Abstract
Cadmium (Cd) water pollution threatens environmental systems and human health. Adsorption is the preferred method for purifying water bodies polluted by Cd, and the development of effective adsorption materials is critical. The performance of original phosphate rock powder (PRP) as an adsorption medium for purifying water bodies polluted by Cd was compared with that of phosphate rock powder modified with fulvic acid, chitosan, MnO2, and sulfhydryl, respectively, and their appearance and adsorption properties were investigated. The surface structures of all modified powders were rougher than the original, and their functional groups were richer. The greatest Cd2+ adsorption capacity, 1.88 mg g−1, was achieved with chitosan-modified PRP (CMPRP). This was 106.59% greater than that of PRP. The capacities of fulvic acid and MnO2 were 15.38% and 4.40% greater than that of the original, respectively. When the fulvic acid-modified PRP, CMPRP, and manganese dioxide PRP reached adsorption equilibrium, the removal rates of Cd2+ were 51.86%, 93.26%, and 46.70%, respectively. Moreover, the removal rate of Cd2+ by CMPRP was 104.43% higher than that of PRP. The main Cd2+ adsorption mechanisms for the MPRPs were electrostatic interactions, ion exchange, co-precipitation, and complexation. Moreover, the processing of the phosphate rock powder was straightforward, harmless to the environment, and could be effectively used for the removal of Cd. These results show that CMPRP is promising as a new adsorption material to treat Cd-contaminated water.
1. Introduction
Heavy metal contamination in water is of widespread concern and has become an environmental problem that cannot be ignored, particularly in developing countries where industry and agriculture have been rapidly developed [1], and large numbers of heavy metal ions drain into the water environment through electroplating, battery processing, dyes, and other industrial and agricultural practices [2]. Cadmium (Cd) is one of these environmental contaminants with severe toxicity; it has been classified as a class I carcinogen [3], and it is not an essential element required for human development or the growth of plants [4]. Water with Cd pollution affects the survival, growth, and development of aquatic organisms, such as fish and phytoplankton. Cd ions (Cd2+) accumulate in multiple organs, causing oxidative damage and immune system suppression [5]. Notably, Cd is easily absorbed by plants and affects their growth and development. The literature indicates that the absorption of mineral nutrients is affected by the interference of enzyme activity and the symbiotic relationship between plants and microorganisms [6,7] and is associated with the disruption of enzymes in the Calvin cycle [7,8], as well as crop yield reduction [9]. Cd2+ is readily soluble and biologically toxic, and it has the ability to enter the human body and accumulate through drinking water and other routes [10,11], where it causes harmful and irreversible damage to the immune system, bones, liver, and kidney, and induces a variety of cancers [12,13]. China and the United States stipulate that Cd heavy metal content should not exceed 0.005 mg L−1 for sources of drinking water [14]. As this level is readily exceeded, there is an urgent need to develop technologies that efficiently remove heavy metal ions from water environments in a simple, convenient, and environmentally friendly manner.
The adsorption method is simple, efficient, economically feasible, environmentally friendly, and safe [15]. As such, it is often applied to remove heavy metals in polluted aqueous environments [3,16]. Many heavy metal adsorbents with excellent adsorption properties have been developed to remove heavy metals from water environments in recent years, and these include biochar [17], natural clay [18], activated sludge [19], zeolite [20], and magnetic graphene oxide [21]. Phosphate rock (PR) powder (PRP) is a phosphorus-containing mineral that provides excellent performance, is simple to process, is environmentally friendly, and can be applied widely at a low cost [22]. Studies have used PRP to adsorb heavy metals from water-polluted environments, and the formation of phosphate precipitates fixed by heavy metal ions from the released phosphate ions has been observed [23,24]. Elouear et al. [25] found that PRP effectively removed Cd2+, Pb2+, Cu2+, and Zn2+ from a polluted water environment, with adsorption capacities of 10.46, 12.78, 9.76, and 8.54 mg g−1, respectively, and that PRP was more effective in adsorbing heavy metals at higher temperatures. Bashir et al. [26] indicated that PRP reduced the effective Cd content in Cd-contaminated red soil by 53.40% to 65.18%, and Sun et al. [27] found that PRP effectively adsorbed uranium (VI) in wastewater and determined its adsorption capacity for uranium to be 5.89 mg g−1.
However, China has limited PR resources, and most of the PR mined is of a low grade [28]. Previous studies have shown that modifying the activation mechanism of PRP can improve its ability to adsorb heavy metals as well as its utilization rate [29]. Chitosan is a polymer material containing active hydroxyl groups and amino, and it can be used as an adsorbent to remove heavy metals in water environments as it has a suitable pore size and volume, has a large surface area, is environmentally friendly, and the associated processing methods are straightforward [30]. Deng et al. [31] indicated that the adsorption capacity of heavy metal ions increased by 19.42% following the application of chitosan-modified biochar. Furthermore, Sun et al. [27] showed that modifying PRP with chitosan effectively reduced uranium pollution, and its adsorption capacity for uranium was 1.5 times that of PRP. Fulvic acid is the main component of humus found in the environment. It can adsorb and precipitate heavy metal pollutants in water environments [32], and heavy metals can also adsorb using fulvic acid-modified materials [33]. Lalas et al. [34] added fulvic acid to a mixed solution of toxic metals containing chromium and copper and found that their concentrations reduced by 60.0–97.8% and 55.1–83.3%, respectively. Manganese dioxide has many adsorption sites, a high surface activity, a good heavy metal ion adsorption performance, and it has also been used for the adsorption of cadmium from water environments [35]. Additionally, it has pore characteristics and redox abilities conducive to passivate heavy metals. Li et al. [36,37] indicated that the adsorption capacity of Pb2+ by MnO2 composite nanofiber materials was higher than that of a nanofiber material alone, which was 46.83 mg g−1, and the Pb2+ removal rate from wastewater reached 194.37%. Furthermore, thiol groups have a high affinity for silver, mercury, Cd, and Pb ions [38], and the adsorption properties of thiol-modified graphene were found to be significantly improved compared to those of the raw materials [36,37]. Sliesarenko et al. [39] indicated that loading thiol functional groups onto polymer membranes was more effective than adding raw materials to remove Pb2+, Cd2+, and Cr3+ from a water environment.
In this study, PRP was modified with xanthovic acid, chitosan, manganese dioxide, and sulfhydryl, respectively, and investigated for its efficacy in treating Cd-contaminated water. The purposes of this present study were as follows: (1) to characterize the morphology, composition, and functional groups of the novel modified PRP (MPRPs); (2) to evaluate their adsorption behaviors towards Cd2+ under the influence of different contact times, Cd2+ concentrations, and pH values, and to clarify their effectors in aqueous solutions; and (3) to elucidate the Cd2+ adsorption mechanisms of the MPRPs in aqueous systems.
2. Materials and Methods
2.1. Materials
PRP was purchased from Zibo Qingda Powder Material Engineering Co., LTD. (Zibo, China). Analytical-grade chemical reagents (hydrochloric acid, acetic acid, and N, and n-dimethylformamide solution) were provided by Kaitong Chemical Reagent Company (Tianjin, China). Analytical-grade fulvic acid, chitosan, manganese dioxide, thioglycolic acid, sodium hydroxide, glutaraldehyde, potassium permanganate, manganese sulfate monohydrate, anhydrous ethanol, acetone, sodium bisulfate monohydrate and sodium sulfide nonahydrate were purchased form Sinophosphoric Chemical Reagent Co., Ltd. (Shanghai, China).
2.2. Preparation of MPRPs
Fulvic acid MPRP (FMPRP): First, 1 L of deionized water was placed in a 5 L beaker and fulvic acid (10 g) was weighed and transferred to the beaker. Then, it was stirred with a magnetic stirrer (78–1, Guohua Instrument, Changzhou, China) until completely dissolved, and 50 g PRP and 2 g cetylammonium bromide were added. We continued to stir it for 5 min, then placed it in an ultrasonic instrument (KH–300E, Guohua Instrument, Changzhou, China) for ultrasonic dispersion for 0.5 h, and then stirred for 6 h. The obtained modified PRP was rinsed thrice with deionized water to remove the excess chemical reagent, then placed in an oven at 60 °C and dried to constant weight.
Chitosan MPRP (CMPRP): To prepare a 4% chitosan solution, 50 mL 2% acetic acid solution was added to 2 g chitosan and a magnetic agitator was applied until it was completely dissolved. Distilled water (100 mL) was added to 10 g PRP, a magnetic agitator was applied for 10 min until completely mixed, and the prepared 4% chitosan solution was then added and stirred for 30 min until fully mixed. The pH value of the above mixed solution was then adjusted to 10.0 with NaOH (0.1 mol L−1) solution. After cooling to 30 °C, 10 mL 2.5% glutaraldehyde solution was added and crosslinked at 30 °C for 24 h. The modified PRP was rinsed with deionized water to adjust its pH value to pH 7.0, then placed in an oven at 60 °C and dried to constant weight.
Manganese dioxide MPRP (MMPRP): First, 0.5 g PRP was soaked in 5% HCl for 18 h, washed five times with distilled water, placed in an oven at 60 °C and dried to constant weight, and then moved to a muffle furnace at 400 °C for high-temperature treatment for 3 h. The pretreated PRP was then placed into a conical bottle containing 100 mL KMnO4 (0.0232 mol L−1) solution, and 100 mL MnSO4·H2O (0.0345 mol L−1) solution was added. The sample was subsequently placed in a thermostatic water bath oscillator (35 °C, 200 rpm) (SHA–B, Yineng, Changzhou, China) for gel treatment for 2 h and then left to stand at room temperature for 10 h. The sample was then placed in a centrifuge and centrifuged (5000 rpm) for 10 min. The modified PRP was rinsed two to three times with deionized water to remove the residual reagent and placed in an oven at 85 °C until a constant sample weight was reached. Finally, the modified PRP was treated in a muffle furnace at high temperature (400 °C) for 3 h, and then taken out to cool to room temperature before grinding.
Mercaptic MPRP (SMPRP): PRP (30 g) was weighed and transferred into a grinding conical bottle. We added 900 mL NaOH (5 mol L−1) solution, sealed the bottle mouth, and performed alkalization for 24 h prior to solid–liquid separation. Subsequently, the pH was adjusted to near 7.0 with anhydrous ethanol and acetone solution, and the modified PRP was placed in an oven at 85 °C and dried to a constant weight. Two grams of the alkali-modified PRP was then placed in a grinding conical flask and 5 mL analytically pure N, and n–dimethylformamide solution was added, followed by 0.05 g NaHSO4·H2O and 11.77 mL mercaptoacetic acid. The sample was kept at 117 °C for 3 h, and 12.94 g Na2S·9H2O and 50 mL anhydrous ethanol solution were then added. The modified PRP was heated for 1 h, then placed in an oven to dry to a constant weight.
2.3. Characterization of Modified PRPs
The morphology and surface elements of the MPRPs were characterized through scanning electron microscopy (SEM) and energy–dispersive X–ray spectroscopy (EDS), respectively; the surface functional group compositions of the MPRPs were analyzed using Fourier transform infrared spectroscopy (FTIR); and the crystallization of the MPRPs was evaluated with X–ray diffraction (XRD).
2.4. Adsorption Experiments
Cd mono-element standard solution (1 mg mL−1) was provided by the National Nonferrous Metal and Electronic Materials Analysis and Test Centre. The solution contained different Cd2+ concentrations required for the adsorption test; it was prepared with Cd mono-element standard solution, and NaNO3 (0.01 mol L−1) solution was used as the supporting electrolyte to dilute Cd single-element standard solution.
2.4.1. Adsorption Kinetics Study
To test the adsorption kinetics, 0.5 g of FMPRP, or CMPRP, or MMPRP, or SMPRP, was placed into a 50 mL centrifuge tube, and then we added 25 mL Cd2+ (40 mg L−1) solution, respectively. The centrifuge tubes containing the samples were oscillated in a thermostatic water bath oscillator (25 °C, 220 rpm) to oscillate, and samples were taken at 10, 20, 40, 60, 80, 120, 240, 360, 720, and 1440 min in triplicate. Each test sample was centrifuged with a centrifuge, and the sample supernatant was filtered. The Cd2+ concentration in the supernatant of the adsorption kinetics test sample was determined through inductively coupled plasma mass spectrometry (ICP–MS; 7500, Agilent Technologies, Santa Clara, CA, USA), and the formula for calculating the Cd2+ adsorption capacity of the MPRPs is shown in Table 1.

Table 1.
Model formulae used in the adsorption kinetics test.
The adsorption kinetics process of Cd2+ on the PRP and MPRPs was quantitatively described using Lagrange’s pseudo-first-order (PFO) and pseudo-second-order (PSO) kinetic models. The PFO model described the adsorption kinetics in the solid-solution system, assuming that one sorption site on the MPRPs surface adsorbs one Cd ion. The kinetics of chemisorption in the liquid solution were analyzed using the pseudo-second-order rate expression. The PSO model assumes that two sorption sites on the MPRP’s surface adsorb one Cd ion [40,41]. Details of the formula for the Lagrange models are shown in Table 1.
Intraparticle diffusion (IPD) and liquid film diffusion (LFD) models were applied to analyze the diffusion mechanisms and rate-controlling step of Cd2+ adsorption with FMPRP, CMPRP, MMPRP, and SMPRP [42]. The detailed formulae of these models are described in Table 1.
The maximum rate of Cd2+ removal by the MPRPs was determined with an adsorption test. The formula used to calculate the Cd2+ removal rate (RR) is shown in Table 1.
2.4.2. Adsorption Isotherm Study
For the isothermal adsorption test, 0.5 g of FMPRP, CMPRP, MMPR, or SMPRP was placed into a 50 mL centrifuge tube, and then we added 25 mL Cd2+ (10–670 mg L−1) solution. The centrifuge tubes containing the samples were then placed in a thermostatic water bath oscillator (at 25 °C, 220 rpm) to oscillate for 24 h. Each test sample was centrifuged with a centrifuge, and the sample supernatant was filtered. Determination of Cd2+ concentration was performed with ICP–MS. The adsorption isotherms of Cd2+ for the MPRPs were quantitatively described using the Langmuir model and Freundlich model. The details formulae of these two models are shown in Table 2.

Table 2.
Model formulae used in the isothermal adsorption test.
The basic characteristics of the adsorption isotherms were analyzed to the constant KL value in the Langmuir model. The separation factor RL is related to the KL value, which was used to describe whether the MPRPs favored the Cd2+ adsorption process. Details of the formula for the RL value are shown in Table 2.
2.4.3. pH Adsorption Study
The Cd2+ adsorption capacities of MPRPs in different pH solutions were also studied. In this respect, 0.5 g samples of the FMPRP, CMPRP, MMPR, or SMPRP were added to 50 mL centrifuge tubes, and then 25 mL Cd2+ (40 mg L−1) solutions with pH values of 2, 4, 6, and 8 were added. The centrifuge tubes containing the samples were then oscillated in a thermostatic water bath oscillator (at 25 °C, 220 rpm) to oscillate for 24 h. Then, each test sample was centrifuged with a centrifuge, and the sample supernatant was filtered. Determination of Cd2+ concentration was performed via ICP–MS.
2.5. Data Analysis
Origin 2021 (version number: 9.80.200) and Statistical Product and Service Solutions 26 (version number: 26.0.0.0) were used for data processing and model analysis. The error bar in the figure represents the standard deviation of multiple measurements of the sample.
3. Results and Discussion
3.1. Characterization of MPRPs
When compared with PRP, the surfaces of FMPRP, CMPRP, MMPRP, and SMPRP were rougher and more irregular (Figure 1). FMPRP, CMPRP, MMPRP, and SMPRP all had partial cavity structures, which had different shapes and sizes and increased their surface areas. Some spherical structures with a small diameter were observed in the SEM images of MMPRP. In addition, some gauzy structures appeared on the surface of SMPRP, and the observed crystal morphology had changed. The energy spectrum analysis showed that the C and O contents in the four MPRPs were higher than those in PRP by 8.31% and 51.22%, 47.12% and 61.34%, 202.40% and 98.52%, and 169.01% and 98.28%, respectively. This phenomenon was related to the functional groups containing C or O in the modified material on the surface of the MPRPs, which provided increased heavy metal ion adsorption sites.
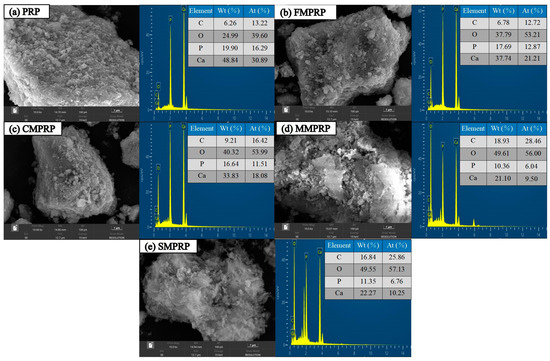
Figure 1.
Scanning electron microscopy (SEM) images and energy-dispersive X-ray spectroscopy (EDS) analysis of (a) PRP, (b) fulvic acid-modified phosphate rock powder (FMPRP), (c) chitosan-modified phosphate rock powder (CMPRP), (d) manganese dioxide-modified phosphate rock powder (MMPRP), and (e) mercaptic-modified phosphate rock powder (SMPRP).
The FTIR images of the different MPRPs and the PRP (Figure 2) show that the spectra of each exhibited seven common characteristic peaks: PO43− bending vibrations at 471, 576, and 603 cm−1; PO43− tensile vibrations at 1043 and 1094 cm−1; H2PO4− tensile vibrations at 1454 cm−1; and –OH tensile vibrations at 3500 cm−1 [43]. Compared to PRP, the FMPRP spectrum showed characteristic vibration peaks at 1630 and 2924 cm−1, representing the carboxyl functional groups and the aliphatic chain C–H in the fulvic acid, respectively [44,45]. The characteristic peaks of C–H and –NH2 appeared at 2882 and 3410 cm−1 in the CMPRP spectra. The reduced smoothness apparent in the CMPRP spectral images was in the range of 3000–3600 cm−1 and was due to the superposition of tensile vibrations of –NH2 and –OH [45,46]. A weak characteristic Mn–O vibration peak appeared at 529 cm−1 in the MMPRP spectrum [47]. However, the SMPRP spectrum had no obvious characteristic –SH peak at 2550 cm−1, possibly because of the severe reduction in the –SH group content of this sample after washing, which resulted in no characteristic peak [48]. The characteristic peak of SMPRP at 1454 cm−1 was weaker than that of the other PRPs, possibly due to the hydrolysis of H2PO4−.

Figure 2.
Fourier transform infrared spectroscopy results for phosphate rock powder (PRP), fulvic acid-modified phosphate rock powder (FMPRP), chitosan-modified phosphate rock powder (CMPRP), manganese dioxide-modified phosphate rock powder (MMPRP), and mercaptic-modified phosphate rock powder (SMPRP).
The XRD images of the different MPRPs and the PRP (Figure 3) show that the characteristic diffraction peaks of MPRPs and PRP were evidently sharp and their crystallinity was good. The main crystalline phases of PRP are fluorapatite [Ca5(PO4)3F] (PDF no. 99–0050), hydroxyapatite [Ca5(PO4)3(OH)] (PDF no. 86–0740), and dolomite [CaMg(CO3)2] (PDF no. 75–1656). There were changes in the XRD pattern of FMPRP at 2θ = 30.92°, and the crystallinity was significantly weaker than that of PRP, which could have been caused by changes in the spatial structure and crystal forms that prevented crosslinking by organic molecules [44]. The SMPRP characteristic diffraction peak at 2θ = 30.92° and 41.12° disappeared. It is possible that the modification of -SH destroyed the spatial structure of dolomite in this PRP and led to a crystal state transformation.

Figure 3.
XRD patterns of phosphate rock powder (PRP), fulvic acid-modified phosphate rock powder (FMPRP), chitosan-modified phosphate rock powder (CMPRP), manganese dioxide-modified phosphate rock powder (MMPRP), and mercaptic-modified phosphate rock powder (SMPRP).
3.2. Adsorption Kinetics
The Cd2+ adsorption capacities of PRP, FMPRP, CMPRP, MMPRP, and SMPRP changed over time (Figure 4). At the initial Cd2+ concentration of 40 mg L−1, the Cd2+ adsorption rates of PRP, FMPRP, CMPRP, MMPRP, and SMPRP increased rapidly within 0–2 h, after which they gradually decreased. FMPRP reached adsorption equilibrium after 6 h, while the adsorption rates of PRP, CMPRP, MMPRP, and SMPRP were slower, reaching adsorption equilibrium after 12 h. When PRP, FMPRP, CMPRP, MMPRP, and SMPRP reached adsorption equilibrium, the Cd2+ removal rates were 45.62%, 51.86%, 93.26%, 46.70%, and 27.58%, respectively. Therefore, the dynamic adsorption processes of the MPRPs comprised three stages: rapid, slow, and equilibrium adsorption. Due to the rich and unreacted adsorption sites on the surface of the MPRPs and the high Cd2+ concentration in the initial solution of the adsorption test [49], the MPRPs underwent rapid adsorption during the incipient stage. However, the adsorption rate of the MPRPs gradually decreased during the adsorption process and finally reached the equilibrium state, which was due to the limited number of adsorption sites on the surface of the MPRPs [50].

Figure 4.
Adsorption kinetics of Cd2+ with phosphate rock powder (PRP), fulvic acid–modified phosphate rock powder (FMPRP), chitosan–modified phosphate rock powder (CMPRP), manganese dioxide–modified phosphate rock powder (MMPRP), and mercaptic-modified phosphate rock powder (SMPRP).
The Cd2+ adsorption capacities of the MPRPs were calculated through nonlinear fitting of the PFO model and PSO model (Table 3, Figure 4). Compared with the two models, the correlation coefficient (R2) of the PSO model was the best, and the calculated Cd2+ adsorption capacity was in good agreement with the measured value. Therefore, the adsorption process of Cd2+ with the MPRPs was suitable to be described by PSO model, which also showed a chemical adsorption-based adsorption process. Moreover, according to the hypothesis of the PSO model, chemical adsorption may limit the rate of valence force through electron exchange or sharing between the MPRPs and Cd2+ [51]. When the initial concentration of Cd2+ was 40 mg L−1, it could be observed in the fitting parameters of the PSO model that the Cd2+ adsorption capacities of FMPRP, CMPRP, and MMPRP were 1.05, 1.88, and 0.95 mg g−1, being 15.38%, 106.59%, and 4.40% higher than that of PRP (0.91 mg g−1), respectively. However, the adsorption capacity of SMPRP for Cd2+ was only 0.54 mg g−1, which was 40.66% lower than that of PRP.

Table 3.
Pseudo-first-order (PFO) and pseudo-second-order (PSO) model parameters of Cd2+ adsorption kinetics on PRP, FMPRP, CMPRP, MMPRP, and SMPRP.
The rate of the MPRPs’ adsorption of Cd2+ during the adsorption kinetics process can be described with the IPD model and LFD model [52], and the different adsorption stages can also be described with the IPD and LFD models. Three stages were identified in the intraparticle diffusion fitting curves of the PRP and MPRP samples (see Supplementary Materials). The relevant fitting parameters had a high R2 (see Supplementary Materials), and there were no zero intercepts in each part, so the IPD model was not the only rate-controlling step of the MPRPs’ Cd2+ adsorption [53]. During the first stage, Cd2+ moved from the solution to the surfaces of the PRP and MPRPs, and this is represented by the LFD model. During the second stage, Cd2+ moved from the surface to the adsorption site and was adsorbed, and this reflected the intraparticle diffusion process. However, adsorption during this stage was slower than during the liquid film diffusion process [54], indicating that the intraparticle diffusion process mainly adjusted the adsorption rate. The last stage involved the adsorption of Cd2+ by the PRP and MPRPs until equilibrium was reached. The LFD model showed that the fitting results had a good linear relationship (see Supplementary Materials), and the fitted curve did not pass through the origin. These results indicate that the LFD model was also one of the rate-controlling steps of the MPRPs’ Cd2+ adsorption. In conclusion, the adsorption of Cd2+ onto PRP and MPRPs occurred as a multistep adsorption process, during which the rate of adsorption was affected by multiple mechanisms.
3.3. Adsorption Isotherms
The adsorption capacities of PRP, FMPRP, CMPRP, MMPRP, and SMPRP for Cd2+ increased with the increase in the concentration of the Cd2+ solution (Figure 5). The Cd2+ removal rates at concentrations of 10–130 mg L−1 for PRP, FMPRP, CMPRP, MMPRP, and SMPRP were 97.50–99.96%, 80.23–99.97%, 78.69–99.98%, 60.44–99.97%, and 60.44–99.97%, respectively. However, when the concentration of Cd2+ in the initial solution was increased to a higher level, the Cd2+ adsorption efficiency of the MPRPs gradually decreased. The removal rates of Cd2+ with PRP, FMPRP, CMPRP, MMPRP, and SMPRP in the range of Cd2+ concentrations of 370–670 mg L−1 were only 28.63–43.26%, 23.10–42.18%, 37.95–49.44%, 15.49–28.16%, and 15.49–28.16%, respectively. The relevant parameters of the Langmuir model and Freundlich model for the Cd2+ adsorption with the five PRPs are shown in Table 4, and the adsorption capacities of PRP, FMPRP, CMPRP, MMPRP, and SMPRP were 11.19, 7.76, 12.73, 5.40, and 1.61 mg g−1, respectively. The Cd2+ adsorption capacity of CMPRP was 13.76% higher than that of PRP. Compared with the two models, the correlation coefficient R2 of the Langmuir model was the best, and the fitting curve data were in good agreement with the measured values. Therefore, the adsorption isotherms of Cd2+ for the MPRPs were better described with the Langmuir model. This shows that the Cd2+ adsorption of the MPRPs was a single-layer adsorption process involving multiple adsorption sites on their surfaces [55]. In addition, the RL value was used to evaluate the degree of Cd2+ adsorption by the MPRPs, and the results were divided into irreversible (RL = 0), favorable (0 < RL < 1), and unfavorable (RL > 1) [56]. The RL values of PRP, FMPRP, CMPRP, MMPRP, and SMPRP were 0.0037–0.1996, 0.0057–0.2786, 0.0563–0.8000, 0.0090–0.3788, and 0.0188–0.5618, respectively, when the Cd2+ concentration of the initial solution ranged from 10 to 670 mg L−1, which indicated that the isothermal adsorption process of the MPRPs’ adsorption of Cd2+ was both favorable and feasible.
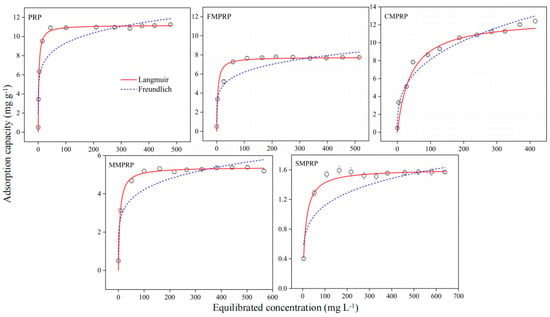
Figure 5.
Adsorption isotherms of Cd2+ on phosphate rock powder (PRP), fulvic acid–modified phosphate rock powder (FMPRP), chitosan–modified phosphate rock powder (CMPRP), manganese dioxide–modified phosphate rock powder (MMPRP), and mercaptic-modified phosphate rock powder (SMPRP).

Table 4.
Langmuir and Freundlich parameters of adsorption isotherms of Cd2+ on PRP, FMPRP, CMPRP, MMPRP, and SMPRP.
3.4. Effect of pH on Cd2+ Adsorption
The morphology of heavy metal ions and the charge on the surface of heavy metal adsorbents are influenced by the pH value [57]. As Cd2+ more readily precipitates in a solution with a higher pH value, the pH value range of the Cd2+ solution in this adsorption test was set to 2–8. The adsorption of Cd2+ by PRP, FMPRP, CMPRP, and MMPRP increased with the increase in pH from 2, and this could have been due to the large amount of H+ in a low-pH environment, which protonates the surface functional groups of the MPRPs, thereby increasing the positive charge on the surface of the MPRPs and producing electrostatic repulsion with positively charged Cd2+, thus affecting the adsorption of Cd2+ by the MPRPs. Therefore, when the pH increases, H+ gradually decreases, and the Cd2+ electrostatic attraction between the MPRPs and Cd2+ in the solution is enhanced (see Supplementary Materials). However, when the pH value of the Cd2+ solution exceeded 6, the Cd2+ adsorption capacity of PRP, FMPRP, CMPRP, and MMPRP began to decrease, which may be related to the gradual formation of OH− and Cd2+ into Cd(OH)2, Cd(OH)3−, and Cd(OH)4−, which reduced the electrostatic attraction between the MPRPs and Cd2+, thereby reducing the Cd2+ adsorption capacity of the MPRPs [58]. The Cd2+ adsorption capacity of SMPRP gradually increased with the increases in initial solution pH value. This is because when the pH increases from 7 to 8, the solution contains more OH−, and the affinity of Cd2+ with the SH group is higher than that with the OH group. Therefore, SMPRP reduced the effect of pH on its ability to adsorb Cd2+. The Cd2+ adsorption capacity of SMPRP increased rapidly when the solution pH increased from 6 to 8. The results showed that, compared to the PRP and other MPRPs, SMPRP is more suitable for Cd removal in environments with a pH greater than 8. The adsorption capacities of CMPRP for Cd2+ were 3.32%, 6.99%, 5.09%, and 11.42% higher than those of PRP at pH 2, 4, 6, and 8, respectively. The adsorption of Cd2+ by FMPRP and MMPRP was 4.98% and 1.33% higher than that of PRP at pH 8, respectively, indicating that electrostatic interactions may be the key mechanism involved in Cd2+ adsorption by CMPRP. The Cd2+ adsorption capacity of the MPRPs was more easily affected than that of PRP at pH 2–6, possibly because Cd2+ has a smaller ionic radius. With the solution at pH 8, the Cd2+ adsorption capacities of PRP, FMPRP, CMPRP, and MMPRP were decreased, but the Cd2+ adsorption capacity of PRP was the lowest, indicating that pH had little effect on the adsorption capacity of the MPRPs, and the modification was effective. The Cd2+ adsorption capacities of PRP, FMPRP, CMPRP, and MMPRP were the largest at an initial pH of 6, and the Cd2+ removal rates were 99.34%, 97.17%, 97.97%, and 97.00%, respectively. SMPRP exhibited the highest adsorption capacity at an initial pH of 8 with 51.96% Cd2+ removal. There was no significant difference between the Cd2+ adsorption of FMPRP and MMPRP at pH 6 and 8, respectively; however, the difference between the adsorption values at pH 2 and 4 was significant, indicating that the optimal pH range for FMPRP and MMPRP in this environment is pH 6–8. The adsorption capacity of CMPRP was significantly different at pH 2, 4, 6, and 8, and its adsorption amount was the highest at pH 6, where the rate of CMPRP’s removal of Cd2+ was 97.97%, indicating that the applicable pH range for CMPRP in this environment is approximately pH = 6.
3.5. Adsorption Mechanisms
Owing to the different types of heavy metals and basic materials, the overall mechanism of the adsorption of heavy metals by materials is highly complex [59], and a combination of multiple mechanisms is employed rather than a single mechanism [60]. The XRD spectra and FTIR images of PRP, FMPRP, CMPRP, MMPRP, and SMPRP after the adsorption of Cd2+ (see Supplementary Materials) were obtained to analyze the possible adsorption mechanisms. The intensity of the peaks at 2θ = 20.84°, 26.62°, and 50.16° increased following Cd2+ adsorption by the five materials, and no new characteristic peaks appeared. As reported in previous studies [61], this may be related to the co-precipitation of Cd2+ and calcium into the PRP structure and the interaction between the surface functional groups of PRP (such as POH) and Cd2+ in the solution [62], or to the presence of phosphorus in the PRP in the form of H2PO4− within the aqueous solution, as it forms phosphate precipitates with Cd2+ to immobilize heavy metals [63]. The EDS images showed that the PRP was rich in calcium, and Zhang et al. [64] found that Ca and divalent metal ions can undergo isomorphous substitution in relation to their electronegativities and ionic radii. The hydration radius of an ion affects the removal of heavy metal ions in water, and the hydration radius is related to the ion radius and charge. The results of the adsorption experiments in this study show that the MPRPs had a certain Cd2+ adsorption capacity, which may be due to the calcium-replacing part of the Cd2+ in the PRP isomorphous structure and the co-precipitation of calcium and Cd2+. This process can be simplified to a generalized adsorption process with the following formulae:
Ca5(PO4)3F + 6H+→5Ca2+ + 3H2PO4− + F−,
5M2+ + 3H2PO4− + F−→M5(PO4)3F + 6H+.
The dissolution of PRP in an aqueous solution releases Ca, H+, and acid radical ions. In this process, Ca is initially exchanged with heavy metals in the aqueous solution and subsequently precipitated [65]. In the FMPRP, CMPRP, MMPRP, and SMPRP Cd2+ adsorption experiments, the Cd2+ adsorption of MPRPs gradually increased with the increase in pH, possibly due to the decrease in the dissolution of PRP at a higher pH. A larger amount of the PRP was used for cation exchange, which indicated that the MPRPs adsorbed Cd2+ on their surfaces during ion exchange reactions. The FTIR images of PR, FMPRP, CMPRP, MMPRP, and SMPRP showed new characteristic peaks at 1430.79 cm−1. Two scattered peaks appeared in the range of 680–800 cm−1 with PR, FMPRP, and MMPRP. For MMPRP, a new characteristic peak emerged at 1629 cm−1, the PO43− characteristic peak disappeared at 1094 cm−1, the characteristic peak intensity of 1043 cm−1 increased, and the characteristic peak of Mn–O at 525 cm−1 weakened. CMPRP showed many small and dispersed peaks between 1300 and 1700 cm−1, which could be credited to the complex-forming reaction of Cd2+ with hydroxyl groups, H2PO4−, and PO43−, or to the surface adsorption of Cd2+ by the PRP. The fulvic acid component of FMPRP undergoes ion exchange and complexation, forming complexes with Cd and activating phosphorus. Its –COOH group can react with Ca2+ in the PRP, thereby releasing HPO42− or H2PO4− to fix the Cd2+. The molecular structure of the chitosan component of CMPRP contains large numbers of active carboxyl groups and amino that can form stable chelates with heavy metals. In addition, the sulfhydryl component of SMPRP can chelate Cd2+ to immobilize metal ions. Therefore, the four types of MPRPs adsorbed Cd2+ mainly through co-precipitation, ion exchange, complexation, and electrostatic interaction.
4. Conclusions
The Cd2+ adsorption processes of FMPRP, CMPRP, MMPRP, and SMPRP were effectively characterized using the Langmuir and PSO kinetic models. The primary mechanisms involved in their adsorption were electrostatic interactions, co-precipitation, ion exchange, and complexation. Notably, FMPRP, CMPRP, and MMPRP (particularly CMPRP) exhibited enhanced purification of the water with respect to the removal of Cd2+. The adsorption capacity of CMPRP for Cd2+ in the solution was significantly greater than that of FMPRP, MMPRP, and SMPRP. Future research should investigate the adsorption efficiencies of MPRPs for other combinations of inorganic and organic pollutants and their adsorption capacities for other heavy metal ions. Moreover, to increase the use of MPRPs, their desorption performances require further research.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/w16060862/s1: Figure S1. Kinetic fitting of Cd2+ on PRP and MPRPs: (a) intraparticle diffusion (IPD) model, (b) liquid-film diffusion (LFD) model. Figure S2. Effect of pH on the Cd2+ adsorption capacity of phosphate rock powder (PRP), fulvic acid modified phosphate rock powder (FMPRP), chitosan modified phosphate rock powder (CMPRP), manganese dioxide modified phosphate rock powder (MMPRP), and sulfhydryl modified phosphate rock powder (SMPRP). Figure S3. XRD patterns of phosphate rock powder (PRP), fulvic acid modified phosphate rock powder (FMPRP), chitosan modified phosphate rock powder (CMPRP), manganese dioxide modified phosphate rock powder (MMPRP), and sulfhydryl modified phosphate rock powder (SMPRP) after adsorption of Cd2+. Figure S4. Fourier transform infrared spectroscopy results of phosphate rock powder (PRP), fulvic acid modified phosphate rock powder (FMPRP), chitosan modified phosphate rock powder (CMPRP), manganese dioxide modified phosphate rock powder (MMPRP), and sulfhydryl modified phosphate rock powder (SMPRP) after adsorption of Cd2+. Table S1. Liquid-film diffusion (LFD) and Intraparticle diffusion (IPD) models for Cd2+.
Author Contributions
S.G.: Data curation, investigation, writing—original draft. X.K.: Investigation, resources. Y.L. (Yaping Li): Data curation, investigation. J.Y.: Investigation. H.W.: Methodology. H.P.: Software. Q.Y.: Formal analysis. Z.Y.: Software. Y.S.: Formal analysis. Y.Z.: Conceptualization, funding acquisition, writing—review and editing. Y.L. (Yanhong Lou): Conceptualization, funding acquisition, project administration, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of Shandong Province, PR China (ZR2022MD073), and the Major Science and Technology Innovation Projects of Shandong Province (2021CXGC010804).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liu, Y.; Luo, H.; Tie, B.; Li, D.; Liu, S.; Lei, M.; Du, H. The Long-Term Effectiveness of Ferromanganese Biochar in Soil Cd Stabilization and Reduction of Cd Bioaccumulation in Rice. Biochar 2021, 3, 499–509. [Google Scholar] [CrossRef]
- Yuan, Z.; Luo, T.; Liu, X.; Hua, H.; Zhuang, Y.; Zhang, X.; Zhang, L.; Zhang, Y.; Xu, W.; Ren, J. Tracing Anthropogenic Cadmium Emissions: From Sources to Pollution. Sci. Total Environ. 2019, 676, 87–96. [Google Scholar] [CrossRef]
- Kang, X.; Geng, N.; Li, X.; Yu, J.; Wang, H.; Pan, H.; Yang, Q.; Zhuge, Y.; Lou, Y. Biochar Alleviates Phytotoxicity by Minimizing Bioavailability and Oxidative Stress in Foxtail Millet (Setaria italica L.) Cultivated in Cd- and Zn-Contaminated Soil. Front. Plant Sci. 2022, 13, 782963. [Google Scholar] [CrossRef]
- Ren, T.; Chen, N.; Wan Mahari, W.A.; Xu, C.; Feng, H.; Ji, X.; Yin, Q.; Chen, P.; Zhu, S.; Liu, H.; et al. Biochar for Cadmium Pollution Mitigation and Stress Resistance in Tobacco Growth. Environ. Res. 2021, 192, 110273. [Google Scholar] [CrossRef]
- Zamora-Ledezma, C.; Negrete-Bolagay, D.; Figueroa, F.; Zamora-Ledezma, E.; Ni, M.; Alexis, F.; Guerrero, V.H. Heavy Metal Water Pollution: A Fresh Look About Hazards, Novel and Conventional Remediation Methods. Environ. Technol. Innov. 2021, 22, 101504. [Google Scholar] [CrossRef]
- Pidlisnyuk, V.; Zgorelec, Ž. Impact of Nutrients and Trace Elements in Soil on Plant Growth: Case of the Second-Generation Energy Crops. Agronomy 2022, 12, 2768. [Google Scholar] [CrossRef]
- Islam, M.M.; Hoque, M.A.; Okuma, E.; Banu, M.N.; Shimoishi, Y.; Nakamura, Y.; Murata, Y. Exogenous Proline and Glycinebetaine Increase Antioxidant Enzyme Activities and Confer Tolerance to Cadmium Stress in Cultured Tobacco Cells. J. Plant Physiol. 2009, 166, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Per, T.S.; Khan, S.; Asgher, M.; Bano, B.; Khan, N.A. Photosynthetic and Growth Responses of Two Mustard Cultivars Differing in Phytocystatin Activity Under Cadmium Stress. Photosynthetica 2016, 54, 491–501. [Google Scholar] [CrossRef]
- Chellaiah, E. Cadmium (Heavy Metals) Bioremediation by Pseudomonas aeruginosa: A Minireview. Appl. Water Sci. 2018, 8, 151. [Google Scholar] [CrossRef]
- Cui, S.; Gao, S.; Zhang, F.; Fu, Q.; Wang, M.; Liu, D.; Li, K.; Song, Z.; Chen, P. Heavy Metal Contamination and Ecological Risk in Sediment from Typical Suburban Rivers. River Res. Appl. 2021, 37, 1080–1088. [Google Scholar] [CrossRef]
- Geng, W.; Xiao, X.; Zhang, L.; Ni, W.; Li, N.; Li, Y. Response and Tolerance Ability of Chlorella vulgaris to Cadmium Pollution Stress. Environ. Tech. 2022, 43, 4391–4401. [Google Scholar] [CrossRef]
- Yu, Z.; Tang, J. Quality Improvement in Vegetable Greenhouse by Cadmium Pollution Remediation. J. Food Qual. 2022, 2022, 8335753. [Google Scholar] [CrossRef]
- Oladoye, P.O.; Olowe, O.M.; Asemoloye, M.D. Phytoremediation Technology and Food Security Impacts of Heavy Metal Contaminated Soils: A Review of Literature. Chemosphere 2022, 288, 132555. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Nan, J.; Mu, Y.; Zu, X.; Guo, M. Study on the Treatment of Sudden Cadmium Pollution in Surface Water by a Polymer Enhanced Ultrafiltration Process. RSC Adv. 2021, 11, 7405–7415. [Google Scholar] [CrossRef] [PubMed]
- Che, N.; Liu, N.; Li, Y.; Li, C.; Liu, Y.; Li, C. Three Dimensional BC/rGA Aerogel: Preparation, Characterization, and Adsorption of Cr(VI). Biochar 2022, 4, 65. [Google Scholar] [CrossRef]
- Seliem, M.K.; Mobarak, M. Cr(VI) Uptake by a New Adsorbent of CTAB–Modified Carbonized Coal: Experimental and Advanced Statistical Physics Studies. J. Mol. Liq. 2019, 294, 111676. [Google Scholar] [CrossRef]
- Kamran, M.; Malik, Z.; Parveen, A.; Zong, Y.; Abbasi, G.H.; Rafiq, M.T.; Shaaban, M.; Mustafa, A.; Bashir, S.; Rafay, M.; et al. Biochar Alleviates Cd Phytotoxicity by Minimizing Bioavailability and Oxidative Stress in Pak Choi (Brassica chinensis L.) Cultivated in Cd-Polluted Soil. J. Environ. Manag. 2019, 250, 109500. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, S.; Ding, D.; Chen, J.; Yang, Y.; Lei, Z.; Feng, C.; Zhang, Z. Effective Adsorption of Cr (VI) from Aqueous Solution Using Natural Akadama Clay. J. Colloid Interface Sci. 2013, 395, 198–204. [Google Scholar] [CrossRef]
- Wang, X.; Xia, S.; Chen, L.; Zhao, J.; Chovelon, J.; Nicole, J.J. Biosorption of cadmium(II) and lead(II) ions from aqueous solutions onto dried activated sludge. Environ. Sci. 2006, 18, 840–844. [Google Scholar] [CrossRef]
- Roshanfekr Rad, L.R.; Anbia, M. Zeolite-Based Composites for the Adsorption of Toxic Matters from Water: A Review. J. Environ. Chem. Eng. 2021, 9, 106088. [Google Scholar] [CrossRef]
- Guo, Y.; Deng, J.; Zhu, J.; Zhou, X.; Bai, R. Removal of Mercury(II) and Methylene Blue from a Wastewater Environment with Magnetic Graphene Oxide: Adsorption Kinetics, Isotherms and Mechanism. RSC Adv. 2016, 6, 82523–82536. [Google Scholar] [CrossRef]
- Ren, W.; Xia, W.; Wei, M.; Du, Y. Research Progress in Remediation of Pb/Zn-Contaminated Soil by Modified Phosphate Rock Powder. J. Nanjing Technol. Natl Sci. 2015, 13. [Google Scholar] [CrossRef]
- Li, Q.; Zhong, H.; Cao, Y. Effects of the Joint Application of Phosphate Rock, Ferric Nitrate and Plant Ash on the Immobility of As, Pb and Cd in Soils. J. Environ. Manag. 2020, 265, 110576. [Google Scholar] [CrossRef]
- Xie, Z.M.; Wang, B.L.; Sun, Y.F.; Li, J. Field Demonstration of Reduction of Lead Availability in Soil and Cabbage (Brassica chinensis L.) Contaminated by Mining Tailings Using Phosphorus Fertilizers. J. Zhejiang Univ. Sci. B. 2006, 7, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Elouear, Z.; Bouzid, J.; Boujelben, N.; Feki, M.; Jamoussi, F.; Montiel, A. Heavy Metal Removal from Aqueous Solutions by Activated Phosphate Rock. J. Hazard. Mater. 2008, 156, 412–420. [Google Scholar] [CrossRef]
- Bashir, S.; Zhu, J.; Fu, Q.; Hu, H. Cadmium Mobility, Uptake and Anti-oxidative Response of Water Spinach (Ipomoea aquatic) Under Rice Straw Biochar, Zeolite and Rock Phosphate as Amendments. Chemosphere 2018, 194, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Chen, D.; Chen, B.; Kong, L.; Su, M. Enhanced Uranium(VI) Adsorption by Chitosan Modified Phosphate Rock. Colloids Surf. A Physicochem. Eng. Asp. 2018, 547, 141–147. [Google Scholar] [CrossRef]
- Gilbert, N. Environment: The Disappearing Nutrient. Nature 2009, 461, 718–761. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Shao, C.; Chen, W.; Lei, Y.; Ke, Q.; Guo, Y. Mesoporous Carbonated Hydroxyapatite/chitosan porous materials for removal of Pb(II) ions under flow conditions. RSC Adv. 2016, 6, 113940–113950. [Google Scholar] [CrossRef]
- Haripriyan, U.; Gopinath, K.P.; Arun, J. Chitosan Based Nano Adsorbents and Its Types for Heavy Metal Removal: A Mini Review. Mater. Lett. 2022, 312, 131670. [Google Scholar] [CrossRef]
- Deng, J.; Liu, Y.; Liu, S.; Zeng, G.; Tan, X.; Huang, B.; Tang, X.; Wang, S.; Hua, Q.; Yan, Z. Competitive Adsorption of Pb(II), Cd(II) and Cu(II) onto Chitosan-Pyromellitic Dianhydride Modified Biochar. J. Colloid Interface Sci. 2017, 506, 355–364. [Google Scholar] [CrossRef]
- Li, W.; Zhang, F.; Ye, Q.; Wu, D.; Wang, L.; Yu, Y.; Deng, B.; Du, J. Composition and Copper Binding Properties of Aquatic Fulvic Acids in Eutrophic Taihu Lake, China. Chemosphere 2017, 172, 496–504. [Google Scholar] [CrossRef]
- Wang, H.; Xing, L.; Zhang, H.; Gui, C.; Jin, S.; Lin, H.; Li, Q.; Cheng, C. Key Factors to Enhance Soil Remediation by Bioelectrochemical Systems (BESs): A Review. Chem. Eng. J. 2021, 419, 129600. [Google Scholar] [CrossRef]
- Lalas, S.; Athanasiadis, V.; Dourtoglou, V.G. Humic and Fulvic Acids as Potentially Toxic Metal Reducing Agents in Water. CLEAN Soil Air Water 2018, 46, 1700608. [Google Scholar] [CrossRef]
- Wang, P.; Cheng, H.; Ding, J.; Ma, J.; Jiang, J.; Huang, Z.; Li, J.; Pang, S.; Guan, C.; Gao, Y. Cadmium Removal with Thiosulfate/Permanganate (TS/Mn(VII)) System: MnO2 Adsorption and/or CdS Formation. Chem. Eng. J. 2020, 380, 122585. [Google Scholar] [CrossRef]
- Li, B.; Gong, J.; Fang, J.; Zheng, Z.; Fan, W. Cysteine Chemical Modification for Surface Regulation of Biochar and Its Application for Polymetallic Adsorption from Aqueous Solutions. Environ. Sci. Pollut. Res. Int. 2021, 28, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, Y.; Liu, J.; Chao, S.; Yang, T.; Li, L.; Wang, C.; Li, X. A Novel Hollow Carbon@MnO2 Electrospun Nanofiber Adsorbent for Efficient Removal of Pb2+ in Wastewater. Chem. Res. Chin. Univ. 2021, 37, 496–504. [Google Scholar] [CrossRef]
- Stolyarchuk, N.V.; Kolev, H.; Kanuchova, M.; Keller, R.; Vaclavikova, M.; Melnyk, I.V. Synthesis and Sorption Properties of Bridged Polysilsesquioxane Microparticles Containing 3-Mercaptopropyl Groups in the Surface Layer. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 694–702. [Google Scholar] [CrossRef]
- Sliesarenko, V.; Tomina, V.; Dudarko, O.; Bauman, M.; Lobnik, A.; Melnyk, I. Functionalization of Polymeric Membranes with Phosphonic and Thiol Groups for Water Purification from Heavy Metal Ions. Appl. Nanosci. 2020, 10, 337–346. [Google Scholar] [CrossRef]
- Boparai, H.K.; Joseph, M.; O’Carroll, D.M. Kinetics and Thermodynamics of Cadmium Ion Removal by Adsorption onto Nano Zerovalent Iron Particles. J. Hazard. Mater. 2011, 186, 458–465. [Google Scholar] [CrossRef]
- Russakova, A.V.; Altynbaeva, L.S.; Barsbay, M.; Zheltov, D.A.; Zdorovets, M.V.; Mashentseva, A.A. Kinetic and Isotherm Study of As(III) Removal from Aqueous Solution by PET Track-Etched Membranes Loaded with Copper Microtubes. Membranes 2021, 11, 116. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, X.; Tang, L.; Zhang, F.; Zeng, G.; Peng, X.; Luo, L.; Deng, Y.; Pang, Y.; Zhang, J. Insight into Highly Efficient Co-removal of p-Nitrophenol and Lead by Nitrogen-Functionalized Magnetic Ordered Mesoporous Carbon: Performance and Modelling. J. Hazard. Mater. 2017, 333, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Khaled, H.; Hafed, E.; Olivier, M.; Christophe, D. Adsorption of Nucleotides on Biomimetic Apatite: The Case of Adenosine 5′-Triphosphate (ATP). Appl. Surf. Sci. 2017, 360, 979–988. [Google Scholar]
- Wang, G.; Bo, W.; Wan, K.; Fan, J.; Miao, Z.; Xue, S. Remediation of the Soil Contaminated by Heavy Metals with Nano-hydroxy Iron Phosphate Coated with Fulvic Acid. Environ. Tech. 2022, 44, 4123–4135. [Google Scholar] [CrossRef]
- Perumal, S.; Atchudan, R.; Yoon, D.H.; Joo, J.; Cheong, I.W. Graphene Oxide-Embedded Chitosan/Gelatin Hydrogel Particles for the Adsorptions of Multiple Heavy Metal Ions. J. Mater. Sci. 2020, 55, 9354–9363. [Google Scholar] [CrossRef]
- Xu, H.; Li, X.; Gao, M.; Hu, X.; Zhang, X.; Li, Y.; Xu, X.; Hu, J.; Tang, C.; Hu, X. Chitosan and Biochar Synergize the Efficient Elimination of Lead from Wastewater by Sulfidised Nano-zero-Valent Iron. J. Environ. Chem. Eng. 2022, 10, 107101. [Google Scholar] [CrossRef]
- Xiong, T.; Yuan, X.; Cao, X.; Wang, H.; Jiang, L.; Wu, Z.; Liu, Y. Mechanistic Insights into Heavy Metals Affinity in Magnetic MnO2@Fe3O4/Poly(m-Phenylenediamine) Core–Shell Adsorbent. Ecotoxicol. Environ. Saf. 2020, 192, 110326. [Google Scholar] [CrossRef]
- Chen, B.; Li, L.; Liu, L.; Cao, J. Effective Adsorption of Heavy Metal Ions in Water by Sulfhydryl Modified Nano Titanium Dioxide. Front. Chem. 2022, 10, 1072139. [Google Scholar] [CrossRef]
- Zeng, M.; Zhou, X.; Guo, J.; Liu, K.; Zhong, C.; Liu, Y. In Situ Remediation of Cd(II) Contaminated Paddy Fields with Activated Ca Si Mineral Material Derived from Potash Feldspar and Its Mechanism. Ecol. Eng. 2020, 158, 106052. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, H.; Sárossy, Z.; Dong, C.; Glarborg, P. Release and Transformation of Chlorine and Potassium During Pyrolysis of KCl Doped Biomass. Fuel 2017, 197, 422–432. [Google Scholar] [CrossRef]
- Wang, Z.; Yin, P.; Qu, R.; Chen, H.; Wang, C.; Ren, S. Adsorption Kinetics, Thermodynamics and Isotherm of Hg(II) from Aqueous Solutions Using Buckwheat Hulls from Jiaodong of China. Food Chem. 2013, 136, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Wang, Y.; Hu, L.; Gao, L.; Du, B.; Wei, Q. Mechanism of Pb(ii) and Methylene Blue Adsorption onto Magnetic Carbonate Hydroxyapatite/Graphene Oxide. RSC Adv. 2015, 5, 9759–9770. [Google Scholar] [CrossRef]
- Rengaraj, S.; Moon, S.H. Kinetics of Adsorption of Co(II) Removal from Water and Wastewater by Ion Exchange Resins. Water Res. 2002, 36, 1783–1793. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Estupiñán, P.; Erto, A.; Giraldo, L.; Moreno-Piraján, J.C. Adsorption of Cd(II) on Modified Granular Activated Carbons: Isotherm and Column Study. Molecules 2017, 22, 2280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gao, S.; Cao, X.; Lin, J.; Feng, J.; Wang, H.; Pan, H.; Yang, Q.; Lou, Y.; Zhuge, Y. Cd Removal from Aqueous Solutions Using a New Modified Zeolite Adsorbent. Minerals 2023, 13, 197. [Google Scholar] [CrossRef]
- Kara, A.; Demirbel, E.; Tekin, N.; Osman, B.; Beşirli, N. Magnetic Vinylphenyl Boronic Acid Microparticles for Cr(VI) Adsorption: Kinetic, Isotherm and Thermodynamic Studies. J. Hazard. Mater. 2015, 286, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Xu, Y.; Huang, Q.; Sun, Y.; Liang, X.; Wang, L.; Qin, X.; Zhao, L. Adsorption characteristics and the removal mechanisms of two novel Fe-Zn composite modified biochars for Cd(II) in water. Bioresour. Technol. 2021, 333, 125078. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, C.; Zhang, L.; Liu, H.; Cao, B.; Liu, L.; Gong, W. Adsorption Studies of Cadmium onto Magnetic Fe3O4@FePO4 and Its Preconcentration with Detection by Electrothermal Atomic Absorption Spectrometry. Talanta 2018, 181, 352–358. [Google Scholar] [CrossRef]
- Qiu, B.; Tao, X.; Wang, H.; Li, W.; Ding, X.; Chu, H. Biochar as a Low-Cost Adsorbent for Aqueous Heavy Metal Removal: A Review. J. Anal. Appl. Pyrol. 2021, 155, 105081. [Google Scholar] [CrossRef]
- Mariana, M.; Hps, A.K.; Mistar, E.M.; Yahya, E.; Alfatah, T. Recent advances in activated carbon modification techniques for enhanced heavy metal adsorption. J. Water Process Eng. 2021, 43, 102221. [Google Scholar] [CrossRef]
- Xu, Y.; Schwartz, F.W.; Traina, S.J. Sorption of Zn2+ and Cd2+ on Hydroxyapatite Surfaces. Environ. Sci. Technol. 1994, 28, 1472–1480. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.; Prasad, M.; Goswami, R.; Ranshore, S.; Kulshreshtha, A.; Kumar Sinha, A.S. Kinetic Model for Sorption of Divalent Heavy Metal Ions on Low Cost Minerals. Korean J. Chem. Eng. 2016, 33, 649–656. [Google Scholar] [CrossRef]
- Du, Y.; Wei, L.; Reddy, K.; Jin, F.; Wu, H.; Liu, Z. New Phosphate-Based Binder for Stabilization of Soils Contaminated with Heavy Metals: Leaching, Strength and Microstructure Characterization. J. Environ. Manag. 2014, 146, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Guo, G.; Wang, M.; Zhang, J.; Wang, Z.; Li, F.; Chen, H. Enhanced Stabilization of Pb, Zn, and Cd in Contaminated Soils Using Oxalic Acid-Activated Phosphate Rocks. Environ. Sci. Pollut. Res. Int. 2018, 25, 2816–2886. [Google Scholar] [CrossRef]
- Saxena, S.; D’Souza, S.F. Heavy Metal Pollution Abatement Using Rock Phosphate Mineral. Environ. Int. 2006, 32, 199–202. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).