Pharmaceutical Contaminants in Wastewater and Receiving Water Bodies of South Africa: A Review of Sources, Pathways, Occurrence, Effects, and Geographical Distribution
Abstract
1. Introduction
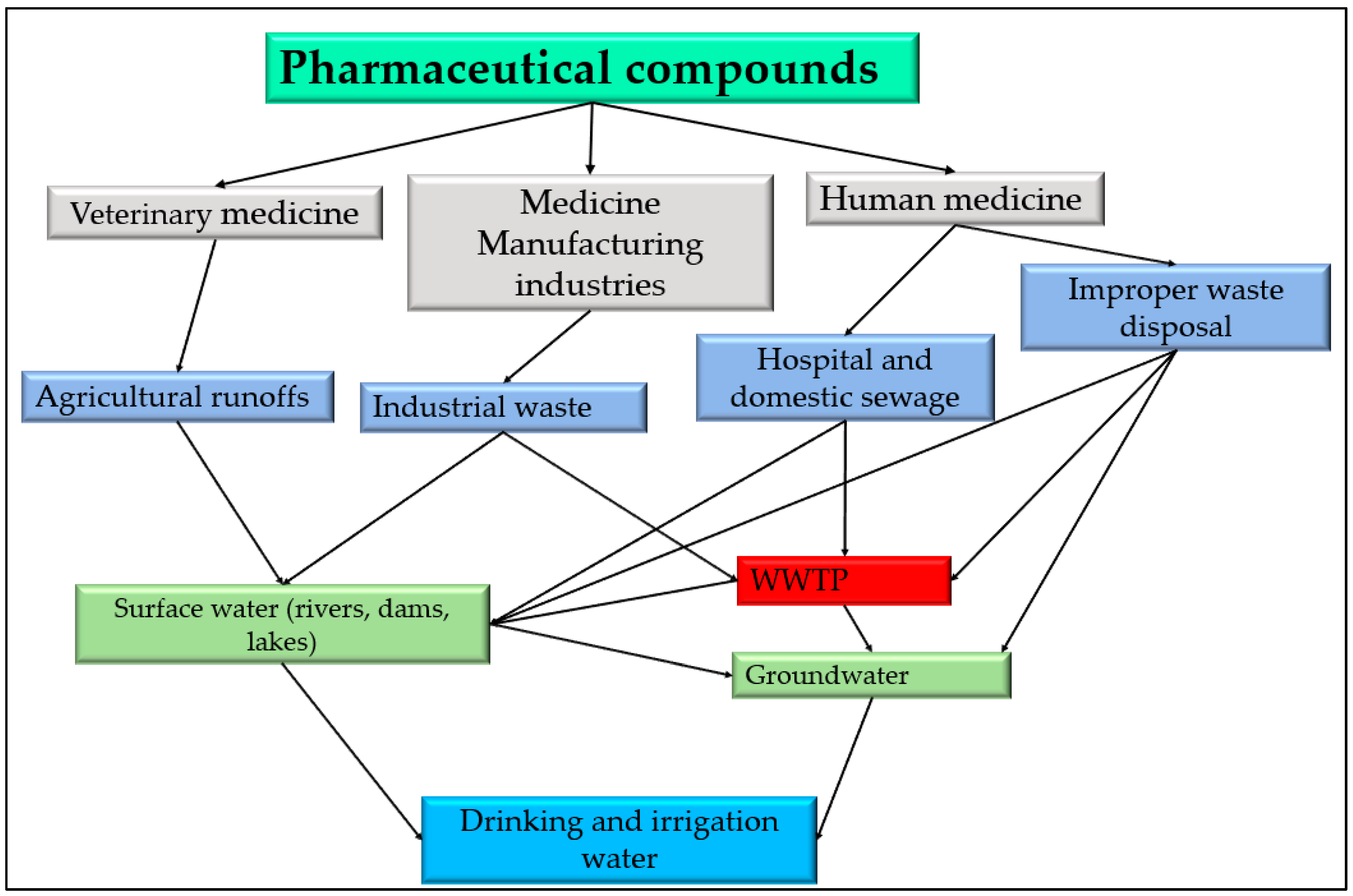
2. Sources and Pathways of Pharmaceutical Contaminants in South African Water Sources
3. Commonly Detected Pharmaceuticals in South Africa’s Wastewater and Water Sources
3.1. Analgesics and NSAIDs
3.2. Antibiotics
3.3. Beta-Blocker Drugs
3.4. Steroid Drugs
3.5. Antiviral Drugs
| Pharmaceuticals | Concentrations (ng/L) | Reference | |||
|---|---|---|---|---|---|
| Antibiotics | Region | WWT | Surface | Tap Water | |
| Erythromycin | KZN, Eastern Cape | LDL–4 | [51,52] | ||
| Tetracycline | KZN, Northwest | LDL–4 | [51,53] | ||
| Streptomycin | KZN | LDL–10 | [51] | ||
| Sulfamethoxazole | KZN, Gauteng; Eastern Cape | LDL–1013.2 | LDL–9 | [51,52] | |
| Acetaminophen | KZN, Gauteng, Gauteng | LDL–135 | [51,52] | ||
| Streptomycin | KZN | LDL–11 | [51,54] | ||
| Tylosin | KZN, Eastern Cape, Gauteng | LDL–11 | [52,55,56] | ||
| Chloramphenicol | KZN | LDL–2.5 | [51] | ||
| Ciprofloxacin | KZN, Gauteng, Eastern Cape, Northwest | LDL–35.5 | LDL–4 | [9,36,51,52,53,57] | |
| Ampicillin | KZN | LDL–5 | [36,51,58] | ||
| Nalidixic acid | KZN, Gauteng | LDL–7 | |||
| Trimethoprim | KZN, Gauteng | LDL–898.7 | LDL–2.8 | [36] | |
| Metronidazole | KZN | LDL–5.77 | |||
| Oxytetracycline | Gauteng | LDL–42 | [32] | ||
| Clarithromycin | Eastern Cape | LDL–3280.4 | [52] | ||
| Ofloxacin | Gauteng, Northwest | LDL–100 | [32,55] | ||
| Oxolinic acid | Gauteng | LDL–37 | LDL–0.25 | [32,59] | |
| Sulfamethazine | Gauteng, Eastern Cape | LDL–56.3 | LDL–0.4 | [32,43,60] | |
| Sulfaguanadin | Gauteng | LDL–17.9 | [32] | ||
| Sulfadoxin | Gauteng | LDL–78.6 | |||
| Sulfadimethoxine | Gauteng | LDL–621.4 | |||
| Enrofloxacin | Gauteng | LDL–0.74 | |||
| Trimethoprim | Gauteng, Eastern Cape | LDL–577.6 | [9,32,44] | ||
| Lincomycin | Gauteng | LDL–20.65 | [32] | ||
| Isoniazid | Gauteng | LDL–93.8 | |||
| Sulfadiazine | Gauteng | LDL–53 | [9,32] | ||
| Sarafloxacin | Gauteng | LDL–8.33 | [32] | ||
| Norfloxacin | Gauteng, Northwest | LDL–319 | [32,55] | ||
| Sulfapyridine | Gauteng | LDL–39 | [32] | ||
| Sulfanilamide | Gauteng | LDL–50 | |||
| Flumequine | Gauteng, Western Cape | LDL–0.25 | [60] | ||
| Lomefloxacin | Gauteng | LDL–0.35 | [59] | ||
| Azithromycin | Gauteng | LDL–24.6 | |||
| Anti-psychotics | |||||
| Clozapine | KZN, Gauteng | 0–2.08 | [36,61] | ||
| Bezafibrate | KZN, Gauteng, Northwest | 85.76–4878 | 0–80.3 | [32,36,43,50,52,62,63,64] | |
| Caffeine | Gauteng, Western Cape, Eastern Cape, Northwest, Mpumalanga | 1170–60,136 | LDL–927 | ||
| Carbamazepine | KZN, Gauteng, Eastern Cape, Northwest, Free State, Mpumalanga | LDL–52.35 | LDL–52.35 | 0.02–0.3 | |
| Mevastatin | Gauteng | LDL–3.32 | [65] | ||
| Simvastatin | Gauteng | LDL–11.7 | [65] | ||
| Clofibric acid | Gauteng | LDL–12.96 | [65] | ||
| Triclocarban | Gauteng, Northwest | 8.973–276.1 | [32,53] | ||
| Pravastatin | Gauteng | LDL–4.82 | [34,65] | ||
| Fluvastatin | Gauteng | LDL–1.97 | |||
| Lovastatin | Gauteng | LDL–8.03 | |||
| Fenofibrate | Gauteng | LDL–0.78 | |||
| Fenofibric acid | Gauteng | LDL-19.9 | |||
| Ifosfamide | Gauteng | LDL–5.43 | [32] | ||
| Lidocaine | Gauteng | LDL–424.6 | |||
| Methylparaben | Gauteng | 1.649–600.4 | |||
| Paraxanthine | Gauteng | 4963–35,286 | |||
| Prednisolone | Gauteng | LDL–36.17 | |||
| Procaine | Gauteng | LDL–14.52 | |||
| Ractopamine | Gauteng | LDL–2.29 | |||
| Salbutamol | Gauteng | LDL–8.60 | |||
| Terbutaline | Gauteng | LDL–1.44 | |||
| Tonalide | Gauteng | 0.21–80.16 | [9] | ||
| Tramadol | Gauteng | 0.718–289.8 | [9,51,65] | ||
| Venlafaxine | Gauteng | LDL–52.35 | LDL–94.6 | [32,66] | |
| Atorvostatin | Gauteng | LDL–3.73 | LDL–150.6 | ||
| Gabapentin | Gauteng | LDL–146.4 | |||
| Gemfibrozil | KZN Gauteng | LDL–598.6 | |||
| Analgesics/anti-inflammatory | |||||
| Aspirin | KZN, Gauteng | LDL–427 | [51] | ||
| Ketoprofen | KZN, Gauteng, Northwest | LDL–57 | [51,53,59,60,64,66,67] | ||
| Diclofenac | KZN, Gauteng, Northwest | LDL–21,100 | LDL–309 | ||
| Ibuprofen | KZN, Gauteng, Northwest | LDL–66,900 | LDL–0.113 | ||
| Naproxen | Gauteng, KZN | LDL–8990 | [32,38,64,66,67] | ||
| Indomethacin | Gauteng | LDL–31.55 | [32,38] | ||
| Mefenamic acid | Gauteng, KZN | 11.30–91.15 | |||
| Paracetamol | Gauteng, KZN | 155.3–22,889 | |||
| Phenacetin | Gauteng, KZN | 0.32–68.58 | |||
| Salicylamide | Gauteng | 5.47–563.50 | [32] | ||
| Tramadol | Gauteng | 0.718–289.8 | |||
| Fenoprofen | KZN | LDL–47,600 | [64] | ||
| Meclofenamic | Gauteng, KZN | LDL–0.849 | [38] | ||
| Beta Blockers | |||||
| Atenolol | KZN, Gauteng | LDL–39.1 | LDL–39.1 | [9,50,68] | |
| Pindolol | Gauteng | LDL–0,03 | LDL–0,03 | [9,30] | |
| Antiretroviral drugs | |||||
| Darunavir | KZN | LDL–43 | [26,61] | ||
| Efavirenz | KZN, Gauteng, Limpopo | LDL–140 | LDL–135 | [14,48,69,70] | |
| Emtricitabine | KZN, Gauteng | LDL–172 | 0–0.13 | [48,50] | |
| Lamivudine | Gauteng | LDL–1001 | LDL–242 | [32,49,50] | |
| Nevirapine | Gauteng, KZN | LDL–1480 | LDL–148 | [32,49] | |
| Penciclovir | Gauteng, KZN | LDL–104.8 | [32,49] | ||
| Zidovudine | KZN, Gauteng, Free State | LDL–243 | LDL–973 | LDL–0.07 | [26,49] |
| Ritonavir | Gauteng | LDL–393.90 | [32] | ||
| Atazanavir | Gauteng | LDL–10.69 | |||
| Famciclovir | Gauteng | LDL–17.67 | [31,32] | ||
| Didanosine | Free State, Gauteng | LDL–54.1 | [49,66] | ||
| Tenofovir disoproxil | Gauteng, KZN, Free State | 0.16–0.19 | LDL–243 | [49,50,66] | |
| Zalcitabine | Gauteng, Free State | LDL–71.3 | LDL–0.008 | [49] | |
| Stavudine | Gauteng | LDL–778 | |||
| Ribavirin | Gauteng | LDL–0.02 | [31] | ||
| Steroid hormones | |||||
| Estriol | Western Cape, Gauteng, Eastern Cape, Northwest, Limpopo | LDL–1313 | [32,46,58,71] | ||
| Estrone | Eastern Cape, Limpopo, Gauteng, Western Cape, Northwest | LDL–60.83 | [32,47] | ||
| Estradiol | Northwest, Western Cape, Limpopo, Gauteng | 154.1–7133 | [32,72] | ||
| Medroxyprogesterone | Mpumalanga, Gauteng | LDL–16.85 | [32,72] | ||
| Mestranol | Gauteng | LDL–123.4 | [32] | ||
| Diethylstilbesterol | Mpumalanga, Gauteng | LDL–547.7 | 0.001–0.01 | [32,72] | |
| Progesterone | Limpopo, Western Cape, KZN, Gauteng | LDL–14.52 | [32,47,71,73] | ||
| Testosterone | KZN, Western Cape, Gauteng, Eastern Cape, Limpopo | LDL–44.09 | [32,47,71,73] | ||
| Other drugs | |||||
| Amphetamine | Gauteng | LDL–37 | |||
| Nicotine | Gauteng | LDL–245.5 | [9] | ||
| Cotinine | Gauteng | LDL–31.7 | |||
| Gliclazide | Gauteng | LDL–53.9 | |||
| Metformin | Gauteng | LDL–81.7 | |||
| Irbesartan | Gauteng | LDL–554.4 | |||
| Valsartan | Gauteng | LDL–924.7 | |||
| Iopromide | Gauteng | LDL–598.3 | |||
| Codeine | Gauteng | LDL–1.61 | [74] | ||
| Morphine | Gauteng | LDL–4.82 | |||
| Meperidine | Gauteng | LDL–3.68 | |||
| Hydrocodone | Gauteng | LDL–10.9 | |||
| Oxycodone | Gauteng | LDL–4.9 | |||
| Heroin | Gauteng | LDL–42.2 | |||
| Hydromorphone | Gauteng | LDL–12.5 | |||
| Oxymorphone | Gauteng | LDL–74.9 | |||
| Thebaine | Gauteng | LDL–21.1 | |||
| Buprenorphine | Gauteng | LDL–22.3 | |||
| Fentanyl | Gauteng | LDL–25.9 | |||
| Ketamine | Gauteng | LDL–11.6 | |||
| Methadone | Gauteng | LDL–147 | |||
| Dihydrocodeine | Gauteng | LDL–4.29 | |||
| Alfentanyl | Gauteng | LDL–4.29 | |||
| Levorphanol | Gauteng | LDL–17.6 | |||
| Tramadol | Gauteng | LDL–24.6 | |||
| Ethylmorphine | Gauteng | LDL–19.9 | |||
| Remifentanyl | Gauteng | LDL–28.9 | |||
3.6. Anti-Depressant and Illicit Drugs
4. Health Impacts of Pharmaceutical Contaminants on Aquatic Organisms
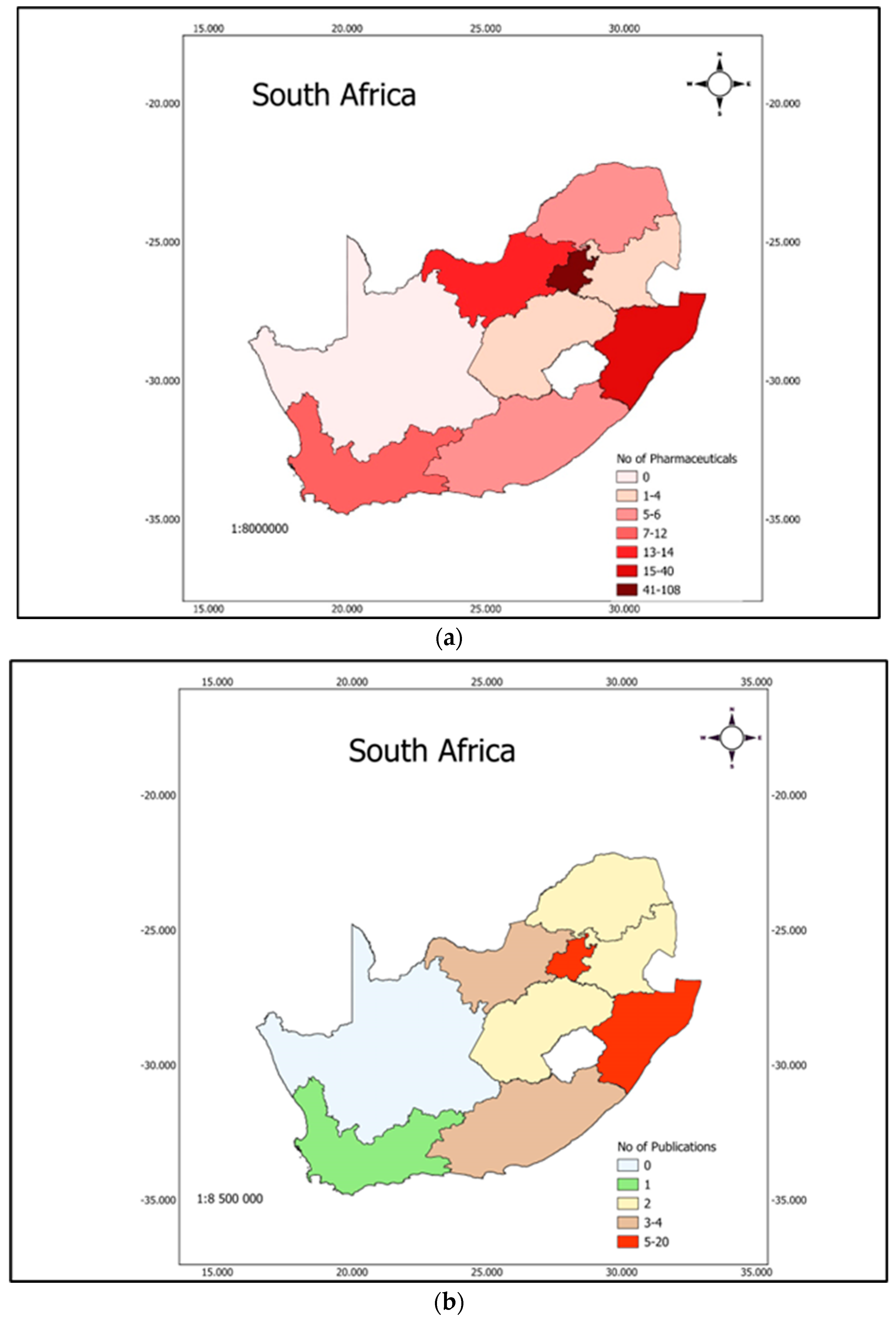
5. Spatial Distribution of PCs in the Aquatic System of South Africa
6. Policy and Regulatory Frameworks for Controlling Pharmaceutical Pollution in South Africa
7. Conclusions and Future Recommendations
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khan, N.A.; Ahmed, S.; Farooqi, I.H.; Ali, I.; Vambol, V.; Changani, F.; Yousefi, M.; Vambol, S.; Khan, S.U.; Khan, A.H.; et al. Occurrence, sources and conventional treatment techniques for various antibiotics present in hospital wastewaters: A critical review. TrAC Trends Anal. Chem. 2020, 129, 115921. [Google Scholar] [CrossRef]
- Deblonde, T.; Cossu-Leguille, C.; Hartmann, P. Emerging pollutants in wastewater: A review of the literature. Int. J. Hyg. Environ. Health 2011, 214, 442–448. [Google Scholar] [CrossRef]
- Desbiolles, F.; Malleret, L.; Tiliacos, C.; Wong-Wah-Chung, P.; Laffont-Schwob, I. Occurrence and ecotoxicological assessment of pharmaceuticals: Is there a risk for the Mediterranean aquatic environment? Sci. Total Environ. 2018, 639, 1334–1348. [Google Scholar] [CrossRef]
- Wu, X.; Dodgen, L.K.; Conkle, J.L.; Gan, J. Plant uptake of pharmaceutical and personal care products from recycled water and biosolids: A review. Sci. Total Environ. 2015, 536, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Sures, B.; Schmidt, T.C. Cephalosporin antibiotics in the aquatic environment: A critical review of occurrence, fate, ecotoxicity, and removal technologies. Environ. Pollut. 2018, 241, 1153–1166. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.N.V.; Vithanage, M.; Kapley, A. Pharmaceuticals, and Personal Care Products: Waste Management and Treatment Technology: Emerging Contaminants and Micropollutants; Butterworth-Heinemann: Oxford, UK, 2019. [Google Scholar]
- Madikizela, L.M.; Ncube, S.; Chimuka, L. Analysis, occurrence and removal of pharmaceuticals in African water resources: A current status. J. Environ. Manag. 2020, 253, 109741. [Google Scholar] [CrossRef]
- Gwenzi, W.; Chaukura, N. Organic contaminants in African aquatic systems: Current knowledge, health risks, and future research directions. Sci. Total Environ. 2018, 619, 1493–1514. [Google Scholar] [CrossRef] [PubMed]
- Archer, E. The fate of pharmaceuticals and personal care products (PPCPs), endocrine disrupting contaminants (EDCs), metabolites and illicit drugs in a WWTW and environmental waters. Chemosphere 2017, 174, 437–446. [Google Scholar] [CrossRef]
- Ngqwala, N.P.; Muchesa, P. Occurrence of pharmaceuticals in aquatic environments: A review and potential impacts in South Africa. S. Afr. J. Sci. 2020, 116, 1–7. [Google Scholar] [CrossRef]
- Gani, K.M.; Hlongwa, N.; Abunama, T.; Kumari, S.; Bux, F. Emerging contaminants in South African water environment-a critical review of their occurrence, sources and ecotoxicological risks. Chemosphere 2021, 269, 128737. [Google Scholar] [CrossRef]
- Karungamye, P.; Rugaika, A.; Mtei, K.; Machunda, R. The pharmaceutical disposal practices and environmental contamination: A review in East African countries. HydroResearch 2022, 5, 99–107. [Google Scholar] [CrossRef]
- Madikizela, L.M.; Rimayi, C.; Khulu, S.; Ncube, S.; Chimuka, L. Pharmaceuticals and Personal Care Products, in Emerging Freshwater Pollutants; Elsevier: Amsterdam, The Netherlands, 2022; pp. 171–190. [Google Scholar]
- Mlunguza, N.Y.; Ncube, S.; Mahlambi, P.N.; Chimuka, L.; Madikizela, L.M. Determination of selected antiretroviral drugs in wastewater, surface water, and aquatic plants using hollow fibre liquid phase microextraction and liquid chromatography-tandem mass spectrometry. J. Hazard. Mater. 2020, 382, 121067. [Google Scholar] [CrossRef] [PubMed]
- Andrews, W.J.; Masoner, J.R.; Cozzarelli, I.M. Emerging contaminants at a closed and operating landfill in Oklahoma. Groundw. Monit. Remediat. 2012, 32, 120–130. [Google Scholar] [CrossRef]
- Anh, H.Q.; Le, T.P.Q.; Da Le, N.; Lu, X.X.; Duong, T.T.; Garnier, J.; Rochelle-Newall, E.; Zhang, S.; Oh, N.H.; Oeurng, C.; et al. Antibiotics in surface water of East and Southeast Asian countries: A focused review on contamination status, pollution sources, potential risks, and future perspectives. Sci. Total Environ. 2021, 764, 142865. [Google Scholar] [CrossRef] [PubMed]
- Penner, N.; Xu, L.; Prakash, C. Radiolabeled absorption, distribution, metabolism, and excretion studies in drug development: Why, when, and how? Chem. Res. Toxicol. 2012, 25, 513–531. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, J.; Khan, S.; Su, J.Q.; Hesham, A.E.L.; Ditta, A.; Nawab, J.; Ali, A. Antibiotics in poultry manure and their associated health issues: A systematic review. J. Soils Sediments 2020, 20, 486–497. [Google Scholar] [CrossRef]
- Mastroianni, N.; Bleda, M.J.; de Alda, M.L.; Barceló, D. Occurrence of drugs of abuse in surface water from four Spanish river basins: Spatial and temporal variations and environmental risk assessment. J. Hazard. Mater. 2016, 316, 134–142. [Google Scholar] [CrossRef]
- Erickson, T.B.; Endo, N.; Duvallet, C.; Ghaeli, N.; Hess, K.; Alm, E.J.; Matus, M.; Chai, P.R. “Waste not, want not”–Leveraging sewer systems and wastewater-based epidemiology for drug use trends and pharmaceutical monitoring. J. Med. Toxicol. 2021, 17, 397–410. [Google Scholar] [CrossRef]
- Riser-Roberts, E. Remediation of Petroleum Contaminated Soils: Biological, Physical, and Chemical Processes; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Awaleh, M.O.; Soubaneh, Y.D. Waste water treatment in chemical industries: The concept and current technologies. Hydrol. Curr. Res. 2014, 5, 1. [Google Scholar]
- Alsaidi, M.; Azeez, F.A.; Al-Hajji, L.A.; Ismail, A.A. Impact of reaction parameters for photodegradation pharmaceuticals in wastewater over gold/titania photocatalyst synthesized by pyrolysis of NH2-MIL-125 (Ti). J. Environ. Manag. 2022, 314, 115047. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Chamorro, S.; Marti, E.; Huerta, B.; Gros, M.; Sànchez-Melsió, A.; Borrego, C.M.; Barceló, D.; Balcázar, J.L. Occurrence of antibiotics and antibiotic resistance genes in hospital and urban wastewaters and their impact on the receiving river. Water Res. 2015, 69, 234–242. [Google Scholar] [CrossRef]
- Olasupo, A.; Suah, F.B.M. Recent advances in the removal of pharmaceuticals and endocrine-disrupting compounds in the aquatic system: A case of polymer inclusion membranes. J. Hazard. Mater. 2021, 406, 124317. [Google Scholar] [CrossRef]
- Abafe, O.A.; Späth, J.; Fick, J.; Jansson, S.; Buckley, C.; Stark, A.; Pietruschka, B.; Martincigh, B.S. LC-MS/MS determination of antiretroviral drugs in influents and effluents from wastewater treatment plants in KwaZulu-Natal, South Africa. Chemosphere 2018, 200, 660–670. [Google Scholar] [CrossRef]
- Letsoalo, M.R.; Sithole, T.; Mufamadi, S.; Mazhandu, Z.; Sillanpaa, M.; Kaushik, A.; Mashifana, T. Efficient detection and treatment of pharmaceutical contaminants to produce clean water for better health and environmental. J. Clean. Prod. 2022, 387, 135798. [Google Scholar] [CrossRef]
- Swanepoel, C.; Bouwman, H.; Pieters, R.; Bezuidenhout, C. Presence, concentrations and potential implications of HIV-anti-retrovirals in selected water resources in South Africa. Water Res. Comm. 2015, 2144, 14. [Google Scholar]
- Ebele, A.J.; Oluseyi, T.; Drage, D.S.; Harrad, S.; Abdallah, M.A.E. Occurrence, seasonal variation and human exposure to pharmaceuticals and personal care products in surface water, groundwater and drinking water in Lagos State, Nigeria. Emerg. Contam. 2020, 6, 124–132. [Google Scholar] [CrossRef]
- Waleng, N.J.; Nomngongo, P.N. Occurrence of pharmaceuticals in the environmental waters: African and Asian perspectives. Environ. Chem. Ecotoxicol. 2022, 4, 50–66. [Google Scholar] [CrossRef]
- Osunmakinde, C.S.; Tshabalala, O.S.; Dube, S.; Nindi, M.M. Verification and Validation of Analytical Methods for Testing the Levels of PPHCPs (Pharmaceutical & Personal Health Care Products) in Treated Drinking Water and Sewage: Report to the Water Research Commission; Water Research Commission: Pretoria, South Africa, 2013. [Google Scholar]
- Mhuka, V.; Dube, S.; Nindi, M.M. Occurrence of pharmaceutical and personal care products (PPCPs) in wastewater and receiving waters in South Africa using LC-Orbitrap™ MS. Emerg. Contam. 2020, 6, 250–258. [Google Scholar] [CrossRef]
- Oluwalana, A.E.; Musvuugwa, T.; Sikwila, S.T.; Sefadi, J.S.; Whata, A.; Nindi, M.M.; Chaukura, N. The screening of emerging micropollutants in wastewater in Sol Plaatje Municipality, Northern Cape, South Africa. Environ. Pollut. 2022, 314, 120275. [Google Scholar] [CrossRef] [PubMed]
- Saikat Sen, S.S.; Raja Chakraborty, R.C.; Biplab De, B.D.; Ganesh, T.; Raghavendra, H.G.; Subal Debnath, S.D. Analgesic and anti-inflammatory herbs: A potential source of modern medicine. Int. J. Pharm. Sci. Res. 2010, 1, 32. [Google Scholar]
- Matongo, S.; Birungi, G.; Moodley, B.; Ndungu, P. Pharmaceutical residues in water and sediment of Msunduzi River, kwazulu-natal, South Africa. Chemosphere 2015, 134, 133–140. [Google Scholar] [CrossRef]
- Henton, M.M.; Eagar, H.A.; Swan, G.E.; Van Vuuren, M. Part VI. Antibiotic management and resistance in livestock production. SAMJ S. Afr. Med. J. 2011, 101, 583–586. [Google Scholar]
- Faleye, A.C.; Adegoke, A.A.; Ramluckan, K.; Fick, J.; Bux, F.; Stenström, T.A. Concentration and reduction of antibiotic residues in selected wastewater treatment plants and receiving waterbodies in Durban, South Africa. Sci. Total Environ. 2019, 678, 10–20. [Google Scholar] [CrossRef]
- Gumbi, B.P.; Moodley, B.; Birungi, G.; Ndungu, P.G. Assessment of nonsteroidal anti-inflammatory drugs by ultrasonic-assisted extraction and GC-MS in Mgeni and Msunduzi river sediments, KwaZulu-Natal, South Africa. Environ. Sci. Pollut. Res. 2017, 24, 20015–20028. [Google Scholar] [CrossRef]
- Kümmerer, K. Antibiotics in the aquatic environment–A review–part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef]
- Agunbiade, F.O.; Moodley, B. Occurrence and distribution pattern of acidic pharmaceuticals in surface water, wastewater, and sediment of the Msunduzi River, Kwazulu-Natal, South Africa. Environ. Toxicol. Chem. 2016, 35, 36–46. [Google Scholar] [CrossRef]
- Khulu, S.; Ncube, S.; Nuapia, Y.; Madikizela, L.M.; Tutu, H.; Richards, H.; Ndungu, K.; Mavhunga, E.; Chimuka, L. Multivariate optimization of a two-way technique for extraction of pharmaceuticals in surface water using a combination of membrane-assisted solvent extraction and a molecularly imprinted polymer. Chemosphere 2022, 286, 131973. [Google Scholar] [CrossRef] [PubMed]
- Agunbiade, F.O.; Moodley, B. Pharmaceuticals as emerging organic contaminants in Umgeni River water system, KwaZulu-Natal, South Africa. Environ. Monit. Assess. 2014, 186, 7273–7291. [Google Scholar] [CrossRef] [PubMed]
- Ohoro, C.R.; Adeniji, A.O.; Semerjian, L.; Okoh, O.O.; Okoh, A.I. Occurrence and distribution of pharmaceuticals in surface water and sediment of Buffalo and Sundays River estuaries, South Africa and their ecological risk assessment. Emerg. Contam. 2021, 7, 187–195. [Google Scholar] [CrossRef]
- Peixoto, R.; Pereira, M.D.L.; Oliveira, M. Beta-blockers and cancer: Where are we? Pharmaceuticals 2020, 13, 105. [Google Scholar] [CrossRef]
- Kubon, C.; Mistry, N.B.; Grundvold, I.; Halvorsen, S.; Kjeldsen, S.E.; Westheim, A.S. The role of beta-blockers in the treatment of chronic heart failure. Trends Pharmacol. Sci. 2011, 32, 206–212. [Google Scholar] [CrossRef]
- Ramiya, P. Chemistry, Manufacturing, and Controls: Active Pharmaceutical Ingredient and Drug Product. Pept. Ther. Strategy Tactics Chem. Manuf. Control. 2019, 72, 97. [Google Scholar]
- Manickum, T.; John, W. Occurrence, fate, and environmental risk assessment of endocrine disrupting compounds at the wastewater treatment works in Pietermaritzburg (South Africa). Sci. Total Environ. 2014, 468, 584–597. [Google Scholar] [CrossRef]
- Manavhela, M.; Sichilima, A.; Samie, A. Distribution and Potential Effects of 17β-Estradiol (E2) on Aeromonas Diversity in Wastewater and Fish Samples. Pak. J. Biol. Sci. PJBS 2020, 23, 278–286. [Google Scholar] [CrossRef]
- Van Zijl, M.C.; Aneck-Hahn, N.H.; Swart, P.; Hayward, S.; Genthe, B.; De Jager, C. Estrogenic activity, chemical levels and health risk assessment of municipal distribution point water from Pretoria and Cape Town, South Africa. Chemosphere 2017, 186, 305–313. [Google Scholar] [CrossRef]
- Mosekiemang, T.T.; Stander, M.A.; de Villiers, A. Simultaneous quantification of commonly prescribed antiretroviral drugs and their selected metabolites in aqueous environmental samples by direct injection and solid phase extraction liquid chromatography-tandem mass spectrometry. Chemosphere 2019, 220, 983–992. [Google Scholar] [CrossRef]
- Wood, T.P.; Duvenage, C.S.; Rohwer, E. The occurrence of anti-retroviral compounds used for HIV treatment in South African surface water. Environ. Pollut. 2015, 199, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Rimayi, C.; Odusanya, D.; Weiss, J.M.; de Boer, J.; Chimuka, L. Contaminants of emerging concern in the Hartbeespoort Dam catchment and the uMngeni River estuary 2016 pollution incident, South Africa. Sci. Total Environ. 2018, 627, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Vumazonke, S.; Khamanga, S.M.; Ngqwala, N.P. Detection of pharmaceutical residues in surface waters of the Eastern Cape Province. Int. J. Environ. Res. Public Health 2020, 17, 4067. [Google Scholar] [CrossRef] [PubMed]
- Kanama, K.M.; Daso, A.P.; Mpenyana-Monyatsi, L.; Coetzee, M.A. Assessment of pharmaceuticals, personal care products, and hormones in wastewater treatment plants receiving inflows from health facilities in North West Province, South Africa. J. Toxicol. 2018, 2018, 3751930. [Google Scholar] [CrossRef]
- Selwe, K.P.; Thorn, J.P.; Desrousseaux, A.O.; Dessent, C.E.; Sallach, J.B. Emerging contaminant exposure to aquatic systems in the Southern African Development Community. Environ. Toxicol. Chem. 2022, 41, 382–395. [Google Scholar] [CrossRef]
- Madikizela, L.M.; Chimuka, L. Occurrence of naproxen, ibuprofen, and diclofenac residues in wastewater and river water of KwaZulu-Natal Province in South Africa. Environ. Monit. Assess. 2017, 189, 348. [Google Scholar] [CrossRef]
- Amdany, R.; Chimuka, L.; Cukrowska, E. Determination of naproxen, ibuprofen, and triclosan in wastewater using the polar organic chemical integrative sampler (POCIS): A laboratory calibration and field application. Water SA 2014, 40, 407–414. [Google Scholar] [CrossRef]
- Madikizela, L.M.; Muthwa, S.F.; Chimuka, L. Determination of triclosan and ketoprofen in river water and wastewater by solid phase extraction and high-performance liquid chromatography. S. Afr. J. Chem. 2014, 67, 143–150. [Google Scholar]
- Farounbi, A.I.; Ngqwala, N.P. Occurrence of selected endocrine disrupting compounds in the eastern Cape province of South Africa. Environ. Sci. Pollut. Res. 2020, 27, 17268–17279. [Google Scholar] [CrossRef] [PubMed]
- Gumbi, B.P.; Moodley, B.; Birungi, G.; Ndungu, P.G. Detection and quantification of acidic drug residues in South African surface water using gas chromatography-mass spectrometry. Chemosphere 2017, 168, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Madikizela, L.M.; Nuapia, Y.B.; Chimuka, L.; Ncube, S.; Etale, A. Target and Suspect Screening of Pharmaceuticals and their Transformation Products in the Klip River, South Africa, using Ultra-High–Performance Liquid Chromatography–Mass Spectrometry. Environ. Toxicol. Chem. 2022, 41, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, R.; Pool, E.J. The effectiveness of sewage treatment processes to remove fecal pathogens and antibiotic residues. J. Environ. Sci. Health Part A 2012, 47, 289–297. [Google Scholar] [CrossRef]
- Matongo, S.; Birungi, G.; Moodley, B.; Ndungu, P. Occurrence of selected pharmaceuticals in water and sediment of Umgeni River, KwaZulu-Natal, South Africa. Environ. Sci. Pollut. Res. 2015, 22, 10298–10308. [Google Scholar] [CrossRef] [PubMed]
- Patterton, H.-G. Scoping Study and Research Strategy Development on Currently Known and Emerging Contaminants Influencing Drinking Water Quality: Main Report; Water Research Commission: Pretoria, South Africa, 2013. [Google Scholar]
- Wanda, E.M.; Nyoni, H.; Mamba, B.B.; Msagati, T.A. Occurrence of emerging micropollutants in water systems in Gauteng, Mpumalanga, and North West Provinces, South Africa. Int. J. Environ. Res. Public Health 2017, 14, 79. [Google Scholar] [CrossRef] [PubMed]
- Hlengwa, N.; Mahlambi, P. SPE-LC-PDA method development, and application for the analysis of selected pharmaceuticals in river and wastewater samples from South Africa. Water SA 2020, 46, 514–522. [Google Scholar] [CrossRef]
- Tete, V.S.; Nyoni, H.; Mamba, B.B.; Msagati, T.A. Occurrence and spatial distribution of statins, fibrates, and their metabolites in aquatic environments. Arab. J. Chem. 2020, 13, 4358–4373. [Google Scholar] [CrossRef]
- Sigonya, S.; Onwubu, S.C.; Mdluli, P.S.; Mokhothu, T.H. Method optimization and application based on solid phase extraction of nonsteroidal anti-inflammatory drugs, antiretroviral drugs, and a lipid regulator from coastal areas of Durban, South Africa. SN Appl. Sci. 2022, 4, 231. [Google Scholar] [CrossRef]
- Amos Sibeko, P.; Naicker, D.; Mdluli, P.S.; Madikizela, L.M. Naproxen, ibuprofen, and diclofenac residues in river water, sediments and Eichhornia crassipes of Mbokodweni river in South Africa: An initial screening. Environ. Forensics 2019, 20, 129–138. [Google Scholar] [CrossRef]
- Osunmakinde, I.O. Towards safety from toxic gases in underground mines using wireless sensor networks and ambient intelligence. Int. J. Distrib. Sens. Netw. 2013, 9, 159273. [Google Scholar] [CrossRef]
- Schoeman, C.; Mashiane, M.; Dlamini, M.; Okonkwo, O.J. Quantification of selected antiretroviral drugs in wastewater treatment works in South Africa using GC-TOFMS. J. Chromatogr. Sep. Tech. 2015, 6, 272. [Google Scholar]
- Wooding, M.; Rohwer, E.R.; Naudé, Y. Determination of endocrine disrupting chemicals and antiretroviral compounds in surface water: A disposable sorptive sampler with comprehensive gas chromatography–time-of-flight mass spectrometry and large volume injection with ultra-high performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2017, 1496, 122–132. [Google Scholar]
- Ohoro, C.R.; Adeniji, A.O.; Okoh, A.I.; Okoh, O.O. Spatial and seasonal variations of endocrine disrupting compounds in water and sediment samples of Markman Canal and Swartkops River Estuary, South Africa, and their ecological risk assessment. Mar. Pollut. Bull. 2021, 173, 113012. [Google Scholar] [CrossRef]
- Kamika, I.; Azizi, S.; Muleja, A.A.; Selvarajan, R.; El-Liethy, M.A.; Mamba, B.B.; Nkambule, T.T. The occurrence of opioid compounds in wastewater treatment plants and their receiving water bodies in Gauteng province, South Africa. Environ. Pollut. 2021, 290, 118048. [Google Scholar] [CrossRef]
- Dart, R.C.; Surratt, H.L.; Cicero, T.J.; Parrino, M.W.; Severtson, S.G.; Bucher-Bartelson, B.; Green, J.L. Trends in opioid analgesic abuse and mortality in the United States. N. Engl. J. Med. 2015, 372, 241–248. [Google Scholar] [CrossRef]
- Campos-Mañas, M.C.; Ferrer, I.; Thurman, E.M.; Pérez, J.A.S.; Agüera, A. Identification of opioids in surface and wastewaters by LC/QTOF-MS using retrospective data analysis. Sci. Total Environ. 2019, 664, 874–884. [Google Scholar] [CrossRef]
- Millar, D.A.; Wright, C.Y. ‘Meet People Where They Are’: An Approach to Opioids and Harm Reduction in South Africa; Academy of Science of South Africa: Pretoria, South Africa, 2020. [Google Scholar]
- Scheibe, A.; Shelly, S.; Gerardy, T.; Von Homeyer, Z.; Schneider, A.; Padayachee, K.; Naidoo, S.B.; Mtshweni, K.; Matau, A.; Hausler, H.; et al. Six-month retention and changes in quality of life and substance use from a low-threshold methadone maintenance therapy program in Durban, South Africa. Addict. Sci. Clin. Pract. 2020, 15, 13. [Google Scholar] [CrossRef] [PubMed]
- Archer, E.; Castrignanò, E.; Kasprzyk-Hordern, B.; Wolfaardt, G.M. Wastewater-based epidemiology and enantiomeric profiling for drugs of abuse in South African wastewaters. Sci. Total Environ. 2018, 625, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Ojemaye, C.Y.; Petrik, L. Occurrences, levels and risk assessment studies of emerging pollutants (pharmaceuticals, perfluoroalkyl, and endocrine disrupting compounds) in fish samples from Kalk Bay harbor, South Africa. Environ. Pollut. 2019, 252, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Madikizela, L.M.; Ncube, S. Health effects and risks associated with the occurrence of pharmaceuticals and their metabolites in marine organisms and seafood. Sci. Total Environ. 2022, 837, 155780. [Google Scholar] [CrossRef]
- Mezzelani, M.; Gorbi, S.; Regoli, F. Pharmaceuticals in the aquatic environments: Evidence of emerged threat and future challenges for marine organisms. Mar. Environ. Res. 2018, 140, 41–60. [Google Scholar] [CrossRef]
- Jacob, R.S.; Araújo, C.V.; de Souza Santos, L.V.; Moreira, V.R.; Lebron, Y.A.R.; Lange, L.C. The environmental risks of pharmaceuticals beyond traditional toxic effects: Chemical differences that can repel or entrap aquatic organisms. Environ. Pollut. 2021, 268, 115902. [Google Scholar] [CrossRef]
- Fenske, M.; Maack, G.; Schäfers, C.; Segner, H. An environmentally relevant concentration of estrogen induces arrest of male gonad development in zebrafish, Danio rerio. Environ. Toxicol. Chem. Int. J. 2005, 24, 1088–1098. [Google Scholar] [CrossRef]
- Miazek, K.; Brozek-Pluska, B. Effect of PHRs and PCPs on microalgal growth, metabolism, and microalgae-based bioremediation processes: A review. Int. J. Mol. Sci. 2019, 20, 2492. [Google Scholar] [CrossRef]
- Cleuvers, M. Mixture toxicity of the anti-inflammatory drugs diclofenac, ibuprofen, naproxen, and acetylsalicylic acid. Ecotoxicol. Environ. Saf. 2004, 59, 309–315. [Google Scholar] [CrossRef]
- Capolupo, M.; Díaz-Garduño, B.; Martín-Díaz, M.L. The impact of propranolol, 17α-ethinylestradiol, and gemfibrozil on early life stages of marine organisms: Effects and risk assessment. Environ. Sci. Pollut. Res. 2018, 25, 32196–32209. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, T.G.; Carriço, T.; Fernandes, E.; Abessa, D.M.S.; Tavares, A.; Bebianno, M.J. Impacts of in vivo and in vitro exposures to tamoxifen: Comparative effects on human cells and marine organisms. Environ. Int. 2019, 129, 256–272. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Pharmaceuticals in Drinking Water; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Naidu, R.; Jit, J.; Kennedy, B.; Arias, V. Emerging contaminant uncertainties and policy: The chicken or the egg conundrum. Chemosphere 2016, 154, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Lamastra, L.; Balderacchi, M.; Trevisan, M. Inclusion of emerging organic contaminants in groundwater monitoring plans. MethodsX 2016, 3, 459–476. [Google Scholar] [CrossRef]
| Drug Class | Types of Pharmaceuticals |
|---|---|
| Analgesics | disprin, ibuprofen, paracetamol, indomethacin |
| codeine, phenazone | |
| Antibiotics | vancomycin, penicillin, amoxicillin, streptomycin, ciprofloxacin |
| sulfamethoxazole, azithromycin | |
| NSAID | diclofenac, ketoprofen, and naproxen |
| Beta-blockers | betacolol, propranolol, atenolol |
| Steroids hormones | 17-beta-oestradiol, 17-alpha-ethinyloestradiol |
| Antiretroviral drugs | efavirenz, zidovudine, darunavir, emtricitabine |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munzhelele, E.P.; Mudzielwana, R.; Ayinde, W.B.; Gitari, W.M. Pharmaceutical Contaminants in Wastewater and Receiving Water Bodies of South Africa: A Review of Sources, Pathways, Occurrence, Effects, and Geographical Distribution. Water 2024, 16, 796. https://doi.org/10.3390/w16060796
Munzhelele EP, Mudzielwana R, Ayinde WB, Gitari WM. Pharmaceutical Contaminants in Wastewater and Receiving Water Bodies of South Africa: A Review of Sources, Pathways, Occurrence, Effects, and Geographical Distribution. Water. 2024; 16(6):796. https://doi.org/10.3390/w16060796
Chicago/Turabian StyleMunzhelele, Elisa Pandelani, Rabelani Mudzielwana, Wasiu Babatunde Ayinde, and Wilson Mugera Gitari. 2024. "Pharmaceutical Contaminants in Wastewater and Receiving Water Bodies of South Africa: A Review of Sources, Pathways, Occurrence, Effects, and Geographical Distribution" Water 16, no. 6: 796. https://doi.org/10.3390/w16060796
APA StyleMunzhelele, E. P., Mudzielwana, R., Ayinde, W. B., & Gitari, W. M. (2024). Pharmaceutical Contaminants in Wastewater and Receiving Water Bodies of South Africa: A Review of Sources, Pathways, Occurrence, Effects, and Geographical Distribution. Water, 16(6), 796. https://doi.org/10.3390/w16060796






