Organic Micropollutants in the Agricultural Chain of Production of Strawberries by Irrigation with Treated Wastewater and Assessment of Human Health Implications
Abstract
1. Introduction
2. Materials and Methods
2.1. Abbreviations of Analytes
2.2. Reagents
2.3. Instrumentation
2.4. Treated Wastewaters
2.5. Strawberry Cultivation
2.6. Extraction Procedures
2.7. Health Risk Assessment
3. Results and Discussion
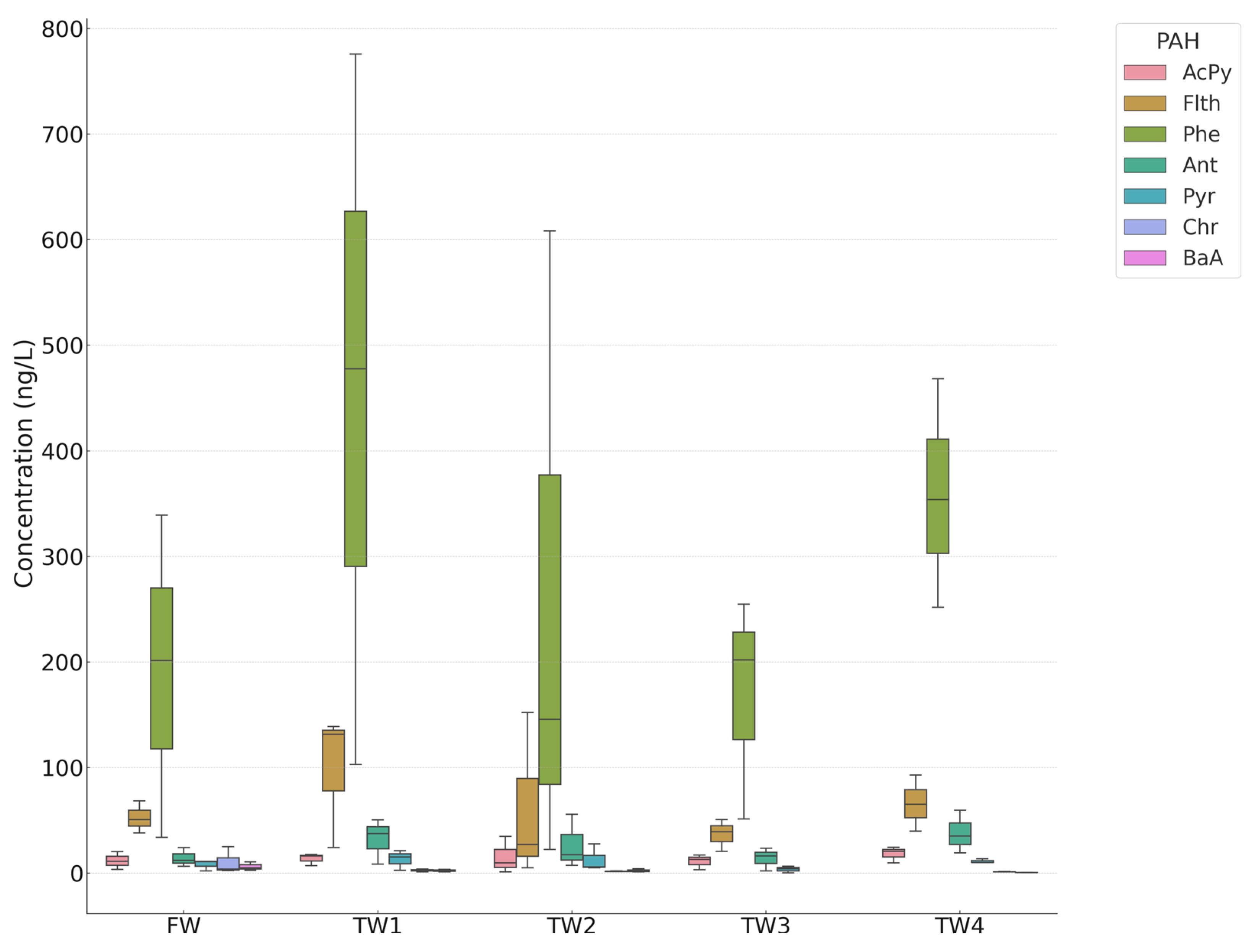
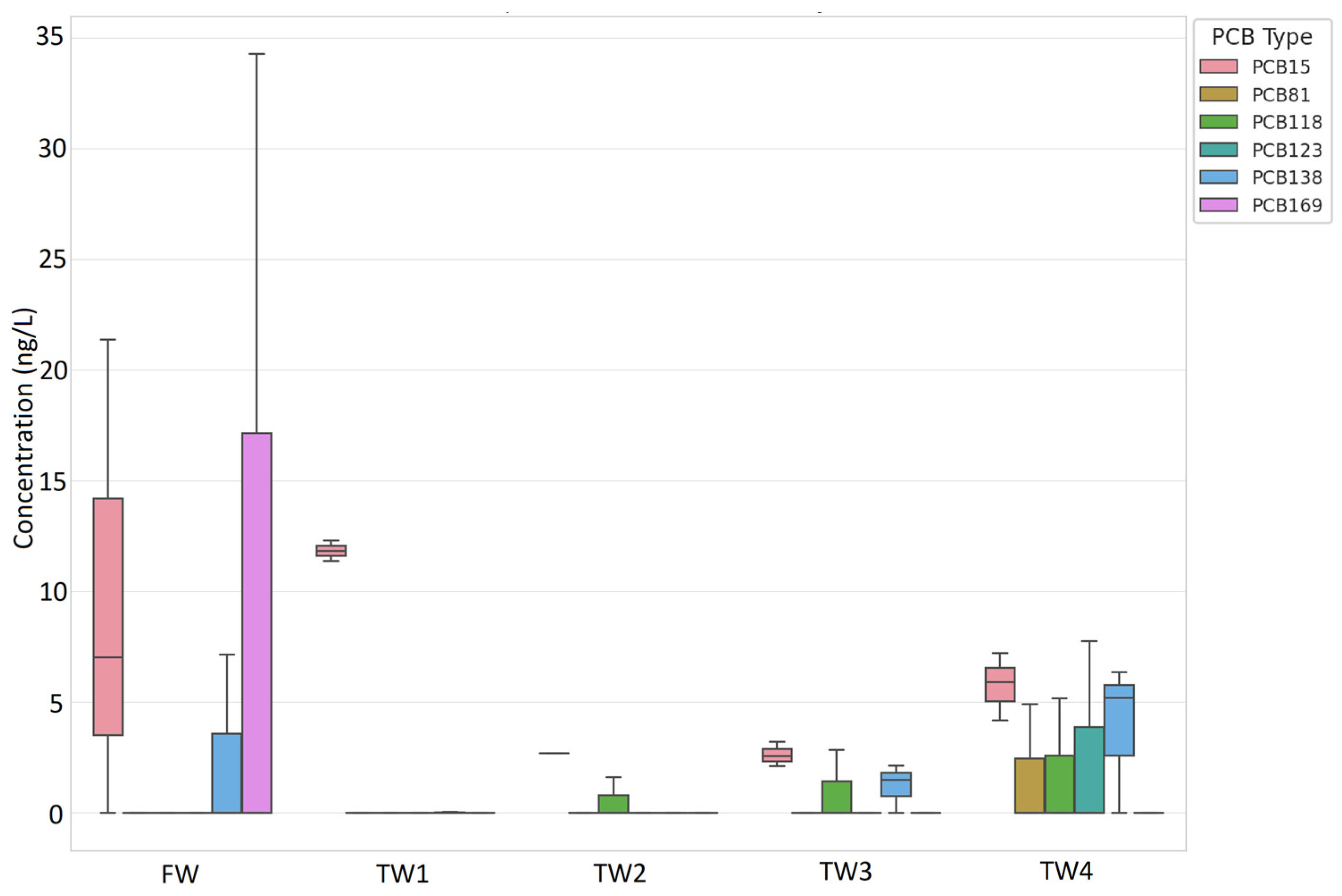
3.1. Treated Wastewaters
3.1.1. Figures of Merit
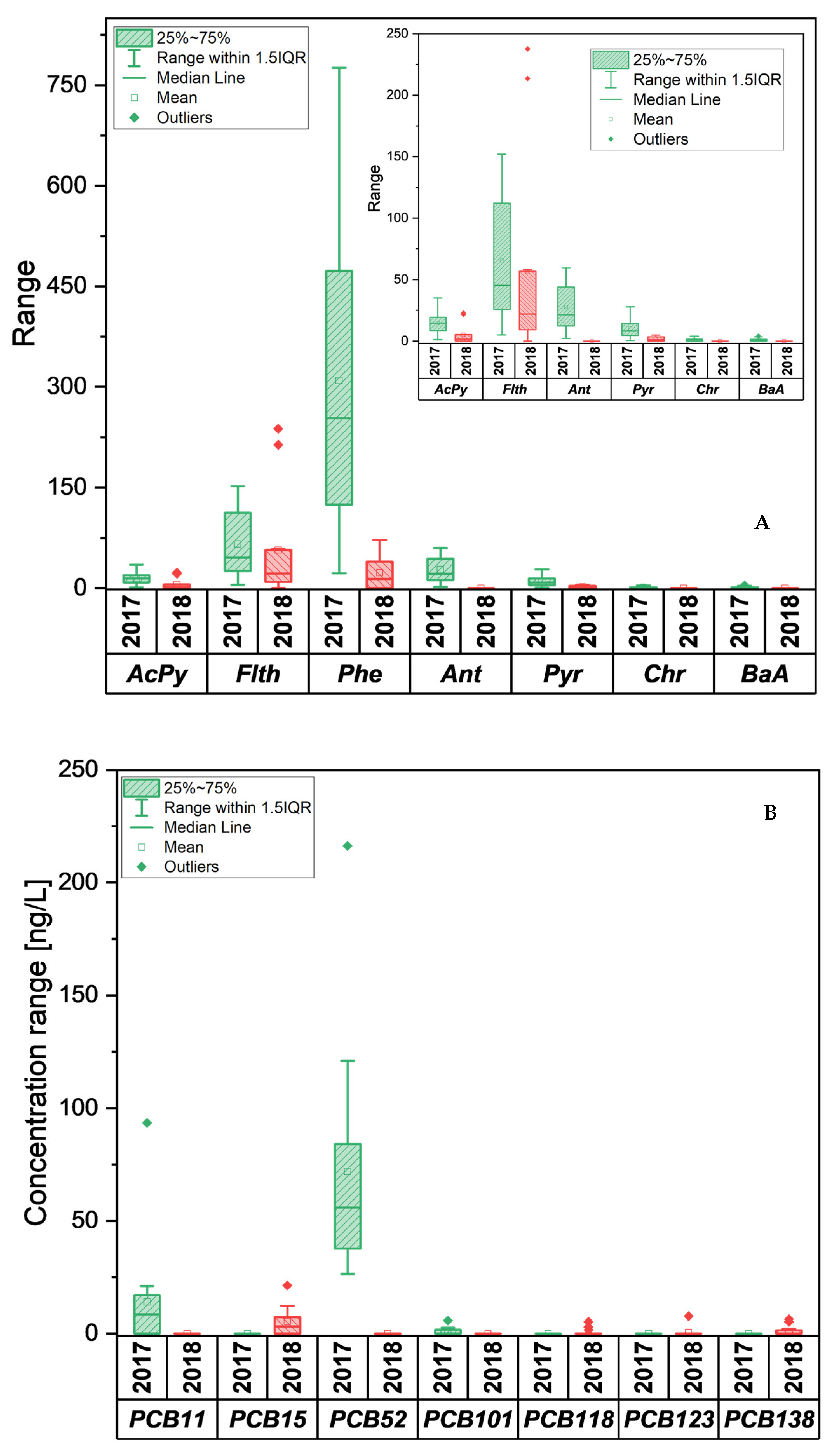
3.1.2. Monitoring of PAHs and PCBs
PAHs
PCBs
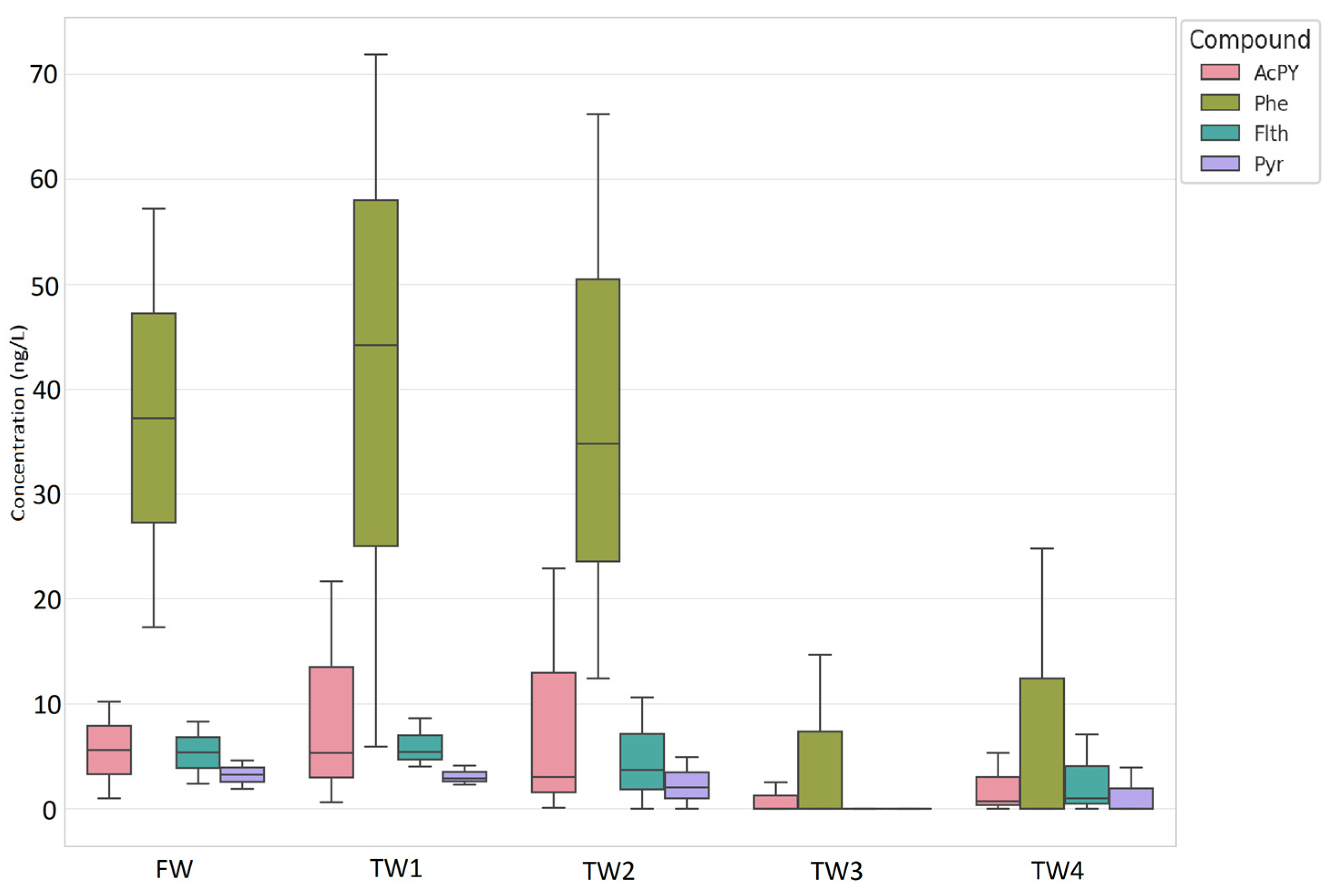
3.2. Strawberries
3.2.1. Figures of Merit
3.2.2. Monitoring of PAHs and PCBs
3.2.3. Health Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tortajada, C. Contributions of recycled wastewater to clean water and sanitation Sustainable Development Goals. NPJ Clean Water 2020, 3, 1–22. [Google Scholar] [CrossRef]
- McDermid, S.; Nocco, M.; Lawston-Parker, P.; Keune, J.; Pokhrel, Y.; Jain, M.; Jägermeyr, J.; Brocca, L.; Massari, C.; Jones, A.D.; et al. Irrigation in the Earth system. Nat. Rev. Earth Environ. 2023, 4, 435–453. [Google Scholar] [CrossRef]
- European Commission. Regulation (EU) 2020/741 of the European Parliament and of the Council of 25 May 2020 on Minimum Requirements for Water. 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/LSU/?uri=CELEX:32020R0741reuse (accessed on 31 January 2024).
- Singh, A. A review of wastewater irrigation: Environmental implications. Resour. Conserv. Recycl. 2021, 168, 105454. [Google Scholar] [CrossRef]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Rivoira, L.; Castiglioni, M.; Kettab, A.; Ouazzani, N.; Al-Karablieh, E.; Boujelben, N.; Fibbi, D.; Coppini, E.; Giordani, E.; Bubba, M.D. Impact of effluents from wastewater treatments reused for irrigation: Strawberry as case study. Environ. Eng. Manag. J. 2019, 18, 2133–2143. [Google Scholar]
- Carpenter, D.O. Exposure to and health effects of volatile PCBs. Rev. Environ. Health 2015, 30, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Vanavermaete, D.; Hostens, K.; Le, H.M.; Lessuise, A.; Ruttens, A.; Waegeneers, N.; De Witte, B. Short-and long-term assessment of PAH, PCB, and metal contamination in the Belgian part of the North Sea. Chemosphere 2023, 310, 136905. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Jia, L.; Li, B.; Yuan, A.; Kong, L.; Qi, H.; Ma, W.; Zhang, A.; Wu, Y. The occurrence and fate of PAHs over multiple years in a wastewater treatment plant of Harbin, Northeast China. Sci. Total Environ. 2018, 624, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Cybulski, J.; Witczak, A.; Pokorska-Niewiada, K. Residues of endocrine-disrupting PCBs in drinking water-influence of water and wastewater treatment in Szczecin (Poland). Urban Water J. 2022, 19, 641–649. [Google Scholar]
- Ferro, G.; Polo-López, M.I.; Martínez-Piernas, A.B.; Fernandez-Ibanez, P.; Agüera, A.; Rizzo, L. Cross-contamination of residual emerging contaminants and antibiotic resistant bacteria in lettuce crops and soil irrigated with wastewater treated by sunlight/H2O2. Environ. Sci. Technol. 2015, 49, 11096–11104. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the Quality of Water Intended for Human Consumption. 2020. Available online: https://eur-lex.europa.eu/eli/dir/2020/2184/oj (accessed on 31 January 2024).
- Singh, L.; Agarwal, T. PAHs in Indian diet: Assessing the cancer risk. Chemosphere 2018, 202, 366–376. [Google Scholar] [CrossRef] [PubMed]
- European Commission—Health and Consumer Protection Directorate-General. Polycyclic Aromatic Hydrocarbons—Occurrence in foods, dietary exposure and health effects. In Background Document to the Opinion of the Scientific Committee on Food on the Risks to Human Health of Polycyclic Aromatic Hydrocarbons in Food (Expressed on 4 December 2002); European Commission: Brussels, Belgium, 2002. [Google Scholar]
- U.S. Environmental Protection Agency (EPA). Example Exposure Scenarios; EPA/600/R-03/036; PB2003-103280; National Center for Environmental Assessment: Washington, DC, USA; National Information Service: Springfield, VA, USA, 2003. Available online: https://cfpub.epa.gov/si/si_public_record_report.cfm?Lab=NCEA&dirEntryId=85843 (accessed on 31 January 2024).
- U.S. Environmental Protection Agency (EPA). Exposure Factors Handbook; EPA/600/R-09/052F; National Center for Environmental Assessment: Washington, DC, USA; National Technical Information Service: Springfield, VA, USA, 2011. Available online: https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=236252 (accessed on 31 January 2024).
- U.S. Environmental Protection Agency (EPA). Risk Assessment Technical Background Document for the Paint and Coatings Hazardous Waste Listing Determination; National Center for Environmental Assessment: Washington, DC, USA; National Information Service: Springfield, VA, USA, 2001.
- Servizio Idrologico Regionale (SIR). Climatologia di Prato 1991–2020. Available online: https://www.lamma.toscana.it/clima-e-energia/climatologia/clima-prato (accessed on 31 January 2024).
- Obaid, H.; Shahid, S.; Basim, K.; Chelliapan, S. Modeling of wastewater quality in an urban area during festival and rainy days. Water Sci. Technol. 2015, 72, 1029–1042. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Hornbuckle, K.C. Inadvertent Polychlorinated Biphenyls in Commercial Paint Pigments. Environ. Sci. Technol. 2010, 44, 2822–2827. [Google Scholar] [CrossRef] [PubMed]
- Frame, G.M.; Cochran, J.W.; Bøwadt, S.S. Complete PCB congener distributions for 17 aroclor mixtures determined by 3 HRGC systems optimized for comprehensive, quantitative, congener-specific analysis. J. High Resolut. Chromatogr. 1996, 19, 657–668. [Google Scholar] [CrossRef]
- Chevreuil, M.; Granier, L.; Chesterikoff, A.; Létolle, R. Polychlorinated biphenyls partitioning in waters from river, filtration plant and wastewater plant: The case for paris (france). Water Res. 1990, 24, 1325–1333. [Google Scholar] [CrossRef]
- Katsoyiannis, A.; Samara, C. Persistent organic pollutants (POPs) in the sewage treatment plant of Thessaloniki, northern Greece: Occurrence and removal. Water Res. 2004, 38, 2685–2698. [Google Scholar] [CrossRef] [PubMed]
- Abu-Shmeis, R.M.; Tarawneh, I.N.; Al-qudah, Y.H.; Dabaibeh, R.N.; Tarawneh, M.N. Evaluation of the Removal Efficiency of PCBs from Five Wastewater Treatment Plants in Jordan. Water Air Soil Pollut. 2020, 231, 114. [Google Scholar] [CrossRef]
- Tozzi, F.; Del Bubba, M.; Petrucci, W.A.; Pecchioli, S.; Macci, C.; García, F.H.; Nicolás, J.J.M.; Giordani, E. Use of a remediated dredged marine sediment as a substrate for food crop cultivation: Sediment characterization and assessment of fruit safety and quality using strawberry (Fragaria x ananassa Duch.) as model species of contamination transfer. Chemosphere 2020, 238, 124651. [Google Scholar] [CrossRef] [PubMed]
- Wennrich, L.; Popp, P.; Zeibig, M. Polycyclic aromatic hydrocarbon burden in fruit and vegetable species cultivated in allotments in an industrial area. Int. J. Environ. Anal. Chem. 2002, 82, 667–690. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (EPA). Risk Assessment Guidance for Superfund: Volume I—Human Health Evaluation Manual (Part B, Development of Risk-Based Preliminary Remediation Goals); EPA/540/R-92/003; Environmental Protection Agency: Washington, DC, USA; National Information Service: Springfield, VA, USA, 1991.


| Wastewater Effluent | Source | Treatment | Point of Discharge |
|---|---|---|---|
| TW1 | mixed urban/industrial | Primary settling, biological oxidation, secondary settling, clariflocculation, and ozonization | River or to TW2 or TW3 plants |
| TW2 | TW1 effluent | Clariflocculation, sand filtration, activated carbon, and disinfection with hypochlorite | Inlet of the industrial aqueduct |
| TW3 | TW1 effluent | Clariflocculation, sand filtration, dilution with river water, and disinfection with hypochlorite | Inlet of the industrial aqueduct |
| TW4 | mixed urban/industrial and pretreated (1) septic tank and landfill leachate sewage | Primary settling, biological oxidation, secondary settling, clariflocculation, and ozonization |
| Analyte | Year | FW | TW1 | TW2 | TW3 | TW4 |
|---|---|---|---|---|---|---|
| BaA | 2017 | - | 1.14 ± 0.05 | - | - | - |
| DBA | 2018 | 1.56 ± 0.09 | 1.00 ± 0.15 | 1.18 ± 0.15 | 1.53 ± 0.16 | 0.85 ± 0.13 |
| Irrigating Water | LADD (mg/kg a Day) | Cancer Risk | ||
|---|---|---|---|---|
| child | adult | child | adult | |
| TW1 | 3.5 × 10−9 | 1.1 × 10−8 | 3.5 × 10−9 | 1.1 × 10−8 |
| Irrigating Water | LADD (mg/kg a Day) | Cancer Risk | ||
|---|---|---|---|---|
| child | adult | child | adult | |
| FW | 9.6 × 10−9 | 3.1 × 10−8 | 9.6 × 10−9 | 3.1 × 10−8 |
| TW1 | 3.9 × 10−8 | 1.0 × 10−7 | 3.9 × 10−8 | 1.0 × 10−7 |
| TW2 | 3.6 × 10−8 | 1.2 × 10−7 | 3.6 × 10−8 | 1.2 × 10−7 |
| TW3 | 4.7 × 10−8 | 1.5 × 10−7 | 4.7 × 10−8 | 1.5 × 10−7 |
| TW4 | 2.6 × 10−8 | 8.5 × 10−8 | 2.6 × 10−8 | 8.5 × 10−8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruzzoniti, M.C.; Del Bubba, M.; Giordani, E.; Fibbi, D.; Beldean-Galea, M.S.; Piesik, D.; Rivoira, L. Organic Micropollutants in the Agricultural Chain of Production of Strawberries by Irrigation with Treated Wastewater and Assessment of Human Health Implications. Water 2024, 16, 830. https://doi.org/10.3390/w16060830
Bruzzoniti MC, Del Bubba M, Giordani E, Fibbi D, Beldean-Galea MS, Piesik D, Rivoira L. Organic Micropollutants in the Agricultural Chain of Production of Strawberries by Irrigation with Treated Wastewater and Assessment of Human Health Implications. Water. 2024; 16(6):830. https://doi.org/10.3390/w16060830
Chicago/Turabian StyleBruzzoniti, Maria Concetta, Massimo Del Bubba, Edgardo Giordani, Donatella Fibbi, Mihail Simion Beldean-Galea, Dariusz Piesik, and Luca Rivoira. 2024. "Organic Micropollutants in the Agricultural Chain of Production of Strawberries by Irrigation with Treated Wastewater and Assessment of Human Health Implications" Water 16, no. 6: 830. https://doi.org/10.3390/w16060830
APA StyleBruzzoniti, M. C., Del Bubba, M., Giordani, E., Fibbi, D., Beldean-Galea, M. S., Piesik, D., & Rivoira, L. (2024). Organic Micropollutants in the Agricultural Chain of Production of Strawberries by Irrigation with Treated Wastewater and Assessment of Human Health Implications. Water, 16(6), 830. https://doi.org/10.3390/w16060830









