River–Spring Connectivity and Hydrogeochemical Processes in a Karst Water System of Northern China: A Case Study of Jinan Spring Catchment
Abstract
1. Introduction
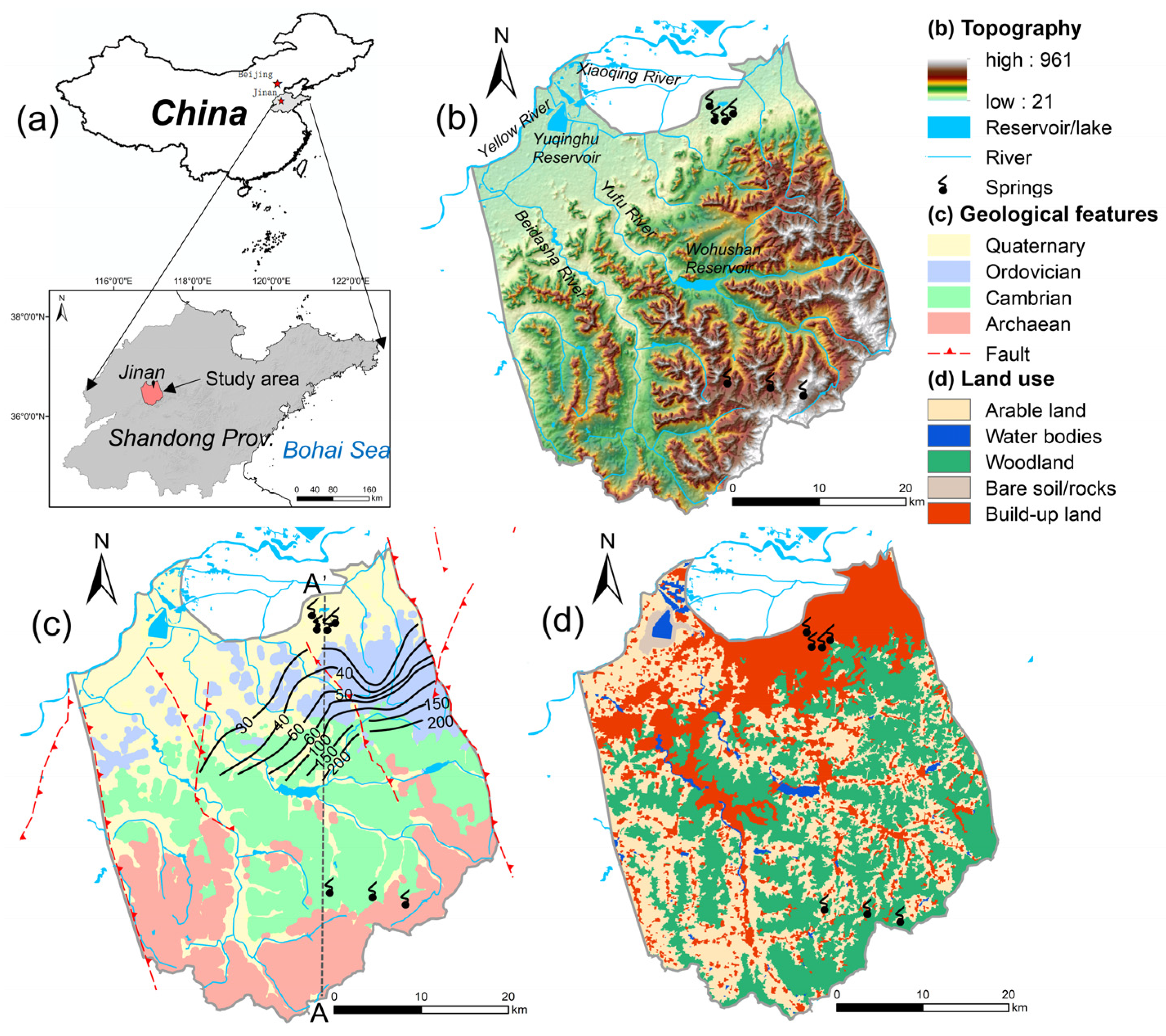
2. Study Area
2.1. Background
2.2. Climate and Hydrology
2.3. Geological and Hydrogeological Setting
2.4. Groundwater Exploitation and History of Springs Drying Up
3. Materials and Methods
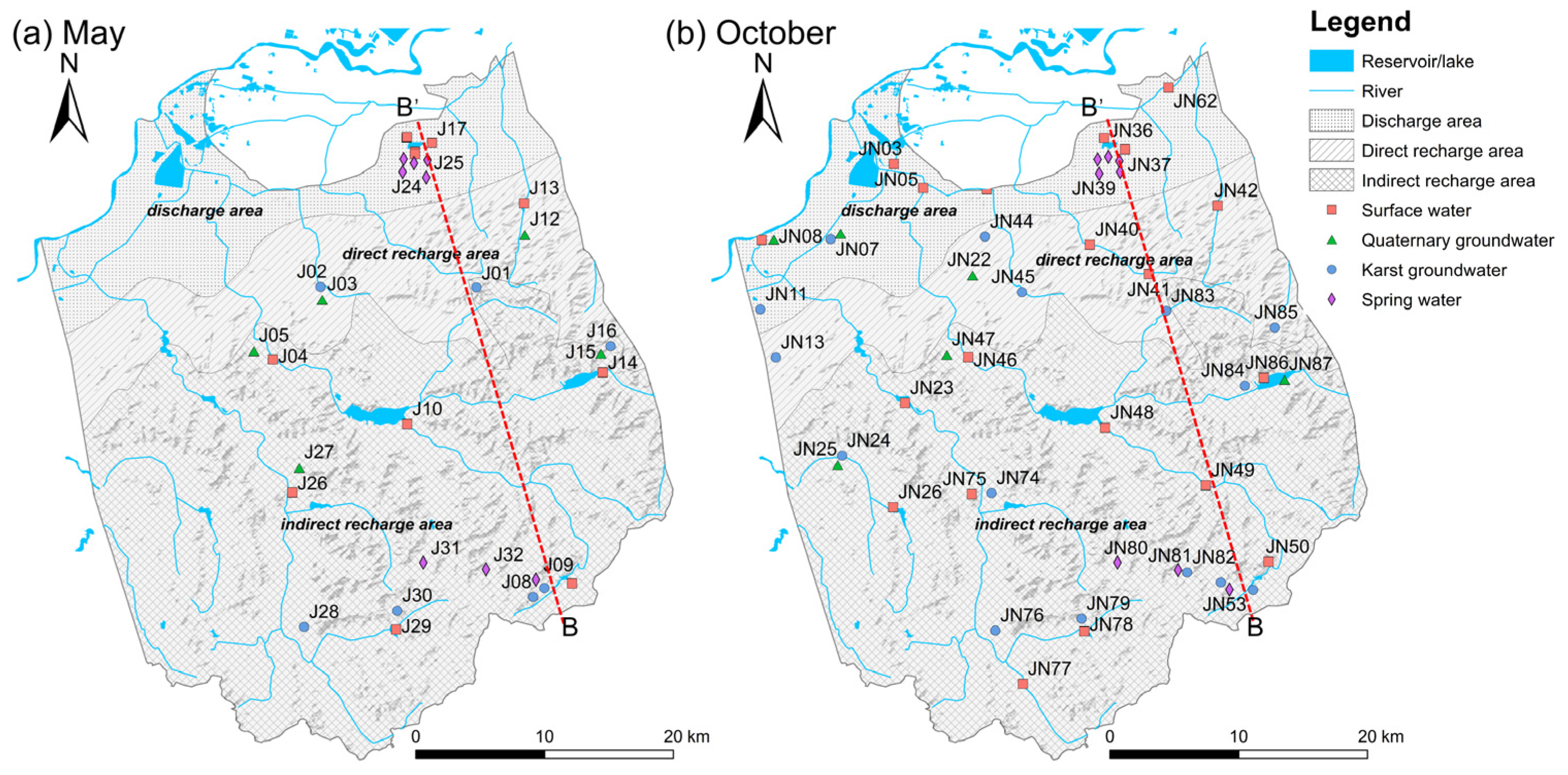
3.1. Water Sampling and Measurement
3.2. Data Analysis Methods
3.3. End-Member Mixing Analysis
4. Results
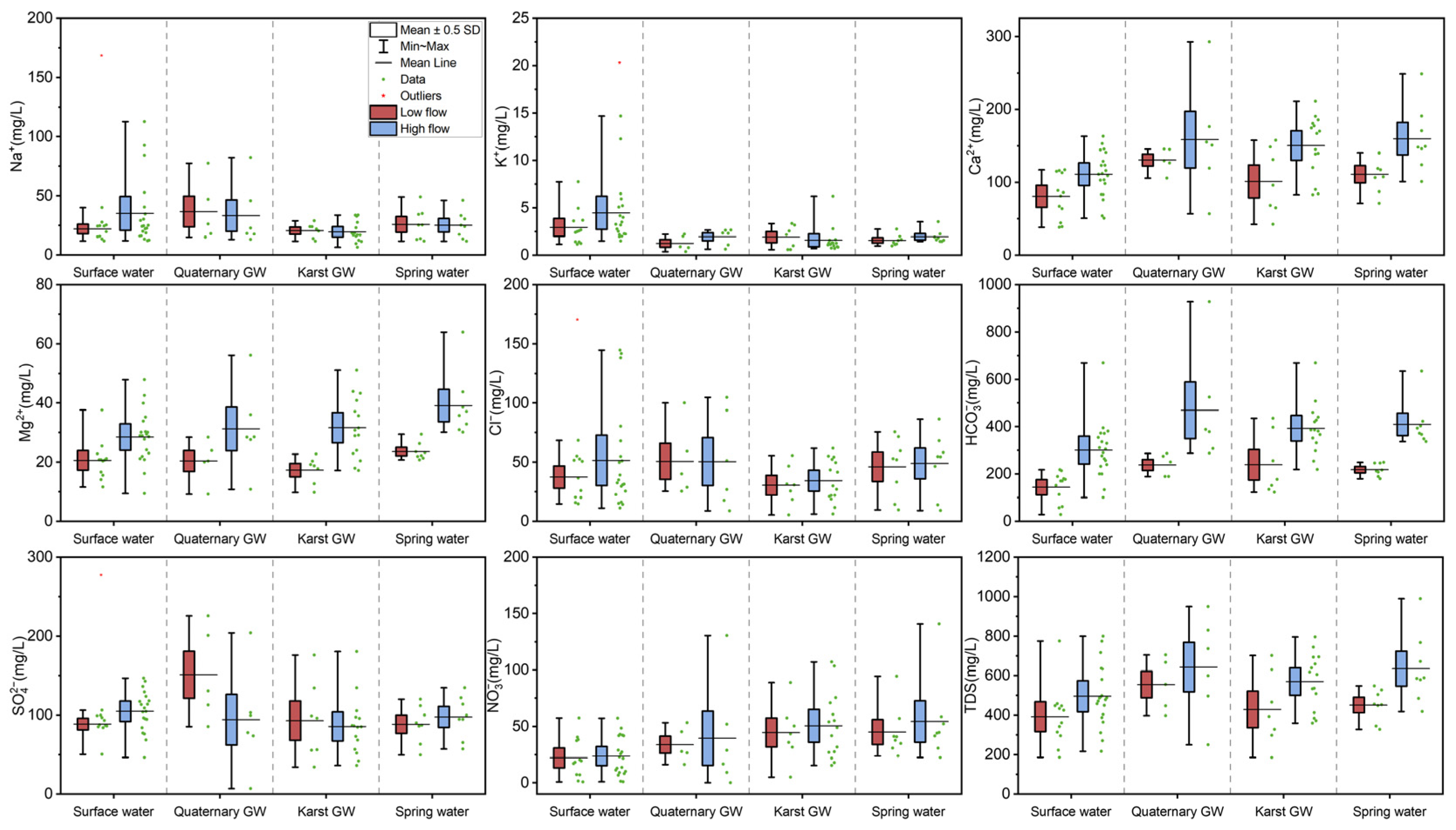
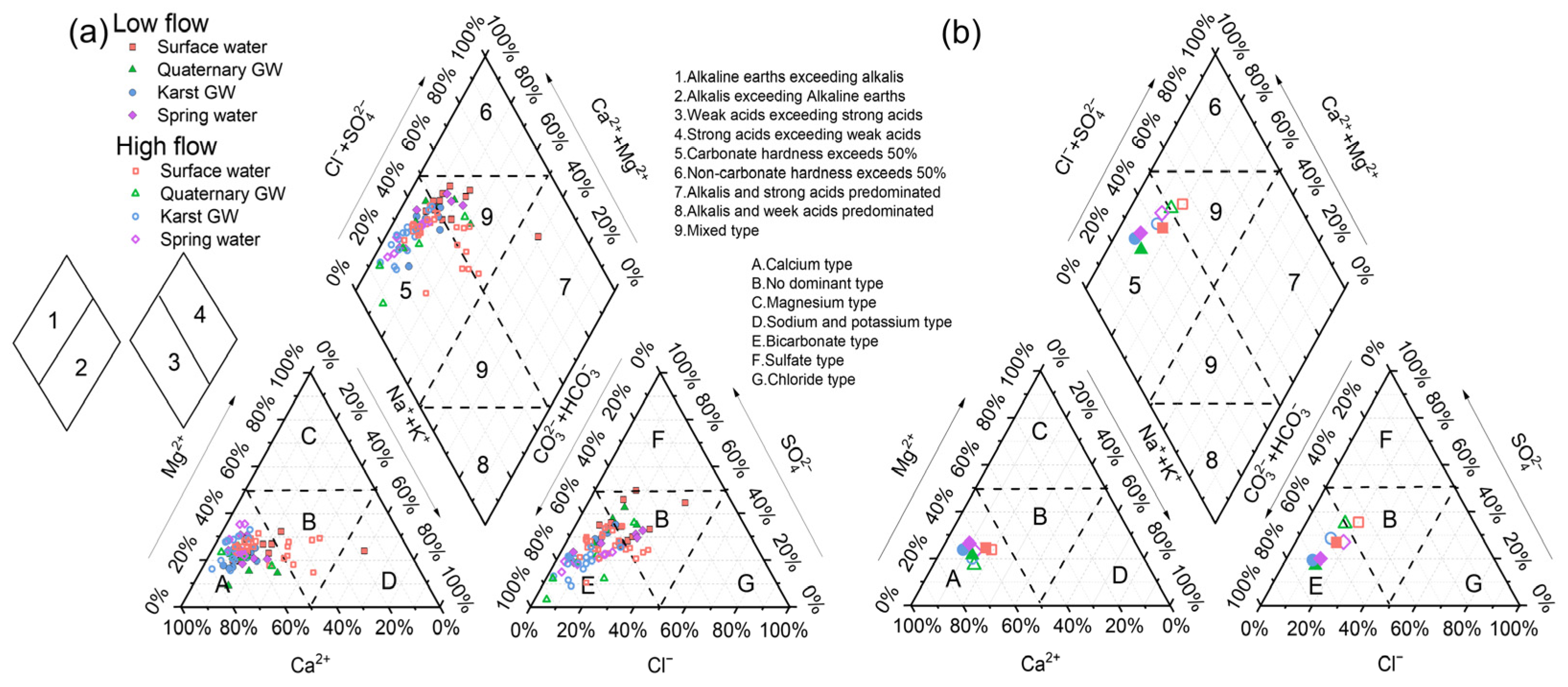
4.1. Physical and Hydrochemical Characteristics of Water Samples
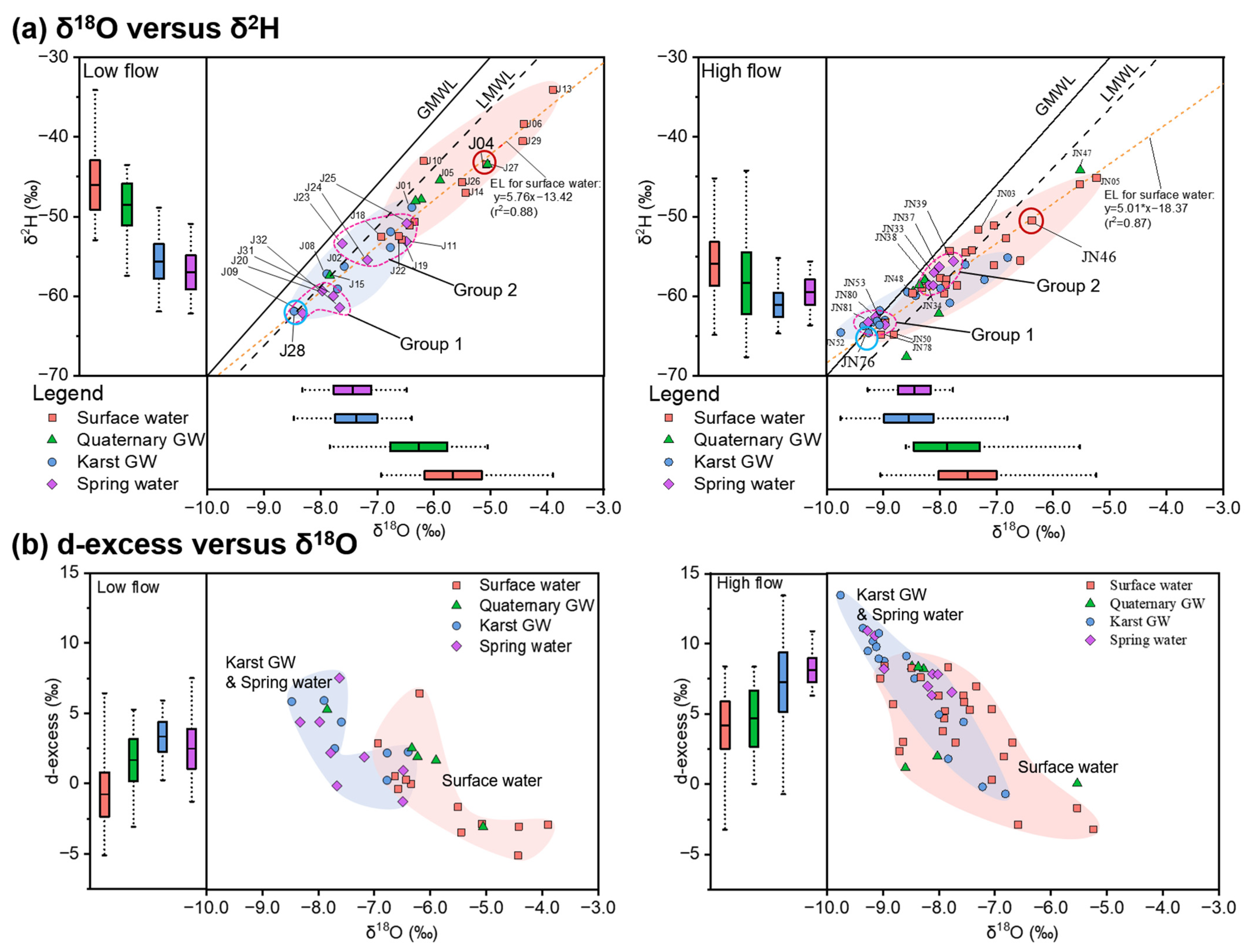
4.2. Stable Isotopes (δ18O and δ2H) in Surface Water and Groundwater
5. Discussion
5.1. Mixing Processes and Karst Water Flow System
5.1.1. Stable Isotopes as Indicators of Water Origins
5.1.2. Mixing Processes in the JSC
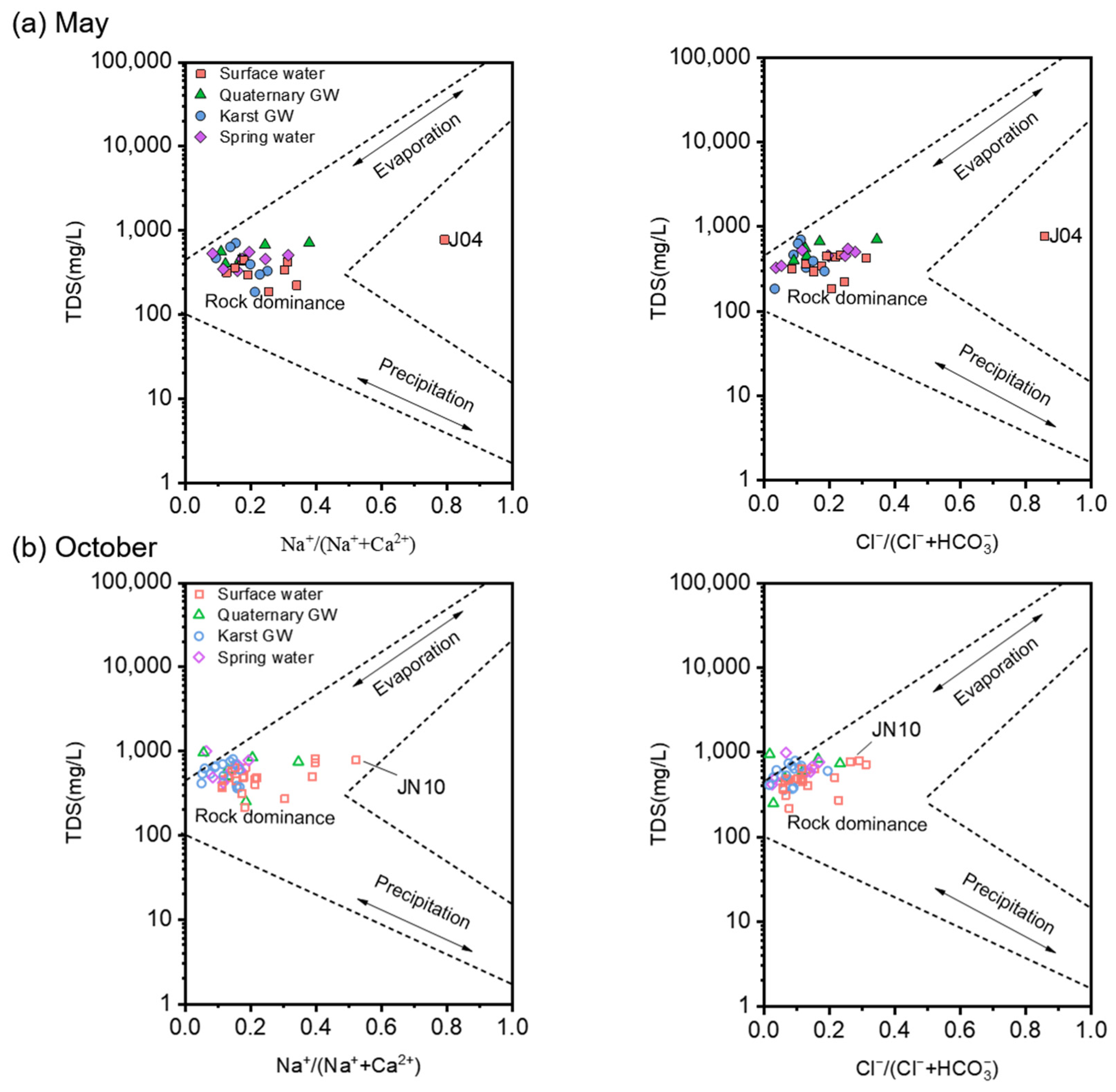
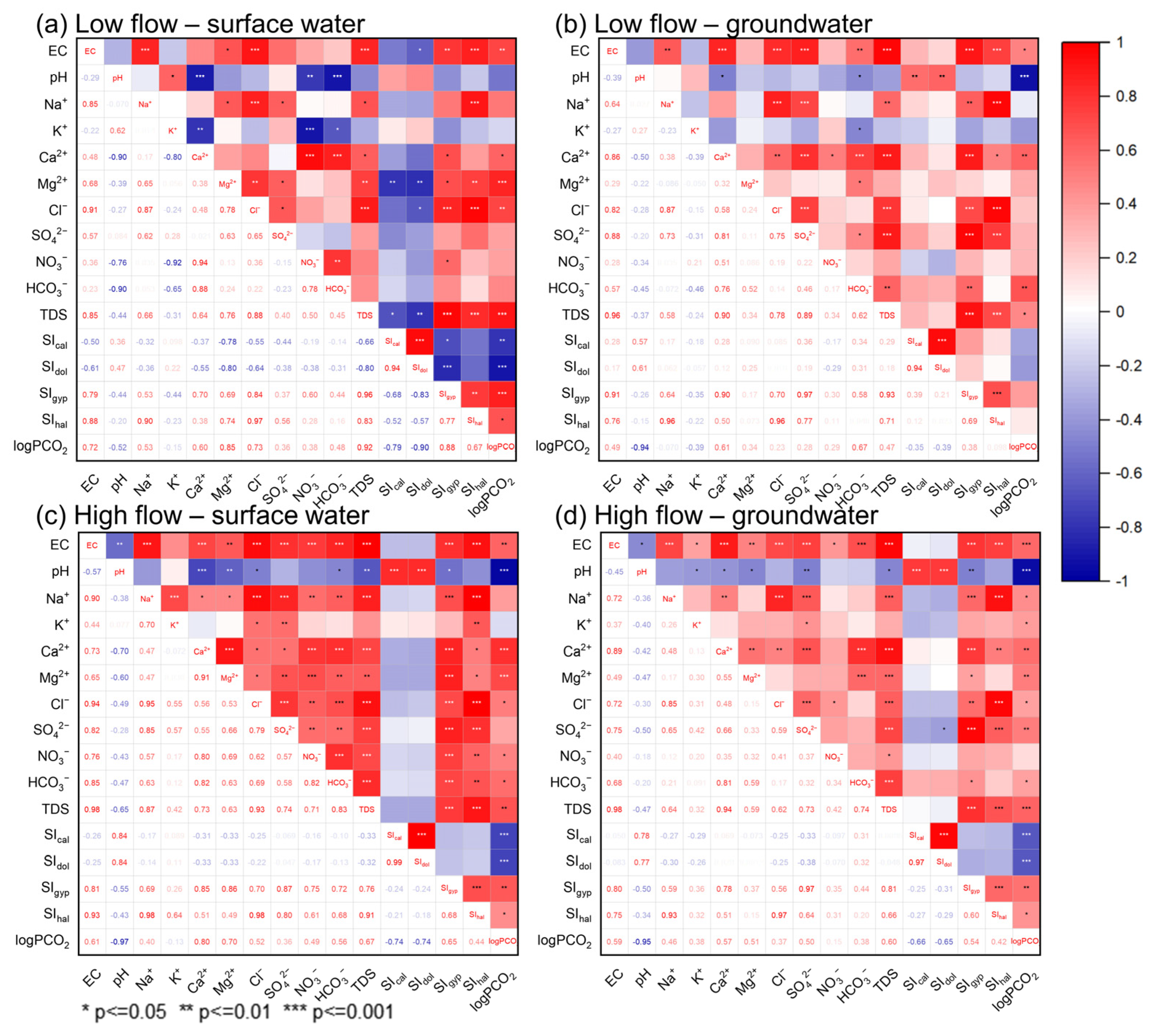
5.2. Factors Controlling the Hydrochemistry of the Jinan Spring Catchment
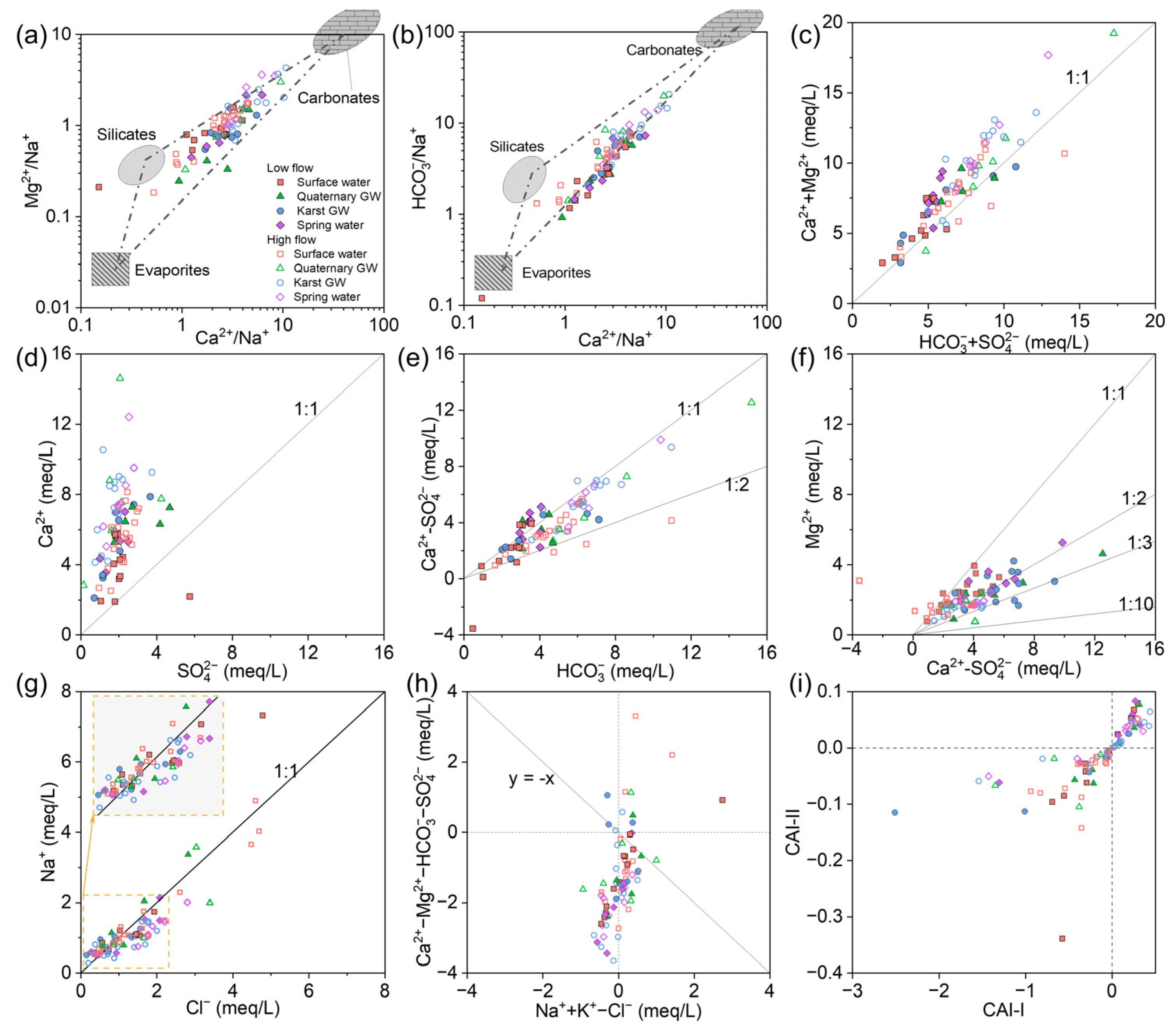
5.3. Sources of Major Ions
5.3.1. Hydrogeochemical Processes
5.3.2. Anthropogenic Influences
5.4. Hydrogeochemical Evolution during Flow Paths
5.5. Conceptual Model and Environmental Implications
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goldscheider, N.; Chen, Z.; Auler, A.S.; Bakalowicz, M.; Broda, S.; Drew, D.; Hartmann, J.; Jiang, G.; Moosdorf, N.; Stevanovic, Z.; et al. Global distribution of carbonate rocks and karst water resources. Hydrogeol. J. 2020, 28, 1661–1677. [Google Scholar] [CrossRef]
- Olarinoye, T.; Gleeson, T.; Marx, V.; Seeger, S.; Adinehvand, R.; Allocca, V.; Andreo, B.; Apaéstegui, J.; Apolit, C.; Arfib, B. Global karst springs hydrograph dataset for research and management of the world’s fastest-flowing groundwater. Sci. Data 2020, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.B.; Kurz, M.J.; Khadka, M.B. Climate control of decadal-scale increases in apparent ages of eogenetic karst spring water. J. Hydrol. 2016, 540, 988–1001. [Google Scholar] [CrossRef]
- Gutiérrez, F.; Parise, M.; De Waele, J.; Jourde, H. A review on natural and human-induced geohazards and impacts in karst. Earth-Sci. Rev. 2014, 138, 61–88. [Google Scholar] [CrossRef]
- An, L.; Hao, Y.; Yeh, T.-C.J.; Liu, Y.; Liu, W.; Zhang, B. Simulation of karst spring discharge using a combination of time–frequency analysis methods and long short-term memory neural networks. J. Hydrol. 2020, 589, 125320. [Google Scholar] [CrossRef]
- Leopold, M.; Gupanis-Broadway, C.; Baker, A.; Hankin, S.; Treble, P. Time lapse electric resistivity tomography to portray infiltration and hydrologic flow paths from surface to cave. J. Hydrol. 2021, 593, 125810. [Google Scholar] [CrossRef]
- Cholet, C.; Steinmann, M.; Charlier, J.-B.; Denimal, S. Characterizing fluxes of trace metals related to dissolved and suspended matter during a storm event: Application to a karst aquifer using trace metals and rare earth elements as provenance indicators. Hydrogeol. J. 2019, 27, 305–319. [Google Scholar] [CrossRef]
- Ni, M.; Li, S. Spectroscopic indices trace spatiotemporal variability of dissolved organic matter in a river system with Karst characteristic. J. Hydrol. 2020, 590, 125570. [Google Scholar] [CrossRef]
- Jiang, Y.; Cao, M.; Yuan, D.; Zhang, Y.; He, Q. Hydrogeological characterization and environmental effects of the deteriorating urban karst groundwater in a karst trough valley: Nanshan, SW China. Hydrogeol. J. 2018, 26, 1487–1497. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, G.; Zhan, H.; Chen, X.; Liu, M.; Wang, M. Identification of hydrogeochemical processes and transport paths of a multi-aquifer system in closed mining regions. J. Hydrol. 2020, 589, 125344. [Google Scholar] [CrossRef]
- White, W.B. A brief history of karst hydrogeology: Contributions of the NSS. J. Cave Karst Stud. 2007, 69, 13–26. [Google Scholar]
- Clark, I.D.; Fritz, P. Environmental Isotopes in Hydrogeology; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Koit, O.; Barberá, J.A.; Marandi, A.; Terasmaa, J.; Kiivit, I.-K.; Martma, T. Spatiotemporal assessment of humic substance-rich stream and shallow karst aquifer interactions in a boreal catchment of northern Estonia. J. Hydrol. 2020, 580, 124238. [Google Scholar] [CrossRef]
- Zhang, J.; Li, T.-Y. Seasonal and interannual variations of hydrochemical characteristics and stable isotopic compositions of drip waters in Furong Cave, Southwest China based on 12 years’ monitoring. J. Hydrol. 2019, 572, 40–50. [Google Scholar] [CrossRef]
- Wang, F.; Chen, H.; Lian, J.; Fu, Z.; Nie, Y. Seasonal recharge of spring and stream waters in a karst catchment revealed by isotopic and hydrochemical analyses. J. Hydrol. 2020, 591, 125595. [Google Scholar] [CrossRef]
- Barberá, J.; Andreo, B. River-spring connectivity and hydrogeochemical interactions in a shallow fractured rock formation. The case study of Fuensanta river valley (Southern Spain). J. Hydrol. 2017, 547, 253–268. [Google Scholar] [CrossRef]
- Carrière, S.D.; Ruffault, J.; Cakpo, C.B.; Olioso, A.; Doussan, C.; Simioni, G.; Chalikakis, K.; Patris, N.; Davi, H.; MartinSt-Paul, N.K. Intra-specific variability in deep water extraction between trees growing on a Mediterranean karst. J. Hydrol. 2020, 590, 125428. [Google Scholar] [CrossRef]
- Filippini, M.; Squarzoni, G.; De Waele, J.; Fiorucci, A.; Vigna, B.; Grillo, B.; Riva, A.; Rossetti, S.; Zini, L.; Casagrande, G. Differentiated spring behavior under changing hydrological conditions in an alpine karst aquifer. J. Hydrol. 2018, 556, 572–584. [Google Scholar] [CrossRef]
- Appelo, C.A.J.; Postma, D. Geochemistry, Groundwater and Pollution; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Bicalho, C.C.; Batiot-Guilhe, C.; Seidel, J.; Van Exter, S.; Jourde, H. Geochemical evidence of water source characterization and hydrodynamic responses in a karst aquifer. J. Hydrol. 2012, 450, 206–218. [Google Scholar] [CrossRef]
- Musgrove, M.; Opsahl, S.P.; Mahler, B.J.; Herrington, C.; Sample, T.L.; Banta, J.R. Source, variability, and transformation of nitrate in a regional karst aquifer: Edwards aquifer, central Texas. Sci. Total Environ. 2016, 568, 457–469. [Google Scholar] [CrossRef]
- Wang, Z.-J.; Yue, F.-J.; Lu, J.; Wang, Y.-C.; Qin, C.-Q.; Ding, H.; Xue, L.-L.; Li, S.-L. New insight into the response and transport of nitrate in karst groundwater to rainfall events. Sci. Total Environ. 2022, 818, 151727. [Google Scholar] [CrossRef]
- Mudarra, M.; Hartmann, A.; Andreo, B. Combining Experimental Methods and Modeling to Quantify the Complex Recharge Behavior of Karst Aquifers. Water Resour. Res. 2019, 55, 1384–1404. [Google Scholar] [CrossRef]
- Wang, W.; Chen, Y.; Wang, W.; Zhu, C.; Chen, Y.; Liu, X.; Zhang, T. Water quality and interaction between groundwater and surface water impacted by agricultural activities in an oasis-desert region. J. Hydrol. 2023, 617, 128937. [Google Scholar] [CrossRef]
- Chu, H.B.; Wei, J.H.; Wang, R.; Xin, B.D. Characterizing the interaction of groundwater and surface water in the karst aquifer of Fangshan, Beijing (China). Hydrogeol. J. 2017, 25, 575–588. [Google Scholar] [CrossRef]
- Rugel, K.; Golladay, S.W.; Jackson, C.R.; Rasmussen, T.C. Delineating groundwater/surface water interaction in a karst watershed: Lower Flint River Basin, southwestern Georgia, USA. J. Hydrol. Reg. Stud. 2016, 5, 1–19. [Google Scholar] [CrossRef]
- Liang, Y.; Gao, X.; Zhao, C.; Tang, C.; Shen, H.; Wang, Z.; Wang, Y. Review: Characterization, evolution, and environmental issues of karst water systems in Northern China. Hydrogeol. J. 2018, 26, 1371–1385. [Google Scholar] [CrossRef]
- Li, C. Analysis on karst resources and preservation of famous springs in Jinan. Carsologica Sin. 1985, 4, 37–45, (In Chinese with English Abstract). [Google Scholar]
- Kang, F.X.; Jin, M.G.; Qin, P.R. Sustainable yield of a karst aquifer system: A case study of Jinan springs in northern China. Hydrogeol. J. 2011, 19, 851–863. [Google Scholar] [CrossRef]
- Guan, Q.; Li, F.; Wang, A.; Feng, P.; Tian, C.; Chen, X.; Liu, D. Hydrochemistry characteristics and evolution of karst spring groundwater system in Jina. Carsologica Sin. 2019, 38, 653–662, (In Chinese with English Abstract). [Google Scholar]
- Qian, J.; Zhan, H.; Wu, Y.; Li, F.; Wang, J. Fractured-karst spring-flow protections: A case study in Jinan, China. Hydrogeol. J. 2006, 14, 1192–1205. [Google Scholar] [CrossRef]
- Luo, Q.; Yang, Y.; Qian, J.; Wang, X.; Chang, X.; Ma, L.; Li, F.; Wu, J. Spring protection and sustainable management of groundwater resources in a spring field. J. Hydrol. 2020, 582, 124498. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, W.; Xiang, H.; Gai, Y.; Li, F. Relationship between Groundwater in Western Jinan and Jinan Spring Area Based on Correlation Degree of Water Table Fluctuation. J. China Hydrol. 2018, 38, 31–36, 96. [Google Scholar]
- Wang, G.-F.; Wu, Y.-X.; Lu, L.; Li, G.; Shen, J.S. Investigation of the geological and hydrogeological environment with relation to metro system construction in Jinan, China. Bull. Eng. Geol. Environ. 2019, 78, 1005–1024. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, J.; Xu, X.; Wang, Q.; Wang, M.; Feng, J.; Fu, T. Temporal variations of spring water in karst areas: A case study of Jinan Spring Area, Northern China. Water 2020, 12, 1009. [Google Scholar] [CrossRef]
- Guo, Y.; Qin, D.; Sun, J.; Li, L.; Li, F.; Huang, J. Recharge of River Water to Karst Aquifer Determined by Hydrogeochemistry and Stable Isotopes. Water 2019, 11, 479. [Google Scholar] [CrossRef]
- Qi, X.; Wang, Y.; Yang, L.; Liu, Z.; Wang, W.; Li, W. Time lags variance of groundwater level response to precipitation of Jinan karst spring watershed in recent 50 years. Carsologica Sin. 2016, 35, 384–393, (In Chinese with English Abstract). [Google Scholar]
- Chi, G.; Xing, L.; Zhu, H.; Hou, X.; Xiang, H.; Xing, X. The study of quantitative relationship between the spring water and the dynamic change of the atmospheric precipitation in Jinan. Ground Water 2017, 39, 8–11, (In Chinese with English Abstract). [Google Scholar]
- Wang, J.L.; Jin, M.G.; Jia, B.J.; Kang, F.X. Hydrochemical characteristics and geothermometry applications of thermal groundwater in northern Jinan, Shandong, China. Geothermics 2015, 57, 185–195. [Google Scholar] [CrossRef]
- Wang, J.L.; Jin, M.G.; Lu, G.P.; Zhang, D.; Kang, F.X.; Jia, B.J. Investigation of discharge-area groundwaters for recharge source characterization on different scales: The case of Jinan in northern China. Hydrogeol. J. 2016, 24, 1723–1737. [Google Scholar] [CrossRef]
- Zhao, Z.; Lu, O.; Qin, D.; Teng, Z.; Dong, Y. Factors Controlling Hydrochemical Characteritics of Karstic Water in Jinan. China Rural Water Hydropower 2012, 7, 31–37, (In Chinese with English Abstract). [Google Scholar]
- Xing, L.; Zhou, J.; Song, G.; Xing, X. Mixing ratios of recharging water sources for the four largest spring groups in Jinan. Earth Sci. Front. 2018, 25, 260–272, (In Chinese with English Abstract). [Google Scholar]
- Wang, Q.; Duan, X.; Gao, Z.; Xu, H.; Yin, X.; Li, W.; Zhou, Y. Reginal groundwater level monitoring in Jinan Karstic Spring Basin. Hydrogeol. Eng. Geol. 2007, 12, 1–7, (In Chinese with English Abstract). [Google Scholar]
- Sun, B.; Peng, Y.; Li, C.; Lin, G. Division of Karst Water System and Hydraulic Connection of Typical Spring Fields in Jinan City. Shandong Land Resour. 2016, 32, 31–34, (In Chinese with English Abstract). [Google Scholar]
- Hong-hai, L.; Cheng, Z. The Variations of Groundwater Quality and Its Relationship with Human Activity. Res. Soil Water Conserv. 2007, 24, 238–240, (In Chinese with English Abstract). [Google Scholar]
- JWRB (Jinan Water Resources Bureau); JURWAB (Jinan Urban and Rural Water Affairs Bureau). Water Resources Bulletin of Jinan; JWRB/JURWAB: Jinan, China, 2017. (In Chinese) [Google Scholar]
- Parkhurst, D.L.; Appelo, C. Description of input and examples for PHREEQC version 3—A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. US Geol. Surv. Tech. Methods 2013, 6, 497. [Google Scholar]
- Mendiguchía, C.; Moreno, C.; García-Vargas, M. Evaluation of natural and anthropogenic influences on the Guadalquivir River (Spain) by dissolved heavy metals and nutrients. Chemosphere 2007, 69, 1509–1517. [Google Scholar] [CrossRef]
- Clark, I. Groundwater Geochemistry and Isotopes; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Gabrovšek, F.; Dreybrodt, W. Role of mixing corrosion in calcite-aggressive H2O-CO2-CaCO3 solutions in the early evolution of karst aquifers in limestone. Water Resour. Res. 2000, 36, 1179–1188. [Google Scholar] [CrossRef]
- Romanov, D.; Gabrovsek, F.; Dreybrodt, W. The impact of hydrochemical boundary conditions on the evolution of limestone karst aquifers. J. Hydrol. 2003, 276, 240–253. [Google Scholar] [CrossRef]
- Kaushal, S.S.; Duan, S.; Doody, T.R.; Haq, S.; Smith, R.M.; Johnson, T.A.N.; Newcomb, K.D.; Gorman, J.; Bowman, N.; Mayer, P.M. Human-accelerated weathering increases salinization, major ions, and alkalinization in fresh water across land use. Appl. Geochem. 2017, 83, 121–135. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines for Drinking-Water Quality; WHO chronicle 2011; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Craig, H. Isotopic variations in meteoric waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Song, X.; Yuan, G.; Sun, X.; Liu, X.; Wang, S. Characteristics of δ18O in precipitation over Eastern Monsoon China and the water vapor sources. Chin. Sci. Bull. 2010, 55, 200–211. [Google Scholar] [CrossRef]
- Cartwright, I.; Weaver, T.; Cendón, D.I.; Swane, I. Environmental isotopes as indicators of inter-aquifer mixing, Wimmera region, Murray Basin, Southeast Australia. Chem. Geol. 2010, 277, 214–226. [Google Scholar] [CrossRef]
- Pang, Z.; Kong, Y.; Li, J.; Tian, J. An isotopic geoindicator in the hydrological cycle. Procedia Earth Planet. Sci. 2017, 17, 534–537. [Google Scholar] [CrossRef]
- Dansgaard, W. Stable isotopes in precipitation. Tellus 1964, 16, 436–468. [Google Scholar] [CrossRef]
- Segura, C.; Noone, D.; Warren, D.; Jones, J.A.; Tenny, J.; Ganio, L.M. Climate, landforms, and geology affect baseflow sources in a mountain catchment. Water Resour. Res. 2019, 55, 5238–5254. [Google Scholar] [CrossRef]
- Xi, D.Y.; Li, X.Z.; Shao, Z. Hydrogeological Survey Report of Keeping Spring Spurting and Water Supply in Jinan City, Shandong Province. 1988. Available online: https://www.ngac.cn/dzzlfw_sjgl/d2d/dse/category/detail.do?method=cdetail&_id=102_81672&tableCode=ty_qgg_edmk_t_ajxx&categoryCode=dzzlk (accessed on 10 January 2024).
- Guo, Y.; Qin, D.; Li, L.; Sun, J.; Li, F.; Huang, J. A Complicated Karst Spring System: Identified by Karst Springs Using Water Level, Hydrogeochemical, and Isotopic Data in Jinan, China. Water 2019, 11, 947. [Google Scholar] [CrossRef]
- Bakalowicz, M. Karst groundwater: A challenge for new resources. Hydrogeol. J. 2005, 13, 148–160. [Google Scholar] [CrossRef]
- Li, C.; Gao, X.; Wang, W.; Zhang, X.; Zhang, X.; Jiang, C.; Wang, Y. Hydro-biogeochemical processes of surface water leakage into groundwater in large scale karst water system: A case study at Jinci, northern China. J. Hydrol. 2021, 596, 125691. [Google Scholar] [CrossRef]
- Drever, J.I. The Geochemistry of Natural Waters: Surface and Groundwater Environments; Wiley Press: Prentice Hall, NJ, USA, 1997; p. 436. [Google Scholar]
- Yuan, R.; Wang, M.; Wang, S.; Song, X. Water transfer imposes hydrochemical impacts on groundwater by altering the interaction of groundwater and surface water. J. Hydrol. 2020, 583, 124617. [Google Scholar] [CrossRef]
- Gibbs, R.J. Mechanisms controlling world water chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef]
- Yuan, R.; Zhang, Y.; Long, X. Deep groundwater circulation in a syncline in Rucheng County, China. J. Hydrol. 2022, 610, 127824. [Google Scholar] [CrossRef]
- Singh, A.K.; Hasnain, S.I. Major ion chemistry and weathering control in a high altitude basin: Alaknanda River, Garhwal Himalaya, India. Hydrol. Sci. J. 1998, 43, 825–843. [Google Scholar] [CrossRef]
- Liu, F.; Wang, S.; Wang, L.; Shi, L.; Song, X.; Yeh, T.-C.J.; Zhen, P. Coupling hydrochemistry and stable isotopes to identify the major factors affecting groundwater geochemical evolution in the Heilongdong Spring Basin, North China. J. Geochem. Explor. 2019, 205, 106352. [Google Scholar] [CrossRef]
- Liu, J.; Peng, Y.; Li, C.; Gao, Z.; Chen, S. Characterization of the hydrochemistry of water resources of the Weibei Plain, Northern China, as well as an assessment of the risk of high groundwater nitrate levels to human health. Environ. Pollut. 2021, 268, 115947. [Google Scholar] [CrossRef]
- Rajmohan, N.; Elango, L. Identification and evolution of hydrogeochemical processes in the groundwater environment in an area of the Palar and Cheyyar River Basins, Southern India. Environ. Geol. 2004, 46, 47–61. [Google Scholar] [CrossRef]
- Jiang, Y.; Gao, J.; Yang, L.; Wu, S.; Dai, E. The interactive effects of elevation, precipitation and lithology on karst rainfall and runoff erosivity. Catena 2021, 207, 105588. [Google Scholar] [CrossRef]
- Class, H.; Bürkle, P.; Sauerborn, T.; Trötschler, O.; Strauch, B.; Zimmer, M. On the Role of Density-Driven Dissolution of CO2 in Phreatic Karst Systems. Water Resour. Res. 2021, 57, e2021WR030912. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, M.; Cao, M.; Huang, X.; Zhang, Z.; Zhang, L. Sources and behaviors of dissolved sulfate in the Jinan karst spring catchment in northern China identified by using environmental stable isotopes and a Bayesian isotope-mixing model. Appl. Geochem. 2021, 134, 105109. [Google Scholar] [CrossRef]
- Panagopoulos, G.; Lambrakis, N.; Tsolis-Katagas, P.; Papoulis, D. Cation exchange processes and human activities in unconfined aquifers. Environ. Geol. 2004, 46, 542–552. [Google Scholar] [CrossRef]
- Gaillardet, J.; Dupré, B.; Louvat, P.; Allegre, C. Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem. Geol. 1999, 159, 3–30. [Google Scholar] [CrossRef]
- Zaidi, F.K.; Nazzal, Y.; Jafri, M.K.; Naeem, M.; Ahmed, I. Reverse ion exchange as a major process controlling the groundwater chemistry in an arid environment: A case study from northwestern Saudi Arabia. Environ. Monit. Assess. 2015, 187, 607. [Google Scholar] [CrossRef]
- Jia, H.; Qian, H.; Zheng, L.; Feng, W.; Wang, H.; Gao, Y. Alterations to groundwater chemistry due to modern water transfer for irrigation over decades. Sci. Total Environ. 2020, 717, 137170. [Google Scholar] [CrossRef] [PubMed]
- Hanshaw, B.B.; Back, W. Major geochemical processes in the evolution of carbonate—Aquifer systems. J. Hydrol. 1979, 43, 287–312. [Google Scholar] [CrossRef]
- Plummer, L. Defining reactions and mass transfer in part of the Floridan aquifer. Water Resour. Res. 1977, 13, 801–812. [Google Scholar] [CrossRef]
- Liu, F.; Song, X.; Yang, L.; Han, D.; Zhang, Y.; Ma, Y.; Bu, H. The role of anthropogenic and natural factors in shaping the geochemical evolution of groundwater in the Subei Lake basin, Ordos energy base, Northwestern China. Sci. Total Environ. 2015, 538, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Carol, E.; Mas-Pla, J.; Kruse, E. Interaction between continental and estuarine waters in the wetlands of the northern coastal plain of Samborombón Bay, Argentina. Appl. Geochem. 2013, 34, 152–163. [Google Scholar] [CrossRef]
- Schoeller, H. Qualitative evaluation of groundwater resources. In Methods and Techniques of Groundwater Investigations and Development; UNESCO: Paris, France, 1965; Volume 5483. [Google Scholar]
- Deutsch, W.J.; Siegel, R. Groundwater Geochemistry: Fundamentals and Applications to Contamination; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Khatri, N.; Tyagi, S. Influences of natural and anthropogenic factors on surface and groundwater quality in rural and urban areas. Front. Life Sci. 2015, 8, 23–39. [Google Scholar] [CrossRef]
- Li, P.; Tian, R.; Xue, C.; Wu, J. Progress, opportunities, and key fields for groundwater quality research under the impacts of human activities in China with a special focus on western China. Environ. Sci. Pollut. Res. 2017, 24, 13224–13234. [Google Scholar] [CrossRef]
- Xu, X.; Liu, J.; Zhang, S.; Li, R.; Yan, C.; Wu, S. China Multi-Period Land Use Remote Sensing Monitoring Dataset; Resource and Environmental Science Data Registration and Publishing System: Beijing, China, 2018. [Google Scholar] [CrossRef]
- White, W.B. Karst hydrology: Recent developments and open questions. Eng. Geol. 2002, 65, 85–105. [Google Scholar] [CrossRef]
- Badruzzaman, M.; Pinzon, J.; Oppenheimer, J.; Jacangelo, J.G. Sources of nutrients impacting surface waters in Florida: A review. J. Environ. Manag. 2012, 109, 80–92. [Google Scholar] [CrossRef]
- Liu, C.-Q.; Li, S.-L.; Lang, Y.-C.; Xiao, H.-Y. Using δ15N-and δ18O-values to identify nitrate sources in karst ground water, Guiyang, Southwest China. Environ. Sci. Technol. 2006, 40, 6928–6933. [Google Scholar] [CrossRef]
- Amiel, R.B.; Grodek, T.; Frumkin, A. Characterization of the hydrogeology of the sacred Gihon Spring, Jerusalem: A deteriorating urban karst spring. Hydrogeol. J. 2010, 18, 1465–1479. [Google Scholar] [CrossRef]
- Anornu, G.; Gibrilla, A.; Adomako, D. Tracking nitrate sources in groundwater and associated health risk for rural communities in the White Volta River basin of Ghana using isotopic approach (δ15N, δ18ONO3 and 3H). Sci. Total Environ. 2017, 603, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Anthonisen, A.; Loehr, R.; Prakasam, T.; Srinath, E. Inhibition of nitrification by ammonia and nitrous acid. J. Water Pollut. Control Fed. 1976, 48, 835–852. [Google Scholar] [CrossRef] [PubMed]
- Vesper, D.J.; Loop, C.M.; White, W.B. Contaminant transport in karst aquifers. Theor. Appl. Karstology 2001, 13, 101–111. [Google Scholar]
- Zhang, P.; Yue, F.-J.; Wang, X.-D.; Chen, S.-N.; Li, X.-Z.; Liu, T.-Z.; Yang, C. Antecedent rainfall and land use controlling the fate of nitrogen in karst urban rivers, elucidated by an isotopic approach. J. Hydrol. 2021, 592, 125803. [Google Scholar] [CrossRef]
- SPBS. Shandong Statistical Yearbook. 2000–2021. Available online: http://tjj.shandong.gov.cn/ (accessed on 10 January 2024).
- Postma, D.; Boesen, C.; Kristiansen, H.; Larsen, F. Nitrate reduction in an unconfined sandy aquifer: Water chemistry, reduction processes, and geochemical modeling. Water Resour. Res. 1991, 27, 2027–2045. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Q.; Sun, H.; Wang, J. Seasonal, diurnal and storm-scale hydrochemical variations of typical epikarst springs in subtropical karst areas of SW China: Soil CO2 and dilution effects. J. Hydrol. 2007, 337, 207–223. [Google Scholar] [CrossRef]
- Herman, J.S.; Lorah, M.M. CO2 outgassing and calcite precipitation in Falling Spring Creek, Virginia, USA. Chem. Geol. 1987, 62, 251–262. [Google Scholar] [CrossRef]
- Crossey, L.J.; Karlstrom, K.E.; Springer, A.E.; Newell, D.; Hilton, D.R.; Fischer, T. Degassing of mantle-derived CO2 and He from springs in the southern Colorado Plateau region—Neotectonic connections and implications for groundwater systems. Geol. Soc. Am. Bull. 2009, 121, 1034–1053. [Google Scholar] [CrossRef]
- Gulley, J.; Martin, J.B.; Screaton, E.J.; Moore, P.J. River reversals into karst springs: A model for cave enlargement in eogenetic karst aquifers. Bulletin 2011, 123, 457–467. [Google Scholar] [CrossRef]
- Zilberbrand, M.; Gimburg, A.; Doroshev, A.; Mirlas, V.; Anker, Y. Chemical evolution of runoff in Eastern Mediterranean mountainous karstic terrains. J. Hydrol. 2022, 605, 127388. [Google Scholar] [CrossRef]
- Lukač Reberski, J.; Terzić, J.; Maurice, L.D.; Lapworth, D.J. Emerging organic contaminants in karst groundwater: A global level assessment. J. Hydrol. 2022, 604, 127242. [Google Scholar] [CrossRef]
- Mahler, B.; Massei, N. Anthropogenic contaminants as tracers in an urbanizing karst aquifer. J. Contam. Hydrol. 2007, 91, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Gale, S.J. The hydraulics of conduit flow in carbonate aquifers. J. Hydrol. 1984, 70, 309–327. [Google Scholar] [CrossRef]


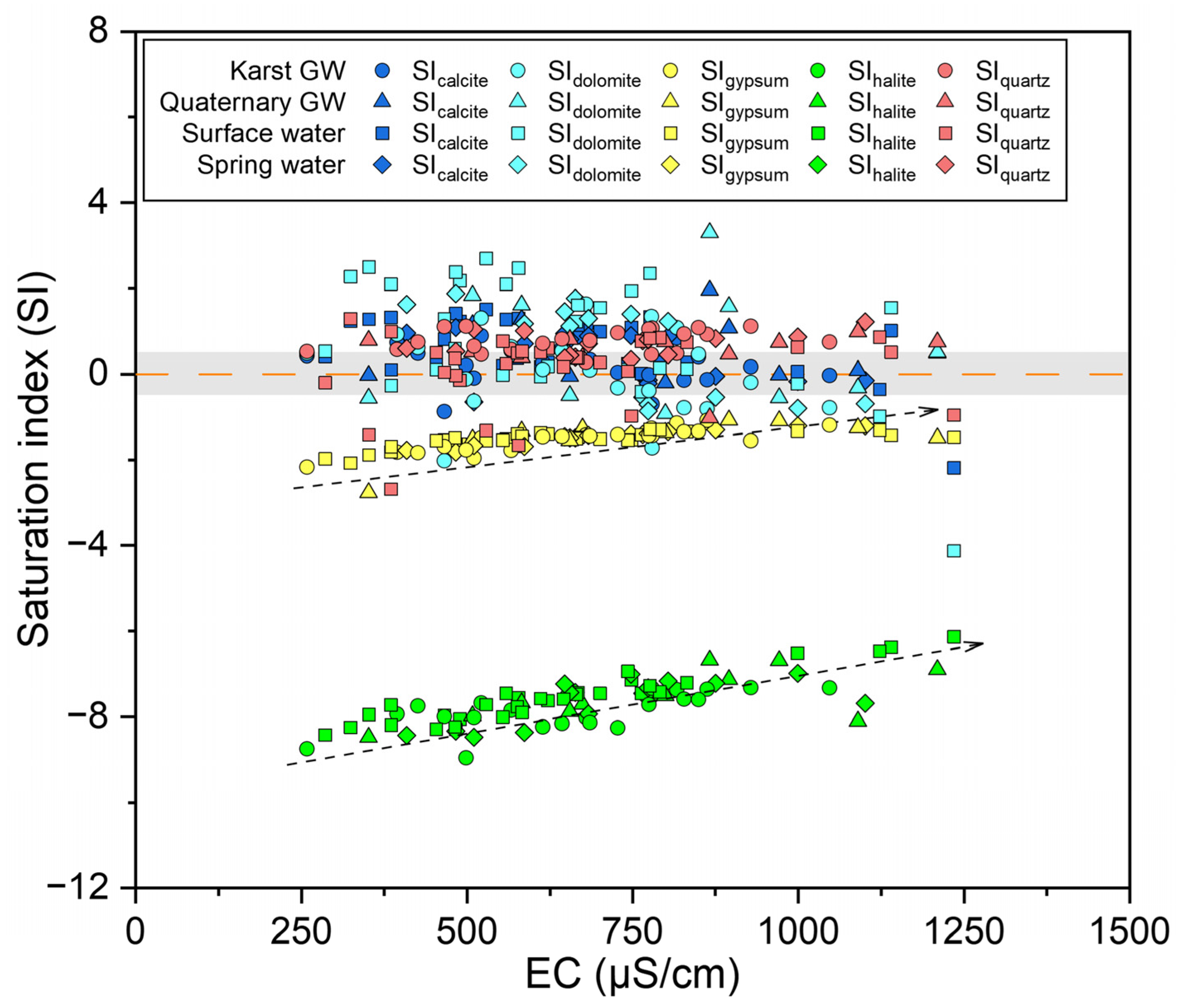
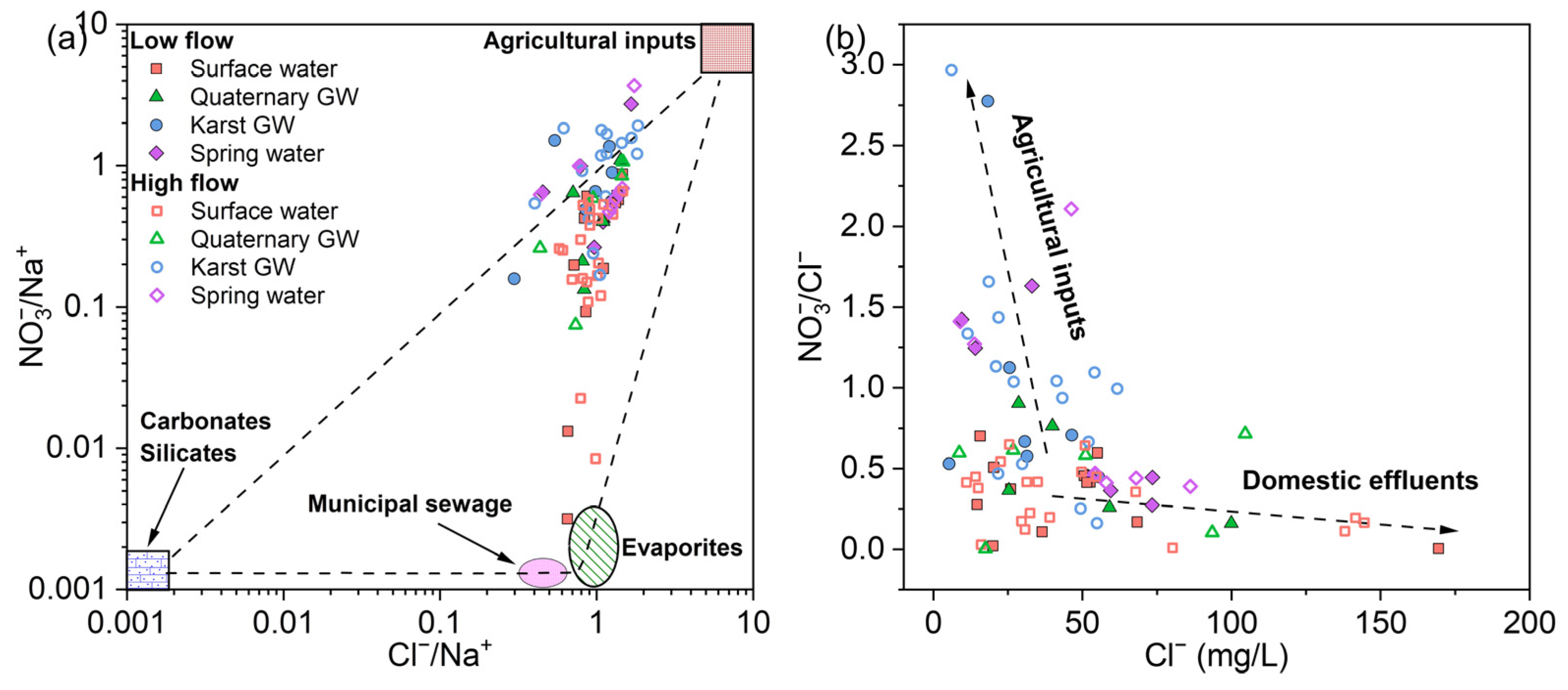

| Sampling Time | Water Type | Statistic | T | pH | EC | ORP | DO | Na+ | K+ | Ca2+ | Mg2+ | Cl− | SO42− | NO3− | HCO3− | TDS | δ18O | δ2H | d-Excess | logPCO2 | SIcalcite | SIdolomite | SIgypsum | SIhalite | SIquartz |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (℃) | (μS/cm) | (mv) | (mg/L) | (‰) | |||||||||||||||||||||
| Low flow (n = 32) | Surface water (n = 12) | Mean | 22.1 | 8.72 | 605.6 | 105.8 | 8.74 | 34.2 | 2.9 | 80.7 | 20.6 | 48.4 | 104.2 | 22.0 | 144.0 | 391.8 | −5.7 | −46 | −0.8 | −3.74 | 1.14 | 1.96 | −1.63 | −7.60 | −0.22 |
| SD | 3.5 | 0.45 | 238.8 | 39.3 | 1.87 | 43.0 | 1.9 | 30.5 | 6.6 | 42.2 | 56.0 | 17.8 | 63.8 | 150.9 | 1.0 | 6 | 3.2 | 1.34 | 0.19 | 0.47 | 0.18 | 0.59 | 0.87 | ||
| Min | 14.6 | 8.08 | 324.0 | 14.6 | 6.39 | 11.7 | 1.1 | 38.3 | 11.6 | 14.7 | 50.4 | 0.7 | 28.4 | 185.6 | −6.9 | −53 | −5.1 | −6.88 | 0.81 | 1.22 | −2.08 | −8.30 | −1.67 | ||
| Max | 26.2 | 9.51 | 1235.0 | 171.7 | 11.53 | 168.5 | 7.7 | 117.0 | 37.6 | 169.5 | 276.4 | 57.4 | 217.5 | 775.3 | −4.0 | −34 | 6.4 | −1.87 | 1.41 | 2.50 | −1.48 | −6.14 | 1.29 | ||
| Quaternary GW (n = 5) | Mean | 15.6 | 8.42 | 706.0 | 124.0 | 6.81 | 36.7 | 1.2 | 130.4 | 20.4 | 50.7 | 151.0 | 33.8 | 237.8 | 554.2 | −6.5 | −49 | 1.7 | −4.05 | 1.30 | 1.98 | −1.28 | −7.45 | 0.14 | |
| SD | 2.9 | 0.26 | 171.0 | 31.6 | 2.21 | 26.0 | 0.8 | 16.4 | 7.1 | 30.6 | 59.7 | 15.1 | 46.4 | 133.9 | 1.0 | 5 | 3.0 | 2.24 | 0.37 | 0.75 | 0.17 | 0.52 | 0.66 | ||
| Min | 13.0 | 8.12 | 509.0 | 81.5 | 4.28 | 14.9 | 0.4 | 105.6 | 9.2 | 25.4 | 85.3 | 16.1 | 189.1 | 397.1 | −7.8 | −57 | −3.1 | −8.04 | 1.06 | 1.57 | −1.53 | −7.99 | −1.03 | ||
| Max | 20.1 | 8.72 | 896.0 | 157.9 | 8.31 | 77.4 | 2.2 | 145.6 | 28.4 | 100.1 | 225.5 | 53.2 | 286.7 | 705.0 | −5.1 | −44 | 5.3 | −2.74 | 1.95 | 3.30 | −1.08 | −6.69 | 0.51 | ||
| Karst GW (n = 7) | Mean | 16.9 | 8.13 | 554.0 | 83.0 | 6.09 | 20.5 | 1.9 | 101.1 | 17.3 | 30.5 | 92.9 | 44.6 | 239.0 | 428.3 | −7.4 | −56 | 3.3 | −2.88 | 0.78 | 1.05 | −1.60 | −7.87 | 0.50 | |
| SD | 2.4 | 0.27 | 210.4 | 45.7 | 0.41 | 6.1 | 1.2 | 45.1 | 4.5 | 16.8 | 49.8 | 25.7 | 128.8 | 184.9 | 0.7 | 4 | 2.1 | 0.48 | 0.25 | 0.41 | 0.36 | 0.45 | 0.14 | ||
| Min | 12.4 | 7.70 | 259.0 | 4.1 | 5.80 | 11.5 | 0.6 | 42.3 | 9.8 | 5.3 | 34.0 | 4.9 | 122.9 | 184.4 | −8.5 | −62 | 0.2 | −3.53 | 0.42 | 0.48 | −2.18 | −8.76 | 0.27 | ||
| Max | 19.7 | 8.51 | 817.0 | 136.1 | 6.38 | 28.9 | 3.3 | 157.6 | 22.7 | 55.5 | 175.9 | 88.7 | 434.9 | 702.0 | −6.4 | −49 | 5.9 | −2.15 | 1.10 | 1.63 | −1.15 | −7.38 | 0.74 | ||
| Spring water (n = 8) | Mean | 17.1 | 8.23 | 637.1 | 114.0 | 6.37 | 25.8 | 1.6 | 110.9 | 23.6 | 46.0 | 88.4 | 45.0 | 218.2 | 450.4 | −7.4 | −56 | 2.5 | −2.98 | 0.94 | 1.46 | −1.54 | −7.64 | 0.47 | |
| SD | 1.6 | 0.17 | 130.4 | 20.0 | 1.15 | 13.2 | 0.6 | 23.9 | 2.9 | 24.8 | 23.4 | 22.1 | 26.6 | 79.8 | 1.0 | 4 | 2.8 | 0.18 | 0.11 | 0.27 | 0.18 | 0.54 | 0.12 | ||
| Min | 14.6 | 8.02 | 409.0 | 93.4 | 4.29 | 11.5 | 1.0 | 71.0 | 20.7 | 9.5 | 49.6 | 23.7 | 179.6 | 327.2 | −9.5 | −62 | −1.3 | −3.16 | 0.80 | 1.11 | −1.83 | −8.45 | 0.33 | ||
| Max | 18.6 | 8.41 | 804.0 | 149.0 | 7.72 | 49.0 | 2.8 | 140.3 | 29.4 | 73.6 | 120.0 | 94.4 | 248.9 | 547.5 | −6.5 | −51 | 7.5 | −2.75 | 1.12 | 1.87 | −1.35 | −7.02 | 0.70 | ||
| High flow (n = 49) | Surface water (n = 20) | Mean | 15.3 | 7.58 | 664.1 | 146.6 | 10.35 | 35.1 | 5.2 | 111.0 | 28.5 | 51.5 | 104.8 | 23.6 | 300.6 | 495.3 | −7.5 | −56 | 4.1 | −2.75 | 0.44 | 0.50 | −1.49 | −7.54 | 0.30 |
| SD | 2.0 | 0.46 | 234.8 | 17.3 | 2.13 | 28.6 | 4.9 | 31.0 | 8.9 | 42.7 | 25.8 | 17.0 | 118.5 | 157.4 | 1.0 | 5 | 3.7 | 2.03 | 0.48 | 0.96 | 0.18 | 0.60 | 0.87 | ||
| Min | 8.7 | 6.66 | 286.0 | 100.8 | 6.02 | 11.9 | 1.5 | 50.6 | 9.4 | 11.1 | 46.2 | 0.8 | 100.1 | 216.2 | −9.0 | −65 | −3.2 | −8.89 | −0.36 | −0.99 | −1.98 | −8.43 | −2.69 | ||
| Max | 18.2 | 8.49 | 1140.0 | 167.5 | 15.74 | 112.6 | 20.0 | 163.1 | 47.9 | 144.6 | 146.7 | 57.2 | 668.8 | 799.5 | −5.2 | −45 | 8.4 | −1.21 | 1.51 | 2.70 | −1.29 | −6.38 | 0.99 | ||
| Quaternary GW (n = 6) | Mean | 16.3 | 6.93 | 847.0 | 153.9 | 6.04 | 33.2 | 1.9 | 158.5 | 31.2 | 50.4 | 94.1 | 39.4 | 468.9 | 643.3 | −7.9 | −58 | 4.7 | −1.38 | 0.04 | −0.40 | −1.60 | −7.61 | 0.80 | |
| SD | 0.4 | 0.34 | 313.5 | 20.6 | 2.07 | 26.6 | 0.9 | 77.8 | 14.7 | 40.5 | 64.1 | 48.1 | 239.6 | 251.5 | 1.2 | 8 | 4.0 | 0.48 | 0.24 | 0.48 | 0.60 | 0.70 | 0.09 | ||
| Min | 15.8 | 6.40 | 352.0 | 123.1 | 3.03 | 13.0 | 0.6 | 56.7 | 10.8 | 8.8 | 6.8 | 9.1 | 287.5 | 249.5 | −8.6 | −68 | 0.0 | −1.94 | −0.21 | −0.93 | −2.78 | −8.49 | 0.73 | ||
| Max | 16.8 | 7.33 | 1211.0 | 171.3 | 8.52 | 82.2 | 2.7 | 292.6 | 56.1 | 104.7 | 204.1 | 130.6 | 927.5 | 949.3 | −5.5 | −44 | 8.4 | −0.54 | 0.48 | 0.50 | −1.10 | −6.70 | 0.98 | ||
| Karst GW (n = 15) | Mean | 15.3 | 6.98 | 719.3 | 156.5 | 8.49 | 19.5 | 1.6 | 150.4 | 31.6 | 34.3 | 85.5 | 50.5 | 392.9 | 569.9 | −8.6 | −61 | 7.3 | −1.49 | 0.03 | −0.40 | −1.50 | −7.87 | 0.91 | |
| SD | 2.2 | 0.37 | 170.2 | 18.6 | 2.13 | 8.8 | 1.4 | 40.8 | 10.2 | 17.7 | 36.7 | 29.1 | 107.9 | 141.1 | 0.9 | 3 | 4.3 | 0.39 | 0.41 | 0.77 | 0.23 | 0.46 | 0.18 | ||
| Min | 9.1 | 6.41 | 466.0 | 125.3 | 5.60 | 6.4 | 0.7 | 82.8 | 17.2 | 6.1 | 36.1 | 15.3 | 218.7 | 359.0 | −9.8 | −65 | −0.7 | −2.30 | −0.88 | −2.03 | −1.97 | −8.97 | 0.57 | ||
| Max | 18.5 | 7.71 | 1048.0 | 200.0 | 14.90 | 33.7 | 6.2 | 211.0 | 51.1 | 61.7 | 180.5 | 107.1 | 668.8 | 795.9 | −6.8 | −55 | 13.4 | −0.99 | 0.60 | 0.63 | −1.09 | −7.33 | 1.11 | ||
| Spring water (n = 8) | Mean | 16.9 | 6.78 | 798.8 | 173.3 | 6.89 | 25.1 | 1.9 | 159.8 | 39.1 | 48.8 | 97.6 | 58.0 | 409.4 | 635.1 | −8.5 | −60 | 8.1 | −1.26 | −0.10 | −0.58 | −1.42 | −7.63 | 0.91 | |
| SD | 1.6 | 0.39 | 195.8 | 25.4 | 1.18 | 11.5 | 0.7 | 45.0 | 11.0 | 26.0 | 26.6 | 46.9 | 95.2 | 178.3 | 0.6 | 3 | 1.7 | 0.42 | 0.38 | 0.78 | 0.18 | 0.54 | 0.16 | ||
| Min | 14.1 | 6.40 | 511.0 | 139.2 | 4.94 | 11.5 | 1.4 | 100.9 | 30.1 | 9.0 | 57.1 | 22.3 | 336.8 | 417.7 | −9.3 | −64 | 6.3 | −2.15 | −0.66 | −1.57 | −1.70 | −8.49 | 0.77 | ||
| Max | 18.2 | 7.67 | 1102.0 | 225.4 | 9.13 | 46.1 | 3.5 | 248.7 | 63.9 | 86.3 | 134.6 | 170.7 | 634.6 | 989.5 | −7.8 | −56 | 10.9 | −0.70 | 0.70 | 1.17 | −1.22 | −7.00 | 1.21 | ||
| T | pH | EC | DO | ORP | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source of variation | df | MS | F | p | MS | F | p | MS | F | p | MS | F | p | MS | F | p |
| Water types (Wt) | 3 | 35.235 | 6.814 | ** | 2.121 | 14.360 | ** | 72,722.923 | 1.589 | ns | 190.994 | 5.196 | * | 2093.917 | 2.858 | * |
| Hydrological conditions (Hc) | 1 | 63.475 | 12.275 | ** | 28.333 | 191.852 | ** | 28,8791.792 | 6.310 | * | 124.551 | 3.388 | ** | 43,146.596 | 58.897 | ** |
| Wt × Hc | 3 | 60.493 | 11.699 | * | 0.151 | 1.020 | ns | 15,438.886 | 0.337 | ns | 195.975 | 5.331 | ns | 1569.102 | 2.142 | ns |
| Residual | 73 | 5.171 | 0.148 | 45,769.803 | 36.758 | 732.574 | ||||||||||
| Na+ | K+ | Ca2+ | Mg2+ | Cl− | ||||||||||||
| Source of variation | df | MS | F | p | MS | F | p | MS | F | p | MS | F | p | MS | F | p |
| Water types (Wt) | 3 | 1027.946 | 1.647 | ns | 34.605 | 4.705 | * | 9431.633 | 6.067 | ** | 187.410 | 2.370 | ns | 1395.253 | 1.254 | ns |
| Hydrological conditions (Hc) | 1 | 19.119 | 0.031 | ns | 9.704 | 1.319 | ns | 25,511.579 | 16.410 | ** | 2463.615 | 31.151 | ** | 96.164 | 0.086 | ns |
| Wt × Hc | 3 | 13.544 | 0.022 | ns | 7.746 | 1.053 | ns | 593.339 | 0.382 | ns | 67.834 | 0.858 | ns | 10.377 | 0.009 | ns |
| Residual | 73 | 624.162 | 7.354 | 1554.606 | 79.086 | 1112.684 | ||||||||||
| SO42− | NO3− | HCO3− | 2H | 18O | ||||||||||||
| Source of variation | df | MS | F | p | MS | F | p | MS | F | p | MS | F | p | MS | F | p |
| Water types (Wt) | 3 | 3033.205 | 1.780 | ns | 3841.047 | 4.999 | * | 66,818.380 | 5.199 | * | 273.985 | 10.976 | ** | 10.016 | 11.165 | ** |
| Hydrological conditions (Hc) | 1 | 3094.559 | 1.816 | ns | 724.910 | 0.944 | ns | 559,437.431 | 43.530 | ** | 863.532 | 34.594 | ** | 31.303 | 34.891 | ** |
| Wt × Hc | 3 | 2749.242 | 1.613 | ns | 112.965 | 0.147 | ns | 4767.358 | 0.371 | ns | 44.247 | 1.773 | ns | 0.736 | 0.821 | ns |
| Residual | 73 | 1704.500 | 768.312 | 12,851.784 | 24.962 | 0.897 | ||||||||||
| d-excess | TDS | logPCO2 | SIcalcite | SIdolomite | ||||||||||||
| Source of variation | df | MS | F | p | MS | F | p | MS | F | p | MS | F | p | MS | F | p |
| Water types (Wt) | 3 | 70.714 | 5.828 | ** | 77,101.839 | 3.026 | * | 6.397 | 3.752 | * | 0.776 | 5.898 | ** | 3.799 | 7.403 | ** |
| Hydrological conditions (Hc) | 1 | 266.265 | 21.945 | ** | 280,740.037 | 11.020 | ** | 47.925 | 28.11 | ** | 14.494 | 110.111 | ** | 55.557 | 108.274 | ** |
| Wt × Hc | 3 | 10.631 | 0.876 | ns | 7455.843 | 0.293 | ns | 1.975 | 1.159 | ns | 0.278 | 2.109 | ns | 0.802 | 1.563 | ns |
| Residual | 73 | 12.133 | 25,475.720 | 1.705 | 0.132 | 0.513 | ||||||||||
| SIgypsum | SIhalite | SIquatz | ||||||||||||||
| Source of variation | df | MS | F | p | MS | F | p | MS | F | p | ||||||
| Water types (Wt) | 3 | 0.050 | 0.744 | ns | 0.433 | 1.406 | ns | 2.344 | 6.797 | ** | ||||||
| Hydrological conditions (Hc) | 1 | 0.002 | 0.034 | ns | 0.008 | 0.026 | ns | 4.251 | 12.324 | ** | ||||||
| Wt × Hc | 3 | 0.148 | 2.198 | ns | 0.033 | 0.106 | ns | 0.041 | 0.119 | ns | ||||||
| Residual | 73 | 0.067 | 0.308 | 0.345 |
| Phase | May | October | Formula | ||
|---|---|---|---|---|---|
| Path A | Path B | Path A | Path B | ||
| IRA → DRA | DRA → DA | IRA → DRA | DRA → DA | ||
| Calcite | 1.172 | −1.612 | 1.635 | −1.037 | CaCO3 |
| Dolomite | 0.023 | −0.093 | 0.686 | 0.660 | CaMg(CO3)2 |
| Halite | 0.967 | 0.696 | 0.181 | 1.034 | NaCl |
| Quartz | −0.053 | −0.061 | −0.271 | 0.026 | SiO2 |
| Gypsum | 1.176 | 0.274 | 0.081 | 0.392 | CaSO4:2H2O |
| CO2(g) | 2.495 | −3.089 | 3.598 | 2.288 | CO2(g) |
| NaX | - | - | 0.016 | −0.076 | NaX |
| CaX2 | - | - | −0.023 | 0.152 | CaX2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, Y.; Song, X.; Yang, L.; Yang, S. River–Spring Connectivity and Hydrogeochemical Processes in a Karst Water System of Northern China: A Case Study of Jinan Spring Catchment. Water 2024, 16, 829. https://doi.org/10.3390/w16060829
Ke Y, Song X, Yang L, Yang S. River–Spring Connectivity and Hydrogeochemical Processes in a Karst Water System of Northern China: A Case Study of Jinan Spring Catchment. Water. 2024; 16(6):829. https://doi.org/10.3390/w16060829
Chicago/Turabian StyleKe, Yunlong, Xianfang Song, Lihu Yang, and Shengtian Yang. 2024. "River–Spring Connectivity and Hydrogeochemical Processes in a Karst Water System of Northern China: A Case Study of Jinan Spring Catchment" Water 16, no. 6: 829. https://doi.org/10.3390/w16060829
APA StyleKe, Y., Song, X., Yang, L., & Yang, S. (2024). River–Spring Connectivity and Hydrogeochemical Processes in a Karst Water System of Northern China: A Case Study of Jinan Spring Catchment. Water, 16(6), 829. https://doi.org/10.3390/w16060829








