technol. 2018, 247, 537–544. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.J.; Zhou, B.; Awasthi, M.K.; Ali, A.; Zhang, Z.; Gaston, L.A.; Lahori, A.H.; Mahar, A. Enhancing Phosphate Adsorption by Mg/Al Layered Double Hydroxide Functionalized Biochar with Different Mg/Al Ratios. Sci. Total Environ. 2016, 559, 121–129. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Liu, D.; Wu, F.; Chen, Y.; He, Z.; Zhang, W.; Xing, S.; Mao, Y. Nitrogen Retention Potentials of Magnesium Oxide- and Sepiolite-Modified Biochars and Their Impacts on Bacterial Distribution under Nitrogen Fertilization. Sci. Total Environ. 2023, 866, 161358. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Chen, Y.; Yang, H.; Wang, S.; Wang, X.; Zhang, S.; Chen, H. Synthesis and Characterization of Magnesium Oxide Nanoparticle-Containing Biochar Composites for Efficient Phosphorus Removal from Aqueous Solution. Chemosphere 2020, 247, 125847. [Google Scholar] [CrossRef]
- Zeng, S.; Kan, E. Sustainable Use of Ca(OH)2 Modified Biochar for Phosphorus Recovery and Tetracycline Removal from Water. Sci. Total Environ. 2022, 839, 156159. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Yan, T.; Qiao, J.; Cao, H. Adsorption of Arsenic, Phosphorus and Chromium by Bismuth Impregnated Biochar: Adsorption Mechanism and Depleted Adsorbent Utilization. Chemosphere 2016, 164, 32–40. [Google Scholar] [CrossRef]
- Tee, K.A.; Ahmed, S.; Badsha, M.A.H.; Wong, K.C.J.; Lo, I.M.C. Comprehensive Review and Future Research Directions on Using Various Lanthanum-Based Adsorbents for Selective Phosphate Removal. Clean Technol. Environ. Policy 2023, 25, 1783–1805. [Google Scholar] [CrossRef]
- Zhang, X.; Xiong, Y.; Wang, X.; Wen, Z.; Xu, X.; Cui, J.; Liu, Z.; Wei, L.; An, X. MgO-Modified Biochar by Modifying Hydroxyl and Amino Groups for Selective Phosphate Removal: Insight into Phosphate Selectivity Adsorption Mechanism through Experimental and Theoretical. Sci. Total Environ. 2024, 918, 170571. [Google Scholar] [CrossRef]
- Zhu, D.; Yang, H.; Chen, X.; Chen, W.; Cai, N.; Chen, Y.; Zhang, S.; Chen, H. Temperature-Dependent Magnesium Citrate Modified Formation of MgO Nanoparticles Biochar Composites with Efficient Phosphate Removal. Chemosphere 2021, 274, 129904. [Google Scholar] [CrossRef]
- Fang, Y.; Ali, A.; Gao, Y.; Zhao, P.; Li, R.; Li, X.; Liu, J.; Luo, Y.; Peng, Y.; Wang, H.; et al. Preparation and Characterization of MgO Hybrid Biochar and Its Mechanism for High Efficient Recovery of Phosphorus from Aqueous Media. Biochar 2022, 4, 40. [Google Scholar] [CrossRef]
- Xu, Q.L.; Wang, L.; Wang, X.L.; Li, J.J.; Tan, M.X.; Geng, H.J. Adsorption Characteristics of Phosphorus in Wastewater by Canna indica Biochar. Wetl. Sci. 2022, 20, 754–761. [Google Scholar]
- Xu, Q.L.; Wang, L.; Tan, M.X.; Wang, X.L.; Li, J.J.; Geng, H.J. Phosphorus Removal from Aqueous Solution by Adsorption Using Wetland-Based Biochar: Batch Experiment. Green Process. Synth. 2022, 11, 555–562. [Google Scholar] [CrossRef]
- Yin, Q.; Wang, R.; Zhao, Z. Application of Mg–Al-Modified Biochar for Simultaneous Removal of Ammonium, Nitrate, and Phosphate from Eutrophic Water. J. Clean. Prod. 2018, 176, 230–240. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Chen, J.; Yang, L. Engineered Biochar Reclaiming Phosphate from Aqueous Solutions: Mechanisms and Potential Application as a Slow-Release Fertilizer. Environ. Sci. Technol. 2013, 47, 8700–8708. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Wang, J.; Wang, X.; Wang, Y.; Guo, Q.; Wang, Z.; Cui, X.; Hu, Y.; Yan, B.; Chen, G. Phosphorus-Enriched Biochar from Biogas Residue of Eichhornia Crassipes: Transformation and Release of Phosphorus. Biochar 2023, 5, 82. [Google Scholar] [CrossRef]
- Suwanree, S.; Knijnenburg, J.T.N.; Kasemsiri, P.; Kraithong, W.; Chindaprasirt, P.; Jetsrisuparb, K. Engineered Biochar from Sugarcane Leaves with Slow Phosphorus Release Kinetics. Biomass Bioenergy 2022, 156, 106304. [Google Scholar] [CrossRef]
- Fang, Q.; Chen, B.; Lin, Y.; Guan, Y. Aromatic and Hydrophobic Surfaces of Wood-Derived Biochar Enhance Perchlorate Adsorption via Hydrogen Bonding to Oxygen-Containing Organic Groups. Environ. Sci. Technol. 2014, 48, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Barrow, C.J. Biochar: Potential for Countering Land Degradation and for Improving Agriculture. Appl. Geogr. 2012, 34, 21–28. [Google Scholar] [CrossRef]
- Ahmad, M.; Lee, S.S.; Dou, X.; Mohan, D.; Sung, J.K.; Yang, J.E.; Ok, Y.S. Effects of Pyrolysis Temperature on Soybean Stover- and Peanut Shell-Derived Biochar Properties and TCE Adsorption in Water. Bioresour. Technol. 2012, 118, 536–544. [Google Scholar] [CrossRef]
- Al-Wabel, M.I.; Al-Omran, A.; El-Naggar, A.H.; Nadeem, M.; Usman, A.R. Pyrolysis Temperature Induced Changes in Characteristics and Chemical Composition of Biochar Produced from Conocarpus Wastes. Bioresour. Technol. 2013, 131, 374–379. [Google Scholar] [CrossRef]
- Aramendıa, M.A.; Benıtez, J.A.; Borau, V.; Jimenez, C.; Marinas, J.M.; Ruiz, J.R.; Urbano, F. Study of MgO and Pt/MgO Systems by XRD, TPR, and 1H. Langmuir 1999, 15, 1192–1197. [Google Scholar] [CrossRef]
- Hao, F.; Zhao, X.; Ouyang, W.; Lin, C.; Chen, S.; Shan, Y.; Lai, X. Molecular Structure of Corncob-Derived Biochars and the Mechanism of Atrazine Sorption. Agron. J. 2013, 105, 773–782. [Google Scholar] [CrossRef]
- Sun, T.; Sun, Y.; Huang, Q.; Xu, Y.; Jia, H. Sustainable Exploitation and Safe Utilization of Biochar: Multiphase Characterization and Potential Hazard Analysis. Bioresour. Technol. 2023, 383, 129241. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.R.; Medeiros, S.L.S.; Paixão, R.L.; Figueredo, I.M.; Mattos, A.L.A.; Rios, M.A.S. Thermogravimetric Pyrolysis of Residual Biomasses Obtained Post-Extraction of Carnauba Wax: Determination of Kinetic Parameters Using Friedman’s Isoconversional Method. Renew. Energy 2023, 207, 703–713. [Google Scholar] [CrossRef]
- Li, X.; Xu, K.; Zhang, Y.; Sun, C.; He, Y. Optical Determination of Lead Chrome Green in Green Tea by Fourier Transform Infrared (FT-IR) Transmission Spectroscopy. PLoS ONE 2017, 12, e0169430. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, M.; Yang, L.; Wu, Y.; Peng, Y.; Dai, L. Influence of Thermal Air Oxidation on the Chemical Composition and Uranium Binding Property of Intrinsic Dissolved Organic Matter from Biochar. Chemosphere 2023, 317, 137896. [Google Scholar] [CrossRef] [PubMed]
- Volkov, D.S.; Krivoshein, P.K.; Proskurnin, M.A. Detonation Nanodiamonds: A Comparison Study by Photoacoustic, Diffuse Reflectance, and Attenuated Total Reflection FTIR Spectroscopies. Nanomaterials 2020, 10, 2501. [Google Scholar] [CrossRef]
- Abdullah, M. Synthesis and Characterizations of Graphene/Copper Ferrite for Efficient Arsenic Removal. Water Air Soil Pollut. 2023, 234, 276. [Google Scholar] [CrossRef]
- Fang, L.; Li, J.; Donatello, S.; Cheeseman, C.R.; Poon, C.S.; Tsang, D.C.W. Use of Mg/Ca Modified Biochars to Take up Phosphorus from Acid-Extract of Incinerated Sewage Sludge Ash (ISSA) for Fertilizer Application. J. Clean. Prod. 2020, 244, 118853. [Google Scholar] [CrossRef]
- Antunes, E.; Jacob, M.V.; Brodie, G.; Schneider, P.A. Isotherms, Kinetics and Mechanism Analysis of Phosphorus Recovery from Aqueous Solution by Calcium-Rich Biochar Produced from Biosolids via Microwave Pyrolysis. J. Environ. Chem. Eng. 2018, 6, 395–403. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, G.; Wu, W.; Ali, A.; Wang, H.; Wang, Q.; Xu, Z.; Qi, W.; Li, R.; Zhang, Z. Magnetic Biochar Composite Decorated with Amino-Containing Biopolymer for Phosphorus Recovery from Swine Wastewater. Colloids Surf. A 2022, 634, 127980. [Google Scholar] [CrossRef]
- Li, Y.; Azeem, M.; Luo, Y.; Peng, Y.; Feng, C.; Li, R.; Peng, J.; Zhang, L.; Wang, H.; Zhang, Z. Phosphate Capture from Biogas Slurry with Magnesium-Doped Biochar Composite Derived from Lycium chinensis branch filings: Performance, mechanism, and effect of coexisting ions. Environ. Sci. Pollut. Res. 2022, 29, 84873–84885. [Google Scholar] [CrossRef] [PubMed]
- Nardis, B.O.; Franca, J.R.; da Silva Carneiro, J.S.; Soares, J.R.; Guilherme, L.R.G.; Silva, C.A.; Melo, L.C.A. Production of Engineered-Biochar under Different Pyrolysis Conditions for Phosphorus Removal from Aqueous Solution. Sci. Total Environ. 2022, 816, 151559. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Badot, P.-M. Application of Chitosan, a Natural Aminopolysaccharide, for Dye Removal from Aqueous Solutions by Adsorption Processes Using Batch Studies: A Review of Recent Literature. Prog. Polym. Sci. 2008, 33, 399–447. [Google Scholar] [CrossRef]
- Deng, W.; Zhang, D.; Zheng, X.; Ye, X.; Zhou, S. Adsorption Recovery of Phosphate from Waste Streams by Ca/Mg-Biochar Synthesis from Marble Waste, Calcium-Rich Sepiolite and Bagasse. J. Clean. Prod. 2020, 288, 125638. [Google Scholar] [CrossRef]
- Koh, K.Y.; Zhang, S.; Chen, J.P. Improvement of Ultrafiltration for Treatment of Phosphorus-Containing Water by a Lanthanum-Modified Aminated Polyacrylonitrile Membrane. ACS Omega 2020, 5, 7170–7181. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Koh, K.Y.; Li, R.; Zhang, H.; Yan, Y.; Chen, J.P. An Innovative Lanthanum Carbonate Grafted Microfibrous Composite for Phosphate Adsorption in Wastewater. J. Hazard. Mater. 2020, 392, 121952. [Google Scholar] [CrossRef]
- Shenmaishang Li, Z.Z.; Xue, W. Adsorption of Lead Ion from Aqueous Solution by Modified Walnut Shell: Kinetics and Thermodynamics. Environ. Technol. 2019, 40, 1810–1820. [Google Scholar]
- Jung, K.-W.; Jeong, T.-U.; Hwang, M.-J.; Kim, K.; Ahn, K.-H. Phosphate Adsorption Ability of Biochar/Mg–Al Assembled Nanocomposites Prepared by Aluminum-Electrode Based Electro-Assisted Modification Method with MgCl2 as Electrolyte. Bioresour. Technol. 2015, 198, 603–610. [Google Scholar] [CrossRef]
- Yi, M.; Li, T.T.; Li, H.H.; Huang, Q.; Yang, J.; Chen, Y.; Yang, Z.M. Characteristics of Phosphorus Adsorption in Aqueous Solution by Ca/MgLoaded Biogas Residue Biochar. Environ. Sci. 2019, 40, 1318–1327. [Google Scholar]



| Biochar | Yield (%) | Ash (%) | pH |
|---|---|---|---|
| BC | 27.23 | 41.00 | 10.95 |
| MBC | 40.11 | 49.12 | 11.18 |
| C (%) | H (%) | N (%) | O (%) | H/C | O/C | (O + N)/C | Mg (%) | P (%) | |
|---|---|---|---|---|---|---|---|---|---|
| BC | 39.24 | 1.343 | 1.39 | 17.02 | 0.034 | 0.433 | 0.469 | 0.06 | 1.43 |
| MBC | 26.50 | 0.900 | 0.55 | 22.93 | 0.033 | 0.486 | 0.507 | 0.39 | 0.72 |
| BET (m2/g) | Average Pore Diameter (nm) | Smicro (m2/g) | Vtotal (cm3/g) | Vmicro (cm3/g) | Vmicro/Vtotal(%) | |
|---|---|---|---|---|---|---|
| BC | 5.759 | 11.141 | 4.027 | 0.0160 | 0.00138 | 0.0863 |
| MBC | 8.147 | 8.325 | 3.472 | 0.0169 | 0.00106 | 0.0631 |
| Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|
| KL | qm | R2 | n | KF | R2 | |
| BC | 0.002 | 46.34 | 0.995 | 0.692 | 0.32 | 0.974 |
| MBC | −0.602 | 291.2 | 0.988 | 0.749 | 1.78 | 0.979 |
| Adsorbents | Adsorption Conditions | Dosage (g/L) | Qmax (mg/g) | References |
|---|---|---|---|---|
| MgCl2-modified sugarcane | C0 = 3–5800 mg/L; pH = 1.9 | 10 | 129.79 | [40] |
| Calcium-doped biochar | C0 = 100–1500 mg/L; pH = 4.5 | 10 | 147 | [41] |
| La(OH)3-supported corn straw magnetic biochar | C0 = 2–100 mg/L; pH = 7 | 0.5 | 116.08 | [42] |
| MgO-doped biochar | C0 = 0.5–2000 mg/L; pH = 7 | 2 | 129.4 | [43] |
| Lanthanum carbonate/biochar | C0 = 30–100 mg/L; pH = 7 | 0.4 | 71.9 | [6] |
| Pig manure, Brazil -N2-Mg | C0 = 10–10,000 mg/L; pH = − | 25 | 226.9 | [44] |
| MgO-modified canna | C0 = 10–1000 mg/L; pH = 7 | 1.67 | 244 | [this work] |
| Biochar | Quasi-First-Order Kinetic | Quasi-Second-Order Kinetic | Elovich Equation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| K1 | Qe | R2 | K2 | Qe | R2 | a1 | K3 | R2 | |
| BC | 0.04 | 6.14 | 0.69 | 0.006 | 6.81 | 0.85 | 0.943 | 0.584 | 0.98 |
| MBC | 0.0226 | 29.33 | 0.98 | 0.001 | 31.6 | 0.96 | 4.496 | 0.485 | 0.83 |
| Diffusion equation in particles | Stage I | Stage II | Stage III | ||||||
| C1 | Kd1 | R2 | C2 | Kd2 | R2 | C3 | Kd3 | R2 | |
| BC | 0.750 | 0.598 | 0.975 | 4.069 | 0.095 | 0.986 | 6.504 | 0.029 | 0.869 |
| MBC | −3.169 | 2.561 | 0.881 | 19.128 | 0.683 | 0.940 | 29.669 | −0.008 | 0.961 |
| BC | MBC | |||
|---|---|---|---|---|
| Initial phosphorus content (mg/L) | 2.06 | 199.8 | 2.06 | 199.8 |
| Phosphorus content after treatment (mg/L) | 0.69~0.76 | 54.06~55.10 | 0.12~0.14 | 1.45~1.55 |
| Average phosphorus removal rate (%) | 63.1~66.6 | 72.4~72.9 | 93.4~93.9 | 99.2~99.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, J.; Long, H.; He, X.; Chen, G.; Yuan, T.; Liu, Y.; Xu, Q. Synthesis of MgO-Coated Canna Biochar and Its Application in the Treatment of Wastewater Containing Phosphorus. Water 2024, 16, 873. https://doi.org/10.3390/w16060873
Xiao J, Long H, He X, Chen G, Yuan T, Liu Y, Xu Q. Synthesis of MgO-Coated Canna Biochar and Its Application in the Treatment of Wastewater Containing Phosphorus. Water. 2024; 16(6):873. https://doi.org/10.3390/w16060873
Chicago/Turabian StyleXiao, Jingjiang, Haiping Long, Xuemei He, Guoyu Chen, Tao Yuan, Yi Liu, and Qiaoling Xu. 2024. "Synthesis of MgO-Coated Canna Biochar and Its Application in the Treatment of Wastewater Containing Phosphorus" Water 16, no. 6: 873. https://doi.org/10.3390/w16060873
APA StyleXiao, J., Long, H., He, X., Chen, G., Yuan, T., Liu, Y., & Xu, Q. (2024). Synthesis of MgO-Coated Canna Biochar and Its Application in the Treatment of Wastewater Containing Phosphorus. Water, 16(6), 873. https://doi.org/10.3390/w16060873





